Abstract
The Wistar Kyoto (WKY) rat is uniquely susceptible to experimentally induced crescentic glomerulonephritis. Two major quantitative trait loci (QTLs) on chromosomes 13 (Crgn1) and 16 (Crgn2) with logarithm of odds >8, as well as five other loci (Crgn3 through 7), largely explain this genetic susceptibility. To understand further the effects of Crgn1 and Crgn2, we generated a double-congenic strain by introgressing these loci from glomerulonephritis-resistant Lewis rats onto the WKY genetic background. Induction of nephrotoxic nephritis in the double-congenic rats (WKY.LCrgn1,2) produced markedly fewer glomerular crescents, reduced macrophage infiltration, and decreased expression of glomerular TNF-α and inducible nitric oxide synthase expression compared with control animals. Bone marrow and kidney transplantation studies between parental and WKY.LCrgn1,2 strains, together with in vitro experiments, demonstrated that Crgn1 and Crgn2 contribute exclusively to circulating cell-related glomerular injury by regulating macrophage infiltration and activation. The residual genetic susceptibility to crescentic glomerulonephritis in WKY.LCrgn1,2 rats associated with macrophage activity (especially with enhanced metalloelastase expression) rather than macrophage infiltration. Taken together, these results demonstrate that a genetic influence on macrophage activation, rather than number, determines glomerular damage in immune-mediated glomerulonephritis.
Glomerulonephritis is a major cause of renal failure. Its most severe form is crescentic glomerulonephritis (Crgn), in which damage to glomerular capillaries leads to accumulation of inflammatory cells and proliferating epithelial cells in Bowman's space. When untreated, Crgn rapidly progresses to irreversible renal scarring and end-stage renal failure.1 In rodents, there are marked strain differences in susceptibility to Crgn.2 To elucidate the genes that control susceptibility we have studied the model of nephrotoxic nephritis (NTN) in the WKY rat. NTN is induced by an intravenous injection of rabbit anti-rat glomerular basement membrane (GBM) antiserum. In the WKY rat strain, this leads to cellular crescents in almost all glomeruli by day 10, whereas Lewis (LEW) rats that share the same MHC haplotype (RT1-l) develop only mild glomerular hypercellularity with no crescents. The model is very reproducible, and the histology closely resembles that seen in human Crgn.3
In bone marrow (BM) and kidney transplant experiments, we previously showed that susceptibility to NTN in the WKY rat depends on both circulating and intrinsic renal factors.4 Indeed, macrophages from WKY rats show a number of differences compared with those from LEW rats, including enhanced Fc receptor–mediated functions such as antibody-dependent cytotoxicity.5,6 In addition, WKY glomerular mesangial cells show increased monocyte chemoattractant protein 1 (MCP-1) synthesis when compared with LEW ones.4 To determine the genes that are responsible for susceptibility to Crgn and for these cellular phenotypes, we carried out a genome-wide linkage analysis of an F2 population derived from WKY and LEW rats and identified seven Crgn quantitative trait loci (QTLs; Crgn1 through 7).5 Two major QTLs, each with highly significant logarithm of odds (LOD) scores >8, mapped on chromosomes 13 (Crgn1) and 16 (Crgn2).5 We have identified genes at each of these QTLs that control macrophage function in vitro. We showed that WKY rats lack an Fc receptor gene, Fcgr3-related sequence on chromosome 13, and that this is, in part, the cause of enhanced macrophage Fc receptor–mediated activity. At the chromosome 16 QTL, we identified the AP-1 transcription factor JunD and showed that there was increased synthesis of JunD in WKY compared with LEW macrophages, and this was responsible for enhanced oxygen burst activity and inducible nitric oxide synthase (iNOS) synthesis in WKY BM-derived macrophages (BMDMs).
Congenic strains have been widely used in the fine mapping of rodent QTLs in various studies using rat models, and they still constitute a powerful tool in QTL positional cloning.7,8 To examine further the effects of these genetic loci on chromosomes 13 and 16, we bred congenic rats. We have already reported the phenotypic effect of Crgn2 on NTN-related phenotypes and demonstrated that congenic rats in which LEW Crgn2 was introgressed into the WKY genetic background (WKY.LCrgn2) showed reduced glomerular crescents, fibrinoid necrosis, and macrophage infiltration.6 We have now investigated the interaction between the two major Crgn loci (Crgn1 and Crgn2) by generating a double-congenic rat strain in which both Crgn1 and Crgn2 from NTN-resistant LEW rats were introgressed into the genetic background of the WKY rat (WKY. LCrgn1,2). Our results show that Crgn1 and Crgn2 have an additive protective effect against glomerular crescent formation. We then used BM and kidney transplants to show that the major effects of these loci are on regulation of macrophage activation/infiltration rather than intrinsic renal cell function. We also show that the residual susceptibility to Crgn in WKY.LCrgn1,2 is associated with differences in macrophage function compared with LEW and, in particular, that there is enhanced macrophage metalloelastase (matrix metalloproteinase 12 [MMP-12]) expression.
