Abstract
Mantle field irradiation has historically been the standard radiation treatment for Hodgkin lymphoma. It involves treating large regions of the chest and neck with high doses of radiation (up to 30 Gy). Previous epidemiological studies on the incidence of second malignancies following radiation therapy for Hodgkin lymphoma have revealed an increased incidence of second tumors in various organs, including lung, breast, thyroid and digestive tract. Multiple other studies, including the Surveillance, Epidemiology and End Results, indicated an increased incidence in digestive tract including stomach cancers following mantle field radiotherapy. Assessment of stomach dose is challenging because the stomach is outside the treatment field but very near the treatment border where there are steep dose gradients. In addition, the stomach can vary greatly in size and position. We sought to evaluate the dosimetric impact of the size and variable position of the stomach relative to the field border for a typical Hodgkin lymphoma mantle field irradiation. The mean stomach dose was measured using thermoluminescent dosimetry for nine variations in stomach size and position. The mean doses to the nine stomach variations ranged from 0.43 to 0.83 Gy when 30 Gy was delivered to the treatment isocenter. Statistical analyses indicated that there were no significant differences in the mean stomach dose when the stomach was symmetrically expanded up to 3 cm or shifted laterally (medial, anterior or posterior shifts) by up to 3 cm. There was, however, a significant (P > 0.01) difference in the mean dose when the stomach was shifted superiorly or inferiorly by ≥ 2.5 cm.
1. Introduction
Hodgkin lymphoma (HL) has historically been treated with mantle fields—large radiation fields covering the lymph system in the chest and neck. It is well known that radiation dose is a risk factor for the incidence of second cancers (Travis et al 2002, 25, Ron 2003, van Leeuwen et al 2003, Lin and Teitell 2005, Bassal et al 2006), and several studies of radiation-induced late effects in survivors of HL have shown that second cancers are prominent, especially in the breast and lung (Travis et al 2002, 2003, van Leeuwen et al 2003). Multiple other studies, including Surveillance, Epidemiology and End Results (SEER), have indicated an increased incidence in digestive tract cancers (including stomach) following mantle field radiotherapy (Boivin et al 1995, Dores et al 2002, Lin and Teitell 2005, Curtis 2006, Ng et al 2010). Ng and Travis (2008) have shown that the majority of second tumors develop within or at the edges of prior radiation fields, and the stomach is in close proximity to the mantle field treatment border. Given that these studies have indicated an increased incidence of digestive tract cancers following radiation therapy for HL, we sought to provide an accurate assessment of stomach dose resulting from the typical fields used for treatment.
The stomach lies outside the mantle field and thus receives a substantially lower radiation dose than that received inside the treatment field. Still, the stomach is in close proximity to the inferior border of the mantle field, placing it in a steep dose gradient. In addition, the size and position of the stomach varies among patients (Mills 1917, Bontrager 1997). These factors potentially complicate an accurate assessment of stomach dose from radiation therapy for HL. This is particularly true for epidemiologic studies where detailed patient anatomy is seldom known and patients are assumed to have standard/typical anatomy (such as a single reference stomach). However, to the authors’ knowledge, the dosimetric impact of this assumption has not been examined. Therefore, we evaluated the dosimetric impact of the size and position variations of the stomach relative to the field border for a typical HL mantle field irradiation. We determined a range of doses received from mantle field irradiations for a typical stomach as well as several variations in size and location from this typical stomach. The mean dose for each stomach variation was then compared with the mean dose for the typical stomach and the effect of these variations was evaluated for statistical significance.
2. Materials and methods
2.1. Hodgkin lymphoma treatment plan
Based on historical standards, we designed a photon treatment using a large irregularly shaped chest field (mantle field). This field was designed to treat the major lymph groups superior to the diaphragm (Bentel 1996), and was shaped with Cerrobend blocks to protect regions of healthy and sensitive tissue: the larynx and lungs. Although advanced-stage HL may require additional fields to treat lymph groups inferior to the diaphragm, such cases are rare, and in this dosimetric study we considered only mantle field radiation therapy.
We used the CIRS ATOM anthropomorphic male reference phantom (CIRS, Inc., Norfolk, VA), which simulated a human torso based on specifications from Report 23 (ICRP-23 1975) of the International Commission on Radiation Protection (ICRP) with a reference height (170 cm) and weight (70 kg) for an adult male. The phantom was composed of tissue-equivalent material with varying densities to represent bone, lung, brain and soft tissue. A computed tomography (CT) scan of the phantom was performed. The CT images were used to delineate organs and calculate a treatment plan. The skeletal system, lungs, brain and spinal cord were contoured on the CT dataset, being easily distinguished from surrounding tissue. Based on these structures, a typical stomach was then contoured in soft-tissue regions of the phantom using a CT anatomy atlas (Fleckenstein and Tranum-Jensen 2001). The typical stomach that was contoured on the CT data set for this study is shown on an axial plane in figure 3(a) and in the frontal and sagittal planes in figure 1. All contours were reviewed by a board-certified gastrointestinal surgeon to ensure that the stomach contours were reasonable based on his expertise and knowledge of gastrointestinal anatomy.
