Abstract
Fibroblast growth factor 23 (FGF23) modulates mineral metabolism by promoting phosphaturia and decreasing the production of 1,25-dihydroxyvitamin D3. FGF23 decreases parathyroid hormone (PTH) mRNA and secretion, but despite a marked elevation in FGF23 in uremia, PTH production increases. Here, we investigated the effect of FGF23 on parathyroid function in normal and uremic hyperplastic parathyroid glands in rats. In normal parathyroid glands, FGF23 decreased PTH production, increased expression of both the parathyroid calcium-sensing receptor and the vitamin D receptor, and reduced cell proliferation. Furthermore, FGF23 induced phosphorylation of extracellular signal–regulated kinase 1/2, which mediates the action of FGF23. In contrast, in hyperplastic parathyroid glands, FGF23 did not reduce PTH production, did not affect expression of the calcium-sensing receptor or vitamin D receptor, and did not affect cell proliferation. In addition, FGF23 failed to activate the extracellular signal–regulated kinase 1/2–mitogen-activated protein kinase pathway in hyperplastic parathyroid glands. We observed very low expression of the FGF23 receptor 1 and the co-receptor Klotho in uremic hyperplastic parathyroid glands, which may explain the lack of response to FGF23 in this tissue. In conclusion, in hyperparathyroidism secondary to renal failure, the parathyroid cells resist the inhibitory effects of FGF23, perhaps as a result of the low expression of FGF23 receptor 1 and Klotho in this condition.
Fibroblast growth factor 23 (FGF23) is produced by bone cells and plays a fundamental role in the regulation of mineral metabolism. FGF23 inhibits tubular resorption of phosphate and decreases 1α hydroxylase activity, which limits 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] production. Both phosphate excess and high 1,25(OH)2D3 stimulate the production of FGF23.1 FGF23 signals through a widely expressed receptor (FGFR) that becomes functional only in cells expressing the Klotho protein.2,3 Klotho, which is expressed in the parathyroid cell, converts FGFR1(IIIc), a canonical receptor for various FGFs, into a specific receptor for FGF23. The tissue-specific unique biological activity of FGF23 is likely to be regulated by the limited local distribution of Klotho. In renal failure, the decrease in glomerular filtration causes phosphate retention, which stimulates the production of FGF23. This elevation in FGF23 levels should help to control phosphate in patients with renal failure.4
Klotho and FGFR are abundantly expressed in parathyroid cells. Some studies5,6 showed that FGF23 decreases parathyroid hormone (PTH) secretion and PTH mRNA. In dialysis patients, FGF23 levels can reach extremely high values4,7 but PTH is not reduced; in fact, the highest PTH values correspond to patients with a marked increase in FGF23 levels.8 Thus, it is not clear whether FGF23 is capable of reducing PTH production in uremia. Our hypothesis is that there may be a resistance to the action of FGF23 in patients with uremia.
This study was designed to evaluate the effect of FGF23 on parathyroid function in normal and hyperplastic parathyroid glands. The study was performed in vivo and in vitro using intact rat parathyroid glands from normal and uremic animals with parathyroid hyperplasia.
Results
FGF23 Reduced PTH Secretion in Normal Rat Parathyroid Glands
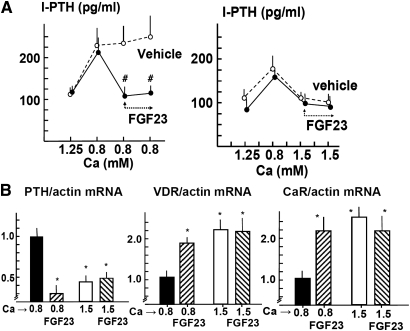
A change in calcium (Ca) concentration in the medium from normal (1.25 mM) to low (0.8 mM) produced a two-fold increase in PTH secretion. Addition of FGF23 (200 nM; 5 × 103 ng/ml) to the low-Ca medium produced a sustained reduction of PTH secretion. With a high Ca concentration in the medium, the addition of FGF23 did not further reduce PTH secretion (Figure 1A).
Figure 1.
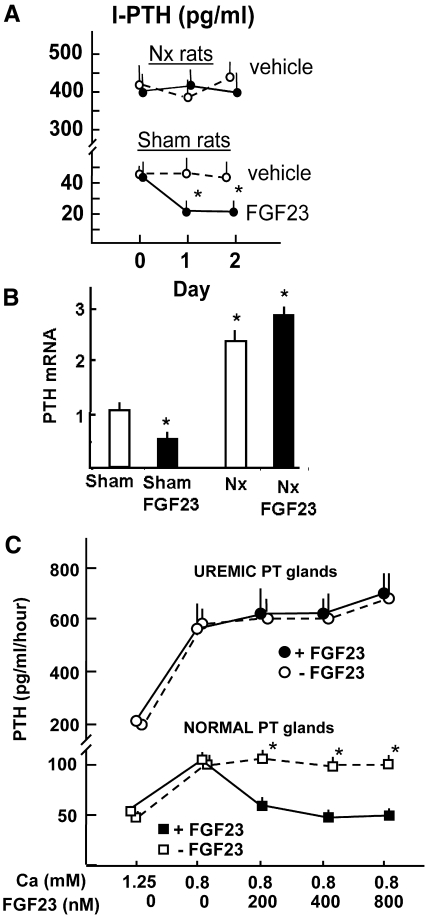
FGF23 reduces PTH secretion in normal rat parathyroid glands. (A) PTH secretion. Parathyroid glands are incubated in 1.25 mM Ca and then shifted to 0.8 mM to show a functional response. Next, the effect of the addition of 200 nM rat FGF23 (5 × 103 ng/ml) is evaluated in low-Ca (0.8 mM) and high-Ca (1.5 mM) concentrations. Data are means ± SEM (n = 4 in each group; the experiment was repeated three times). #P < 0.01 versus vehicle-treated glands. (B) Regulation of PTH, VDR, and CaR mRNA levels by FGF23 in vitro. Intact rat parathyroid glands are incubated for 6 hours in Ca concentrations of 0.8 and 1.5 mM with or without FGF23 (200 nM). Data are means ± SEM (n = 4 in each group; the experiments were repeated three times). *P < 0.05 versus 0.8 mM Ca with no added FGF23.
The expression of PTH mRNA in high-Ca medium (1.5 mM) was reduced as compared with low-Ca medium (0.8 mM; Figure 1B). The addition of FGF23 to the low-Ca medium markedly decreased PTH mRNA, whereas FGF23 did not further reduce PTH mRNA in high-Ca medium. Furthermore, both vitamin D receptor (VDR) and Ca-sensing receptor (CaR) mRNA were significantly increased in the high-Ca medium as compared with the low-Ca medium (Figure 1B); addition of FGF23 to the low-Ca medium increased VDR and CaR mRNA to levels not different from those observed in the high-Ca concentration.