Results
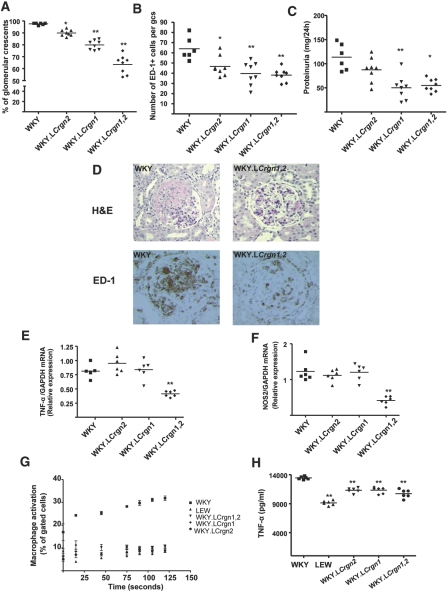
We previously generated a single-congenic strain for the chromosome 16 congenic interval (WKY.LCrgn2) and assessed Crgn susceptibility in this strain compared with WKY rats.6 Here, we analyzed the single effect sizes of Crgn1 and Crgn2 individually and investigated the combined effect of these two loci on Crgn susceptibility. A double-congenic strain for Crgn1 and Crgn2 was generated by introgressing the corresponding chromosome 13 and chromosome 16 segments from the donor LEW strain into the genetic background of the WKY strain (Figure 1). NTN was induced in WKY, WKY.LCrgn2, WKY. LCrgn1, and the double-congenic rats (WKY.LCrgn1,2; Figure 2). Introgression of LEW Crgn2 into the WKY recipients reduced glomerular crescents by 8%, and the single phenotypic effect of LEW Crgn1 corresponded to a reduction of 18%. Introgression of both LEW Crgn1 and Crgn2 into the WKY genetic background reduced glomerular crescents by 34%, demonstrating an additive effect of both Crgn loci on glomerular crescent formation (Figure 2, A and D). Macrophage infiltration, assessed by the measurement of ED-1–positive cells per glomerular cross-section, showed a significant reduction for WKY. LCrgn1,2 rats when compared with parental WKY strain (Figure 2, B and D). The interstitial macrophage numbers were not different between the WKY and all congenic strains (Supplemental Figure 1). Proteinuria was also measured in WKY and single- and double-congenic rats, and we confirmed our previous finding that introgression of LEW Crgn2 on a WKY genetic background did not significantly reduce proteinuria levels; however, introgression of Crgn1 led to reduced proteinuria levels in both WKY.LCrgn1 and WKY.LCrgn1,2 rats (Figure 2C). Because infiltration of macrophages into the glomeruli is the main source of iNOS9,10 and TNF-α,11,12 we then investigated iNOS and TNF-α expression in the glomeruli extracted 10 days after NTN induction, as a measure of macrophage activation within the inflamed glomeruli. The results showed that WKY.LCrgn1,2 animals had significantly reduced glomerular TNF-α (Figure 2E) and iNOS expression (Figure 2F) compared with parental WKY rats and single-congenic rats 10 days after NTN induction, confirming the combined protective effect of Crgn1 and Crgn2 on glomerular inflammation. To examine the effect of these loci on macrophage function, we studied macrophage activation in primary parental and congenic macrophages by Fc receptor–mediated phagocytosis and oxidation (Fc oxyBURST assay; Figure 2G). These results showed that when LEW Crgn1 and/or Crgn2 is introgressed to the WKY genetic background, the Fc receptor–mediated macrophage activation is similar to that observed in LEW BMDMs (Figure 2G). Furthermore, BMDMs from single- and double-congenic animals secrete reduced levels of TNF-α after LPS stimulation when compared with WKY rats (Figure 2H). We additionally assessed macrophage activation by measuring mRNA levels of IL-6 and iNOS after LPS stimulation. Quantitative real-time PCR (qRT-PCR) results confirmed increased BMDM iNOS expression in the WKY macrophages when compared with LEW strain6 and showed that both Crgn1 and Crgn2 control macrophage activation, because WKY. LCrgn1, WKY.LCrgn2, and WKY.LCrgn1,2 animals had similar BMDM iNOS (Supplemental Figure 2a) and IL-6 (Supplemental Figure 2b) expression as the NTN-resistant LEW rats.
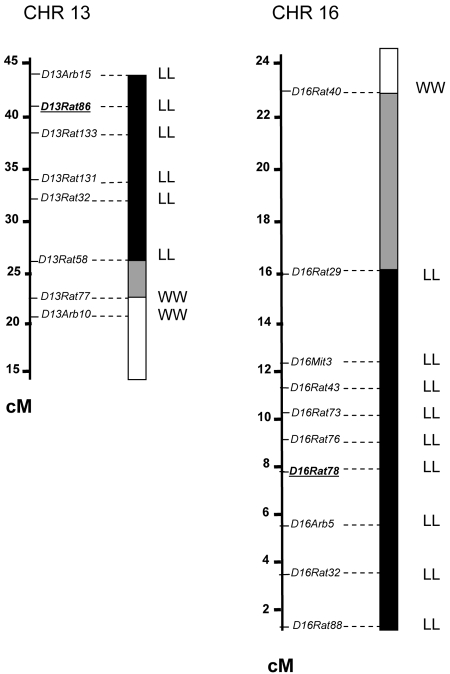
Figure 1.
Genetic map showing the transferred chromosomal segments in congenic lines. Map distances are based on the SHRSP × BN genetic map (http://rgd.mcw.edu/) and are in cM. ■, Chromosomal region transferred from the donor LEW strain (designated as LL); □, chromosomal region transferred from the recipient WKY strain (designated as WW); ▩, recombination zone. Microsatellite markers D13Rat86 and D16Rat78 are underlined and represent the peak of linkage for Crgn1 and Crgn2, respectively.
Figure 2.