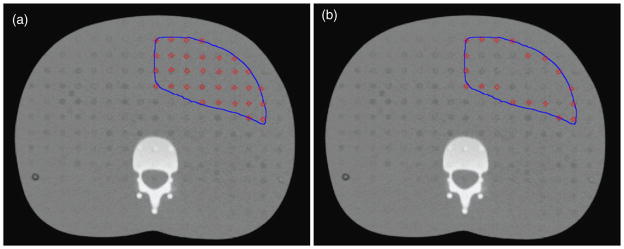
Figure 3.
Axial plane from the CT scan of the phantom of the phantom showing the typical stomach contour in blue and TLD locations indicated by red squares. (a) Contour of typical stomach and all TLD locations within that contour used to estimate dose to the entire stomach. (b) Contour of typical stomach and TLD locations around the periphery used to estimate dose to the stomach wall.
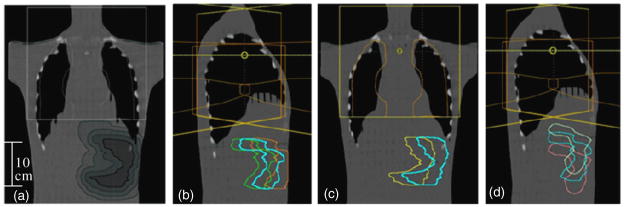
Figure 1.
Three expansions and five shifts of typical stomach relative to the treatment field. (a) Three expansions: the typical stomach is the smallest contour, shaded dark gray. The progressively lighter shaded contours are the 1 cm, 2 cm and 3 cm 3D symmetric expansions. Five shifts of the typical stomach relative to the treatment field (typical stomach, cyan): (b) 3 cm posterior, green; 3 cm anterior, orange; (c) 3 cm medial, yellow; (d) 2.5 cm superior, white; and 2.5 cm inferior, pink.
The radiation therapy plan was calculated using the Eclipse (Version 8.6) treatment planning system (Varian Medical Systems, Palo Alto, CA). The treatment plan included two opposed 6 MV photon beam mantle fields, one anterior and one posterior. The fields (15 cm × 27 cm) extended from the inferior border of the phantom’s chin to vertebra T10, consistent with clinical practice (Bentel 1996). In the lateral direction, the field covered the ribcage.
2.2. Variations in stomach size and position
The typical stomach contour was shifted and symmetrically expanded in the treatment planning system to create variations in the size and position of the stomach. First, the typical stomach contour was expanded to create three new stomachs based on a 1 cm, 2 cm and 3 cm isotropic expansion of the typical stomach. These three new stomach contours (typical stomach is also shown) are shown in figure 1(a) relative to the anterior-to-posterior (AP) treatment field. Next, the typical stomach was shifted (separately) by 3 cm each in the medial, posterior and anterior directions as well as shifted (separately) by 2.5 cm each in the superior and inferior directions. Each of these shifts represented a distinctive stomach location within the phantom. Figures 1(b)–(d) show the five new stomach contours representing the shifted positions (typical stomach is also shown). In total, this study considered nine stomach variations: the typical stomach, three expanded stomachs and five shifted stomachs. Although these expansions and shifts are large, they are anatomically reasonable and were intended to represent extreme variations in stomach size and position. Thermoluminescent dosimetry (TLD) locations within each stomach variation were mapped for assessment of dose within each stomach variation. In addition, the distance from each TLD location (within the organ map) to the nearest field edge was determined.
2.3. Phantom irradiation
The phantom is horizontally transected into 39 2.5-cm slices. Each slice has a 1.5 cm × 1.5 cm grid pattern of tissue-equivalent plugs that can be replaced by TLD capsules. To measure the dose throughout the stomach region (including the typical stomach and all variations), >550 LiF TLD-100 (Quantaflux Radiological Services, San Jose, CA) capsules were loaded into appropriate locations. The number of TLD capsules within the stomach region was maximized by filling each position within the grid pattern throughout each of the contoured stomach variations. This was done to improve the statistical power of the analysis.
The phantom, once loaded with TLD capsules, was irradiated using the mantle field treatment plan. A total of 30 Gy was delivered to the treatment isocenter in a single irradiation using 6 MV photons on a Varian Clinac 2100 at The University of Texas M D Anderson Cancer Center, Houston, TX. The experimental set-up for the phantom irradiation is shown in figure 2.