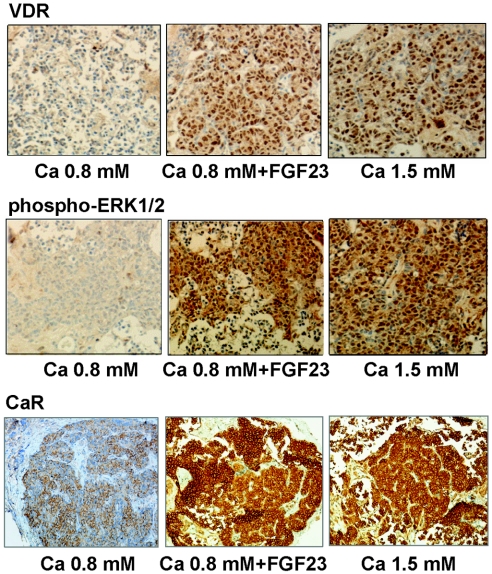
Parathyroid VDR protein expression decreased in low-Ca concentration as compared with high-Ca concentration (Figure 2). Addition of FGF23 to the low-Ca medium increased both VDR and CaR staining (expressed as the label index [LI]; see the Concise Methods section for an explanation) to a level similar to that observed with a high-Ca concentration (VDR-LI: 167 ± 10 in 0.8 mM Ca versus 510 ± 51 and 520 ± 60 in 0.8 mM Ca + FGF23 and 1.5 mM Ca, respectively [P < 0.01]; CaR-LI [relative to 0.8 mM Ca]: 1.00 ± 0.23 in 0.8 Ca mM versus 3.03 ± 0.41 and 3.34 ± 0.32 in 0.8 mM Ca + FGF23 and 1.5 mM Ca respectively [P < 0.01]).
Figure 2.
FGF23 increases VDR and CaR expression and ERK1/2 phosphorylation in normal rat parathyroid glands. Intact normal rat parathyroid glands are incubated for 6 hours with low Ca (0.8 mM), with or without FGF23 (200 nM; 5 × 103 ng/ml), or high Ca (1.5 mM) for immunohistochemical evaluation of VDR, CaR, and phospho-ERK1/2. Sections were counterstained with hematoxylin. Magnification, ×200.
FGF23 Increased VDR and CaR Expression and Extracellular Signal–Regulated Kinase 1/2 Activation in Normal Rat Parathyroid Glands
FGF23 has been shown to activate the extracellular signal–regulated kinase 1/2 (ERK1/2)–mitogen-activated protein kinase pathway in parathyroid glands.5 We evaluated this signaling pathway and observed that the FGF23-dependent changes in VDR and CaR expression were paralleled by the activation (phosphorylation) of ERK1/2 (phospho-ERK1/2; Figure 2); phospho-ERK1/2 was decreased in low- as compared with high-Ca concentration, and FGF23 induced ERK1/2 phosphorylation to the level of high-Ca concentration (phospho-ERK1/2-LI [relative to Ca 0.8 mM]: 1.00 ± 0.26 in 0.8 mM Ca versus 4.45 ± 0.23 and 4.27 ± 0.27 in 0.8 mM Ca + FGF23 and 1.5 mM Ca, respectively; P < 0.01).
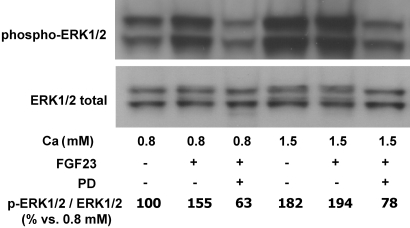
Phosphorylation of ERK1/2 by FGF23 was also evaluated by Western blot in bovine parathyroid tissue (Figure 3). The stimulation of ERK1/2 phosphorylation by FGF23 was clearly observed in the low-Ca concentration. High-Ca concentration stimulated ERK1/2 phosphorylation to almost the same extent as FGF23; the combination of FGF23 and high Ca did not cause a further increase in phospho-ERK1/2 (Figure 3). The addition of PD98059 (10 μM), an inhibitor of ERK1/2 phosphorylation, reduced the density of the band.
Figure 3.
FGF23 increases ERK1/2 phosphorylation in normal rat parathyroid glands. Western blot of phospho-ERK1/2 in bovine parathyroid glands incubated for 6 hours with low-Ca (0.8 mM) and high-Ca (1.5 mM) concentration with and without FGF23 (200 nM) added. PD98059 (10 μM), an inhibitor of ERK1/2 phosphorylation, is added as control. Quantification is performed by measurement of the integrated OD. Image is representative of three different experiments. The values presented in the figure are related to that specific experiment; the statistical data of all of the experiments (means ± SEM) are 100 ± 9 in 0.8 mM Ca, 185 ± 11* in 0.8 mM Ca + FGF23, 60 ± 7*# in 0.8 mM Ca + FGF23 + PD, 182 ± 8* in 1.5 mM Ca, 200 ± 12* in 1.5 mM Ca + FGF23, and 66 ± 5*# in 1.5 mM Ca + FGF23 + PD; *P < 0.01 versus 0.8 mM Ca; #P < 0.01 versus 0.8 or 1.5 mM Ca with added FGF23.
FGF23 Decreased Parathyroid Cell Proliferation in Normal Rat Parathyroid Glands in Low-Ca Medium
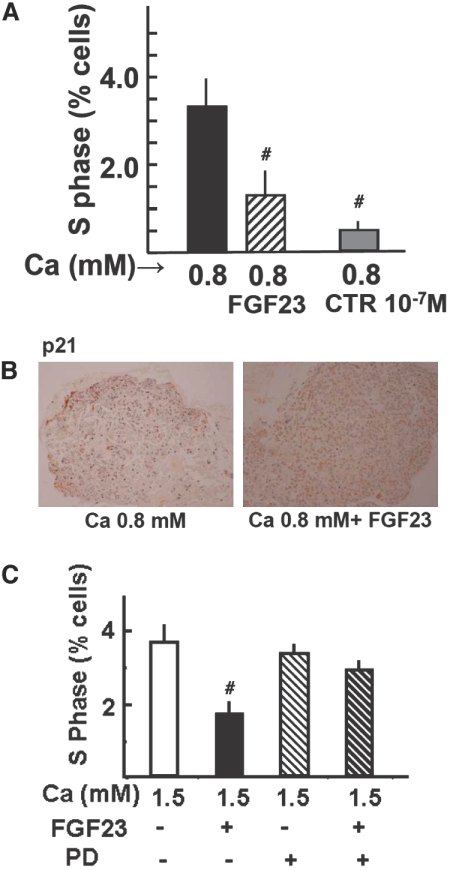
As shown in Figure 4 A and Supplemental Figure SI-1, the addition of FGF23 to the low-Ca medium decreased the percentage of cells in S phase to less than half. A marked decreased in proliferation was also observed with high calcitriol concentration in the medium (10−7 M), a positive control of parathyroid cell proliferation inhibition. Furthermore, immunohistochemical analysis revealed that expression of p21, an inhibitory protein of the cell cycle, was increased in parathyroid glands incubated with FGF23 (p21-LI: 206 ± 69 in 0.8 mM Ca versus 797 ± 46 in 0.8 mM Ca + FGF23; P < 0.01; Figure 4B). In addition, the expression of PCNA (Supplemental Figure SI-1), a marker of cycling cells, decreased after incubation of parathyroid glands with FGF23 (PCNA-LI: 267 ± 22 in 0.8 mM Ca versus 178 ± 25 in 0.8 mM Ca + FGF23; P < 0.05).
Figure 4.