NTN-related phenotypes and macrophage activation in single- and double-congenic lines (WKY, n = 6 rats; all congenics, n = 8 rats per strain). (A) Percentage of glomerular crescents in single-congenic (WKY.LCrgn1, WKY.LCrgn2) and double-congenic (WKY.LCrgn1,2) rats in comparison with parental WKY rats. (B) Macrophage infiltration assessed by quantitative measurement of percentage of ED-1+ area per glomerular cross-section (gcs). (C) Proteinuria levels measured in WKY and single- and double-congenic lines. (D) Histology showing a crescentic glomerulus in a WKY rat and a mildly hypercellular glomerulus in a WKY.LCrgn1,2 rat (hematoxylin and eosin). ED-1 immunohistochemistry demonstrates extensive glomerular monocyte and macrophage infiltration in WKY, whereas WKY.LCrgn1,2 rats display reduced staining. (E and F) Glomerular TNF-α (E) and iNOS (F) expression as assessed by qRT-PCR 10 days after injection of NTS (n = 6 rats per strain). (G) Macrophage activation was assessed in WKY, LEW, and congenic BMDMs by Fc receptor–mediated phagocytosis and oxidization (n = 5 rats per strain, without NTN induction). BMDMs are stimulated with Fc oxyBURST, and the WKY rat shows significantly more activation than all of the other strains at all time points (P < 0.001; error bars, SEM). (H) Sandwich ELISA for secretion of TNF-α in LPS-stimulated (100 ng/ml) parental and single- and double-congenic BMDMs (n = 6 rats per strain). **P < 0.01, *P < 0.05 versus WKY. Magnification, ×200 in D.
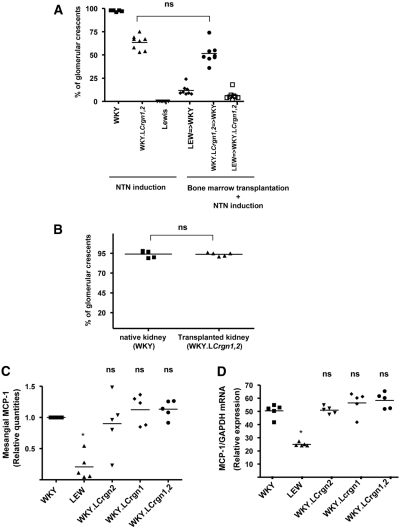
To assess the relative effects of these loci on circulating cells and on intrinsic renal cells, we carried out BM and kidney transplant experiments. We previously showed that in WKY rats that were given isologous WKY BM, glomerular crescent formation in NTN was similar to that in unmanipulated WKY rats and that no crescents were seen in LEW rats given isologous BM.4 When BM was transferred from WKY. LCrgn1,2 to WKY rats (WKY.LCrgn1,2 → WKY), followed by induction of NTN, the rats showed similar glomerular crescent formation to WKY.LCrgn1,2 animals that did not receive a transplant (P = 0.28), suggesting that Crgn1 and Crgn2 exert their effect primarily through effects on BM-derived cells and not intrinsic renal cells (Figure 3A). In keeping with this, NTN induction in WKY.LCrgn1,2 kidneys transplanted to WKY rats led to as many crescents in the transplanted kidney as in the native WKY kidney (Figure 3B), demonstrating that there is no effect of these loci on intrinsic renal susceptibility to crescent formation. We also tested the effect of Crgn1 and Crgn2 on mesangial cell MCP-1 production by ELISA (Figure 3C) and qRT-PCR (Figure 3D). Although we confirmed the previously shown MCP-1 production differences between inbred WKY and LEW rats, these results demonstrate that neither Crgn1 nor Crgn2 contributes to mesangial cell MCP-1 production (Figure 3, C and D), suggesting that these loci do not affect intrinsic renal cell function.
Figure 3.
Crgn1 and Crgn2 control the circulating cell-related glomerular inflammation in the WKY rat. (A) Percentage of glomerular crescents in parental and WKY.LCrgn1,2 rats 10 days after the injection of NTS with or without BM transplantation. When BM is transferred from WKY.LCrgn1,2 to WKY rats (WKY.LCrgn1,2 → WKY, n = 8 rats), the reduction in crescent formation is not significantly different from WKY.LCrgn1,2 animals that do not receive a transplant (P = 0.28, n = 8 rats) 10 days after NTN induction. All rats show significant reduction (P < 0.01) in percentage of glomerular crescents compared with WKY, and WKY.LCrgn1,2 → WKY rats show significantly increased percentage of glomerular crescents (P < 0.01) when compared with LEW → WKY rats (n = 8 rats are used in all groups). (B) Kidneys from WKY. LCrgn1,2 rats transplanted into the WKY recipients (n = 5 rats) do not show any reduction in the glomerular crescent formation. (C and D) Mesangial cell MCP-1 production assessed in cell supernatants by sandwich ELISA (C) and cell layers by qRT-PCR (D) in parental (WKY, LEW), single-congenic (WKY.LCrgn1, WKY.LCrgn2), and double-congenic lines (WKY.LCrgn1,2) 24 hours after TNF-α (2 ng/ml) stimulation. Mesangial MCP-1 quantities are the result of five independent experiments and expressed as relative quantities compared with WKY rats. *P < 0.05, **P < 0.01 versus WKY; ns, nonsignificant compared with WKY.
