Abstract
Emerging evidence suggests a role for resistin in inflammation and vascular dysfunction, which may contribute to the pathogenesis of hypertension, but the association between resistin levels and incident hypertension is unknown. We examined the association between plasma resistin levels and the risk for incident hypertension among 872 women without a history of hypertension or diabetes from the Nurses' Health Study. We identified 361 incident cases of hypertension during 14 years of follow-up. After adjustment for potential confounders, resistin levels in the highest tertile conferred a 75% higher risk for hypertension than the lowest tertile (relative risk [RR] 1.75; 95% confidence interval [CI] 1.19 to 2.56). Further adjustment for other adipokines did not change the RR substantially. In stratified analysis, resistin levels in the highest tertile significantly increased the risk for hypertension among women aged ≥55 years (adjusted RR 2.40; 95% CI 1.55 to 3.73) but not among women aged <55 years (adjusted RR 0.64; 95% CI 0.25 to 1.62). In a subset analysis of 362 women who also had measurements of inflammatory and endothelial biomarkers, plasma resistin levels significantly correlated with IL-6, soluble TNF receptor 2, intercellular adhesion molecule 1, vascular adhesion molecule 1, and E-selectin after controlling for age and body mass index. After further adjustment for these biomarkers and C-reactive protein, resistin levels remained significantly associated with incident hypertension. In conclusion, higher plasma resistin levels independently associate with an increased risk for incident hypertension among women without diabetes.
Resistin, a polypeptide derived almost exclusively from adipose tissue in rodents, was originally described as providing a link between obesity and insulin resistance1; however, the putative involvement of resistin in obesity and/or insulin resistance in humans is largely controversial.2,3 In contrast to rodents, human resistin is expressed primarily in inflammatory cells.4–7 Emerging evidence suggests a role for resistin in inflammatory states. For example, in vitro experiments on human peripheral blood mononuclear cells demonstrate that resistin upregulates proinflammatory cytokines; in vivo, resistin can induce inflammation in animal models.8 Furthermore, recombinant resistin upregulates endothelin 1 and vascular adhesion molecule expression on human endothelial cells,9,10 as well as proliferation of human aortic smooth muscle cells,11 suggesting a possible role in vascular dysfunction for this adipokine.
It has been hypothesized that hypertension may in part be a disorder of inflammation and vascular dysfunction.12,13 A recent cross-sectional study from Japan suggested that resistin was an independent predictor of both systolic (SBP) and diastolic BP (DBP) in patients with diabetes, even after adjustment for body mass index (BMI) and fasting glucose.14 A cross-sectional study from Greece demonstrated that “healthy” individuals with prehypertension had significantly higher plasma resistin levels compared with healthy individuals with normotension.15 To the best of our knowledge, however, no study has prospectively investigated whether plasma resistin levels influence the risk for developing hypertension; therefore, we prospectively investigated the association between plasma resistin levels and the risk for incident hypertension among 872 women without diabetes or hypertension from the Nurses' Health Study (NHS).
Results
During the 14 years (9398 person-years) of follow-up of 872 women without hypertension, 361 incident cases of physician-diagnosed hypertension were reported. Participant characteristics by tertiles of plasma resistin level are presented in Table 1. Women with higher plasma resistin were younger; had higher BMI; were less physically active; and had lower intakes of alcohol, magnesium, and calcium. Current smoking was more frequent within higher tertiles of resistin. Although the high molecular weight (HMW)–total adiponectin ratio and leptin were associated with resistin at baseline in crude analysis (Table 1), these adipokines were no longer associated with resistin levels after adjustment for age and BMI (data not shown).
Table 1.