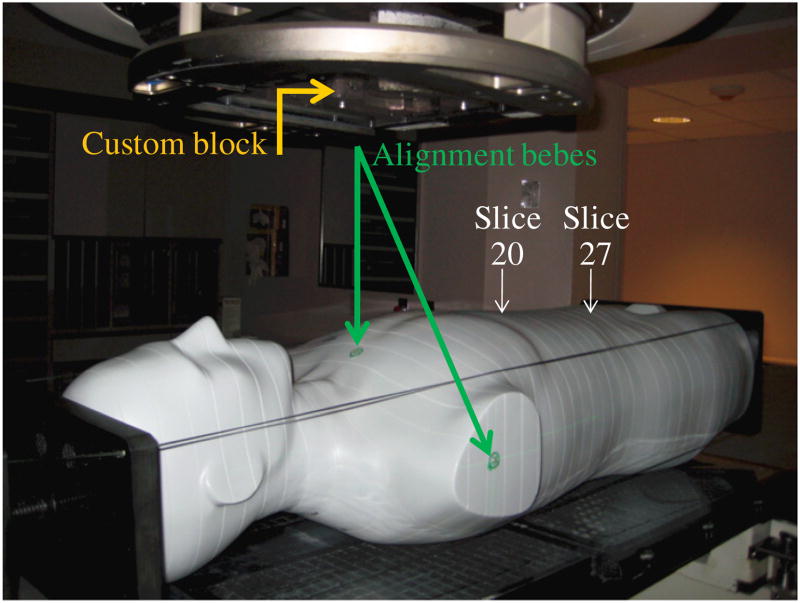
Figure 2.
The anthropomorphic male phantom used in this study placed in the treatment position. The figure shows gantry positioned for the anterior treatment field, custom block used to shape the treatment field and alignment bebes used to align phantom according to planned treatment isocenter. The phantom was loaded with lithium fluoride thermoluminescent dosimeters in the specified locations (in slices 20–27 of the phantom).
The TLDs were analyzed using a well-established protocol developed at the Radiological Physics Center (Houston, TX) (Kirby et al 1986). This protocol accounts for energy response, linearity and dosimeter fading. The system has an uncertainty of ≤3% (Kirby et al 1986, 1992). TLDs were read in batches of 60 TLDs per session. Each reading session included two background TLDs, four sets of standards and one set of controls (3 TLDs per set). Standards and controls were both irradiated with known but different doses from two different Co-60 units. The Co-60 unit used to irradiate the standards has been certified and maintained by an Accredited Dosimetry Calibration Laboratory, with standards linked to the National Institute of Standards and Technology. The Co-60 unit used to irradiate the controls was cross-calibrated within our institution.
The TLD readings were converted to dose in muscle using an nC-to-cGy calibration factor from a set of reference TLDs irradiated with ADCL-certified Co-60 units. The readings from the TLD capsules in the phantom were normalized and reported as cGy per Gy at isocenter in muscle. Mean doses (and standard deviations) for each of the stomach variations (including the typical stomach) were calculated from the dose to the TLDs within the organ map for each of the stomachs.
2.4. Statistical analysis
Statistical analyses were performed to compare the mean dose for the typical stomach with the mean dose for each of the stomach variations to test the null hypothesis that the mean doses were not statistically different. All statistical analyses were performed using SPSS v16 statistical software (SPSS 2007). P values ≤ 0.05 were taken to indicate significance.
Our first step in the analyses was to examine whether the individual TLD data that comprised the mean stomach dose for each stomach variation were normally distributed. This was done using the Shapiro–Wilks test for normality. We observed that we had two types of data, groups of normally distributed data and groups of non-normally distributed data. Next, data were divided into four data sets for statistical testing: (1) typical and expanded stomachs (four stomach variations); (2) typical and laterally shifted stomachs (four stomach variations); (3) typical stomach and superiorly shifted stomach (two stomach variations); and (4) typical stomach and inferiorly shifted stomach (two stomach variations). Finally, we applied either parametric or non-parametric statistical tests of the null hypothesis, depending on whether data were normally or non-normally distributed, respectively.
The data set that included the typical stomach and the expanded stomachs was normally distributed. A one-way analysis of variance (ANOVA), a parametric test, was performed after adjusting for multiple comparisons using a Bonferroni correction to determine if the mean dose to the stomach was statistically different from the mean doses to the expanded stomachs. For the non-normally distributed data, nonparametric tests must be used to compare the means (Rosner 2006, Kinnear and Gray 2009). We used the non-parametric Kruskal–Wallis chi-square test to compare the means of the typical stomach and the three laterally shifted stomachs (the 3 cm medial, anterior and posterior shifts). This test was used because these data sets only contained multiple samples. To compare the means of the typical stomach to the superiorly shifted stomach and the typical stomach to the inferiorly shifted stomach, the non-parametric Mann–Whitney U (M-WU)-test was used. This test was used because these data sets contained only two samples. Additional details regarding the statistical tests used in this study can be found in Rosner (2006) and Kinnear and Gray (2009).
