Abstract
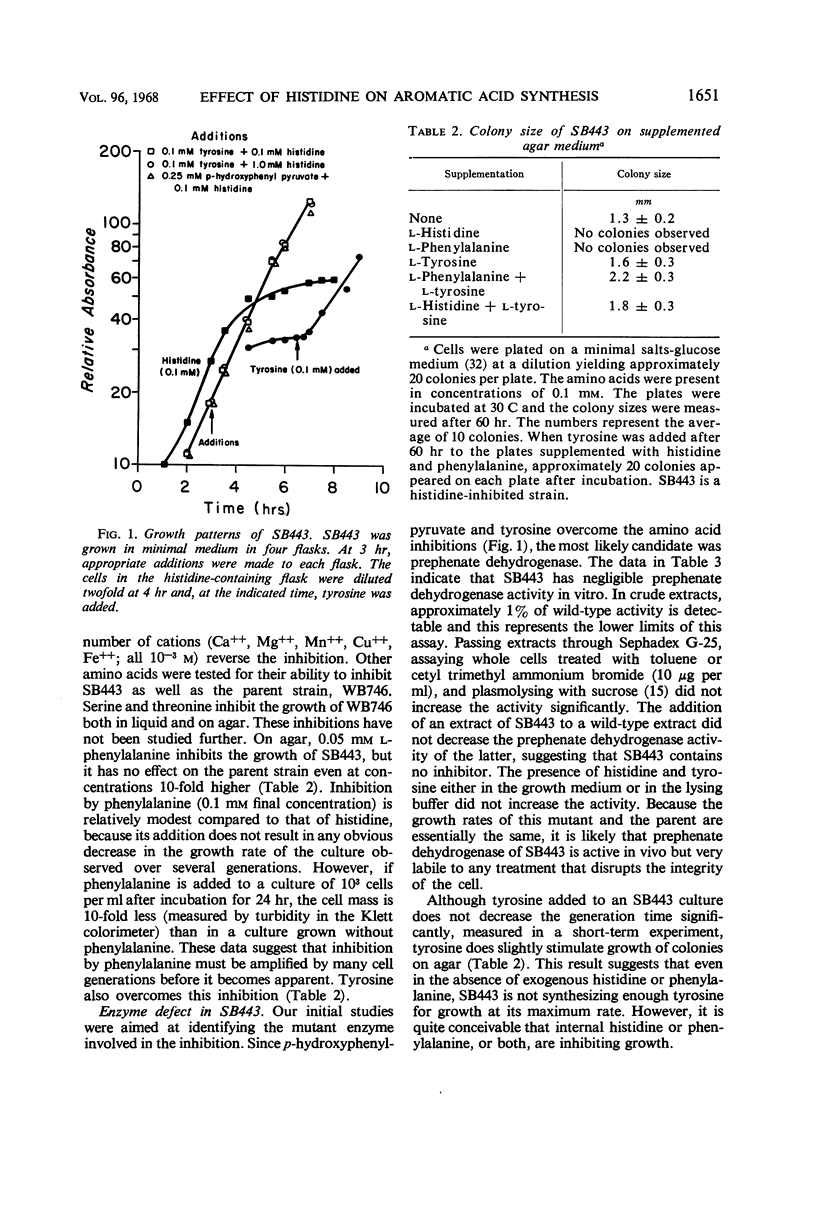
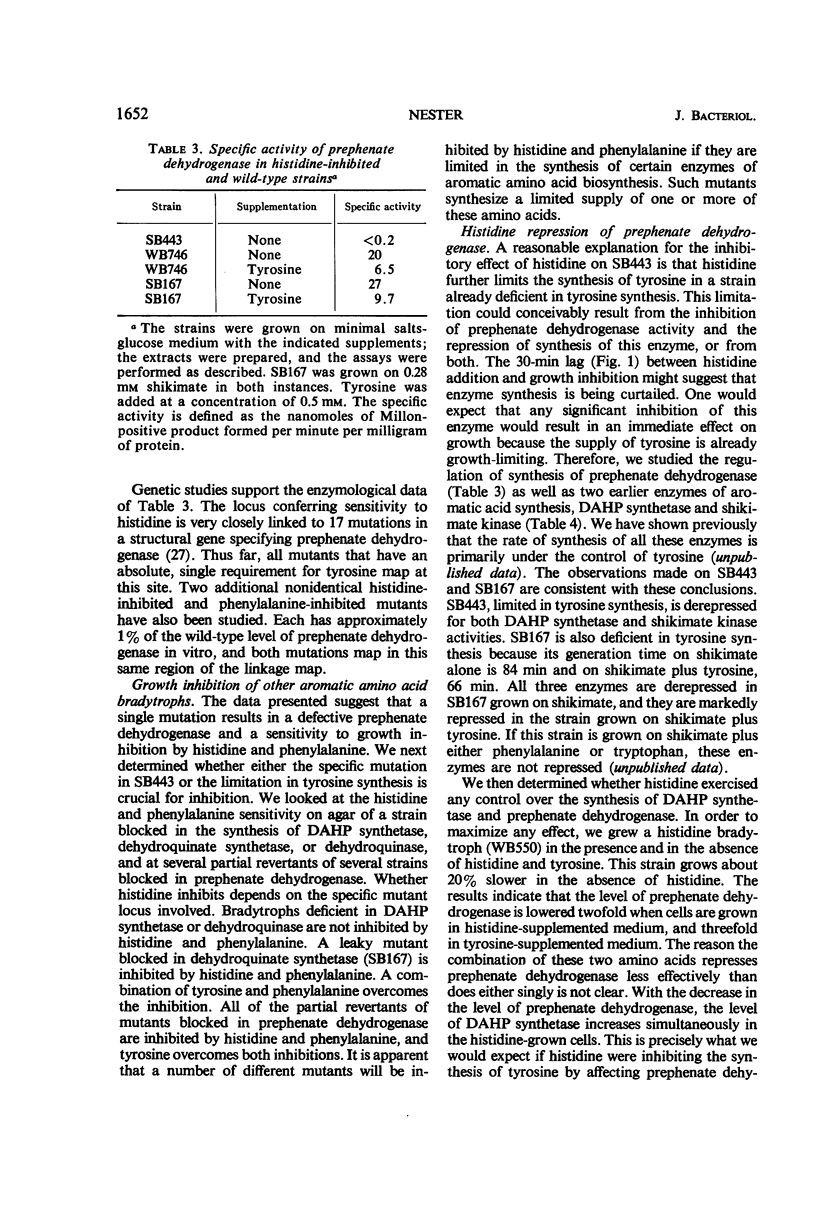
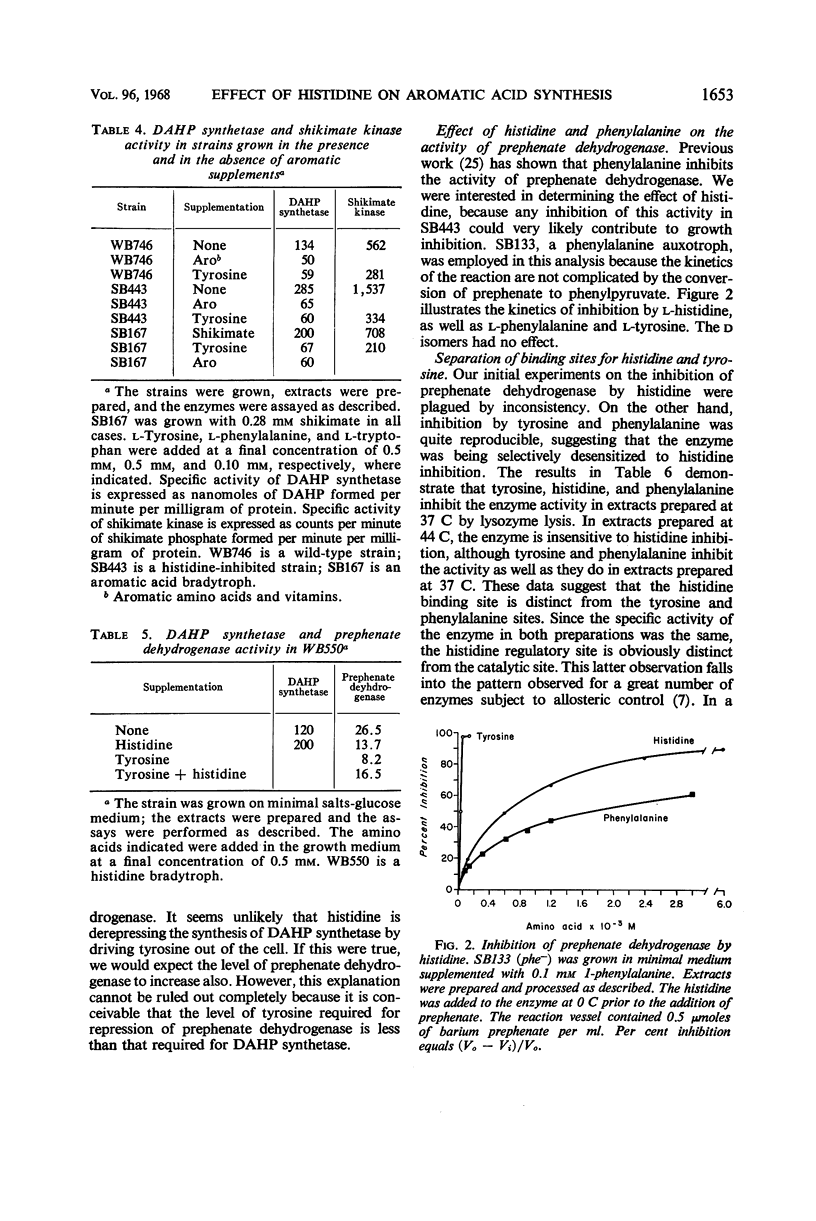
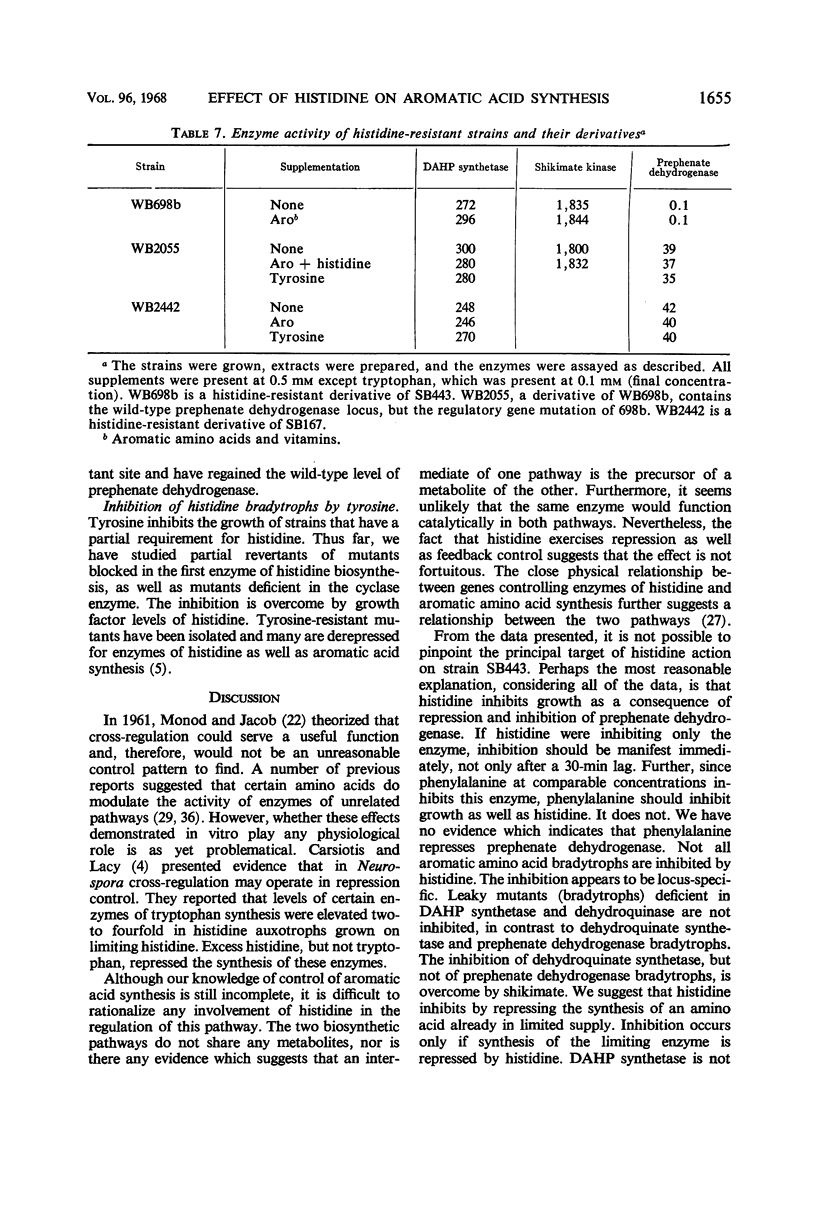
l-Histidine and, to a lesser degree, l-phenylalanine at concentrations of 10−4m inhibit the growth of leaky mutants (bradytrophs) of Bacillus subtilis that are deficient in the synthesis of p-hydroxyphenylpyruvate, the first intermediate specific to tyrosine synthesis. The inhibition can be overcome by growth factor amounts of l-tyrosine and p-hydroxyphenylpyruvate. Histidine and phenylalanine are capable of inhibiting the synthesis of tyrosine in several ways, and the major physiological effect which results in growth inhibition has not been established. Both l-histidine and l-phenylalanine inhibit the activity of prephenate dehydrogenase at concentrations about 100-fold higher than the inhibitory concentration of l-tyrosine. Histidine also appears to repress the synthesis of prephenate dehydrogenase because a histidine bradytroph growing in histidine-supplemented medium has a twofold lower level of this enzyme than the same cells growing in unsupplemented medium. These same two amino acids also inhibit the growth of a bradytroph deficient in dehydroquinate synthetase, an early enzyme in the pathway of tyrosine, phenylalanine, and tryptophan synthesis. The inhibition is overcome by a combination of tyrosine and phenylalanine. Histidine-resistant derivatives of both the prephenate dehydrogenase