Abstract
Some reports suggest that autologous hematopoietic stem cell transplantation holds potential for treatment of renal diseases such as lupus nephritis, but the safety of delivering various stem cell types (hematopoietic, mesenchymal, and endothelial precursors) is not well established. Here, we report a case of lupus nephritis treated by direct renal injection of autologous stem cells recovered from peripheral blood. The patient developed masses at the sites of injection and hematuria. We suspected transitional cell carcinoma but nephrectomy revealed that the masses were angiomyeloproliferative lesions. We believe that this previously undescribed pathologic entity is stem cell–derived or –induced. The biologic potential, including the neoplastic potential, of this lesion is unknown. This case illustrates that the development of angiomyeloproliferative lesions is a possible complication of stem cell therapy.
To date, stem cell transplantation has seen only limited application for treatment of chronic renal disease in humans, whereas results from animal models have provided optimism for possible application to patients.9,13,21 Autologous hematopoietic stem cell (HSC) transplantation has been used to treat lupus nephritis.3,6,10 The rationale in this situation is not to reconstitute the kidney with new cells that effect repair but rather to “reset” the immune system by replacing autoreactive lymphocytes with populations from precursor cells that are not autoreactive.10 Most protocols use peripheral blood with stem cell mobilization and CD34+ cell selection; the cells are then administered systemically. Complete remission of renal disease has been reported, but about one third of patients relapse or progress.3,6,10
We present the unusual case of a 46-year-old female patient who had suffered from systemic lupus erythematosus for over 20 years with diffuse proliferative lupus nephritis diagnosed by biopsy in 2000. At that time, ultrasound of the kidneys was normal. Despite treatment with cyclophosphamide and prednisolone, the patient progressed to end-stage renal disease. In 2006, she elected to undergo stem cell therapy for treatment of renal disease at a private clinic. For this treatment, autologous HSCs were mobilized by G-CSF and collected from peripheral blood. The stem cell preparation was later injected percutaneously into the regions of both kidneys via multiple blind passes during a single procedure. Additional details of this treatment are not available. There was no improvement in renal function and the patient began hemodialysis 3 months after stem cell therapy. Six months after therapy, the patient presented with left flank pain and hematuria. Ultrasound and magnetic resonance imaging studies showed a 4.0-cm enhancing mass in the left renal pelvis, with smaller lesions in the left kidney, the liver, and right adrenal gland. A chest x-ray was negative. A magnetic resonance image of the brain showed diffuse cerebral atrophy but no mass lesions. The clinical impression was urothelial cell carcinoma with metastatic spread to the right adrenal and liver. The patient underwent a left nephrectomy 11 months after stem cell therapy. The patient continued on hemodialysis over the next year but gradually deteriorated and died of sepsis after infection of the arteriovenous shunt. Erythropoietin had been started before 2006 and was continued up to the time of the patient's demise, but hemoglobin always remained at pre-erythropoetin levels in the range of 9.3 to 11.5 g/dl (normal 12 to 16). Complete blood count results from the time of diagnosis of lupus nephritis up to the time of the patient's death showed normal leukocyte counts and differential and normal platelet count, with no evidence of immature forms on peripheral smears. No bone marrow biopsy was performed.
The resected left kidney weighed 200 g and the renal pelvis and calyces were dilated. There was a well-circumscribed mass in the renal pelvis, involving adipose tissue and impinging on the pelvicalyceal system (Figure 1 ). The mass measured 3.5 cm with a red-brown spongy cut surface. In addition, three similar lesions measuring up to 5 mm were found in the renal parenchyma, one in the cortex and two in the medulla. Elsewhere, the renal parenchyma showed a granular appearance with areas of scarring and by light microscopy the kidney showed end-stage damage with diffuse glomerulosclerosis but no active lupus nephritis. The red solid lesions consisted of a proliferation of vascular channels separated by varying amounts of loose fibrous connective tissue (Figure 2). The channels were lined by a single layer of endothelial cells that lacked atypia and mitotic activity. Focal areas of necrosis and recent hemorrhage were noted. By immunohistochemistry (Figure 3), the endothelial cells expressed the endothelial markers CD31 and CD34, but not D2-40 (a lymphatic marker). Both VEGF receptor 1 (Flt-1) and VEGF receptor 2 (Flk-1) were diffusely expressed. No cycling of endothelial cells was detected by MIB1 (Ki67) expression. Within the vascular channels, there was a striking number of hematopoietic precursor cells predominantly from the megakaryocytic and erythroid lineages (Figure 4). Intimately mixed with these cells was a population of macrophages. Macrophages were also noted in the fibrous connective tissue of the lesion. The hematopoietic cells did not appear cytologically atypical but mitotic activity was apparent and virtually 100% of these cells were cycling as determined by MIB1 (Ki67) expression. By immunohistochemistry, the intravascular cells expressed CD15 (focal in myeloid cells), CD117 (focal in myeloid cells), and CD61 (megakaryocytes), CD44 (all), CD68 (macrophages), and Flk1 (focal in myeloid cells); Flt1 staining was negative.