2.5. Comparison of stomach dose and stomach wall dose
The new adult computational reference phantoms from the International Commission on Radiation Protection (IRCP-110 2009) as well as other computational phantoms (Zankl et al 2002, Zhang et al 2009) have delineated separate structures for the stomach wall and the stomach contents. It is important to consider the stomach wall separately from the contents of the stomach in studies where the radiation does not uniformly penetrate the entire volume and is of particular relevance for internal dosimetry studies for radioisotopes. In this study, we considered the entire stomach rather than only the stomach wall because out-of-field dose from megavoltage photon radiation therapy is relatively uniform. However, to confirm this assumption, we compared the mean dose to the stomach wall to the mean dose from the entire stomach volume for four stomach variations (the typical stomach and the 1 cm, 2 cm and 3 cm isotropic expansions of the typical stomach). This methodology is illustrated in figure 3, which shows the contour of the typical stomach for an axial plane on the CT scan of the phantom. Figure 3(a) shows the TLD positions used to calculate the mean dose to the typical stomach volume, and figure 3(b) shows the TLD positions around the periphery of the contour used to estimate dose to the stomach wall. In addition, we quantitatively assessed the variation of dose as a function of distance from the field edge by evaluating the standard deviation of doses from all TLD within each slice of the phantom for the largest stomach variation considered in this study (3 cm isotropic expansion of the typical stomach).
3. Results
3.1. Dose distribution across the stomach region
Measured TLD data points were distributed uniformly throughout the volume of the stomachs. Consequently, the stomach expansions contained more data points than the shifted stomachs. The mean, minimum and maximum dose (cGy/Gy, normalized to the dose at isocenter), relative standard deviation, total mean stomach dose for a full course of treatment (30 Gy to isocenter), and the number of TLD data points for each of the nine stomach variations are shown in table 1. The volume (cm3) of each stomach variation is also provided in table 1.
Table 1.
Dose to stomachs: mean, minimum and maximum stomach dose; mean stomach dose per full treatment course; relative standard deviation, number of TLDs; and stomach volume for each of the nine stomach variations.
| Mean dose (cGy/GyRx) | Min dose (cGy/GyRx) | Max dose (cGy/GyRx) | Relative standard deviationb | Total mean stomach (%) dosec (cGy) | Number of TLDs | Volume (cm3) | |
|---|---|---|---|---|---|---|---|
| Stomacha | 2.06 | 0.86 | 4.22 | 44.2 | 61.8 | 68 | 433.77 |
| Stomach + 1 cm | 2.28 | 0.83 | 8.29 | 62.6 | 63.6 | 191 | 1049.33 |
| Stomach + 2 cm | 2.41 | 0.77 | 8.63 | 67.6 | 72.3 | 315 | 1784.83 |
| Stomach + 3 cm | 2.47 | 0.67 | 8.72 | 70.9 | 74.1 | 466 | 2641.40 |
| Stomach shifted 3 cm midline | 2.19 | 0.94 | 4.54 | 45.2 | 65.7 | 67 | 433.29 |
| Stomach shifted 3 cm posterior | 2.01 | 0.88 | 4.21 | 43.3 | 60.3 | 74 | 433.26 |
| Stomach shifted 3 cm anterior | 2.01 | 0.83 | 4.54 | 44.8 | 60.3 | 67 | 432.77 |
| Stomach shifted 2.5 cm superior | 2.76 | 1.15 | 7.19 | 48.6 | 82.8 | 63 | 395.65 |
| Stomach shifted 2.5 cm inferior | 1.43 | 0.71 | 3.29 | 44.1 | 42.9 | 72 | 395.11 |
TLD: thermoluminescent dosimeter, Gy/GyRx: Gy per prescribed Gy.
Refers to typical stomach.
Relative standard deviation of the mean dose.
Total mean doses are for a complete course of treatment, 30 Gy.