Baseline characteristics by tertiles of resistin
| Variable | Tertile 1 | Tertile 2 | Tertile 3 | P |
|---|---|---|---|---|
| No. of participants | 310 | 289 | 273 | |
| Age (years; median [IQR]) | 57 (49 to 61) | 55 (49 to 62) | 53 (48 to 59) | 0.008 |
| BMI (kg/m2; median [IQR]) | 23.6 (21.6 to 25.8) | 24.8 (22.5 to 28.3) | 25.7 (22.8 to 30.9) | <0.001 |
| Alcohol consumption (g/d; median [IQR]) | 1.8 (0.0 to 7.0) | 1.1 (0.0 to 5.8) | 1.1 (0.0 to 4.7) | 0.04 |
| Physical activity (METs/wk; median [IQR]) | 11.5 (5.1 to 25.7) | 9.2 (3.4 to 21.5) | 7.9 (3.1 to 17.3) | <0.001 |
| Family history of hypertension (%) | 43.2 | 47.4 | 43.2 | 0.97 |
| Current smoker (%) | 8.7 | 10.4 | 15.8 | 0.009 |
| Postmenopausal (%) | 78.1 | 72.0 | 70.7 | 0.04 |
| Dietary factors (median [IQR]) | ||||
| Folate (μg/d) | 372 (276 to 590) | 366 (279 to 573) | 344 (251 to 562) | 0.17 |
| Sodium (mg/d) | 1856 (1645 to 2045) | 1815 (1623 to 2027) | 1797 (1601 to 2044) | 0.37 |
| Potassium (mg/d) | 2897 (2522 to 3261) | 2824 (2544 to 3138) | 2809 (2450 to 3114) | 0.09 |
| Magnesium (mg/d) | 303 (264 to 344) | 300 (255 to 347) | 287 (246 to 330) | 0.009 |
| Calcium (mg/d) | 713 (563 to 900) | 691 (550 to 885) | 656 (523 to 866) | 0.08 |
| Total adiponectin (μg/ml; median [IQR]) | 19.1 (13.7 to 22.9) | 18.4 (13.2 to 22.6) | 17.1 (12.9 to 22.8) | 0.33 |
| HMW–total adiponectin ratio (median [IQR]) | 0.41 (0.33 to 0.48) | 0.39 (0.32 to 0.47) | 0.37 (0.30 to 0.47) | 0.04 |
| Leptin (pg/ml; median [IQR]) | 14.2 (8.1 to 24.9) | 17.0 (10.4 to 27.0) | 20.5 (10.3 to 32.9) | <0.001 |
IQR, interquartile range; MET, metabolic equivalent.
Plasma resistin levels were positively associated with the risk for incident hypertension in age-adjusted and multivariable-adjusted analyses (Table 2). Compared with those in the bottom tertile of resistin, the multivariable relative risk (RR) for incident hypertension for those in the top tertile of resistin was 1.75 (95% confidence interval [CI] 1.19 to 2.56; P < 0.001 for trend). Further adjustment for other adipokines did not change the RRs substantially (1.78 [95% CI 1.20 to 2.65] for the top tertile). To avoid misclassification by including women with undiagnosed hypertension at baseline, we performed two additional analyses. First, we excluded women who never had a physical examination for screening purposes during the follow-up period (n = 70). The results were not markedly changed, and the multivariable RR for the top tertile of resistin was 1.79 (95% CI 1.19 to 2.70). Second, we limited our primary analysis to participants who reported hypertension 1 year after 1990 (n = 36). The multivariable RR for the top tertile of resistin attenuated slightly, which was 1.73 (95% CI 1.16 to 2.60).
Table 2.
Plasma resistin levels and risk of incident hypertension
| Parameter | Plasma Resistin Levels |
P for Trend | ||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| Total | ||||
| person-years | 3415 | 3161 | 2822 | |
| no. of cases | 115 | 115 | 131 | |
| age-adjusted RR (95% CI) | 1.00 (reference) | 1.18 (0.84 to 1.66) | 1.69 (1.20 to 2.37) | <0.001 |
| age- and BMI-adjusted RR (95% CI) | 1.00 (reference) | 1.12 (0.79 to 1.58) | 1.54 (1.08 to 2.18) | <0.001 |
| model A (RR [95% CI])a | 1.00 (reference) | 1.18 (0.82 to 1.70) | 1.75 (1.19 to 2.56) | <0.001 |
| model A + other adipokines (RR [95% CI])b | 1.00 (reference) | 1.25 (0.86 to 1.82) | 1.78 (1.20 to 2.65) | <0.001 |
| Age ≥55 yearsc | ||||
| person-years | 2505 | 2208 | 1771 | |
| no. of cases | 94 | 93 | 107 | |
| age-adjusted RR (95% CI) | 1.00 (reference) | 1.24 (0.85 to 1.81) | 1.94 (1.32 to 2.86) | <0.001 |
| age- and BMI-adjusted RR (95% CI) | 1.00 (reference) | 1.20 (0.82 to 1.77) | 1.97 (1.31 to 2.94) | <0.001 |
| model A (RR [95% CI])a | 1.00 (reference) | 1.24 (0.82 to 1.85) | 2.40 (1.55 to 3.73) | <0.001 |
| model A + other adipokines (RR [95% CI])b | 1.00 (reference) | 1.31 (0.85 to 1.99) | 2.46 (1.55 to 3.90) | <0.001 |
aModel A was adjusted for age; BMI; physical activity; family history of hypertension; current smoking; menopause status; fasting status; and intakes of alcohol, sodium, potassium, magnesium, calcium, and folate.