FGF23 decreases parathyroid cell proliferation in normal rat parathyroid glands in low-Ca medium. Intact normal rat parathyroid glands are incubated for 24 h with low Ca (0.8 mM) with or without FGF23 (200 nM; 5 × 103 ng/ml), and an additional group is treated with calcitriol 10−7 M as a positive control of parathyroid cell proliferation inhibition. (A and B) Proliferation is assessed by the number of cells entering the S phase of the cell cycle by flow cytometry (data are means ± SEM; n = 4 in each group; the experiment is repeated three times; #P < 0.01 versus 0.8 Ca mM; A) and by the immunohistochemical determination of p21 (an inhibitory protein of the cell cycle; B). Images are representative of four glands per three different experiments. (C) Intact normal rat parathyroid glands are treated as in A but with high Ca (1.5 mM) with or without the specific ERK1/2 inhibitor PD98059 (10 μM). Data are means ± SEM (n = 4 in each group; the experiment is repeated three times). #P < 0.01 versus 1.5 mM Ca. Magnification, ×200.
We performed additional experiments to assess the effect of FGF23 on cell proliferation in high Ca. Because both FGF23 and high Ca stimulate ERK1/2 activation, we also evaluated the effect of its inhibition by PD98059 (10 μM). As shown in Figure 4C, in high Ca, FGF23 inhibited cell proliferation, but the addition of PD98059 precluded the effect of FGF23. PD98059 alone did not produce a significant effect.
FGF23 Reduced PTH Secretion and Synthesis in Normal but not in Uremic Parathyroid Glands
As compared with sham-operated control rats, uremic (5/6 nephrectomized) rats had increased serum creatinine levels (1.40 ± 0.04 versus 0.58 ± 0.02 mg/dl; P < 0.01). The serum phosphate levels in uremic and control rats were 15.5 ± 0.4 and 6.4 ± 0.3 mg/dl, respectively (P < 0.01), and the serum ionized Ca levels were 0.83 ± 0.07 and 1.24 ± 0.03 mM, respectively (P < 0.01). In uremic rats, the serum levels of FGF23 were much higher than in sham rats (1835 ± 694 versus 325 ± 59 pg/ml; P < 0.01). Administration of FGF23 to uremic rats (15 μg/d per rat; by the injection of 0.3 ml of FGF23 50 × 106 pg/ml) for 2 consecutive days increased serum FGF23 levels to 12,100 ± 652 pg/ml, but it did not produce a significant change in the serum concentration of PTH (394 ± 45 versus 445 ± 35 pg/ml in the vehicle-treated rats; NS). This result contrasted with the reduction in serum PTH level observed in sham-operated rats after administration of the same dosage of FGF23 for 2 days (43 ± 7 versus 21 ± 4 pg/ml, respectively; P < 0.01; Figure 5A). The 2-day administration of FGF23 to sham-operated rats increased the serum FGF23 levels (11,630 ± 933 pg/ml). Uremic rats showed an increase in PTH mRNA as compared with normal rats (Figure 5B). Administration of FGF23 for 2 consecutive days to normal rats produced a significant decrease in PTH mRNA; however, in uremic rats with hyperparathyroidism, the administration of FGF23 did not reduce PTH mRNA levels (2.89 ± 0.14 versus 2.31 ± 0.15; NS by two-way ANOVA). Treatment with FGF23 did not modify serum levels of Ca or phosphate in uremic rats.
Figure 5.
FGF23 reduces PTH secretion and synthesis in normal but not in uremic parathyroid glands. In vivo studies: Renal failure in rats is induced by two-step 5/6 Nx. (A and B) Sham-operated and 5/6 Nx rats are administered FGF23 (15 μg/d per rat) or vehicle for 2 consecutive days, and PTH secretion (A) and PTH mRNA (B) are evaluated. Data are means ± SEM (n = 4 in each group; the experiments are repeated three times). *P < 0.05 versus sham with vehicle. (C) In vitro studies: After a stabilization period of 6 hours, parathyroid glands from normal and uremic rats are incubated with increasing concentrations of FGF23. The incubation medium is replaced hourly, and PTH secretion is evaluated. Data are means ± SEM (n = 4 in each group; the experiments are repeated three times). *P < 0.05 versus 0.8 mM Ca with added FGF23.
As shown in Figure 5C, in vitro, increasing concentrations of FGF23 hourly above 200 nM (5 × 103 ng/ml) did not produce further inhibition of PTH secretion in normal parathyroid glands. Hyperplastic parathyroid glands secrete more PTH, and the addition of increasing concentrations of FGF23 (up to 800 nM) was unable to reduce PTH secretion.
The effect of FGF23 was also assessed in vitro under various phosphate concentrations. Incubation of normal intact rat parathyroid glands with normal Ca (1.25 mM) and a high-phosphate (4 mM) concentration resulted in increased PTH levels as compared with the normal phosphate (1 mM) concentration (310 ± 30 versus 103 ± 13 pg/104 cells per h, respectively; P < 0.01). The addition of FGF23 200 nM (5 × 103 ng/ml) to the high-phosphate medium reduced PTH secretion from 314 ± 43 to 183 ± 47 pg/104 cells per h (P < 0.01).
We performed experiments to determine whether the high FGF23 levels observed in uremic rats may impair the ability of exogenous FGF23 to produce a further inhibition of PTH. Normal rats were given FGF23 to increase the serum FGF23 from 275 ± 71 to 2200 ± 466 pg/ml, which decreased the serum PTH levels from 43 ± 7 to 24 ± 5 pg/ml (P < 0.01). Additional administration of FGF23, which increased serum FGF23 to 10,780 ± 1028 pg/ml, did not result in a significant further reduction in PTH (Supplemental Figure SI-2).
FGF23 Had No Effect on the Parathyroid Cell Proliferation in Uremic Rats
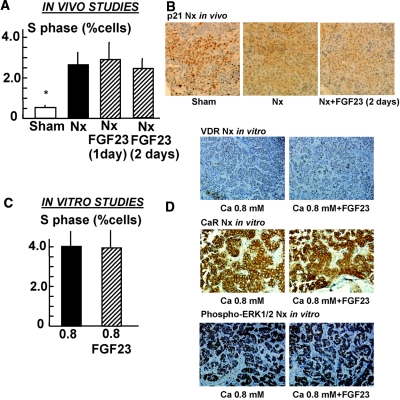
The percentage of cells in the S phase in the parathyroid glands from uremic rats was increased as compared with those of sham rats (2.6 ± 0.5 versus 0.5 ± 0.1; P < 0.01). The administration of FGF23 (15 μg/d for 2 consecutive days) to uremic rats did not produce a change in the number of cells entering the S phase of cell cycle (Figure 6A, Supplemental Figure SI-3). In these uremic rats, immunohistochemical analysis showed that the administration of FGF23 did not affect p21 expression (p21-LI: 641 ± 61 in sham rats; 365 ± 32 in nephrectomized rats and 331 ± 27 in nephrectomized rats + FGF23; P < 0.01 versus shams; Figure 6B) or PCNA expression (PCNA-LI: 114 ± 26 in sham rats; 405 ± 33 in nephrectomized rats and 374 ± 29 in nephrectomized rats + FGF23; P < 0.01 versus Shams; Supplemental Figure SI-3).
Figure 6.