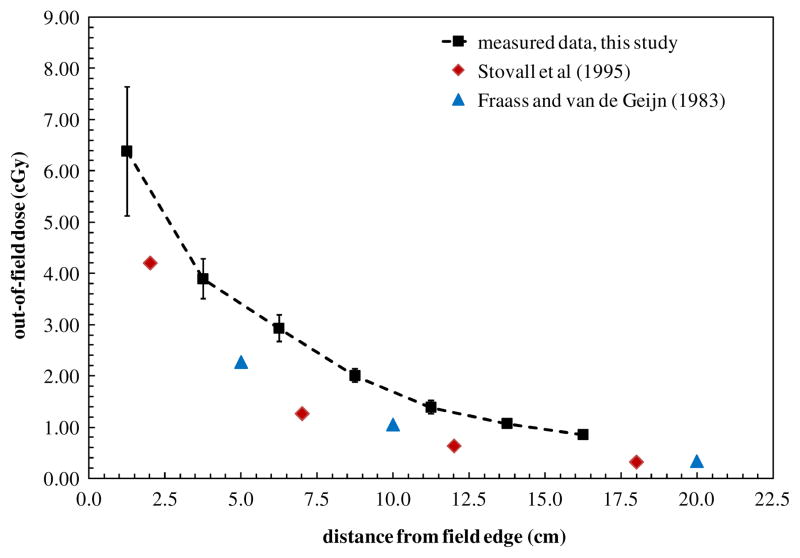
The variation in the mean dose for each phantom slice through the stomach region at a depth of 9.5 cm in tissue is shown in figure 4. The error bars on each data point in figure 4 are the standard deviation in the measured dose among all TLDs at that depth in each slice of the phantom. Figure 4 also compares our data with those reported by American Association of Physicists in Medicine (AAPM) Task Group Number 36 (Stovall et al 1995), which show out-of-field dose measured at a depth of 10 cm in water phantom for a 25 cm × 25 cm 6 MV field. Fraass and van de Geijn measured out of field dose from a 6 MV beam, 20 cm × 20 cm field size. These data are also shown in figure 4.
Figure 4.
The variation in the mean dose through the stomach region at a depth of 9.5 cm in tissue as a function of distance from the field edge. Data are compared with measured dose values from Stovall et al (1995) and from Fraass and van de Geijn (1983).
3.2. Statistical analyses of stomach variations
All data sets were evaluated for outliers or extreme cases. None of the stomach variations contained extreme cases. However, the three stomach expansions and the superior stomach shift contained outliers (values >1.5 times the difference between the 25th and 75th percentile) (Kinnear and Gray 2009). All of the outliers had values that were greater than the mean value, and all outliers were included in the data sets for statistical analyses.
The mean organ doses (μ, in cGy/Gy) to the three stomach expansions (μ1 = 2.12, μ2 = 2.41 and μ3 = 2.47) and the typical stomach (μT = 2.06) were compared using a one-way ANOVA. The one-way ANOVA showed no significance at the 0.05 level among the four samples (P = 0.199). These results indicate that changes in size of the stomach do not significantly affect the mean dose to the stomach.
The K–W chi-square test was performed to compare the mean dose of the typical stomach (μT = 2.06) with that of the laterally shifted stomachs, including the stomachs that were shifted toward the midline (μM = 2.19), posterior (μP = 2.01) and anterior (μA = 2.01) by 3 cm. The results of the K–W test were not significant at the 0.05 level (P = 0.186). These results indicate that lateral, anterior or posterior shifts in the stomach position do not affect the mean dose to the stomach.
The stomach that was shifted 2.5 cm in the superior direction contained data points with higher doses than the typical stomach because of the closer proximity to the treatment field. The maximum dose in this stomach was 7.19 cGy per Gy to isocenter, 70% higher than the maximum dose in the typical stomach. The mean dose to the superior stomach (μS = 2.76, σS = 1.34) was correspondingly and significantly higher than the mean dose to the typical stomach (μT = 2.06, σT = 0.91). A Mann–Whitney U-test (the nonparametric equivalent of the t-test) was used to compare the means of these two samples, and the difference was found to be significant (P < 0.01, two tailed). Using the same method, the mean dose to the inferior stomach (μI = 1.43, σI = 0.63) was significantly lower than the dose to the typical stomach (P < 0.01). These results indicate that the mean dose to the stomach does depend on the superior and inferior position of the stomach.
3.3. Comparison of stomach dose and stomach wall dose
The dose to the stomach wall was estimated by calculating the mean dose from TLDs around the periphery of the organ volume for four stomach variations (the typical stomach and the 1 cm, 2 cm and 3 cm isotropic expansions of the typical stomach). These data were compared to the mean dose to the entire organ calculated from all TLDs within the organ contours in table 2. Even the largest difference (approximately 5%) was not significantly different.
Table 2.
