Abstract
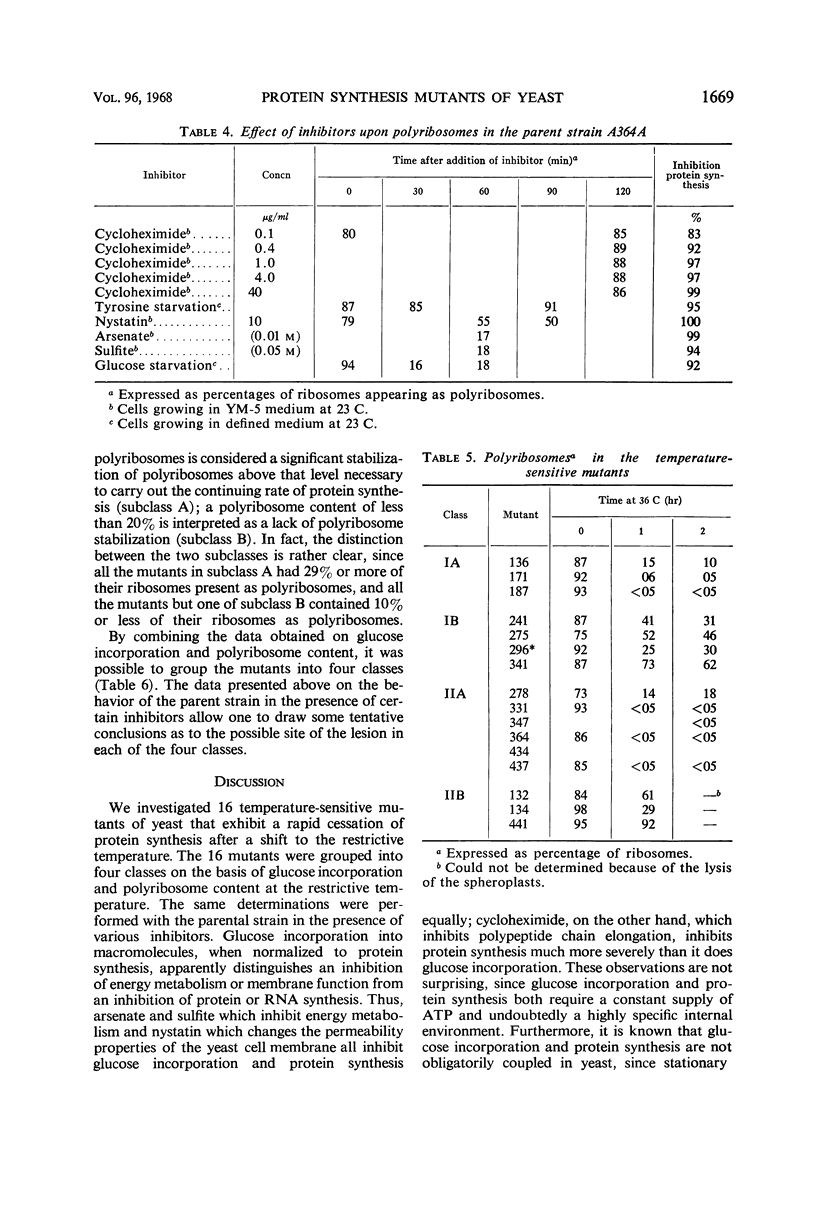
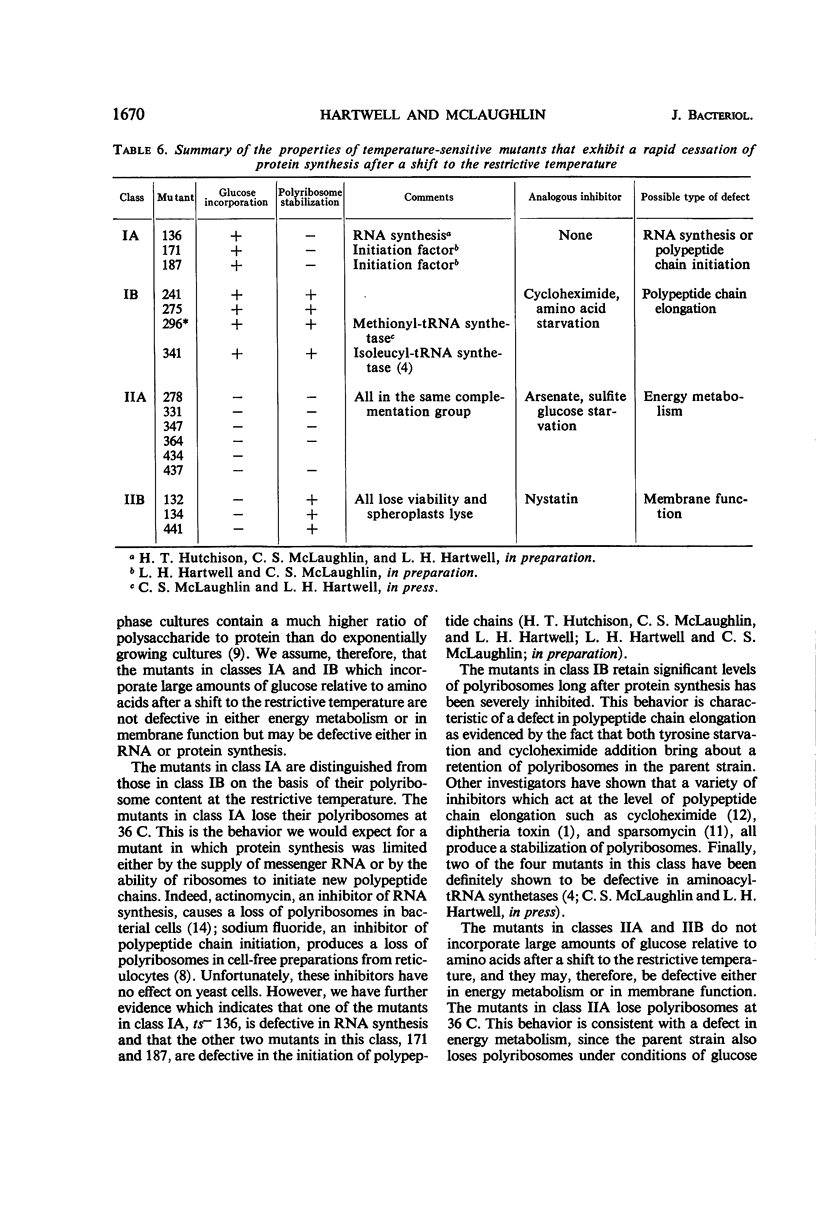
Certain temperature-sensitive (ts−) mutants of yeast which cannot be corrected by nutritional supplementation exhibited a rapid cessation of protein synthesis after a shift to the restrictive temperature. Genetic and biochemical tests permitted a division of these mutants into four classes. This division was based upon genetic complementation patterns among the mutants and an investigation of glucose incorporation into macromolecules and polyribosome content in the mutants after a shift to the restrictive temperature. A study of these parameters in the parent strain (ts+) in the presence of certain well-characterized inhibitors allowed a tentative identification of the biochemical defects in each of the four classes. The properties of the mutants in class IA were consistent with the hypothesis that they result from a defect in the initiation of polypeptide chains or in ribonucleic acid synthesis; mutants in class IB from a defect in the elongation of polypeptide chains; mutants in class IIA from a defect in energy metabolism; and mutants in class IIB from a lesion affecting membrane function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- Dresden M. H., Hoagland M. B. Polyribosomes of Escherichia coli. Breakdown during glucose starvation. J Biol Chem. 1967 Mar 10;242(5):1065–1068. [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Mutants of yeast with temperature-sensitive isoleucyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1968 Feb;59(2):422–428. doi: 10.1073/pnas.59.