Abstract
The peritoneal cavity is a membrane-bound and fluid-filled abdominal cavity of mammals, which contains the liver, spleen, most of the gastro-intestinal tract and other viscera. It harbors a number of immune cells including macrophages, B cells and T cells. The presence of a high number of naïve macrophages in the peritoneal cavity makes it a preferred site for the collection of naïve tissue resident macrophages (1). The peritoneal cavity is also important to the study of B cells because of the presence of a unique peritoneal cavity-resident B cell subset known as B1 cells in addition to conventional B2 cells. B1 cells are subdivided into B1a and B1b cells, which can be distinguished by the surface expression of CD11b and CD5. B1 cells are an important source of natural IgM providing early protection from a variety of pathogens (2-4). These cells are autoreactive in nature (5), but how they are controlled to prevent autoimmunity is still not understood completely. On the contrary, CD5+ B1a cells possess some regulatory properties by virtue of their IL-10 producing capacity (6). Therefore, peritoneal cavity B1 cells are an interesting cell population to study because of their diverse function and many unaddressed questions associated with their development and regulation. The isolation of peritoneal cavity resident immune cells is tricky because of the lack of a defined structure inside the peritoneal cavity. Our protocol will describe a procedure for obtaining viable immune cells from the peritoneal cavity of mice, which then can be used for phenotypic analysis by flow cytometry and for different biochemical and immunological assays.
Keywords: JoVE Immunology, Issue 35, Immune cells, Peritoneal cavity, Macrophage, B cell, B1 cell, isolation procedure
Protocol
- Before starting the process, the following items need to be to collected and prepared:
- Ice
- 5ml syringe with 27g needle
- 5ml syringe with 25g needle
- Styrofoam block and pins for mounting the mouse
- Tray on which the mounting block can be placed
- Scissors and forceps
- Collection tubes
- 70% ethanol
- PBS with 3% fetal calf serum (FCS) (Pre chilled and kept on ice)
Euthanize the mouse, spray it with 70% ethanol and mount it on the styrofoam block on its back.
Using a scissors and forceps cut the outer skin of the peritoneum and gently pull it back to expose the inner skin lining the peritoneal cavity.
Inject 5 ml of ice cold PBS (with 3% FCS) into the peritoneal cavity using a 27g needle. Push the needle slowly in the peritoneum being careful not to puncture any organs.
After injection, gently massage the peritoneum to dislodge any attached cells into the PBS solution.
Insert a 25 g needle, bevel up, attached to a 5 ml syringe in the peritoneum and collect the fluid while moving the tip of the needle gently to avoid clogging by the fat tissue or other organs. Collect as much fluid as possible and deposit the collected cell suspension in tubes kept on ice after removing the needle from the syringe.Optional: Repeat step 4-6
Make an incision in the inner skin of the peritoneum and while holding up the skin with a forceps use a plastic Pasteur pipette to collect the remaining fluid from the cavity.
If in step 6 or 7 visible blood contamination is detected then the contaminated sample should be discarded.
Spin the collected cell suspension at 1500 RPM for 8 minutes, discard the supernatant and resuspend the cells in desired media or PBS for counting.
Alternative protocol to obtain thioglycollate elicited macrophages:
This method is used to obtain a higher yield of macrophages.
Inject 5 ml of 3% (w/v) Brewer thioglycollate medium (7) into the peritoneal cavity of each mouse.
Wait for 3-5 days, and proceed to step 1 above for the collection of the cells.
Compared to the nonelicited approach, approximately 10 times more macrophages can be collected. The only concern is that macrophages obtained by this procedure differ in some of their physiological properties.
Representative results:
From an unmanipulated mouse, 5-10 million peritoneal cavity cells can be obtained with a good isolation protocol. Among all live cells, 50-60% are B cells, ~30% macrophages and 5-10% are T cells (Fig 1).
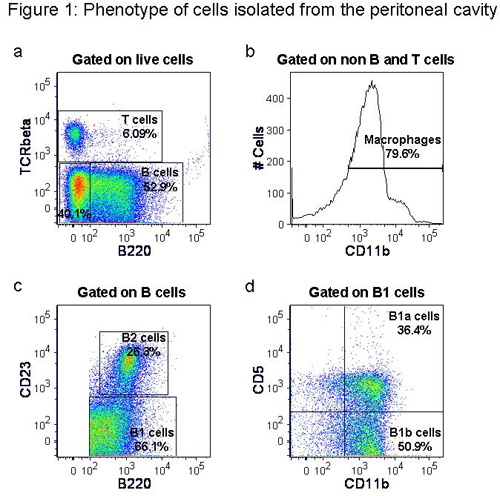 Figure 1. Phenotype of cells isolated from the peritoneal cavity. Following isolation of peritoneal cavity cells they were stained with anti-mouse TCRβ-FITC, B220-PE-Texas red, CD11b-Pacific blue, CD23-PE-Cy7 and CD5-APC. Representative flow cytometric plots show percentages of B and T cells (a), macrophages (b), B1 and B2 cells (c) and B1a and B1b cells (d).