FGF23 has no effect on parathyroid cell proliferation in uremic rats. (A through C) Parathyroid cell proliferation is studied both in vivo (A and B) and in vitro (C). (A) In the in vivo studies, the uremic rats receive FGF23 (15 μg/d for 2 consecutive days). Rats are killed and parathyroid glands are removed for analysis of cell proliferation (number of cells entering the S phase of the cell cycle). Data are means ± SEM (n = 8 rats in each group; the experiment is repeated three times). *P < 0.05 versus 5/6 Nx rats. (B) Immunohistochemical determination of p21. (C) Intact parathyroid glands from uremic animals are incubated for 24 hours with low Ca (0.8 mM) with and without FGF23 (200 nM; 5 × 103 ng/ml). The proliferation is assessed by number of cells entering the S phase. Data are means ± SEM (n = 4 in each group; experiments are repeated at least three times). (D) Immunohistochemical determination of VDR, CaR, and phospho-ERK1/2 (activation of ERK1/2). Images are representative of three rats per three different experiments. Sections are counterstained with hematoxylin. Magnification, ×200.
The in vitro effect of FGF23 on cell proliferation, VDR, CaR, and phospho-ERK1/2 in parathyroid glands from uremic rats is shown in Figure 6, C and D. FGF23 was unable to reduce cell proliferation and did not have an effect on VDR or CaR expression (VDR-LI: 43 ± 7 in 0.8 mM Ca versus 39 ± 9 in 0.8 mM Ca + FGF23 [NS]; CaR-LI [relative to 0.8 mM Ca]: 1.00 ± 0.21 in 0.8 mM Ca versus 1.3 ± 0.35 in 0.8 mM Ca + FGF23 [NS]) and did not enhance phosphorylation of ERK1/2 (phospho-ERK1/2-LI [relative to 0.8 mM Ca]: 1.00 ± 0.33 in 0.8 mM Ca versus 0.95 ± 0.22 in 0.8 mM Ca + FGF23; NS).
FGF23 Did not Increase VDR Expression in Parathyroid Glands from Uremic Rats
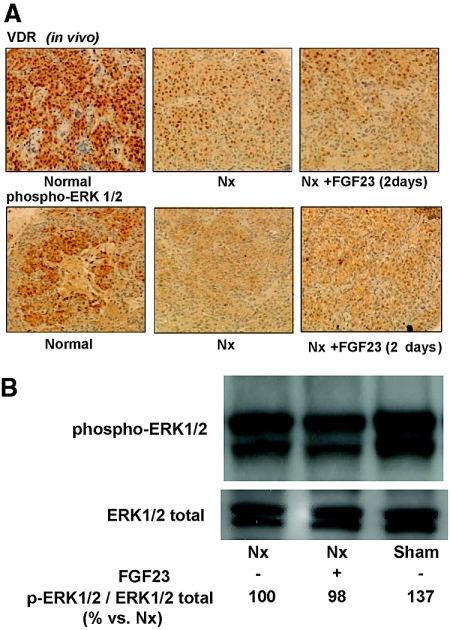
Immunohistochemical analysis of the uremic parathyroid glands showed a decreased expression of VDR as compared with sham (455 ± 27 versus 766 ± 44; P < 0.01), and FGF23 administration did not upregulate parathyroid VDR expression in uremic rats (409 ± 60; Figure 7 A). VDR mRNA levels were also lower in uremic than in normal rats (0.270 ± 0.040 versus 1.000 ± 0.045; P < 0.01), and, again, administration of FGF23 to the uremic rats did not affect the level of VDR mRNA (0.300 ± 0.025). Similar results were obtained with the CaR mRNA expression, which was decreased in uremic as compared with normal rats (0.44 ± 0.04 versus 1.00 ± 0.04; P < 0.01) and did not increase after FGF23 administration (0.29 ± 0.07 versus 1.00 ± 0.04; P < 0.01).
Figure 7.
FGF23 does not increase VDR expression or activate ERK1/2 in parathyroid glands from uremic rats. Nx rats receive FGF23 (15 μg/d for 2 consecutive days). (A and B) Parathyroid glands are removed for immunohistochemical analysis of VDR expression and phospho-ERK1/2 (ERK1/2 activation; images are representative of four rats from three different experiments; A) and Western blot evaluation of ERK1/2 phosphorylation (B). Quantification is performed by measuring the integrated OD. Image is representative of three different experiments. The values presented in the figure are related to that specific experiment; the statistical data of all of the experiments (means ± SEM) are 100 ± 9 in Nx, 94 ± 4 in Nx + FGF23, and 144 ± 21* in Sham. *P < 0.01 versus Nx. Magnification, ×200.
FGF23 Did not Activate ERK1/2 in Uremic Parathyroid Glands
Immunohistochemical evaluation revealed that parathyroid glands from uremic rats showed low ERK1/2 activation (phospho-ERK1/2) as compared with normal rats (333 ± 36 versus 579 ± 72; P < 0.05), and the administration of FGF23 was not able to increase ERK1/2 activation in parathyroid glands from uremic rats (375 ± 41; Figure 7A). The immunohistochemical data were confirmed by Western blot of phospho-ERK1/2 (Figure 7B); nephrectomized rats showed a moderate but significant reduction of phospho-ERK1/2 (phospho-ERK1/2-ERK1/2 ratio, as relative to nephrectomy: 100 ± 9 versus 144 ± 21; P < 0.05), and the administration of FGF23 did not produce an increase in phospho-ERK1/2.
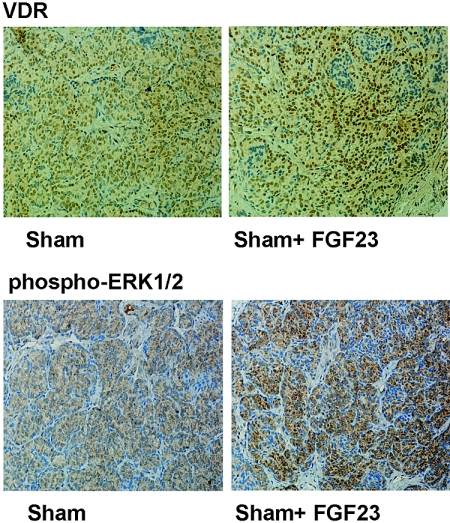
FGF23 Increased VDR Expression and Activated ERK1/2 in Normal Rats In Vivo
In vivo studies also showed that administration of FGF23 produced upregulation of VDR (VDR-LI: 317 ± 39 in control versus 437 ± 36 in FGF23-treated rats; P < 0.05; Figure 8) and phosphorylation of ERK1/ 2 (phospho-ERK1/2-LI [relative to controls]: 1.00 ± 0.19 in control versus 2.66 ± 0.21 in FGF23-treated rats; P < 0.01).
Figure 8.
FGF23 increases VDR expression and activates ERK1/2 in normal rats in vivo. Normal rats receive FGF23 (15 μg/d for 2 consecutive days). The glands are processed for immunohistochemical determination of VDR and ERK1/2 phosphorylation (activation). Images are representative of four glands per three different experiments. Magnification, ×200.