and dehydroquinate synthetase-deficient strains, which simultaneously have gained resistance to phenylalanine, have been isolated. Most of these resistant mutants synthesize additional tyrosine compared with the parent strain. One class of resistant mutants excretes tyrosine and has a number of enzymes of aromatic acid synthesis which are no longer repressible by any combination of the aromatic amino acids. Tyrosine inhibits the growth of histidine bradytrophs. Histidine, at growth factor levels, overcomes the inhibition.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- CARSIOTIS M., LACY A. M. INCREASED ACTIVITY OF TRYPTOPHAN BIOSYNTHETIC ENZYMES IN HISTIDINE MUTANTS OF NEUROSPORA CRASSA. J Bacteriol. 1965 Jun;89:1472–1477. doi: 10.1128/jb.89.6.1472-1477.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman L. F., Nester E. W. Common element in the repression control of enzymes of histidine and aromatic amino acid biosynthesis in Bacillus subtilus. J Bacteriol. 1968 Nov;96(5):1658–1663. doi: 10.1128/jb.96.5.1658-1663.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats J. H., Nester E. W. Regulation reversal mutation: characterization of end product-activated mutants of Bacillus subtilis. J Biol Chem. 1967 Nov 10;242(21):4948–4955. [PubMed] [Google Scholar]
- Cohen G. N. Regulation of enzyme activity in microorganisms. Annu Rev Microbiol. 1965;19:105–126. doi: 10.1146/annurev.mi.19.100165.000541. [DOI] [PubMed] [Google Scholar]