bAdditional adjustment for plasma total adiponectin, the high molecular weight/total adiponectin ratio, and leptin.
cP = 0.05 for interaction between tertile of resistin and age (≥55 versus <55 years).
After adjustment for age and BMI, total adiponectin (RR for bottom compared with top tertile 0.95 [95% CI 0.65 to 1.38]), the HMW–total adiponectin ratio (RR for bottom compared with top tertile 0.83 [95% CI 0.57 to 1.21]), and leptin (RR for top compared with bottom tertile 1.22 [95% CI 0.72 to 2.06] were not associated with risk for incident hypertension. The results remained NS after additional adjustment for other covariates.
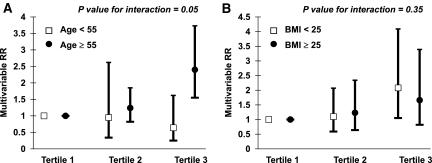
The association between plasma resistin level and risk for incident hypertension was greater among women who were aged ≥55 years (P = 0.05 value for interaction; Figure 1). The multivariable RR for the top tertile of resistin was 2.40 (95% CI 1.55 to 3.73) among older women and was 0.64 (95% CI 0.25 to 1.62) among women who were younger than 55 years (Table 2). We did not observe effect modification by BMI (P = 0.35 value for interaction).
Figure 1.
Increased association between plasma resistin level and risk for incident hypertension among women aged 55 or older. Incident hypertension by tertiles of plasma resistin levels are stratified by age (P = 0.05 for interaction; A) and BMI (P = 0.35 for interaction; B). Multivariate RRs were adjusted for age; BMI; physical activity; family history of hypertension; current smoking; menopause status; fasting status; and intakes of alcohol, sodium, potassium, magnesium, calcium, and folate.
Altogether, 362 women were included in the secondary analysis, with 145 incident hypertension cases through 14 years of follow-up. At baseline, plasma resistin levels were correlated with levels of IL-6 (correlation coefficient 0.24; P < 0.001) and TNF receptor 2 (TNF R2; r = 0.22, P < 0.001) after controlling for age and BMI but not with C-reactive protein (CRP; r = 0.07, P = 0.18; Table 3). Plasma resistin levels were significantly correlated with biomarkers of endothelial dysfunction including intercellular adhesion molecule 1 (ICAM-1; r = 0.15, P = 0.005), vascular adhesion molecule 1 (VCAM-1; r = 0.10, P = 0.05), and E-selectin (r = 0.12, P = 0.02; Table 3).
Table 3.
Correlation between plasma resistin levels and other biomarkers
| Parameter | ra | P | rb | P |
|---|---|---|---|---|
| CRP (mg/L) | 0.18 | <0.001 | 0.07 | 0.18 |
| IL-6 (pg/ml) | 0.30 | <0.001 | 0.24 | <0.001 |
| TNF R2 (pg/ml) | 0.26 | <0.001 | 0.22 | <0.001 |
| ICAM-1 (ng/ml) | 0.16 | 0.003 | 0.15 | 0.005 |
| VCAM-1 (ng/ml) | 0.10 | 0.07 | 0.10 | 0.05 |
| E-selectin (ng/ml) | 0.18 | <0.001 | 0.12 | 0.02 |
aControlled for age.
bControlled for age and BMI.