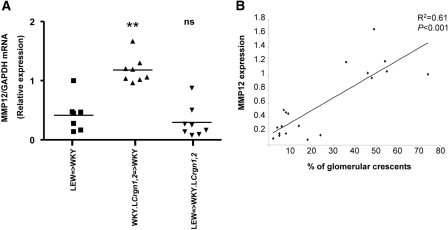
The BM transplant experiments also shed light on the role of Crgn genes outside of Crgn1 and Crgn2. We compared crescent formation in WKY rats that received a transplant of BM from LEW rats or from double-congenic WKY.LCrgn1,2 rats. We found that, although WKY.LCrgn1,2 → WKY rats developed a significantly increased number of glomerular crescents when compared with LEW → WKY rats (P < 0.01; Figure 3A), glomerular macrophage numbers were not different (P = 0.32) in the two groups (Figure 4 A). This suggests that there is a difference in the phenotype of the macrophages that leads to more glomerular injury even though the numbers of macrophages are similar. MMP-12 is predominantly expressed in mature tissue macrophages13 and is a major factor for glomerular injury in anti-GBM nephritis.14,15 On the basis of these findings, we hypothesized that MMP-12 expression differences in parental and congenic primary macrophages could partly explain the observed macrophage infiltration–independent glomerular damage. MMP-12 expression was significantly increased in WKY and WKY.LCrgn1,2 BMDMs when compared with LEW (Figure 4B). We then investigated whether MMP-12 expression correlated with crescent formation. We measured MMP-12 expression in glomeruli from three groups of BM-transplanted rats after NTN induction: LEW → WKY, WKY. LCrgn1,2 → WKY, and LEW → WKY.LCrgn1,2 (Figure 5A). The MMP-12 expression profile was found to mirror the severity of glomerular crescent formation in the three groups (Figure 3A), and this was confirmed by a strong positive correlation (R = 0.61, P < 0.001) between MMP-12 expression and percentage of glomerular crescents (Figure 5B). We also studied MMP-9 because this was previously reported for its capacity to degrade constituents of GBM such as type IV collagen16 and to play a protective role in anti-GBM nephritis model in the mouse.17 Although WKY and WKY.LCrgn1,2 BMDMs express relatively increased MMP-9 when compared with LEW, we found that glomerular MMP-9 expression did not correlate with the glomerular crescent formation in the transplant groups (Supplemental Figure 3).
Figure 4.
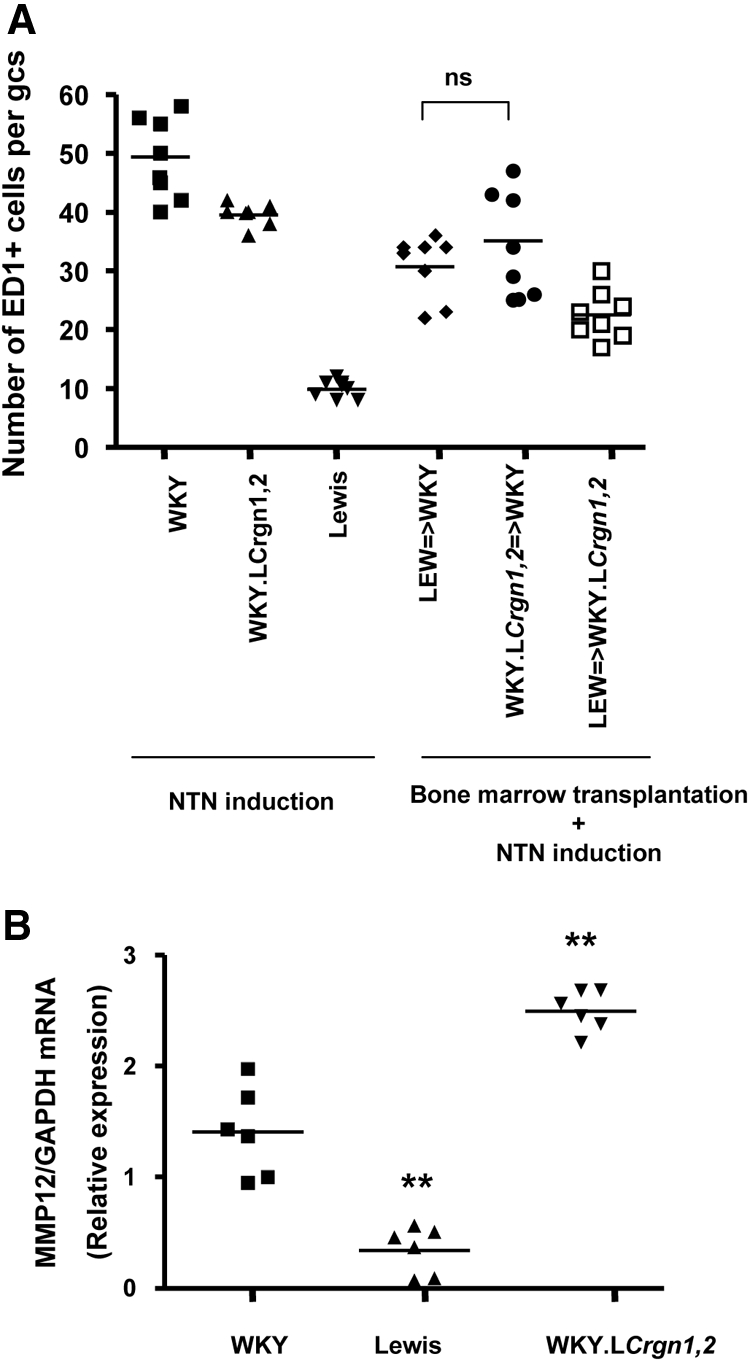
Macrophage activation (and not infiltration) controls genetic susceptibility to Crgn encoded by loci outside of Crgn1 and Crgn2. (A) Number of ED-1+ cells per glomerular cross-section (gcs) in parental and WKY.LCrgn1,2 rats 10 days after the injection of NTS with or without BM transplantation. Although WKY.LCrgn1,2 → WKY and LEW → WKY rats develop 52 and 12% glomerular crescents, respectively (P < 0.01; Figure 3), ED-1+ cells per gcs are similar (P = 0.32) in the two groups. The ED-1+ cells per gcs are significantly reduced (P < 0.05) in all groups when compared with WKY rats (n = 8 rats in all groups). (B) WKY and WKY.LCrgn1,2 BMDMs express significantly higher levels of MMP-12 when compared with LEW BMDMs (n = 6 rats per strain), indicating the genetically determined macrophage activation status in primary macrophages. WKY.LCrgn1,2 BMDMs also show increased MMP-12 expression when compared with WKY ones. **P < 0.01 versus WKY.