Comparison of the mean doses to the entire volume and to the lining of the volume for four of the stomach variations. The standard deviations about the mean are shown to the right of the values in parenthesis.
| Mean dose (cGy/GyRx)
|
||||
|---|---|---|---|---|
| Stomach | Stomach+1 | Stomach+2 | Stomach+3 | |
| Entire volume | 2.06 (0.90) | 2.28 (1.43) | 2.41 (1.62) | 2.47 (1.75) |
| Volume wall | 2.02 (0.88) | 2.28 (1.73) | 2.37 (1.48) | 2.33 (1.83) |
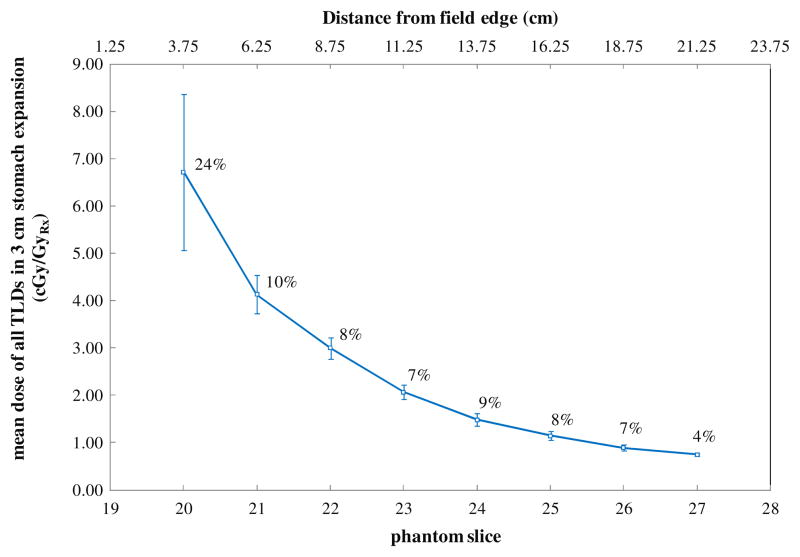
The lack of the difference between mean doses to the stomach and stomach wall can be understood by the data presented in figure 5, which shows the mean dose for all TLD within each slice of the phantom for the largest stomach variation considered in this study (3 cm isotropic expansion). The data plotted in figure 5 include all TLD points that were considered in this study and thus provide a good indication of the variation of the dose at a given distance from the field edge. The average deviation in dose to all points in a given phantom slice (as indicated by the error bars) was less than 10%, and not large enough to translate into a statistical difference between the mean doses for the wall structures and the mean doses for the entire volumes. Thus using the entire stomach rather than the stomach wall for dosimetric analysis of out-of-field dose is appropriate because out-of-field dose from megavoltage photon radiation therapy is relatively uniform.
Figure 5.
The mean dose for all TLD within each slice of the phantom for the largest stomach variation considered in this study (3 cm isotropic expansion). The error bars represent one standard deviation and the percent standard deviation for each phantom slice is indicated to the right of each data point (average deviation for all slices = 9.6%).
4. Discussion
In our study, the measured TLD data showed that the radiation dose throughout the stomach decreased rapidly with increasing distance from the field edge during mantle field. Additionally, the stomach dose became more uniform as the distance from the field edge increased. The radiation dose distribution throughout the stomach was compared to out-of-field trends reported in the literature in figure 4, including the AAPM Task Group Number 36 Report (Stovall et al 1995) and measured peripheral dose from various megavolt beams (Fraass and van de Geijn 1983). The doses reported in the current study were generally in good agreement with previously published data by Stovall et al and Fraass and van de Geijn, but were slightly higher, by 39% on average, but up to 45% for very low doses. Several factors could account for this discrepancy, including differences in the measurement technique, phantom set-up, and head design in modern linear accelerators (particularly the introduction of the MLC). Additionally, while the measurements by Stovall et al and Frass and van de Geijn were taken in a rectangular water phantom with an open field, our measurements were taken in an anthropomorphic phantom that included soft tissue, bone and lung heterogeneities. Furthermore, both sets of previously published data are for open field arrangements, whereas our fields include Cerrobend blocking which may increase scattered radiation, although the impact of blocks has not been thoroughly evaluated. Regardless, a recent study evaluating the agreement between anthropomorphic phantom measurements of clinical treatments with modern accelerators and TG-36 estimated data found that TG-36 underestimated the measured dose by 31% on average (Kry et al 2007) for those treatments, consistent with the findings of this study. Further, a review article by Xu et al (2008) noted that there has been a large variation in published out-of-field dose data.
For a complete course of radiation treatment using conventional mantle fields (30 Gy to the isocenter), the mean dose received by the stomach ranged from 0.43 to 0.84 Gy. The most superior stomach received the highest mean dose. However, the largest stomach (3 cm expansion) had the largest maximum point dose of 2.62 Gy. Shifts in stomach position in the inferior and superior directions affected the mean organ dose, while changes in size and shifts in the lateral and anterior/posterior direction did not affect the mean organ dose. The implications of these results demonstrate that for most variations in stomach size or position, there is no significant impact on mean dose. Only in situations where the organ is known to move closer to or farther from the field border will the mean dose be significantly affected.