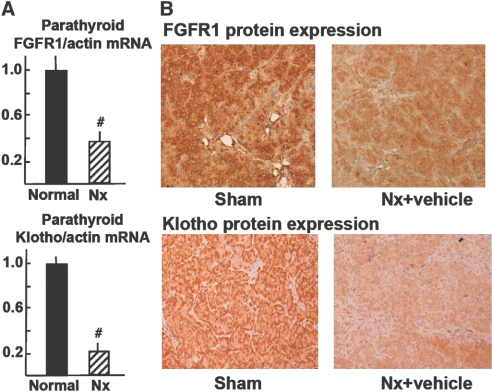
FGFR1 and Klotho Expression Were Reduced in Parathyroid Glands from Uremic Rats In Vivo
The levels of FGFR1 mRNA in the hyperplastic parathyroid glands of the uremic rats were markedly reduced as compared with control sham-operated rats (P < 0.01; Figure 9A). The levels of Klotho mRNA in the uremic hyperplastic parathyroid glands were also reduced (to approximately 25% of those in control sham-operated rats; P < 0.01). Immunohistochemical analysis revealed that FGFR1 and Klotho were also markedly reduced in the uremic hyperplastic parathyroid glands as compared with control sham-operated rats (FGFR1-LI [relative to sham]: 1.00 ± 0.17 in sham versus 0.30 ± 0.10 in nephrectomized rats [P < 0.01]; Klotho-L I [% versus sham]: 1.00 ± 0.04 in sham versus 0.38 ± 0.02 in nephrectomized rats [P < 0.01]; Figure 9B).
Figure 9.
FGFR1 and Klotho expression are reduced in parathyroid glands from uremic rats in vivo. (A) FGFR1 and Klotho mRNA levels are measured by quantitative real-time reverse transcriptase–PCR (versus β-actin mRNA). Data are means ± SEM (n = 4 in each group; the experiment is repeated three times). #P < 0.01 versus normal (sham) rats. (B) FGFR1 and Klotho protein expression in hyperplastic parathyroid glands from uremic rats. Parathyroid glands are processed for immunohistochemical evaluation of FGFR1 and Klotho protein. Images are representative of four glands from three different experiments. Positive reaction (brown deposits) is revealed by the diaminobenzidine-tetrachloride system. Sections are counterstained with hematoxylin. Magnification, ×200.
Effect of Extracellular Ca, Phosphate, and FGF23 on FGFR1 and Klotho Expression in Parathyroid Glands from Uremic Rats In Vitro
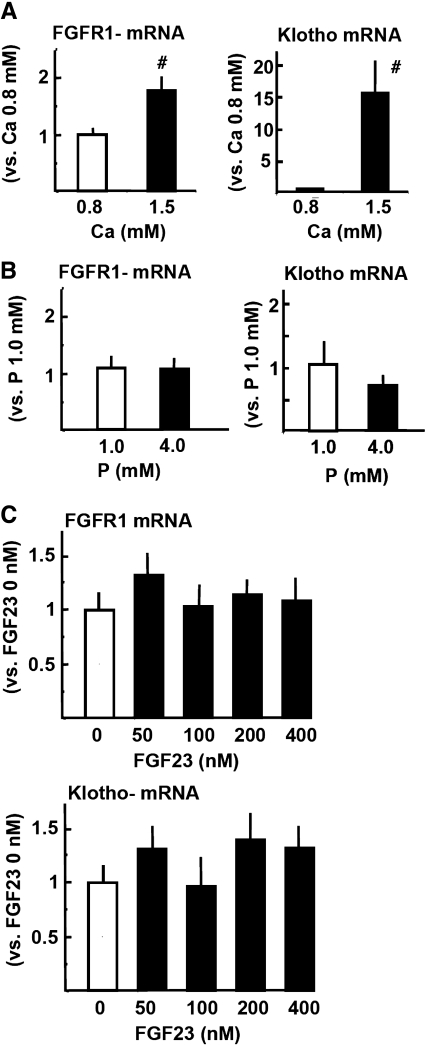
Both FGFR1 and Klotho mRNA were significantly greater in high-Ca (1.5 mM) than low-Ca (0.8 mM) concentration in the medium (Figure 10A). There was a marked effect of Ca concentration on parathyroid Klotho expression. As shown in Figure 10B, FGFR1 mRNA was not modified by extracellular phosphate; however, Klotho mRNA tended to be lower in high-phosphate (4.0 mM) than normal-phosphate (1.0 mM) concentration in the medium with normal (1.25 mM) Ca, although no significance was obtained. Furthermore, increasing concentrations of FGF23 in the medium with normal Ca (1.25 mM) did not produce a significant change in FGFR1 or Klotho mRNA expression (Figure 10C).
Figure 10.
Effect of extracellular Ca (A), phosphate (B), and FGF23 (C and D) on FGFR1 and Klotho expression in parathyroid glands from uremic rats in vitro. (A through C) Intact normal rat parathyroid glands are incubated for 6 hours with low-Ca (0.8 mM) or high-Ca (1.5 mM) concentration in the medium with normal (1.0 mM) phosphate (A), high (4.0 mM) or normal (1.0 mM) phosphate concentration in the medium with normal (1.25 mM) Ca (B), and increasing concentrations of FGF23 in the medium with normal Ca (1.25 mM) and phosphate (1.0 mM; C). FGFR1 and Klotho mRNA levels are measured by quantitative real-time reverse transcriptase–PCR (versus β-actin mRNA). Data are means ± SEM (n = 4 in each group; the experiment is repeated three times). #P < 0.01 versus 0.8 mM Ca.
Discussion
This study was designed to investigate the effect of FGF23 on parathyroid glands from normal rats and hyperplastic parathyroid glands from rats with renal failure. The results obtained demonstrate that in normal parathyroid glands, FGF23 inhibits parathyroid function; however, in hyperplastic parathyroid glands, FGF23 did not decrease parathyroid cell activity. In normal parathyroid glands, FGF23 decreased PTH secretion and PTH mRNA and increased the expression of both CaR and VDR. By contrast, in hyperplastic parathyroid glands, FGF23 did not produce a significant effect on PTH secretion, PTH mRNA, CaR, or VDR. Furthermore, whereas in normal parathyroid glands cell proliferation was significantly reduced by FGF23, in hyperplastic parathyroid cells, FGF23 was not able to reduce cell proliferation. In normal parathyroid glands, FGF23 produced phosphorylation of ERK1/2. By contrast, in hyperplastic parathyroid glands, FGF23 did not cause phosphorylation of ERK1/2. The lack of response to FGF23 in hyperplastic parathyroid glands prompted us to analyze the expression of receptors for FGF23 and the level of Klotho expression. In hyperplastic parathyroid glands, the expression of FGFR1 and Klotho was markedly reduced as compared with normal parathyroid glands. These results may explain why uremic rats fail to respond to FGF23.