Figure 5.
Glomerular MMP-12 expression correlates with the severity of crescent formation following BM transplantation. (A) MMP-12 expression is assessed by qRT-PCR in cultured glomeruli 10 days after the injection of NTS and after BM transplantation (n = 8 rats in each group). (B) Linear regression analysis shows positive correlation (R2 = 0.61, P < 0.01) with the percentage of glomerular crescents shown in Figure 3A. **P < 0.01 versus LEW → WKY; ns, nonsignificant compared with LEW → WKY. LEW=>WKY, LEW BM transplanted to WKY; WKY.LCrgn1,2=> WKY, double congenic BM transplanted to WKY; LEW=>WKY.LCrgn1,2, LEW BM transplanted to double congenic.
It was previously reported that CD8+ cells infiltrate the glomerulus at an early stage in the course of NTN, and our previous studies showed that they represent a subset of ED-1+ macrophages.3 We therefore asked whether there was a difference in the infiltration of CD8+ cells in WKY.LCrgn1,2 → WKY kidneys compared with LEW → WKY. We found similar numbers of CD8+ cells in the WKY.LCrgn1,2 → WKY and LEW → WKY glomeruli after NTN induction (Supplemental Figure 4), and CD8 infiltration was markedly reduced compared with ED-1+ cells 10 days after the nephrotoxic serum (NTS) injection.
Discussion
Our previous work identified seven QTLs that control susceptibility to Crgn in the WKY rat.5 The QTLs with the highest LOD scores were on chromosomes 13 and 16, and we have designated these Crgn1 and Crgn2. We have now generated congenic rats in which we introgressed these loci from the resistant LEW strain into the WKY genetic background and also generated a double-congenic strain, WKY.LCrgn1,2. This has allowed us to assess the magnitude of the effect of these QTLs on Crgn susceptibility, to examine the effect on cellular phenotypes, and to elucidate the possible role of the other QTLs (Crgn3 through 7). Our previous studies showed a modest protective effect of Crgn2 on NTN-related phenotypes, confirming the previously established linkage of Crgn2 on chromosome 16.5,6 This work has provided direct evidence of additive protective effects of Crgn1 and Crgn2 on glomerular crescent formation. In the double-congenic rats, we found a reduction of 34% in crescent formation compared with WKY rats.
We previously showed that susceptibility to Crgn in the WKY rat was associated with differences in the phenotypes of macrophages and of glomerular mesangial cells.4–6 Our results in the congenic rats suggest that Crgn1 and Crgn2 exert their effects predominantly on BM-derived cells and specifically on macrophages. Thus, when WKY rats were given BM transplants from double-congenic rats, they developed similar numbers of crescents in NTN to double-congenic animals, whereas kidneys from double-congenic animals transplanted into WKY rats developed the same number of crescents in NTN as the native kidneys.
There is a clear effect of Crgn1 and Crgn2 on macrophage phenotype, because both loci regulate glomerular macrophage infiltration and control IL-6 and iNOS expression in BM-derived macrophages. In contrast, neither Crgn1 nor Crgn2 controls the enhanced mesangial cell MCP-1 synthesis seen in WKY rats. These findings are entirely consistent with our positional cloning studies focusing on Crgn1 and Crgn2. As we previously showed, Crgn1 includes the gene coding for the α subunit of the activatory Fcγ receptor, Fcgr3. We showed that most laboratory rat strains express two forms of Fcgr3 but that in the WKY rat, a newly identified paralogue, Fcgr3-related sequence (Fcgr3-rs), was absent.5 We then established an inhibitory role for Fcgr3-rs in macrophage activation, because COS7 cells co-transfected with Fcgr3 and Fcgr3-rs showed 70% inhibition of Fcgr3-mediated phagocytosis. This suggests that the deletion of Fcgr3-rs from the WKY genome explains partly the unchecked activation in the macrophages of this strain.5 The locus on chromosome 16 (Crgn2) contains the AP-1 transcription factor Jund, which is highly overexpressed in the macrophages (but not in mesangial cells) of the NTN-susceptible WKY rat.6 We also established an important role for Jund in macrophage activation, because knockdown of Jund expression levels by small interfering RNA in WKY BM-derived macrophages reduces Fc receptor–mediated macrophage activation.6 Together these data indicate that Fcgr3 in Crgn1 and Jund in Crgn2 are susceptibility genes for Crgn and act by regulating macrophage activation.