- Dalal F. R., Gots R. E., Gots J. S. Mechanism of adenine inhibition in adenine-sensitive mutants of Salmonella typhimurium. J Bacteriol. 1966 Feb;91(2):507–513. doi: 10.1128/jb.91.2.507-513.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudney C. O., Wagner R. P. Threonine Inhibition in a Strain of Neurospora. Proc Natl Acad Sci U S A. 1952 Mar;38(3):196–205. doi: 10.1073/pnas.38.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros F., Gallant J., Weisberg R., Cashel M. Decryptification of RNA polymerase in whole cells of Escherichia coli. J Mol Biol. 1967 May 14;25(3):555–557. doi: 10.1016/0022-2836(67)90206-9. [DOI] [PubMed] [Google Scholar]
- JENSEN R. A., NESTER E. W. THE REGULATORY SIGNIFICANCE OF INTERMEDIARY METABOLITES: CONTROL OF AROMATIC ACID BIOSYNTHESIS BY FEEDBACK INHIBITION IN BACILLUS SUBTILIS. J Mol Biol. 1965 Jun;12:468–481. doi: 10.1016/s0022-2836(65)80270-4. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. I. Purification and properties of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3365–3372. [PubMed] [Google Scholar]
- KINDLER S. H., BEN-GURION R. LETHAL EFFECT OF L-LEUCINE ON E. COLI, IN VIVO CORROBORATION OF CODING AMBIGUITY. Biochem Biophys Res Commun. 1965 Feb 3;18:337–340. doi: 10.1016/0006-291x(65)90709-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorence J. H., Nester E. W. Multiple molecular forms of chorismate mutase in Bacillus subtillis. Biochemistry. 1967 May;6(5):1541–1553. doi: 10.1021/bi00857a041. [DOI] [PubMed] [Google Scholar]