In this subset of 362 women, the highest compared with lowest tertile of plasma resistin was associated with an increased risk for incident hypertension after multivariable adjustment (RR 2.78 [95% CI 1.07 to 7.23]; Table 4). Further adjustment for either CRP or all inflammatory biomarkers (CRP, IL-6, and TNF R2) simultaneously did not materially alter the RR (Table 4). Similarly, adjustment for endothelial markers including ICAM-1, VCAM-1, and E-selectin did not change RR markedly (RR 2.99 [95% CI 1.09 to 8.16] for the top tertile). Adding all inflammatory and endothelial biomarkers simultaneously into the multivariable model resulted in an RR for the top tertile of 3.01 (95% CI 1.06 to 8.83; Table 4). Because we observed effect modification by age in the primary analysis, we also performed a stratified analysis in this subset of women despite that the P value for interaction was not statistically significant (P = 0.67). After adjustment for all inflammatory and endothelial biomarkers, the RR for the top tertile of resistin was 4.03 (95% CI 1.03 to 18.84) among women aged ≥55 years.
Table 4.
Plasma resistin levels and risk for incident hypertension adjusting for other inflammatory biomarkers
| Parameter | Plasma Resistin Levels |
||
|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |
| Person-years | 1431 | 1372 | 1053 |
| No. of cases | 49 | 43 | 53 |
| Model A (RR [95% CI])a | 1.00 (reference) | 1.34 (0.56 to 3.19) | 2.78 (1.07 to 7.23) |
| Model A + CRP (RR [95% CI]) | 1.00 (reference) | 1.36 (0.57 to 3.27) | 2.98 (1.12 to 7.94) |
| Model A + CRP + IL-6 + TNF R2 (RR [95% CI]) | 1.00 (reference) | 1.34 (0.55 to 3.25) | 2.74 (0.99 to 7.56) |
| Model A + endothelial biomarkers (RR [95% CI])b | 1.00 (reference) | 1.71 (0.65 to 4.47) | 2.99 (1.09 to 8.16) |
| Model A + other biomarkers (RR [95% CI])c | 1.00 (reference) | 1.71 (0.65 to 4.53) | 3.01 (1.06 to 8.83) |
aModel A was adjusted for age; BMI; physical activity; family history of hypertension; current smoking; menopause status; fasting status; and intakes of alcohol, sodium, potassium, magnesium, calcium, and folate.
bEndothelial biomarkers include ICAM-1, VCAM-1, and E-selectin.
cBiomarkers include CRP, IL-6, TNF R2, ICAM-1, VCAM-1, and E-selectin.
Discussion
We present the first prospective study to examine the relation between plasma resistin levels and the risk for incident hypertension. We found that higher plasma resistin levels were independently associated with the risk for incident hypertension. The association was statistically significant only among women aged ≥55 years in stratified analysis. Finally, the association between resistin and hypertension was robust even after controlling for inflammatory and endothelial biomarkers.
Experimental studies suggest a possible role for resistin in the pathogenesis of hypertension. Resistin is primarily involved in the inflammatory process by strongly upregulating IL-6 and TNF-α, probably via the NF-κB pathway.8 Resistin exerts direct effects to promote endothelial cell activation by inducing endothelin 1 release9 and also the expression of adhesion molecules such as ICAM-1 and VCAM-1.9,10 Furthermore, resistin leads to proliferation of human vascular smooth muscle cells.11 All of these processes can induce inflammation within the vascular wall and lead to vascular remodeling and injury. At the functional level, resistin has been shown to reduce endothelium-dependent and -independent vasorelaxation, possibly by increasing superoxide radical production and by decreasing endothelial nitric oxide synthase expression in endothelial cells.16
In this study, plasma resistin levels were strongly and independently correlated with IL-6 and TNF R2, as well as biomarkers of endothelial dysfunction, which corresponds with results of in vitro studies. In contrast, plasma CRP was not correlated with resistin after adjustment for age and BMI in our study, which is consistent with another report of participants without diabetes,17 yet adding these biomarkers to our multivariable models did not eliminate the association between plasma resistin and risk for hypertension, suggesting that other mechanisms explaining the resistin–hypertension link might exist.