Although we have shown that Crgn1 and Crgn2 control susceptibility to Crgn through effects on macrophage infiltration and activation, the double-congenic (WKY.LCrgn1,2) strain still showed significant glomerular crescent formation, indicating effects of other loci. In our linkage analysis, we identified five other QTLs, Crgn3 through 7. This study provides insights into the role of these QTLs. In our BM transplant experiments, we found that WKY rats given LEW BM developed 12% glomerular crescents in NTN, whereas those that received a transplant of WKY.LCrgn1,2 BM developed 52% crescents; however, the numbers of infiltrating glomerular macrophages were very similar. This suggests that the macrophages from the double-congenic animals have a more proinflammatory phenotype. We investigated this by focusing on macrophage activation molecules previously reported to be involved in GBM degradation. MMPs, particularly MMP-12, was previously described to cause glomerular injury in the WKY anti-GBM nephritis model.14 Unlike MMP-12, MMP-9 showed a protective effect in the accelerated model of crescentic nephritis in the mouse as MMP-9 knockout mice showed exacerbated nephritis with increased crescent formation and fibrin deposits compared with wild-type controls.17 We showed that MMP-9 and MMP-12 expressions were significantly increased in WKY.LCrgn1,2 and WKY BMDMs when compared with LEW, demonstrating that they are under the control of genes outside of Crgn1 and Crgn2; however, only MMP-12 expression correlated with the percentage of crescents in WKY glomeruli after transplantation with either LEW or WKY.LCrgn1,2 BM and after NTN induction. Because MMP-12 is mainly produced by macrophages infiltrating the glomerulus in anti-GBM nephritis,14 our results suggest that macrophage activation status, partly explained by increased MMP-12, contributes to the susceptibility to glomerular injury encoded by Crgn3 through 7. We also investigated whether infiltration of CD8+ cells was the cause of relatively more glomerular crescents in WKY.LCrgn1,2 → WKY kidneys compared with LEW → WKY but found that (1) their infiltration was markedly reduced compared with ED-1+ cells 10 days after the NTS injection and (2) there was no difference in CD8+ cell number between the WKY. LCrgn1,2 → WKY and LEW → WKY glomeruli after NTN induction. Given that our previous studies have shown that CD8+ cells represent a subset of ED-1+ macrophages,3 these results support the finding that macrophage phenotype is the more likely pathologic source of glomerular injury.
Our studies of the WKY rat clearly demonstrate the importance of macrophage phenotype in susceptibility to glomerulonephritis. Previous work has also provided evidence on the heterogeneity of the macrophage activation, suggesting that macrophage activation status is as important as the number of macrophages in the outcome of inflammatory diseases.18–21 Here, we have shown that there are multiple genetically determined differences between macrophages from WKY and LEW rats and that they are controlled at different genetic loci. In summary, WKY macrophages show enhanced antibody-dependent cytotoxicity and Fc receptor–mediated phagocytosis controlled by Crgn1, enhanced cytokine and iNOS expression, and respiratory burst controlled by Crgn2, and, as we demonstrate here, enhanced protease synthesis controlled by loci outside Crgn1 or Crgn2. We have now shown that the loci controlling these various macrophage phenotypes have independent and additive effects on Crgn susceptibility. In future work, we aim to identify the genes outside of Crgn1 and Crgn2 that control the residual Crgn susceptibility seen in the double-congenic rats and to determine how they control macrophage accumulation and activation. We also need to identify which genes are responsible for the differences in mesangial cell phenotype between the two strains and how this contributes to susceptibility to Crgn in vivo.
In conclusion, our work emphasizes the importance of macrophage activation in the pathophysiology of Crgn. Understanding the mechanisms of macrophage infiltration and activation within the inflamed glomeruli may ultimately facilitate the design of more rational and targeted treatment of human Crgn.
Concise Methods
Congenic and Control Rat Strains
WKY (WKY/NCrl) rats were purchased from Charles River. Single-congenic rats were generated as described previously.6 We constructed a double-congenic line (i.e., a single strain in which both Crgn1 and Crgn2 were on the WKY genetic background) as follows: WKY.LCrgn1 and WKY.LCrgn2 strains were crossed to produce an F1 generation. The F1 rats were backcrossed with WKY.LCrgn1. The F2 rats heterozygous for Crgn2 and homozygous for Crgn1 were crossed by brother–sister mating to obtain an F3 generation double congenic for LEW Crgn1 and LEW Crgn2 on a WKY background. All procedures were performed in accordance with the United Kingdom Animals (Scientific Procedures) Act.
Nephrotoxic Nephritis
NTS was prepared in rabbits by standard methods. NTN was induced in male rats by intravenous injection of 0.1 ml of NTS. Nine days later, urine was collected by placing rats into metabolic cages for 24 hours with free access to food and water. Proteinuria was determined by the sulfosalicylic acid method.22 On day 10 after induction of NTN, rats were killed under isoflurane anesthesia. Samples of kidney were fixed in 10% formal saline, processed, and embedded in paraffin wax. In some cases, glomeruli were isolated by sieving as described previously,23 and 2000 glomeruli were plated in six-well plates (Nunc, Roskilde, Denmark) in DMEM (Life Technologies, Paisley, UK). After 48 hours of incubation, glomeruli were collected for qRT-PCR.
RNA Extraction and qRT-PCR
Total RNA was extracted from isolated glomeruli, mesangial cells, or macrophages using the Trizol method. Total RNA concentration was determined by using Nanodrop spectrophotometer (Labtech Int., Ringmer, UK). TNF-α, iNOS, MCP-1, and IL-6 primers were as follows: TNF-α forward 5′-TGACCCCCATTACTCTGACC-3′ and reverse 5′-GGCCACTACTTCAGCGTCTC-3′, iNOS forward 5′-GGACCACCTCTATCAGGAA-3′ and reverse 5′-GGAGCACGCTGAACACCT-3′; MCP-1 forward 5′-ATGCAGTTAATGCCCCACTC-3′ and reverse 5′-TTCCTTATTGGGGTCAGCAC-3′; IL-6 forward 5′-CCGGAGAGGAGACTTCACAG-3′ and reverse 5′-ACAGTGCATCATCGCTGTTC-3′; and MMP-12 forward 5′-TGCAGCTGTCTTTGATCCAC-3′ and reverse 5′-GCATCAATTTTTGGCCTGAT-3′.
Real-time RT-PCR was performed on a ABI 7500 Sequence Detection System (Applied Biosystems, Warrington, UK) using SYBR Green (Stratagene, Cambridge, UK). A total of 100 ng of total RNA was used for qRT-PCR, and all of the samples were amplified in triplicate. After the initial reverse transcription (30 minutes at 50°C and 10 minutes at 95°C), the samples were cycled 40 times at 95°C for 15 seconds and 60°C for 1 minute. Results were then exported to 7500 Fast system SDS software (ABS), and Ct values were determined for all of the genes analyzed. The relative expression levels normalized to glyceraldehyde-3-phosphate dehydrogenase gene expression were then determined by using the 2−ΔΔCt method.