A previous investigation by Csendes and Burgos (2005) on variations in stomach size among individuals revealed that there is no correlation between stomach size or shape and body mass index (BMI). Csendes and Burgos also found that the stomachs of morbidly obese patients have the same variations in size and position as individuals of average BMI. Therefore, BMI should not be used as an indicator of stomach size and position. However, the results of the present study indicate that the assumption of a typical anatomical position and size for the stomach is acceptable practice for most studies involving out-of-field dose. The only variation that may necessitate consideration of multiple stomachs is variations toward or away from the field border. Mills (1917) defined three basic stomach categories that could be used to classify the inferior/superior position of the stomach relative to the mantle field treatment border: (1) a hypersthenic stomach, which is located between vertebral bodies T9 and T11/12 and usually has a transverse shape; (2) a hyposthenic/asthenic stomach, which is located between vertebral bodies T11 and L5 and typically has a J shape; and (3) an asthenic stomach, located between vertebral bodies T10/11 and L2 and typically J shaped.
While this study was specific for a particular organ and treatment technique delivered on a specific linac, similar trends are likely to be observed for different organs, treatments and linacs although the magnitude of the out-of-field dose might vary substantially. The measured data reported in this work were determined for a Varian Clinic linear accelerator and differences may exist in the stomach dose received by irradiation using equipment from different manufacturers. It should also be noted that this study considered only historical mantle field irradiation. Newer treatment techniques often employ MLCs rather than Cerrobend blocks. The out-of-field dose (close to the treatment field, where scatter radiation is a larger component than leakage radiation) for these treatments may be lower than those reported here because of decreased scatter due to the closer proximity of the Cerrobend blocks to the patient compared to MLC. In addition, Yahalom (2005) suggested the use of intensity-modulated radiation therapy (IMRT) for selected patients with very bulky residual disease or for re-irradiation of relapsed HL. IMRT results in higher beam-on times than are used in conventional radiation therapy. It is anticipated that the out-of-field dose would be higher for IMRT than that reported here. Recently, Weber et al (2009) completed a comparative treatment planning study of volumetric-modulated arc therapy and IMRT for involved-field and involved-node radiotherapy for HL. That study relied on the treatment planning system to evaluate dose to organs at risk (OAR), including an OAR located outside the treatment field. The dose to the out-of-field OAR reported by Weber et al may be underestimated because of limitations in the treatment planning system’s ability to accurately predict dose outside the treatment field. Future work to compare conventional and more modern radiation therapy techniques should consider the dose to peripheral organs using either measurement techniques or Monte Carlo calculations with models benchmarked for out-of-field dose.
5. Conclusion
The results of this study indicate that isotropic variations in the size of the stomach do not affect the mean stomach dose from mantle field radiation therapy. In addition, variations in the position of the stomach of up to 3 cm in directions parallel to the treatment field border do not affect the mean radiation dose. However, shifts in the stomach position toward or away from the treatment field do significantly alter the mean organ dose. Although the difference between the mean doses in the superior and inferior stomach was statistically significant, it should be noted that the mean stomach dose was <1 Gy for a full treatment course for both stomach positions.
The important finding of this study is that most variations in organ size or position have no significant impact on mean dose. Only in situations where the organ is known to move closer to or farther from the field border will the mean dose be significantly affected. This result could potentially impact the design of epidemiologic studies of a radiation-induced late effect for organs that are known to vary in size and or position between individuals.
Acknowledgments
This work was supported in part by the National Cancer Institute K01 Career Development Grant (5K01CA125204-04; Howell) and by an American Legion Auxiliary Fellowship (Scarboro).