Human studies of the association between resistin and hypertension are limited. A cross-sectional study of patients with diabetes from Japan14 revealed that serum resistin levels were higher in patients with hypertension compared with those without hypertension. Moreover, resistin was independently associated with both SBP and DBP. In another small cross-sectional study from Japan, 33 patients with essential hypertensive were classified into as having insulin resistance or not.18 Their plasma resistin levels were compared with 18 volunteers with normotension.18 Plasma resistin levels were nonsignificantly slightly higher in the groups that had essential hypertension without insulin resistance (21.9 ± 8.9 μg/L) and with insulin resistance (20.2 ± 8.2 μg/L) than in the volunteer group (19.0 ± 9.6 μg/L). A report from Greece15 indicated that individuals without diabetes and with prehypertension had significantly higher plasma resistin levels compared with healthy individuals with normotension (10.62 ± 3.17 versus 6.72 ± 3.15 μg/L). Furthermore, plasma resistin levels were found to be higher in healthy offspring of patients with essential hypertension compared with those without a family history of hypertension. These findings suggest that resistin might be associated with hypertension in the population without diabetes and that increased resistin levels may exist before the occurrence of clinical hypertension. Results from this study provide the first prospective evidence of the association between plasma resistin and risk for incident hypertension during 14 years of follow-up.
Although classified as an adipokine, resistin in humans is mainly produced by inflammatory cells, both within and outside the adipose tissue,1,5 distinguishing resistin biologically from adiponectin and leptin. After controlling for total adiponectin, the HMW–total adiponectin ratio (reflecting the proportion of biologically most active form of adiponectin) and leptin, the association between plasma resistin and hypertension did not change markedly, suggesting the resistin–hypertension link is independent of other adipokines.
The association was statistically significant only among women aged ≥ 55 years in stratified analysis. Because the prevalence of hypertension in women who are older than 55 to 60 years is markedly increased and exceeds the prevalence of hypertension in men,19 the strong association among older women may have significant public health implications. The mechanisms that may underlie this finding are unclear, although sex hormones might be one explanation. For example, studies have documented higher resistin levels in premenopausal women compared with men20 and with postmenopausal women.21 Although speculative, the possibility that sex hormones also influence the function of resistin could not be excluded. Unfortunately, there were too few premenopausal women in our study to be able to analyze effect modification by menopausal status.
In our secondary analysis (n = 362), the RRs were larger and the CIs were wider compared with the RRs and CIs in the primary analysis (n = 872). This seeming discrepancy may simply be due to the smaller sample size and smaller number of cases, increasing the instability of the model. Indeed, the RR estimates from the secondary analysis were within the CIs from the primary analysis, so we believe the results were consistent.
Our study has limitations. First, our study population was derived from a preexisting case-control study of diabetes. Although we only included control subjects to avoid bias, the generalizability of results from this study might be limited. Second, BP was not directly measured and hypertension was self-reported; however, all participants are registered nurses, and we demonstrated that hypertension reporting by participants of this cohort is highly sensitive.22 Another concern might be ascertainment bias. For example, overweight and obese individuals, who are susceptible to developing health problems, would have been more likely than normal-weight participants to be screened for and receive a diagnosis of clinical hypertension; however, excluding participants who never had a screening physical examination did not change the results in our analysis. Third, we lacked information on renal function. It has been reported that higher plasma resistin levels were associated with lower estimated GFR,23,24 yet, because all women in our study were free from diabetes and hypertension at baseline, it is unlikely that many women had impaired renal function. Indeed, other studies have indicated a very low prevalence of renal dysfunction in the NHS.25,26 Fourth, we had a single measurement of plasma resistin. Nonetheless, a pilot study indicated that individual plasma resistin levels do not significantly change during at least a period of 1 year.27 Although during 14 years the changes in resistin levels may be more substantial, this type of misclassification would tend to bias our study toward not finding an association; therefore, it is possible that we underestimated the true association. Fifth, because our study was observational, the possibility of residual confounding by some unmeasured covariate exists; however, the association between resistin and hypertension was robust after adjustment for numerous lifestyle and dietary covariates, as well as inflammatory and endothelial biomarkers. Finally, our study was entirely female and mostly white; therefore, the results may not be generalizable to nonwhite individuals or to men.