Histology and Immunohistochemistry
Crescent formation was assessed by counting the number of crescents in 100 consecutive glomeruli in periodic acid-Schiff–stained sections. Macrophages were identified by immunoperoxidase staining with mAb ED-1 (Serotec, Oxford, UK). Quantification of ED-1–stained macrophages in the renal interstitium was performed by photographing five randomly selected cortical interstitial areas using an Olympus BX40 microscope (Olympus Optical, London, UK) mounted with a Photonic Science Color Coolview digital camera (Photonic Science, East Sussex, UK). The percentage of each of the stained cross-sectional areas was calculated using Image Pro-Plus software (Media Cybernetics, Silver Spring, MD) and was expressed as the mean percentage area stained.
BMDM Culture and Fc oxyBURST Assay
Femurs from adult WKY and LEW rats were isolated and flushed with Hanks buffer (Life Technologies). Total BM-derived cells were plated and cultured for 7 days in DMEM (Life Technologies) that contained 25 mM HEPES (Sigma), 25% L929 conditioned medium, 25% decomplemented FBS (Biosera), penicillin (100 U/ml; Invitrogen), streptomycin (100 μg/ml; Invitrogen), and l-glutamine (2 mM; Invitrogen). These cells were characterized as macrophages by ED-1 staining. BMDMs were made quiescent in serum-free medium for 24 hours and then stimulated with LPS (100 ng/ml). Control macrophages were unstimulated, and iNOS and IL-6 mRNA levels were measured by qRT-PCR. We carried out sandwich ELISA for rat TNF-α (BD Biosciences), in accordance with the manufacturer's specifications, with supernatants from BMDMs plated in six-well plates at a density of 106 cells per well and incubated in 2 ml of culture medium for 24 hours with LPS (100 ng/ml). For Fc oxyBURST assay, 106 cells (in triplicate) were suspended in Krebs' Ringer PBS with 1.0 mM Ca2+, 1.5 mM Mg2+, and 5.5 mM glucose; warmed to 37°C; and stimulated with Fc oxyBURST reagent (240 μg/ml; Invitrogen). Individual data points consisting of 10,000 fluorescence events were collected at 0, 15, 45, 75, 90, 105, and 120 seconds in a FACSCalibur after a baseline fluorescence reading was taken to determine the intrinsic fluorescence of unstimulated cells. Percentage of fluorescence BMDMs corresponds to percentage of activated gated cells after Fc receptor–mediated phagocytosis.
Mesangial Cell Culture
Glomeruli from LEW and WKY rats were isolated by sieving. Purified glomeruli were digested with collagenase type 1 (750 U/ml; Sigma) for 20 minutes. Partially digested glomeruli were cultured in 25-cm2 tissue culture flasks at 600 glomeruli/ml in RPMI 1640 medium (Invitrogen) that contained 20% decomplemented FBS (F-539), penicillin (100 U/ml; Invitrogen), streptomycin (100 μg/ml; Invitrogen), and l-glutamine (2 mM; Invitrogen) and was supplemented with insulin-transferrin-selenite (Sigma). The cultures were maintained at 37°C with 5% CO2 for 6 days, allowing glomerular mesangial cells to grow out. Medium was changed every 2 to 3 days thereafter. By days 21 to 28, when the cell outgrowth reached confluence, cells were subcultured. These cells were characterized by immunofluorescence staining using cells that were cultured on coverslips. They were positive for Thy-1.1 antigen, myosin, and desmin and negative for pancytokeratin, OX-1, ED-1, and OX-23.
To make the culture conditions comparable, passage 8 mesangial cells from different strains (n = 4 rats per strain) of rats were plated into six-well culture plates (106 cells/well) at the same time. Confluent mesangial cells were stimulated with TNF-α (2 ng/ml; R&D) in serum-free medium. After stimulation for 24 hours, mesangial cell supernatants were harvested and centrifuged to remove cellular contaminants. These supernatants were either examined immediately with sandwich ELISA or stored at −20°C. MCP-1 was measured in the supernatant by sandwich ELISA according to manufacturer's instructions (BD Biosciences).
BM and Kidney Transplantation
Femurs were removed from donor rats (n = 8 rats). BM was flushed out using RPMI with 10% FBS (Sigma-Aldrich, Poole, UK), 100 U/ml penicillin, and 100 g/ml streptomycin (Invitrogen, Paisley, UK). Cells were then washed, resuspended in fresh medium at 5 × 107 cells/ml, and kept on ice. Recipient rats were irradiated with 12 Gy at 0.74 Gy/min, using gamma rays from a cesium source irradiator (IBL 637; CIS Bio Int., Saclay, France). They were administered an intravenous injection of 0.2 ml of BM cell preparation within 2 hours of irradiation. Rats were left for 12 weeks to recover and to reconstitute their BM. Assessment of BM chimerism was performed as described previously,4 and the average percentage of donor DNA was >97% in all three groups of recipients. Orthotopic transplantation of the left kidney was performed as described previously4 with removal of the recipient's own left kidney at the time. After transplantation, rats were allowed to recover for 6 to 8 days before induction of NTN.
Statistical Analysis
Statistical differences in mean values between all of the congenic strains and parental WKY rats were compared using one-way ANOVA followed by Bonferroni multiple comparison posttest; P < 0.05 was considered statistically significant. Differences in relative MCP-1 quantities were tested for significance with the nonparametric Wilcoxon signed-rank test. Comparisons between native and transplanted kidney groups were analyzed by Mann-Whitney U test. Correlation between MMP-12 expression and glomerular crescents was analyzed by linear regression.