References
- Bassal M, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2006;24:476–83. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- Bentel GC. Radiation Therapy Planning. 2. New York: McGraw-Hill; 1996. [Google Scholar]
- Boivin J-F, et al. Incidence of second cancers in patients treated for Hodgkin’s disease. J Natl Cancer Inst. 1995;87:732–41. doi: 10.1093/jnci/87.10.732. [DOI] [PubMed] [Google Scholar]
- Bontrager KL. Textbook of Radiographic Positioning and Related Anatomy. St Louis, MO: Mosby; 1997. [Google Scholar]
- Csendes A, Burgos A. Size, volume and weight of the stomach in patients with morbid obesity compared to controls. Obes Surg. 2005;15:1133–6. doi: 10.1381/0960892055002158. [DOI] [PubMed] [Google Scholar]
- Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF. New Malignancies among Cancer Survivors, SEER Cancer Registries, 1973–2000. Bethesda, MD: NIH Publication No. 05-5302; 2006. [Google Scholar]
- Dores GM, et al. Second malignant neoplasms among long-term survivors of Hodgkin’s disease: a population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–94. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- Fleckenstein P, Tranum-Jensen J. Anatomy in Diagnostic Imaging. 2. Copenhagen: Munksgaard; 2001. [Google Scholar]
- Fraass BA, van de Geijn J. Peripheral dose from megavolt beams. Med Phys. 1983;10:809–18. doi: 10.1118/1.595359. [DOI] [PubMed] [Google Scholar]
- ICRP. ICRP Publication. Vol. 23. Oxford: Pergamon; 1975. International Commission on Radiological Protection Report of the Task Group on Reference Man. [Google Scholar]
- IRCP. International Commission on Radiological Protection Report 110, adult reference computational phantoms. Ann ICRP. 2009;39(2) doi: 10.1016/j.icrp.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Kinnear PR, Gray CD. SPSS 16 Made Simple. New York: Psychology Press; 2009. [Google Scholar]
- Kirby TH, Hanson WF, Gastorf RJ, Chu CH, Shalek RJ. Mailable TLD system for photon and electron therapy beams. Int J Radiat Oncol Biol Phys. 1986;12:261–5. doi: 10.1016/0360-3016(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Kirby TH, Hanson WF, Johnston DA. Uncertainty analysis of absorbed dose calculations from thermoluminescence dosimeters. Med Phys. 1992;19:1427–33. doi: 10.1118/1.596797. [DOI] [PubMed] [Google Scholar]
- Kry SF, Starkschall G, Antolak JA, Salehpour M. Evaluation of the accuracy of fetal dose estimates using TG-36 data. Med Phys. 2007;34:1193–7. doi: 10.1118/1.2710332. [DOI] [PubMed] [Google Scholar]
- Lin H-MJ, Teitell MA. Second malignancy after treatment of pediatric Hodgkin disease. J Pediatr Hematol Oncol. 2005;27:28–36. doi: 10.1097/01.mph.0000150740.80690.d4. [DOI] [PubMed] [Google Scholar]
- Mills WR. Relation of bodily habitus to visceral form, position, tonus, and motility. Am J Roentgenology 1917 [Google Scholar]
- Ng AK, Kenney LB, Gilbert ES, Travis LB. Secondary malignancies across the age spectrum. Semin Radiat Oncol. 2010;20:67–78. doi: 10.1016/j.semradonc.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AK, Travis L. Subsequent malignant neoplasms in cancer survivors. Cancer J. 2008;14:429–34. doi: 10.1097/PPO.0b013e31818d8779. [DOI] [PubMed] [Google Scholar]
- Ron E. Cancer risks from medical radiation. Health Phys. 2003;85:47–59. doi: 10.1097/00004032-200307000-00011. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 6. Boston, MA: Cengage Learning; 2006. [Google Scholar]
- SPSS I. SPSS 160 for windows. 2007. [Google Scholar]
- Stovall M, Blackwell CR, Cundiff J, Novack DH, Palta JR, Wagner LK, Webster EW, Shalek RJ. Fetal dose from radiotherapy with photon beams: report of AAPM radiation therapy committee task group no. 36. Med Phys. 1995;22:63–82. doi: 10.1118/1.597525. [DOI] [PubMed] [Google Scholar]
- Travis LB, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94:182–92. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- Travis LB, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. J Am Med Assoc. 2003;290:465–75. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FE, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95:971–80. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- Weber DC, Peguret N, Dipasquale G, Cozzi L. Involved-node and involved-field volumetric modulated arc vs. Fixed beam intensity-modulated radiotherapy for female patients with early-stage supra-diaphragmatic Hodgkin lymphoma: a comparative planning study. Int J Radiat Oncol Biol Phys. 2009;75:1578–86. doi: 10.1016/j.ijrobp.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Xu XG, Bednarz B, Paganetti H. A review of dosimetry studies on external-beam radiation treatment with respect to second cancer induction. Phys Med Biol. 2008;53:R193–241. doi: 10.1088/0031-9155/53/13/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahalom J. Transformation in the use of radiation therapy of Hodgkin lymphoma: new concepts and indications lead to modern field design and are assisted by PET imaging and intensity modulated radiation therapy (IMRT) Eur J Haematol. 2005;75:90–7. doi: 10.1111/j.1600-0609.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Zankl M, Fill U, Petoussi-Henss N, Regulla D. Organ dose conversion coefficients for external photon irradiation of male and female voxel models. Phys Med Biol. 2002;47:2367–85. doi: 10.1088/0031-9155/47/14/301. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Na YH, Caracappa PF, Xu XG. RPI-AM and RPI-AF, a pair of mesh-based, size-adjustable adult male and female computational phantoms using ICRP-89 parameters and their calculations for organ doses from monoenergetic photon beams. Phys Med Biol. 2009;54:5885–908. doi: 10.1088/0031-9155/54/19/015. [DOI] [PMC free article] [PubMed] [Google Scholar]