Abstract
The GELFREE 8100 Fractionation System is a novel protein fractionation system designed to maximize protein recovery during molecular weight based fractionation. The system is comprised of single-use, 8-sample capacity cartridges and a benchtop GELFREE Fractionation Instrument. During separation, a constant voltage is applied between the anode and cathode reservoirs, and each protein mixture is electrophoretically driven from a loading chamber into a specially designed gel column gel. Proteins are concentrated into a tight band in a stacking gel, and separated based on their respective electrophoretic mobilities in a resolving gel. As proteins elute from the column, they are trapped and concentrated in liquid phase in the collection chamber, free of the gel. The instrument is then paused at specific time intervals, and fractions are collected using a pipette. This process is repeated until all desired fractions have been collected. If fewer than 8 samples are run on a cartridge, any unused chambers can be used in subsequent separations.
This novel technology facilitates the quick and simple separation of up to 8 complex protein mixtures simultaneously, and offers several advantages when compared to previously available fractionation methods. This system is capable of fractionating up to 1mg of total protein per channel, for a total of 8mg per cartridge. Intact proteins over a broad mass range are separated on the basis of molecular weight, retaining important physiochemical properties of the analyte. The liquid phase entrapment provides for high recovery while eliminating the need for band or spot cutting, making the fractionation process highly reproducible1.
Protocol
1. Sample Protein Preparation
In a 500 μl microfuge tube, combine the following:
| Reagent | Volume to add: |
| *Protein sample (up to 1 mg) | Up to 112 μL |
| Sample Buffer (5X) provided in cartridge kit | 30 μL |
| DTT 1M | 8μL |
| 18 Ω H2O | x μL to a total of sample volume of 150μL** |
Denature the samples at 95°C for 5 minutes. Cool to room temperature.
*The total amount of protein to be separated will depend on the complexity of the sample. For more complex samples, such as tissue homogenate, 150-200 μg per channel is recommended.
**If the sample volume is less than 112 μL, add 18 Ω H2O to bring the final volume to 150 μL.
2. Loading the GELFREE Cartridge
Remove the GELFREE 8100 Cartridge from the foil pouch.
Remove and discard the plate sealer.
Remove the storage buffer from the cartridge compartments using a pipette. If all 8 chambers of the cartridge will be used, the cartridge can be inverted to drain the storage buffer from the compartments.
Prepare the cartridge for loading by adding GELFREE Running Buffer to each of the cartridge chambers as follows:
| GELFREE Cartridge Chamber | Volume of GELFREE Running Buffer to add |
| Anode buffer reservoir | 8 mL |
| Cathode buffer reservoir | 6 mL |
| Collection chambers | 100 μL each |
Remove and discard any buffer from the sample loading chamber.
Load the 150 μL samples into the loading chambers using an 8-channel pipettor.
Place the loaded cartridge into the GELFREE 8100 Fractionation Station. Lower the electrode arrays and close the lid.
3. Running the cartridge
Using the instrument s touch screen display, press the method button to view the list of pre-programmed methods. Each of the pre-programmed methods consists of set voltages and time-based pauses designed to resolve proteins within a specific range of molecular weights. The GELFREE 8100 will automatically pause for fraction collection at the intervals specified in the method.
Once the appropriate method has been identified, press the retrieve button, and enter the number of the desired pre-programmed method using the on-screen keypad. Press ok, then press the apply button. The method that has been applied will appear at the bottom of the main screen.
To select the channels (number of samples) that will be used in the run, press the channel button on the main screen. To select all eight channels, press select all. Then, press done to return to the main screen.
Ensure that the safety lid is closed and that the indicator light is green at the bottom of the screen.
To begin the protein separation, press start. The Fractionation Station will automatically pause when it is time to collect fractions. Note: While the system is running, the circles shown in the box on the left side of the screen depict the status of each channel. A green circle indicates that the channel is active, a yellow circle indicates that the instrument has paused, and a red circle indicates that the channel has failed. The applied voltages and currents are displayed to the right of the channel status circles. Fractions should only be removed when the status circles have turned yellow and a text alert appears on the instrument s screen.
To remove fractions, open the lid of the instrument and use an 8-channel pipette to withdraw 150 μL from each of the collection chambers.
Once the fractions have been removed, wash the collection chamber twice with GELFREE Running Buffer by adding 100 μl per channel and pipetting up and down twice.
After washing, add 100uL of GELFREE Running Buffer back into the collection chambers, close the lid, and press the resume button.
The GELFREE 8100 will run until the next time interval. The total time remaining in the experiment is shown on the right hand side of the screen. To toggle between time remaining, time elapsed, and time to pause, press the button above the box displaying time.
Once all of the fractions have been collected, they can immediately be used for further downstream preparation, such as isoelectric focusing or gel electrophoresis, or for analysis using liquid chromatography mass spectrometry or immunoaffinity.
4. Representative Results
A 200 μg of bovine liver homogenate was partitioned into 12 fractions each, using the GELFREE Fractionation System. 6 μL of each fraction was run on a standard 1D SDS PAGE gel and silver stained. As shown in Figure 1, the protein sample was fractionated into 12 distinct fractions, spanning the molecular weight range from 3.5kDa to 150kDa. A molecular weight standard (ColorBurst, Sigma) are also shown in lanes 1 and 14. The gel shown here highlights the high-recovery rate achieved using the GELFREE 8100 system for protein fractionation. The gel shown here represents 4% of the protein recovered from 200 μg.
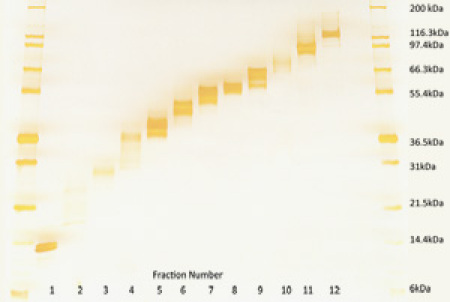 Figure 1. Proteins from bovine liver homogenate separated using the GELFREE Fractionation system. 6 μL of each 150 μL fraction was loaded and run on a 1D SDS PAGE gel and silver stained. A molecular weight (MW) standard was also loaded as indicated. Please click here to see a larger version of figure 1.
Figure 1. Proteins from bovine liver homogenate separated using the GELFREE Fractionation system. 6 μL of each 150 μL fraction was loaded and run on a 1D SDS PAGE gel and silver stained. A molecular weight (MW) standard was also loaded as indicated. Please click here to see a larger version of figure 1.
Discussion
The GELFREE 8100 Fractionation System provides a quick, simple and reproducible method for partitioning complex protein samples into discrete molecular weight-based fractions. This novel method of fractionation eliminates the need for band or spot cutting, making it possible to isolate proteins over a broad mass range in 90 minutes. The ability to load relatively large amounts of sample and recover fractions in liquid phase is particularly useful in the isolation and analysis of low-abundance proteins, which are typically more difficult to detect. The resulting fractions can then be used in a wide array of applications, such as LC-MS, MALDI-TOF, and immunoblotting.
References
- Tran JC, Doucette AA. Multiplexed Size Separation of Intact Proteins in Solution Phase for Mass Spectrometry. Anal Chem. 2009 doi: 10.1021/ac900729r. [DOI] [PubMed] [Google Scholar]