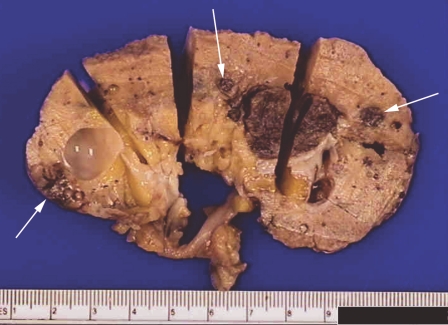
Figure 1.
Macroscopic appearance of resected kidney. A solid hemorrhagic mass is present in the renal sinus, external to which is atrophic renal parenchyma. In addition, three similar smaller lesions are present (arrows), separate from the main lesion.
Figure 2.
Microscopic appearance of lesion. (A) The lesion is composed of numerous vascular channels, containing red cells and mixtures of hematopoietic cells. (B) The vascular component of the lesion consists of channels of varying caliber lined by benign-appearing endothelial cells. (C) and (D) The hematopoietic cells are mainly erythroid precursors with a smaller number of myeloid and megakaryocytic precursors mixed with numerous macrophages (larger foamy cells). (Hematoxylin and eosin; magnification: ×200 in A; ×600 in B through D.)
Figure 3.
Immunohistochemical profile of endothelial cells. Endothelial cells express (A) CD31, (B) VEGF receptor 1/Flt-1, and (C) VEGF receptor 2/Flk-1. (Immunoperoxidase; magnification: ×600 in A through C.)
Figure 4.
Immunohistochemical panel of hematopoietic cells. (A) There is widespread expression of CD44, marking the erythroid precursors but not megakaryocytic cells. (B) Virtually 100% of hematopoietic cells were cycling as demonstrated by MIB1 expression, whereas endothelial cells were not cycling. (C) CD68 staining highlights the large number of macrophages present both in vascular channels and in the intervening stroma. (Immunoperoxidase; magnification: ×600 in A and B; ×200 in C.)
Thus, the lesions in this patient showed both angioproliferative and myeloproliferative components, with an appearance not typical of any tumor or reactive condition. Given that there are multiple lesions in the left kidney and this patient received multiple injections of stem cells into this area, the logical conclusion is that these lesions are stem cell–derived or stem cell–induced. Because the exact number of injections is not known other than “multiple,” the number of lesions in the left kidney may or may not have exceeded the number of injections. We cannot rule out that one or more of the smaller lesions could have spread from the main lesion. Although the lesions in the liver and right adrenal were never biopsied, we postulate that these are the same as those in the left kidney. All injections were given blindly and the liver and right adrenal may have been injected instead of the right kidney, particularly because no lesions were detected in the right kidney.
Autologous HSCs have been used in bone marrow transplant patients and occasionally in lupus patients, but never have lesions occurred after therapy as were seen in this patient. We believe this may be related to the route of administration because the multiple masses almost surely correspond to the sites of injection of cells, whereas the usual approach for lupus treatment would be to inject the stem cells systemically. In this particular case, the patient derived no benefit from this therapy; there was no improvement in renal function and there was no improvement in renal histology; nephrons adjacent to the lesions remained atrophic.
Although the histology of these lesions appears to be benign, it is unknown if these are truly neoplastic or localized proliferations of normal stem cells. If they are stem cell–derived or –induced, the proliferative and recurrence potentials are unknown and the classic concepts of benign and malignant may therefore not apply. Although the lesions contained precursor hematopoietic cells, no changes were noted on any peripheral blood smears with respect to absolute cell counts or the presence of immature forms. Thus, it would appear these cells were not entering the circulation in detectable numbers. Continued follow-up would be critical to establish the natural course of such lesions. Unfortunately, the patient died and no autopsy was performed to determine if such lesions persisted or had spread to noninjected sites.