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H. Macromolecule synthesis in yeast spheroplasts. J Bacteriol. 1967 Nov;94(5):1697–1705. doi: 10.1128/jb.94.5.1697-1705.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Mosteller R. D., Hardesty B. The mechanism of sodium fluoride and cycloheximide inhibition of hemoglobin biosynthesis in the cell-free reticulocyte system. J Mol Biol. 1966 Oct 28;21(1):51–69. doi: 10.1016/0022-2836(66)90079-9. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in dry weight, protein, deoxyribonucleic acid, ribonucleic acid and reserve and structural carbohydrate during the aerobic growth cycle of yeast. Biochem J. 1966 Mar;98(3):883–887. doi: 10.1042/bj0980883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL M. R., SISLER H. D. SITE OF ACTION OF CYCLOHEXIMIDE IN CELLS OF SACCHAROMYCES PASTORIANUS. II. THE NATURE OF INHIBITION OF PROTEIN SYNTHESIS IN A CELL-FREE SYSTEM. Biochim Biophys Acta. 1964 May 18;87:83–89. [PubMed] [Google Scholar]
- Trakatellis A. C. Effect of sparsomycin on protein synthesis in the mouse liver. Proc Natl Acad Sci U S A. 1968 Mar;59(3):854–860. doi: 10.1073/pnas.59.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., NOLL H., PENMAN S. EFFECT OF CYCLOHEXIMIDE ON RIBOSOMAL AGGREGATES ENGAGED IN PROTEIN SYNTHESIS IN VITRO. Biochim Biophys Acta. 1964 Jul 22;87:525–528. doi: 10.1016/0926-6550(64)90131-8. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Levinthal C. Messenger RNA and RNA transcription time. J Mol Biol. 1967 Dec 14;30(2):349–370. [PubMed] [Google Scholar]