In conclusion, our prospective analysis suggests that higher plasma resistin levels are independently associated with an increased risk for incident hypertension among women without diabetes. We further confirmed the association noted in experimental studies between resistin and inflammation and endothelial dysfunction; however, these pathways do not fully explain the association between resistin and hypertension. Our findings may increase the understanding of the pathophysiology of hypertension in older individuals and should be tested in other cohorts.
Concise Methods
Source Population
The NHS cohort was assembled in 1976, when 121,700 female nurses aged 30 to 55 years returned a mailed questionnaire. Participants are followed via biennial questionnaires that gather updated information on health-related behaviors and medical events. From 1989 to 1990, 32,826 consenting women provided blood samples returned with a cold pack by overnight mail; 97% of samples were received within 24 hours of collection. All blood samples were stored in liquid nitrogen (≤−130°C) until laboratory analysis. This study was approved by the institutional review board at Brigham and Women's Hospital. Receipt of each questionnaire implied participant's consent.
Study Population
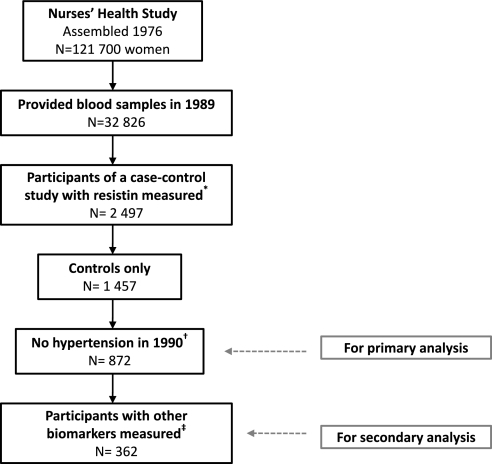
In a nested case-control study in the NHS designed to examine incident diabetes,28 2497 participants had resistin measured and were therefore included in this study (Figure 2). The exclusion criteria were (1) women who developed diabetes between 1990 and June 2004 (“the cases”) to minimize potential bias; (2) participants with prevalent hypertension at the time of blood collection so that our study would be prospective; (3) participants with history of cancer (except for nonmelanoma skin cancer) at the time of blood collection; and (4) participants with missing information on BMI, physical activity, dietary information, or fasting status at baseline (n = 172). These exclusions resulted in 872 eligible participants for our primary analysis. As a secondary analysis, we further limited our study population to women who also had inflammatory and endothelial biomarkers measured (Figure 2).
Figure 2.
Study population for analysis of plasma resistin levels and risk for incident hypertension. *A nested case-control study of diabetes, which is described elsewhere.28 †Participants were also excluded when they reported a history of cancer (except for nonmelanoma skin cancer) at baseline or had missing information on BMI, physical activity, dietary information, or fasting status at baseline. ‡Other biomarkers (including CRP, IL-6, and TNF R2, as well as ICAM-1, VCAM-1, and E-selectin) were measured among women selected as control subjects for those who developed diabetes before June 2000.
Assessment of Plasma Resistin Levels
Resistin concentration was measured by using an ELISA (Linco Research, St. Charles, MO) with a minimum detectable limit of 0.16 ng/ml. On the basis of blinded quality-control samples, the coefficient of variation (CV) for resistin was 2.5%. One study29 indicated that the overall intraclass correlation between resistin measured on blood samples frozen at −70°C for 4 years, 2 years, and 1 year was 0.95, suggesting that long-term storage might not have a major effect on resistin levels.
Assessment of Other Covariates
BMI (calculated as weight in kilograms divided by height in meters squared), physical activity (metabolic equivalent tasks), smoking status, and menopause status were ascertained by questionnaire at baseline. Intakes of alcohol, sodium, potassium, calcium, magnesium, and folate were ascertained from a food frequency questionnaire in 1990. Except for intake of alcohol, nutrient values were adjusted for total energy intake by the residual method.30 The reproducibility and validity of the questionnaire has been documented.31 Other plasma adipokines, including total adiponectin, HMW adiponectin, and leptin, were also measured. The methods for measuring these adipokines are described elsewhere.28,32 In blinded quality-control samples, the CVs for total adiponectin, HMW adiponectin, and leptin were 8.9, 9.9, and 8.3%, respectively.