Before addressing the effect of FGF23 on hyperplastic parathyroid glands from uremic rats, we characterized the response of normal parathyroid glands to FGF23. Although the article by Ben-Dov et al.5 demonstrated an effect of FGF23 on parathyroid gland function, changes in parathyroid cell receptor expression were not evaluated. Furthermore, to have a measure of the inhibitory effect of FGF23 on parathyroid function, we considered it important to test the effect of FGF23 under low- and high-Ca concentration, because Ca concentration is the main regulator of parathyroid function. Our experiments demonstrated that FGF23 decreased PTH secretion and PTH mRNA. These results confirm previous data reported by Ben-Dov et al.5 and Krajisnik et al.6 It is important to note that the degree of inhibition of PTH secretion and PTH mRNA by FGF23 is similar to that observed by high Ca. In vitro experiments showed that in high-Ca concentration, FGF23 was unable to reduce further the PTH secretion. Our interpretation is that FGF23 is able to prevent an increase in PTH secretion and synthesis when cells are being stimulated by hypocalcemia. In vivo, FGF23 reduced PTH secretion in normal rats with normal serum Ca, suggesting that high FGF23 levels inhibit parathyroid function even with a normal serum Ca concentration. High phosphate has been shown to stimulate PTH production and secretion9–11; in vivo, an elevation of FGF23 induced by high phosphate may ameliorate the stimulation of PTH secretion by phosphate. It is intriguing that in our study, acute administration of exogenous FGF23 suppressed parathyroid gland function in normal rats, whereas transgenic mice overexpressing FGF23 developed secondary hyperparathyroidism; however, there are elements that can be affected by chronic elevation of FGF23 and that may contribute to secondary hyperparathyroidism such as low 1,25(OH)2D3 and perhaps low serum Ca. In uremic rats with parathyroid hyperplasia, even very high levels of FGF23 did not decrease PTH secretion or PTH mRNA either in vivo or in vitro. Thus. the lack of response to exogenous FGF23 in nephrectomized rats demonstrates a resistance of the hyperplastic parathyroid gland to the action of FGF23.
Uremic rats had high serum phosphate levels; we explored a potential effect of high serum phosphate concentration on the parathyroid response to FGF23. Thus, parathyroid glands were incubated in high phosphate concentration. As expected, high phosphate stimulated PTH secretion, and FGF23 was effective in reducing PTH secretion toward normal levels; high phosphate does not interfere with the inhibitory action of FGF23 on the parathyroid glands. These results suggest that the hyperphosphatemia associated with renal failure was not the cause of the lack of parathyroid response to FGF23. Furthermore, in vitro experiments showed that high phosphate did not decrease FGFR1 or Klotho mRNA significantly.
Another significant effect of FGF23 was the upregulation of CaR and VDR expression in normal parathyroid glands. The activation of these receptors results in the inhibition of parathyroid cell activity; therefore, the upregulation of CaR and VDR may represent another way to reduce parathyroid function. An upregulation of VDR and CaR was also observed in parathyroid glands incubated with a high Ca concentration; these results confirm previous published observations.12,13 The addition of FGF23 to high Ca did not further increase the expression of parathyroid CaR and VDR. In uremic hyperplastic parathyroid glands, the expression of VDR and CaR is reduced14–16; the administration of FGF23 to rats with renal failure failed to increase parathyroid ERK1/2 activity and did not have an effect on the expression of VDR or CaR.
One important question addressed in this study was whether FGF23 had an effect on parathyroid cell proliferation. In normal parathyroid glands, FGF23 decreased the number of cells in S phase and enhanced expression of p21. Furthermore, ERK1/2 phosphorylation may mediate the reduction of cell proliferation induced by FGF23, because the specific inhibition of ERK1/2 by PD98059 precluded the effect of FGF23. By contrast, in vivo and in vitro studies showed that FGF23 failed to suppress parathyroid cell proliferation in uremic hyperplastic parathyroid glands.
Ben-Dov et al.5 reported that FGFR1 and FGFR3 were coexpressed with Klotho in parathyroid glands. FGFR1 has been shown to be the primary physiologic receptor for FGF23; FGFR1 is associated to a heterodimeric, high-affinity receptor for FGF23.2,3 Liu et al.17 reported co-localization of FGFR1 and Klotho in the distal tubule, which suggests that this may be an effector site of FGF23. In this study, we evaluated the expression of both FGFR1 and Klotho. The inability of hyperplastic parathyroid glands to respond to FGF23 may be explained by the fact that hyperplastic parathyroid cells from uremic rats had very low expression of both FGFR1 and Klotho mRNA and there was a clear decrease in FGFR1 protein level. Thus, decreased expression of either FGFR1 or Klotho would have been enough to obtain a resistance of uremic rats to the action of FGF23. This is the first report showing a decrease in FGFR1 and Klotho in hyperplastic parathyroid glands from uremic rats. A similar decrease in FGFR1 and Klotho was also observed in the remnant kidney (data not shown), supporting a previous observation by others18; however, this study does not rule out that the resistance to FGF23 in uremic hyperparathyroidism could be in part due to the persistent elevation of FGF23.
Björklund et al.19 reported that Klotho mRNA expression is decreased in primary parathyroid adenomas; they suggested that the high serum Ca may be causing the decrease in Klotho mRNA expression. We show a decrease in Klotho expression in secondary parathyroid hyperplasia, which begs the question of whether increased parathyroid cell proliferation (hyperplasia) is associated with a decrease in Klotho expression. It should be stressed that the autonomous parathyroid proliferation and function in the primary hyperparathyroidism are different from the uremic hyperparathyroidism. Primary and secondary hyperparathyroidism both are characterized by parathyroid hyperplasia, but in primary hyperparathyroidism the serum Ca is high and in secondary hyperparathyroidism the serum Ca is low. Furthermore, Björklund et al.19 showed in vitro in bovine parathyroid cells in culture that high Ca in a dose-response manner decreased Klotho protein. Nevertheless, we found that, in vitro, low Ca was associated with a decrease in Klotho mRNA expression; however, we did not perform experiments using Ca levels >1.5 mM.
In mice, Imura et al.20 found a molecular association of Klotho and Na+, K+-ATPase; the lack of Klotho in a kl−/− mouse impairs the secretion of PTH in response to low Ca, suggesting that Klotho may be required for Na+,K+-ATPase–dependent PTH release. In our study, we showed a decreased expression of Klotho in hyperplastic parathyroid glands, which would prevent an inhibitory effect of FGF23 on PTH production. The relevance of Klotho in parathyroid cell function is illustrated by the marked effect of extracellular Ca on the regulation of parathyroid Klotho expression.
Increased serum FGF23 levels in animal models of renal failure and in patients with uremia has been mainly attributed to the accumulation of phosphate, which is known to stimulate FGF23 production by bone cells.4,7,8,21,22 Despite the progressive increase in FGF23 levels observed in renal failure, phosphate accumulates, PTH secretion is increased, and parathyroid proliferation persists. From our results, we deduce that the poor expression of parathyroid FGFR and Klotho impairs the ability of FGF23 to control mineral metabolism in renal failure. It suggests that the decrease in FGFR1-Klotho would facilitate the phosphaturic activity of PTH.
Resistance of rat uremic hyperplastic parathyroid glands to the action of FGF23 can be explained by the decrease in FGFR expression. A number of factors could be responsible for the parathyroid resistance to FGF23 and the decrease in FGFR expression. Changes in mineral metabolism or other metabolic changes derived from the uremic status may cause a decrease in FGFR and klotho expression. Our study cannot rule out a direct effect of uremic toxins on FGFR and/or Klotho expression. High phosphate did not seem to affect the expression of parathyroid FGFR and klotho in vitro; however, low Ca concentration was associated with a decrease in both FGFR and klotho. In normal rats exposed to high levels of FGF23, a further increase in FGF23 did not result in an additional decrease in PTH; persistent elevation of FGF23 may be responsible for a parathyroid resistance to FGF23. Finally, the decrease in FGFR may be the result of an increase in the rate of cell proliferation.