One possibility is that these lesions are foci of extramedullary hematopoiesis (EMH). We believe this is unlikely for two reasons. First, the rare reports of EMH in the kidney (reviewed in5) have occurred in the setting of myelofibrosis or severe marrow impairment, for which there was no evidence in our patient. Second, each lesion was diffusely angiomatous, whereas the myeloproliferative portion was less prominent, present in about one third of the total vascular area. This is not the appearance expected for EMH and does not match the illustrations in previous reports of EMH in the kidney.5
Experimental systems may provide some clues regarding pathogenesis of these lesions. Direct injection of autologous HSCs into nonischemic muscle has been used to treat ischemic limbs.24 Vessel proliferation occurred in the ischemic tissue and continued for 2 months after injection, implying HSCs can initiate angiogenesis and the process can be self-sustaining. However, there was no angiogenesis at the site of injection, suggesting that ischemia was necessary for endothelial cell proliferation. Although it is possible that in our patient the kidney tissue was relatively ischemic due to end-stage damage, other lesions developed outside of the kidney, indicating organ ischemia was not necessary. Angiogenic lesions have also occurred in experiments that have used transient VEGF gene expression to increase the vascular supply to ischemic myocardium and ischemic limbs.8,17,18,22,23,25 Ectopic angiogenesis in the liver, spleen, and lung and excessive angiogenesis at the injection site has been noted.23 There is also a report of a spider angioma appearing on the skin of a patient treated by intra-arterial delivery of a VEGF-expressing plasmid for an ischemic leg.7 The angioma resolved by 8 weeks, by which time expression of VEGF had ceased. These lesions, however, do not match the pathology seen in our case. However, a good match is seen in lesions that follow constitutive expression of VEGF. Myoblasts constitutively expressing VEGF implanted into ischemic mouse skeletal muscle15,17 and mouse myocardium8 resulted in hemangioma formation at the implantation site within 15 days. Similarly, a capsule containing these mouse myoblasts implanted subcutaneously or intraperitoneally resulted in a tissue mass of dilated capillaries and solid areas of endothelial cells, infiltrated by myeloid cells and macrophages attributed to the fact that these cells express VEGF receptors.16,30
The lesions in our patient showed these same histologic features. However, because our patient received no VEGF therapy, it must be possible for stem cells from peripheral blood to give rise to such lesions if injected directly. It is reasonable to expect large numbers of hematopoietic cells in the lesions because the injected cells would include mainly HSCs. Macrophages could be derived from these same cells or could be recruited to the lesion. The basis of the angioproliferative component is less clear but one possible source could be endothelial progenitor cells (EPCs).1 EPCs facilitate angiogenesis by activating mature endothelial cells.14,21,29 EPCs express CD34 (as do HSCs) and can be isolated from bone marrow and peripheral blood21 and thus could have been injected into our patient after enrichment for CD34+ cells. In the angioproliferative lesions that followed constitutive VEGF expression, VEGF had recruited HSCs and EPCs from the marrow to sites of angiogenesis.26,30 VEGF-induced recruitment would not be necessary in our patient because direct injection of stem cells would eliminate the need for HSCs and EPCs to travel. Nevertheless, if the animal models of constitutive VEGF expression are predictive, then sustained levels of VEGF would be needed for these lesions to continue to develop. Macrophages are a source of VEGF2 and macrophages are also recruited by VEGF.15,17 Because macrophages were plentiful in the patient's lesions, they theoretically could be a source for locally increased VEGF, but this remains speculative.
The histologic appearance of the lesions is also reminiscent of the blood lakes in the human yolk sac, a site of early hematopoiesis in the embryo.12,19,20 In the yolk sac, vascular endothelial cells, or a precursor of them, are able to differentiate into myeloid cells.4,11,19,27,28 This might account for the combined angioproliferative and myeloproliferative features of the lesions observed in our patient. However, these lesions are not completely explained as a recapitulation of blood lakes. First, the histology is not identical in that myeloid cells in blood lakes are attached to the endothelium in clumps,12,19,20 whereas this was not observed in our patient's lesions. Second, the myeloid cells in blood lakes are CD34+CD45− as are the endothelial cells (in keeping with a common precursor),19,20 whereas the myeloid cells in the renal lesions were CD34−CD45+, indicating a more mature phenotype. Finally, the majority of cells injected into our patient were HSCs and there is no evidence that HSCs can differentiate into vasculogenic cells or dedifferentiate into the multipotential precursor cells present in the yolk sac.
In conclusion, results from animal models studying various organs including kidney have concluded that therapy using HSCs and other stem cells is safe, with clinical trials planned to expand this type of treatment for human disease. Our case shows that it is possible for a proliferative lesion of unknown biologic potential to occur after HSC therapy, sounding a note of caution for those embarking on such therapy before the causative mechanisms are elucidated.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Stem Cell Therapy for the Kidney: A Cautionary Tale,” on pages 1070–1072.