For the secondary analysis, other measured biomarkers included CRP, IL-6, soluble TNF R2, ICAM-1, VCAM-1, and E-selectin. Assays used for measuring these biomarkers are described elsewhere,33 and CVs ranged from 2.1% for CRP to 9.8% for VCAM-1.
Assessment of Hypertension
The baseline and biennial follow-up questionnaires inquired about physician-diagnosed hypertension and the year of diagnosis. Self-reported hypertension was found to be highly reliable in the NHS.34 In a subset of women (n = 51) who reported hypertension, medical record review confirmed a documented SBP ≥140 mmHg or DBP ≥90 mmHg in 100% of participants.34 Among another subset of women without previous self-reported hypertension (n = 161), only 6.8% of them had recorded BP ≥140/90 mmHg, and none of them had BP ≥160/95 mmHg.34 A participant was considered to have prevalent hypertension when she reported this diagnosis on any questionnaire up to and including the 1990 questionnaire and therefore was excluded from this study. Incident cases included individuals who first reported hypertension on subsequent questionnaires and whose year of diagnosis was after the return of the 1990 questionnaire.
Statistical Analysis
Person-time was truncated at the date of hypertension diagnosis, at the date of death, or at the date of cancer diagnosis (except for nonmelanoma skin cancer), whichever came first. Plasma resistin levels were analyzed in tertiles, using the lowest tertile as the reference group. The relationship between resistin and other covariates at baseline (in 1990) were analyzed using the Kruskall-Wallis test for continuous variables and the Mantel-Hanzel χ2 test of trend for categorical variables. The association between resistin and hypertension through 14 years of follow-up was analyzed using Cox proportional hazards regression models to estimate RRs and 95% CIs. Multivariable models were constructed to adjust for potential confounding variables that have been previously associated with incident hypertension: Age (continuous); BMI (six categories); current smoking (cigarettes per day); family history of hypertension (yes/no); menopause status (yes/no); physical activity (quintiles); fasting status (<8 versus ≥8 hours since last meal); and intakes of alcohol (continuous), sodium, potassium, calcium, magnesium, and folate (all continuous). Total adiponectin, the HMW–total adiponectin ratio, and leptin (all in tertiles) were further added to the multivariable model. We determined P values for trend for each of the exposures of interest by using the median for each category.
We also investigated whether the association between plasma resistin and hypertension varied according to age (<55 or ≥55 years) and BMI (<25 or ≥25 kg/m2). Stratified multivariable analyses were performed, and appropriate interaction terms were generated to test whether interactions were statistically significant.
In the secondary analyses limited to the 362 women who also had inflammatory and endothelial biomarkers measured, we used age- and BMI-controlled Spearman correlation coefficients to evaluate associations between resistin and other biomarkers. We then added CRP, IL-6, and TNF R2, as well as ICAM-1, VCAM-1, and E-selectin individually and together (all as continuous variables) in the multivariate model of resistin to observe whether the association between plasma resistin levels and incident hypertension changed and became insignificant after controlling for those biomarkers.
All P values are two-tailed. Statistical tests were performed using SAS 9.1 for UNIX statistical software package (SAS Institute, Cary, NC).
Disclosures
None.
Acknowledgments
This study was funded by American Heart Association grant 0535401T, National Institutes of Health grants CA87969 and HL079929, and the Beijing NOVA program from the Beijing Municipal Science and Technology Commission. In addition, this work was made possible through the International Society of Nephrology–funded Fellowship (L.Z.).
Footnotes
See related editorial, “Hypertension: Shall We Focus on Adipose Tissue?” on pages 1067–1068.