In clinical studies, high FGF23 levels have been shown to predict mortality in patients with renal failure23; therefore, a high FGF23 level may be a surrogate for phosphate accumulation with no capacity to reduce the phosphate load in patients with renal failure. It is difficult to predict what the extent of mineral metabolism abnormalities in rats with advanced renal failure and ablated FGF23 production will be; most likely, the abnormal mineral metabolism is not affected by the presence of FGF23, but, of course, this has to be demonstrated experimentally.
In conclusion, in normal rats, FGF23 reduces parathyroid cell function and parathyroid cells proliferation; however, in hyperplastic parathyroid glands from rats with renal failure, there is a reduction in receptors for FGF23 and in Klotho that likely makes the tissue resistant to the action of FGF23.
Concise Methods
Animals
Male Wistar rats (250 g) were purchased from the Animal Breeding Facility of the University of Cordoba (Cordoba, Spain). Rats were housed on a 12-hour/12-hour light/dark cycle and given ad libitum access to food (Ca 0.6%, P 0.6%, 100 IU/100 g vitamin D). The animals were anesthetized with pentobarbital (50 mg/kg), and blood was drained by aortic puncture; within 2 minutes, the parathyroid glands were dissected free of the thyroid gland under a dissecting microscope. Normal bovine parathyroid tissue sliced in small pieces (<1 mm3) were used when measurements required a large amount of tissue protein. These glands were obtained from Covap Slaughterhouse (Pozoblanco, Cordoba, Spain). The experimental protocols were reviewed and approved by the Ethics Committee for Animal Research of the University of Cordoba, and all rats received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science.
In Vitro Parathyroid Tissue Culture
Parathyroid tissue culture was performed following the method described by Almaden et al.9 Briefly, 10 intact rat parathyroid glands or small (1 mm3) pieces of bovine parathyroid tissue were placed inside a nylon basket in individual wells with constant shaking at 37°C in a humid atmosphere. The incubation medium was buffered (7.4) and contained (in mM) 125 NaCl, 5.9 KCl, 0.5 MgCl2, 1 Na pyruvate, 4 glutamine, 12 glucose, and 25 HEPES. Insulin 0.1 IU/ml, BSA 0.1%, penicillin G 100 IU/ml, and streptomycin 100 μg/ml were added to the medium. CaCl2 was added to medium to achieve the desired Ca concentration. Ionized Ca concentration was measured using a selective electrode (634 Ca/pH analyzer; Ciba-Corning, Essex, UK). The experiments were performed using normal (1 mM) phosphate (NaH2PO4:Na2HPO4, 1:2 ratio) concentrations in the medium. In specific experiments, the phosphate concentration was modified to 4 mM. After a stabilization period of 6 hours in 1.25 mM Ca, the glands were transferred to various Ca concentrations with specific experimental treatments. The incubation medium was replaced hourly, and the medium removed was stored for biochemical determinations. In other in vitro experiments, parathyroid glands were maintained in culture for 6 or 24 hours in a Ca concentration of 0.8 or 1.5 mM with or without the addition FGF23 or other compounds. Hyperplastic glands for in vitro experiments were obtained from 5/6 nephrectomized rats. Cell viability was assessed in mechanically dispersed cells labeled with two fluorochrome dyes: Green fluordiacetate (Sigma) and red propidium iodine (Sigma), which stain live and dead cells, respectively. Stained cells were assessed by flow cytometry (FACScan; Becton-Dickinson, San Jose, CA) using the software LYSIS II. Only tissue samples with >90% viable cells were used in the experiments.
In Vivo Studies
Renal failure in rats was induced by two-step 5/6 nephrectomy (5/6 Nx) as follows. On day 1, renal mass ablation of two thirds of the left kidney and on day 7 nephrectomy of the right kidney was performed. Sham-operated rats underwent the same procedures without renal resection. The experimental diet contained normal Ca (0.6%) and high phosphate (1.2%).
On day 7, the rats were assigned to the experimental groups: 5/6 Nx + vehicle and 5/6 Nx + FGF23, which were administered FGF23 (15 μg/d per rat intraperitoneally [by the injection of 0.3 ml of FGF23 50 × 106 pg/ml] for 2 consecutive days (FGF23 was administered on days 8 and 9 after completion of 5/6 Nx). Sham rats fed a normal phosphorus diet (0.6%) was used as a control. Blood samples were obtained 24 hours after the last injection of FGF23. Rats were exsanguinated by aortic puncture, and parathyroid glands were removed and processed immediately. Each experiment included eight rats in each group. Experiments were repeated at least three times.
Serum ionized Ca levels was determined using a Ciba-Corning 634 ISE Ca2/pH Analyzer (Ciba-Corning). Intact rat PTH levels were quantified using an Elisa Kit (Immutopics, San Clemente, CA). Plasma creatinine and phosphate were measured by spectrophotometry (Sigma Diagnostics, St. Louis, MO). FGF23 levels were determined using a rat FGF23 ELISA Kit (Kainos Laboratories, Tokyo, Japan).
Determination of Cell Proliferation by Flow Cytometry
All experiments designed to assess cell proliferation were made in intact rat parathyroid glands. At the end of the experiment (in vivo or in vitro), isolated cells were obtained from the glands under the inverted microscope using No. 5 Dumont tweezers-Biology Tips followed by gentle pipetting in a 50-μl volume of PBS; immediately after, the cells were processed for flow cytometric analysis. The cell cycle was analyzed by the method described by Vindelov and Christensen.24 A total of 10,000 clean cell nuclei inside the analysis region (the debris was discarded) per sample were acquired by the flow cytometer and analyzed using the CELLFIT software (Becton-Dickinson) with doublet discrimination module to discriminate cell aggregates. The percentage of cells in the S phase was used as a marker of cell proliferation.
RNA Analysis
Total RNA was extracted following a modification of Chomczynski and Sacchi's protocol.25 The RNA was dissolved in nuclease-free water (Promega, Madison, WI) and heated for 10 minutes at 60°C. Total RNA was quantified by spectrophotometry (ND-1000; Nanodrop Technologies, Wilmington, DE). PTH, VDR, CaR, FGFR1, and Klotho mRNA levels were determined versus β-actin mRNA by quantitative real-time reverse transcriptase–PCR (Light cycler; Roche Diagnostics, Basel, Switzerland). Amplifications versus actin were performed using a reverse transcriptase–PCR kit (Qiagen Sybr Green). The primers used for PCR are shown in Table 1.
Table 1.