- MONOD J., JACOB F. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Symp Quant Biol. 1961;26:389–401. doi: 10.1101/sqb.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. INHIBITION OF THE BIOSYNTHESIS OF THE PYRIMIDINE PORTION OF THIAMINE BY ADENOSINE. J Bacteriol. 1964 Oct;88:1024–1029. doi: 10.1128/jb.88.4.1024-1029.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYED H. S. Interference with feedback control of enzyme activity. Cold Spring Harb Symp Quant Biol. 1961;26:323–329. doi: 10.1101/sqb.1961.026.01.039. [DOI] [PubMed] [Google Scholar]
- Meuris P., Lacroute F., Slonimski P. P. Etude systematique de mutants inhibes par leurs propres metabolites chez la levure Saccharomyces cerevisiae. I. Obtention et caracterisation des differentes classes de mutants. Genetics. 1967 May;56(1):149–161. doi: 10.1093/genetics/56.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Lorence J. H., Nasser D. S. An enzyme aggregate involved in the biosynthesis of aromatic amino acids in Bacillus subtilis. Its possible function in feedback regulation. Biochemistry. 1967 May;6(5):1553–1563. doi: 10.1021/bi00857a042. [DOI] [PubMed] [Google Scholar]
- Patte J. C., Cohen G. N. Isolement et propriétés d'un mutant d'Escherichia coli depourvu d'aspartokinase sensible à la lysine. Biochim Biophys Acta. 1965 Jun 22;99(3):561–563. [PubMed] [Google Scholar]
- Patte J. C., Loviny T., Cohen G. N. Effets inhibiteurs cooperatifs de la L-lysine avec d'autres amino acides sur une aspartokinase de Escherichia coli. Biochim Biophys Acta. 1965 Jun 22;99(3):523–530. [PubMed] [Google Scholar]