REFERENCES
- 1. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM: Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW: Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res 62: 7042–7049, 2002 [PubMed] [Google Scholar]
- 3. Chen J, Wang Y, Kunkel G, Zhao H, Xue H, Xie X, Li L, Xu C, Shen L, Gu L: Use of CD34+ autologous stem cell transplantation in the treatment of children with refractory systemic lupus erythematosus. Clin Rheumatol 24: 464–468, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Dzierzak E: Ontogenic emergence of definitive hematopoietic stem cells. Curr Opin Hematol 10: 229–234, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Gibbins J, Pankhurst T, Murray J, McCafferty I, Baiden-Amissahk K, Shafeek S, Lipkin GW: Extramedullary haematopoiesis in the kidney: A case report and review of literature. Clin Lab Haematol 27: 391–394, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Gratwohl A, Passweg J, Bocelli-Tyndall C, Fassas A, van Laar JM, Farge D, Andolina M, Arnold R, Carreras E, Finke J, Kotter I, Kozak T, Lisukov I, Lowenberg B, Marmont A, Moore J, Saccardi R, Snowden JA, van den Hoogen F, Wulffraat NM, Zhao XW, Tyndall A: Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant 35: 869–879, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes JF: Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 348: 370–374, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM: VEGF gene delivery to myocardium: Deleterious effects of unregulated expression. Circulation 102: 898–901, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Little MH: Regrow or repair: Potential regenerative therapies for the kidney. J Am Soc Nephrol 17: 2390–2401, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Marmont AM, Gualandi F, van Lint MT, Guastoni C, Bacigalupo A: Long term complete remission of severe nephrotic syndrome secondary to diffuse global (IV-G) lupus nephritis following autologous, haematopoietic peripheral stem (CD34+) cell transplantation. Lupus 15: 44–46, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Oberlin E, Tavian M, Blazsek I, Peault B: Blood-forming potential of vascular endothelium in the human embryo. Development 129: 4147–4157, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Palis J, Yoder MC: Yolk-sac hematopoiesis: The first blood cells of mouse and man. Exp Hematol 29: 927–936, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Sagrinati C, Ronconi E, Lazzeri E, Lasagni L, Romagnani P: Stem-cell approaches for kidney repair: Choosing the right cells. Trends Mol Med 14: 277–285, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Shantsila E, Watson T, Lip GY: Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol 49: 741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM: VEGF gene delivery to muscle: Potential role for vasculogenesis in adults. Mol Cell 2: 549–558, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Springer ML, Hortelano G, Bouley DM, Wong J, Kraft PE, Blau HM: Induction of angiogenesis by implantation of encapsulated primary myoblasts expressing vascular endothelial growth factor. J Gene Med 2: 279–288, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Springer ML, Ozawa CR, Banfi A, Kraft PE, Ip TK, Brazelton TR, Blau HM: Localized arteriole formation directly adjacent to the site of VEGF-induced angiogenesis in muscle. Mol Ther 7: 441–449, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Takeshita S, Pu LQ, Stein LA, Sniderman AD, Bunting S, Ferrara N, Isner JM, Symes JF: Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation 90: II228–II234, 1994 [PubMed] [Google Scholar]
- 19. Tavian M, Hallais MF, Peault B: Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development 126: 793–803, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Tavian M, Peault B: The changing cellular environments of hematopoiesis in human development in utero. Exp Hematol 33: 1062–1069, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Tongers J, Losordo DW: Frontiers in nephrology: The evolving therapeutic applications of endothelial progenitor cells. J Am Soc Nephrol 18: 2843–2852, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, Horowitz JR, Symes JF, Isner JM: Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation 94: 3281–3290, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Vajanto I, Rissanen TT, Rutanen J, Hiltunen MO, Tuomisto TT, Arve K, Narvanen O, Manninen H, Rasanen H, Hippelainen M, Alhava E, Yla-Herttuala S: Evaluation of angiogenesis and side effects in ischemic rabbit hindlimbs after intramuscular injection of adenoviral vectors encoding VEGF and LacZ. J Gene Med 4: 371–380, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Van Huyen JP, Smadja DM, Bruneval P, Gaussem P, Dal-Cortivo L, Julia P, Fiessinger JN, Cavazzana-Calvo M, Aiach M, Emmerich J: Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod Pathol 21: 837–846, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J: Vascular endothelial growth factors: Biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol 49: 1015–1026, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Zacchigna S, Pattarini L, Zentilin L, Moimas S, Carrer A, Sinigaglia M, Arsic N, Tafuro S, Sinagra G, Giacca M: Bone marrow cells recruited through the neuropilin-1 receptor promote arterial formation at the sites of adult neoangiogenesis in mice. J Clin Invest 118: 2062–2075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zambidis ET, Peault B, Park TS, Bunz F, Civin CI: Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood 106: 860–870, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zambidis ET, Sinka L, Tavian M, Jokubaitis V, Park TS, Simmons P, Peault B: Emergence of human angiohematopoietic cells in normal development and from cultured embryonic stem cells. Ann N Y Acad Sci 1106: 223–232, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zampetaki A, Kirton JP, Xu Q: Vascular repair by endothelial progenitor cells. Cardiovasc Res 78: 413–421, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, Giacca M: Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood 107: 3546–3554, 2006 [DOI] [PubMed] [Google Scholar]