Figure 1. Phenotype of cells isolated from the peritoneal cavity. Following isolation of peritoneal cavity cells they were stained with anti-mouse TCRβ-FITC, B220-PE-Texas red, CD11b-Pacific blue, CD23-PE-Cy7 and CD5-APC. Representative flow cytometric plots show percentages of B and T cells (a), macrophages (b), B1 and B2 cells (c) and B1a and B1b cells (d).
Discussion
Isolation of peritoneal cavity cells is an important technique for the study of different immune cells, primarily macrophages and specific B cell subsets. Although this is a simple process, there are some critical steps involved. Presence of contaminating blood in the collected sample should be avoided to obtain a pure peritoneal cavity cell population. Euthanasia by cervical dislocation should be done carefully to avoid blood contamination in the peritoneal cavity. Alternatively CO2 can be used. In addition, during the procedure proper care should be taken to avoid puncturing the bladder or any other organs in the peritoneal cavity. Recovering most of the injected fluid is also important to the cell yield.
This procedure is widely used to study macrophage biology since moderate numbers of resident, unstimulated macrophages can easily be obtained from the peritoneal cavity, in contrast to the laborious task of differentiating myeloid progenitor cells into mature macrophages in vitro, using macrophage colony stimulating factor (8,9). The macrophage yield from the peritoneal cavity can be improved by using the thioglycollate elicitation method, although thioglycollate use might alter physiological properties of the macrophages (7).
B cells are an important part of our immune system playing crucial roles in both innate and adaptive immunity. Among different B cell subpopulations, B1 cells comprise a unique subset of B cells involved in innate immunity, autoimmunity and immune regulation. B1 cells are primarily located in the peritoneal cavity and are self replenishing. They are one of the primary producers of natural IgM which provides the first line of protection against a number of virus and bacteria (10-12). B1 cells are also a major source of IL-10 (6), which is an important cytokine involved in immune regulation. Although several studies have been pursued with the B1 cells, still there is scope for further evaluation of their contrasting functions, specially their regulatory role. Peritoneal cavity cell isolation method provides the unique opportunity to study the functions of B1 cells.
Acknowledgments
This work was supported in part by NIH grant AI069358 and the BloodCenter Research Foundation.
References
- Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Current Protocols in Immunology. 2008. [DOI] [PMC free article] [PubMed]
- Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Daniel LS, Benjamin WH, Forman C, Briles DE. Blood clearance by anti-phosphocholine antibodies as a mechanism of protection in experimental pneumococcal bacteremia. J. Immunol. 1984;133:3308–3312. [PubMed] [Google Scholar]
- Paciorkowski N, Porte P, Shultz LD, Rajan TV. B1 B lymphocytes play a critical role in host protection against lymphatic filarial parasites. J. Exp. Med. 2000;191:731–736. doi: 10.1084/jem.191.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercolino TJ, Arnold LW, Hawkins LA, Haughton G. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline: relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J. Exp. Med. 1988;168:687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell derived interleukin 10. Eur. J. Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- Hoover DL, Nacy CA. Macrophage activation to kill Leishmania tropica: Defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J. Immunol. 1984;132:1487–1491. [PubMed] [Google Scholar]
- Austin PE, McCulloch EA, Till JE. Characterization of the factor in L-cell conditioned medium capable of stimulating colony formation by mouse marrow cells in culture. J. Cell Physiol. 1971;77:121–134. doi: 10.1002/jcp.1040770202. [DOI] [PubMed] [Google Scholar]
- Stanley ER. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Baumgarth N, Chen J, Herman OC, Jager GC, Herzenberg LA. The role of B-1 and B-2 cells in immune protection from influenza virus infection. Curr. Top. Microbiol. Immunol. 2000;252:163–169. doi: 10.1007/978-3-642-57284-5_17. [DOI] [PubMed] [Google Scholar]
- Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]