- SCHWINCK I., ADAMS E. Aromatic biosynthesis. XVI. Aromatization of prephenic acid to p-hydroxyphenylpyruvic acid, a step in tyrosine biosynthesis in Escherichia coli. Biochim Biophys Acta. 1959 Nov;36:102–117. doi: 10.1016/0006-3002(59)90074-5. [DOI] [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- STURANE E., DATTA P., HUGHES M., GEST H. REGULATION OF ENZYME ACTIVITY BY SPECIFIC REVERSAL OF FEEDBACK INHIBITION. Science. 1963 Sep 13;141(3585):1053–1054. doi: 10.1126/science.141.3585.1053. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Allosteric regulation of enzyme activity. Adv Enzymol Relat Areas Mol Biol. 1966;28:41–154. doi: 10.1002/9780470122730.ch2. [DOI] [PubMed] [Google Scholar]
- TOMKINS G. M., YIELDING K. L. Regulation of the enzymic activity of glutamic dehydrogenase mediated by changes in its structure. Cold Spring Harb Symp Quant Biol. 1961;26:331–341. doi: 10.1101/sqb.1961.026.01.040. [DOI] [PubMed] [Google Scholar]
- WILKINS J. H., BARNES J. H. Effects of phencyclidine on the radiosensitivity of mice. Nature. 1962 Sep 22;195:1173–1175. [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Genetic and biochemical analysis of the isoenzymes concerned in the first reaction of aromatic biosynthesis in Escherichia coli. J Bacteriol. 1967 Jan;93(1):237–244. doi: 10.1128/jb.93.1.237-244.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