Primers used for quantitative reverse transcriptase–PCR
| Primer | Forward | Reverse |
|---|---|---|
| CaSR | 5′-TGGAGAGACAGATGCGAGTG-3′ | 5′-GTCCACGCCAGAAACTCAAT-3′ |
| VDR | 5′-ACAGTCTGAGGCCCAAGCTA-3′ | 5′-TCCCTGAAG TCAGCGTAGGT-3′ |
| β-Actin | 5′-TGTCACCAACTGGGACGATATGGAG-3′ | 5′-ACAATGCCAGTGGTACGACCAGA-3′ |
| PTH | 5′-TTGTCTCCTTACCCAGGCAGAT-3′ | 5′-TTTGCCCAGGTTGTGCATAA-3′ |
| FGFR1 | 5′-CAATGTCTCAGATGCACTGCCA-3′ | 5′-ACAGGCCTACGGTTTGGTTTG-3′ |
| Klotho | 5′-GAAAATGGCTGGTTTGTCTCG-3′ | 5′-CCTGATGGCTTTTAAGCTTTC-3′ |
Immunohistochemistry
Parathyroid glands were fixed in 4% formalin and embedded in paraffin. Three-micrometer sections were deparaffinized and incubated in 0.3% H2O2 in methanol for 30 minutes. Next, sections were microwave-treated in 0.01 mmol/L citrate buffer (pH 6) for 20 minutes. Sections were blocked with goat serum 10% for 40 minutes, then sections were incubated overnight at 4°C in a humidified chamber with primary affinity-purified rabbit anti–phospho-ERK1/2 (T-202/Y204) antibody (R&D Systems, Minneapolis, MN; goat anti-mouse antibody, 1:100 dilution), rat anti-VDR mAb (Chemicon Int., Temecula, CA; 1:100 dilution), mouse anti-FGFR1 mAb (GeneTex, Irvine, CA; 1:200 dilution), mouse anti-p21waf1 mAb (Chemicon Int.; 1:50 dilution), mouse anti-PCNA (PC10) mAb (Santa Cruz Biotechnology, Santa Cruz, CA; 1:100 dilution), or rabbit anti-klotho antibody (Alpha Diagnostic, Int., San Antonio, TX; 1:50 dilution). After rinsing, the sections were incubated for 40 minutes at room temperature with peroxidase-labeled polymer conjugated to goat anti-mouse/rabbit Igs and treated with 3,3′ diaminobenzidine-tetrachloride chromogen solution for 10 to 20 minutes (En-Vision + System-HRP [DAB]; DakoCytomation, Glostrup, Denmark; 1:100 dilution). Every step was followed by three washes with PBS for 5 minutes. Sections were counterstained with hematoxylin (Dako). Immunoreactivity was assessed using NIH image freeware 1.62. Distinct positive staining was quantified in randomly selected areas on each specimen, in a blind manner, over a minimum of five fields in more than three sections. The number of cells expressing VDR, PCNA, and p21 was determined by counting a minimum of 1000 nuclei per slide and reported as the number of positive nuclei per 1000. The expression of CaR, phospho-ERK1/2, Klotho, and FGR1 was measured by calculating the average OD per section of tissue by dividing the sum integrated OD by the sum area (background staining was subtracted from this value). Then, the staining was expressed relative to that of controls. For avoiding the influence of nonspecific positive staining, plots of <30 pixels were excluded. Values are expressed as the LI: Positive nuclei per 1000 (VDR-LI, PCNA-LI, and p21-LI) or staining relative to controls (CaR-LI, phospho-ERK1/2-LI, Klotho-LI, and FGFR1-LI). Repeat counts showed variability of LIs <10%.
Chemicals
All chemical products of culture medium were obtained from Sigma (St. Louis, MO). PD98059 (ERK1/2–mitogen-activated protein kinase inhibitor) was purchased from Calbiochem (San Diego, CA). The rat FGF23 used in this work was obtained from the 293F cells and was supplied by Amgen, Inc. (Thousand Oaks, CA).
Statistical Analysis
Values are expressed as means ± SEM. The difference between means for two different groups was determined by t test; the difference between means for three or more groups was assessed by ANOVA followed by post hoc Bonferroni correction analysis or two-way ANOVA for multiple dependent variables. P < 0.05 was considered significant.
Disclosures
V.S. is an employee and shareholder of Amgen; and M.R. receives research funding from Amgen.
Acknowledgments
This study was supported by Instituto Carlos III (FIS 07-0287, FIS 07/0315) and Junta de Andalucia (0025/07). Y.A. is a senior researcher supported by the Fundacion Progreso y Salud, Consejeria de Salud (Junta de Andalucia). We thank Juan A. Ballesteros from COVAP for technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98: 6500–6505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblat KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M: Regulation of fibroblast growth factor-23 signaling by Klotho. J Biol Chem 281: 6120–6123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goet R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J: The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE: Fibroblast growth factor-23 regulates parathyroid hormone and 1α-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195: 125–131, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB: Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T, Fukagawa M: Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients Kidney Int 67: 1171–1178, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Almaden Y, Canalejo A, Hernandez A, Ballesteros E, Garcia-Navarro S, Torres A, Rodriguez M: Direct effect of phosphorus on PTH secretion from whole rat parathyroid gland in vitro. J Bone Miner Res 11: 970–976, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Almadén Y, Hernández A, Torregrosa V, Canalejo A, Sabate L, Fernández-Cruz L, Campistol JM, Torres A, Rodriguez M: High phosphorus directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol 9: 1845–1852, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Slatopolsky E, Finch J, Denda M, Ritter C, Zhong M, Dusso A, MacDonald P, Brown A: Phosphorus restriction prevents parathyroid gland growth: High phosphate directly stimulates PTH secretion in vitro. J Clin Invest 97: 2534–2540, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garfia B, Canadillas S, Canalejo A, Luque F, Siendones E, Quesada M, Almaden Y, Aguilera-Tejero E, Rodriguez M: Regulation of parathyroid vitamin D receptor expression by extracellular calcium. J Am Soc Nephrol 13: 2945–2952, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Mendoza FJ, Lopez I, Canalejo R, Almaden Y, Martin D, Aguilera-Tejero E, Rodriguez M: Direct upregulation of parathyroid calcium-sensing receptor and vitamin D receptor by calcimimetics in uremic rats. Am J Physiol Renal Physiol 296: F605–F613, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Merke J, Hügel U, Zlotkowsk A, Szabó A, Bommer J, Mall G, Ritz E: Diminished parathyroid 1,25(OH)2D3 receptors in experimental uremia. Kidney Int 32: 350–353, 1987 [DOI] [PubMed] [Google Scholar]
- 15. Szabó A, Ritz E, Schmidt-Gayk H, Reichel H: Abnormal expression and regulation of vitamin D receptor in experimental uremia. Nephron 73: 619–628, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Brown AJ, Ritter CS, Finch JL, Slatopolsky EA: Decreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: role of dietary phosphate. Kidney Int 55: 1284–1292, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD: FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol 19: 2342–2350, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y: Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Björklund P, Krajisnik T, Akerström G, Westin G, Larsson TE: Type I membrane klotho expression is decreased and inversely correlated to serum calcium in primary hyperparathyroidism. J Clin Endocrinol Metab 93: 4152–4157, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y: Alpha-Klotho as a regulator of calcium homeostasis. Science 316: 1615–1618, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N: Circulating FGF-23 is regulated by 1alpha, 25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280: 2543–2549, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Koiwa F, Kazama JJ, Tokumoto A, Onoda N, Kato H, Okada T, Nii-Kono T, Fukagawa M, Shigematsu T: Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial 9: 336–339, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vindelov L, Christensen IJ: Detergent and proteolytic enzyme-based techniques for nuclear isolation and DNA content analysis. In: Methods in Cell Biology, Vol. 41, 2nd Ed., Flow Cytometry, edited by Darzynkiewicz Z, Robinson JP, Crissman HA, New York, Academic Press, 1994, pp 219–229 [DOI] [PubMed] [Google Scholar]
- 25. Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium isothyocianate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]