Disclosures
None.
Acknowledgments
We thank Raphaële Castagné for excellent technical assistance. We also thank Ruth Tarzi and Richard Hull for editing the manuscript. J.B. gratefully acknowledges his Imperial College Junior Research Fellowship for funding to perform this research. We acknowledge intramural funding from the Clinical Sciences Centre and support from the FP6 EURATools (European Union contract LSHG-CT-2005-019015), the UK Medical Research Council, and the Wellcome Trust.
Footnotes
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Jennette JC, Heptinstall RH, Ovid Technologies Inc. : Heptinstall's Pathology of the Kidney, 6th Ed., Philadelphia, Lippincott Williams & Wilkins, 2007 [Google Scholar]
- 2. Tipping PG: Crescentic nephritis: Is it in your genes? Nephrol Dial Transplant 23: 3065–3066, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Tam FW, Smith J, Morel D, Karkar AM, Thompson EM, Cook HT, Pusey CD: Development of scarring and renal failure in a rat model of crescentic glomerulonephritis. Nephrol Dial Transplant 14: 1658–1666, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Smith J, Lai PC, Behmoaras J, Roufosse C, Bhangal G, McDaid JP, Aitman T, Tam FW, Pusey CD, Cook HT: Genes expressed by both mesangial cells and bone marrow-derived cells underlie genetic susceptibility to crescentic glomerulonephritis in the rat. J Am Soc Nephrol 18: 1816–1823, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey CD, Cook HT: Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature 439: 851–855, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Behmoaras J, Bhangal G, Smith J, McDonald K, Mutch B, Lai PC, Domin J, Game L, Salama A, Foxwell BM, Pusey CD, Cook HT, Aitman TJ: Jund is a determinant of macrophage activation and is associated with glomerulonephritis susceptibility. Nat Genet 40: 553–559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aitman TJ, Critser JK, Cuppen E, Dominiczak A, Fernandez-Suarez XM, Flint J, Gauguier D, Geurts AM, Gould M, Harris PC, Holmdahl R, Hubner N, Izsvak Z, Jacob HJ, Kuramoto T, Kwitek AE, Marrone A, Mashimo T, Moreno C, Mullins J, Mullins L, Olsson T, Pravenec M, Riley L, Saar K, Serikawa T, Shull JD, Szpirer C, Twigger SN, Voigt B, Worley K: Progress and prospects in rat genetics: A community view. Nat Genet 40: 516–522, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Aitman TJ, Petretto E, Behmoaras J: Genetic mapping and positional cloning. Methods Mol Biol 597: 13–32, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Cook HT, Jansen A, Lewis S, Largen P, O'Donnell M, Reaveley D, Cattell V: Arginine metabolism in experimental glomerulonephritis: Interaction between nitric oxide synthase and arginase. Am J Physiol 267: F646–F653, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Erwig LP, Stewart K, Rees AJ: Macrophages from inflamed but not normal glomeruli are unresponsive to anti-inflammatory cytokines. Am J Pathol 156: 295–301, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang XR, Kitching AR, Tipping PG, Holdsworth SR: Interleukin-10 inhibits macrophage-induced glomerular injury. J Am Soc Nephrol 11: 262–269, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Tipping PG, Leong TW, Holdsworth SR: Tumor necrosis factor production by glomerular macrophages in anti-glomerular basement membrane glomerulonephritis in rabbits. Lab Invest 65: 272–279, 1991 [PubMed] [Google Scholar]
- 13. Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD: Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A 93: 3942–3946, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaneko Y, Sakatsume M, Xie Y, Kuroda T, Igashima M, Narita I, Gejyo F: Macrophage metalloelastase as a major factor for glomerular injury in anti-glomerular basement membrane nephritis. J Immunol 170: 3377–3385, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Ma FY, Flanc RS, Tesch GH, Bennett BL, Friedman GC, Nikolic-Paterson DJ: Blockade of the c-Jun amino terminal kinase prevents crescent formation and halts established anti-GBM glomerulonephritis in the rat. Lab Invest 89: 470–484, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Parks WC, Mecham RP: Matrix Metalloproteinases, xii, San Diego, Academic Press, 1998 [Google Scholar]
- 17. Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, Ronco PM: Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J Exp Med 193: 793–802, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allen AR, McHale J, Smith J, Cook HT, Karkar A, Haskard DO, Lobb RR, Pusey CD: Endothelial expression of VCAM-1 in experimental crescentic nephritis and effect of antibodies to very late antigen-4 or VCAM-1 on glomerular injury. J Immunol 162: 5519–5527, 1999 [PubMed] [Google Scholar]
- 19. Andersson A, Kokkola R, Wefer J, Erlandsson-Harris H, Harris RA: Differential macrophage expression of IL-12 and IL-23 upon innate immune activation defines rat autoimmune susceptibility. J Leukoc Biol 76: 1118–1124, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kluth DC: Pro-resolution properties of macrophages in renal injury. Kidney Int 72: 234–236, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Kluth DC, Erwig LP, Rees AJ: Multiple facets of macrophages in renal injury. Kidney Int 66: 542–557, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Baker FJ, Silverton RE: Introduction to Medical Laboratory Technology, x, London, Butterworths, 1985 [Google Scholar]
- 23. Cook HT, Cattell V, Smith J, Salmon JA, Moncada S: Effect of a thromboxane synthetase inhibitor on eicosanoid synthesis and glomerular injury during acute unilateral glomerulonephritis in the rat. Clin Nephrol 26: 195–202, 1986 [PubMed] [Google Scholar]