REFERENCES
- 1. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA: The hormone resistin links obesity to diabetes. Nature 409: 307–312, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Koerner A, Kratzsch J, Kiess W: Adipocytokines: Leptin—the classical, resistin—the controversial, adiponectin—the promising, and more to come. Best Pract Res Clin Endocrinol Metab 19: 525–546, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Rea R, Donnelly R: Resistin: An adipocyte-derived hormone—Has it a role in diabetes and obesity? Diabetes Obes Metab 6: 163–170, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Nagaev I, Smith U: Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun 285: 561–564, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA: Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 300: 472–476, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Yura S, Sagawa N, Itoh H, Kakui K, Nuamah MA, Korita D, Takemura M, Fujii S: Resistin is expressed in the human placenta. J Clin Endocrinol Metab 88: 1394–1397, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW: Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun 310: 927–935, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A: Resistin, an adipokine with potent proinflammatory properties. J Immunol 174: 5789–5795, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA: Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation 108: 736–740, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R: Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: A new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun 314: 415–419, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Calabro P, Samudio I, Willerson JT, Yeh ET: Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation 110: 3335–3340, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Grundy SM: Inflammation, hypertension, and the metabolic syndrome. JAMA 290: 3000–3002, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Oparil S, Zaman MA, Calhoun DA: Pathogenesis of hypertension. Ann Intern Med 139: 761–776, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Takata Y, Osawa H, Kurata M, Kurokawa M, Yamauchi J, Ochi M, Nishida W, Okura T, Higaki J, Makino H: Hyperresistinemia is associated with coexistence of hypertension and type 2 diabetes. Hypertension 51: 534–539, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Papadopoulos DP, Makris TK, Krespi PG, Poulakou M, Stavroulakis G, Hatzizacharias AN, Perrea D, Votteas VV: Adiponectin and resistin plasma levels in healthy individuals with prehypertension. J Clin Hypertens (Greenwich) 7: 729–733, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kougias P, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C: Adipocyte-derived cytokine resistin causes endothelial dysfunction of porcine coronary arteries. J Vasc Surg 41: 691–698, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ: Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 111: 932–939, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Furuhashi M, Ura N, Higashiura K, Murakami H, Shimamoto K: Circulating resistin levels in essential hypertension. Clin Endocrinol (Oxf) 59: 507–510, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ: Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension 52: 818–827, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Yannakoulia M, Yiannakouris N, Bluher S, Matalas AL, Klimis-Zacas D, Mantzoros CS: Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab 88: 1730–1736, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Sowers MR, Wildman RP, Mancuso P, Eyvazzadeh AD, Karvonen-Gutierrez CA, Rillamas-Sun E, Jannausch ML: Change in adipocytokines and ghrelin with menopause. Maturitas 59: 149–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA: Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 161: 1581–1586, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Dimitriadis K, Tsioufis C, Selima M, Tsiachris D, Miliou A, Kasiakogias A, Andrikou E, Tousoulis D, Stefanadis C: Independent association of circulating resistin with glomerular filtration rate in the early stages of essential hypertension. J Hum Hypertens 23: 668–673, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Ellington AA, Malik AR, Klee GG, Turner ST, Rule AD, Mosley TH, Jr, Kullo IJ: Association of plasma resistin with glomerular filtration rate and albuminuria in hypertensive adults. Hypertension 50: 708–714, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Knight EL, Rimm EB, Pai JK, Rexrode KM, Cannuscio CC, Manson JE, Stampfer MJ, Curhan GC: Kidney dysfunction, inflammation, and coronary events: A prospective study. J Am Soc Nephrol 15: 1897–1903, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Knight EL, Stampfer MJ, Rimm EB, Hankinson SE, Curhan GC: Moderate alcohol intake and renal function decline in women: A prospective study. Nephrol Dial Transplant 18: 1549–1554, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Weikert C, Westphal S, Luley C, Willich SN, Boeing H, Pischon T: Within-subject variation of plasma resistin levels over a 1-year period. Clin Chem Lab Med 45: 899–902, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB: Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med 149: 307–316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaplan RC, Ho GY, Xue X, Rajpathak S, Cushman M, Rohan TE, Strickler HD, Scherer PE, Anastos K: Within-individual stability of obesity-related biomarkers among women. Cancer Epidemiol Biomarkers Prev 16: 1291–1293, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Willett W, Stampfer MJ: Total energy intake: Implications for epidemiologic analyses. Am J Epidemiol 124: 17–27, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE: Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122: 51–65, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Williams CJ, Hu FB, Patel SR, Mantzoros CS: Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care 30: 1233–1240, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Hu FB, Meigs JB, Li TY, Rifai N, Manson JE: Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 53: 693–700, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE: Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 123: 894–900, 1986 [DOI] [PubMed] [Google Scholar]