Abstract
A sensitive analytical method for simultaneous quantification of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), and 11-nor-9-carboxy-THC (THCCOOH) in plasma is presented for monitoring cannabinoid pharmacotherapy and illicit cannabis use. Analytes were extracted from 1 mL plasma by solid phase extraction, derivatized with N, O,-bis(trimethylsilyl) trifluoroacetamide with 1% trimethylchlorosilane, and analyzed by two-dimensional gas chromatography mass spectrometry (2D-GCMS) with cryofocusing. The lower calibration curve was linear from 0.25–25 ng/mL for CBD and THC, 0.125–25 ng/mL for 11-OH-THC and 0.25–50 ng/mL for THCCOOH. A second higher linear range from 5–100 ng/mL, achieved through modification of injection parameters, was validated for THC, 11-OH-THC and THCCOOH and was only implemented if concentrations exceeded the lower curve upper limit of linearity. This procedure prevented laborious re-extraction by allowing the same specimen to be re-injected for quantification on the high calibration curve. Intra- and inter-assay imprecision, determined at four quality control concentrations, were <7.8% CV. Analytical bias was within ±9.2% of target and extraction efficiencies were >72.9% for all analytes. Analytes were stable when stored at 22°C for 16h, 4°C for 48h, after three freeze-thaw cycles at −20°C and when stored on the autosampler for 48h. This sensitive and specific 2D-GCMS assay provides a new means of simultaneously quantifying CBD, THC and metabolite biomarkers in clinical medicine, forensic toxicology, workplace drug testing, and driving under the influence of drugs programs.
Keywords: Δ9-tetrahydrocannabinol, cannabidiol, Sativex®, two-dimensional chromatography, plasma
INTRODUCTION
Cannabis sativa contains over sixty cannabinoids, including cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC). Although THC is the principal euphoric chemical in cannabis, its therapeutic properties include analgesia, muscle relaxation, anti-emesis, and appetite stimulation. CBD, a non-psychoactive cannabinoid, is an analgesic, anti-convulsant, anxiolytic, anti-oxidant, anti-psychotic, and muscle relaxant [1]. Smoked cannabis provides rapid delivery of THC to the brain [2–3], increasing its abuse potential [4], and exposing individuals to carcinogenic pyrolytic compounds [5], making smoking an unattractive drug delivery system. Sativex® is a cannabis plant extract formulated as an oromucosal spray containing approximately 2.5 mg CBD and 2.7 mg THC per 100 μL actuation [6]. In 2005, Health Canada approved Sativex® for treatment of neuropathic pain associated with multiple sclerosis. In the US, the Food and Drug Administration (FDA) approved phase III clinical trials of this cannabis extract as an adjunct to opioid analgesia in advanced-stage cancer patients. Oromucosal THC delivery results in a slow rise in THC plasma concentrations with low peak levels [7]. Other cannabinoid pharmacotherapies include FDA-approved oral synthetic THC (Marinol®) and synthetic THC analogue (Nabilone). Investigational therapeutics such as the oral cannabis extract (Cannador®) have been explored for a variety of medical indications [8]. These new synthetic and whole-plant cannabinoid extracts require pharmacokinetic characterization and in the future, perhaps, routine monitoring of cannabinoids with sensitive analytical methods.
Phase I hydroxylation of THC at C9 by hepatic cytochrome P450 enzymes 2C9 & 2C19 yields the equipotent monohydroxylated compound, 11-hydroxy-THC (11-OH-THC). This is further oxidized to the non-psychoactive 11-nor-9-carboxy-THC metabolite (THCCOOH) [9–10]. Two hours after 10 mg THC and CBD administration to 24 individuals by sublingual spray, mean CBD, THC and 11-OH-THC concentrations were 1.2, 1.7 and 1.9 ng/mL, respectively. Concentrations decreased to 0.5, 0.5 and 1.0 ng/mL, respectively, 9h post-dose [7].
Few human plasma cannabinoid gas chromatography mass spectrometry (GCMS) methods achieved sub-nanogram concentrations of THC [11–14], and only one included CBD [14]. Separation of highly lipophilic cannabinoids from co-eluting matrix is complex. Two-dimensional GCMS (2D-GCMS) is an analytical tool that can help separate analytes from co-eluting matrix, increase signal to noise and improve sensitivity. 2D-GCMS was first employed for forensic purposes to quantify cannabinoids in whole blood [15], and shortly thereafter, applied to plasma [13], oral fluid [16–18], hair [19], and meconium [20].
We report the development and validation of the first 2D-GCMS method with enhanced sensitivity and simultaneous analysis of CBD, THC, 11-OH-THC and THCCOOH in plasma. This method will be utilized for specimen analysis in future oral THC and Sativex® controlled administration research.
MATERIALS AND METHODS
Chemicals and reagents
THC, 11-OH-THC, THCCOOH, and CBD (1 mg/mL) and deuterated internal standards THC-d3, 11-OH-d3, THC-d3, THCCOOH-d3 and CBD-d3 (100 μg/mL) were purchased from Cerilliant (Round Rock, TX, USA). N, O,-bis(trimethylsilyl) trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) was obtained from Regis Technologies, Inc. (Morton Grove, IL, USA), Styre Screen® SSTHC06Z solid phase extraction (SPE) columns (10 mL/60 mg) from United Chemical Technologies (Bristol, PA, USA), and blank human plasma was acquired from the National Institutes of Health Clinical Center. HPLC-grade water, glacial acetic acid and ammonium hydroxide were obtained from JT Baker (Phillipsburg, NJ, USA). Ethyl acetate and hexane were acquired from Sigma-Aldrich (St. Louis, MO, USA) and all solvents were HPLC-grade.
Calibrators, quality control samples and internal standards
Individual stock solutions (1 mg/mL) were diluted in methanol and combined to prepare an intermediate calibration standard (10 μg/mL) containing CBD, THC, 11-OH-THC and THCCOOH. Methanolic working calibrator solutions at 10, 100 and 1000 ng/mL were prepared by dilution of the 10 μg/mL intermediate cannabinoid standard. Daily calibration curves were prepared by fortifying 1.0 mL blank plasma with appropriate amounts of working calibrator solution. Multi-analyte calibrators (0.125, 0.25, 0.5, 1.0, 5.0, 10, 25, 50 and 100 ng/mL) were assayed with each batch. Quality control (QC) solutions (containing all analytes) were prepared from different source lots and diluted to 10, 500 and 1000 ng/mL with methanol. Plasma QCs (0.35, 7.5, 20, 75 ng/mL) were prepared by fortifying blank plasma with appropriate QC solutions.
Stock ampoules of CBD-d3, THC-d3, 11-OH-THC-d3, and THCCOOH-d3 (100 μg/mL) were combined and diluted in methanol to produce a working 200 ng/mL internal standard solution containing all analogs. A 5 ng/mL final internal standard concentration was achieved by adding 25 μL of working internal standard to each calibrator, control or specimen. All cannabinoid solutions were stored in amber vials at −20°C.
PROCEDURES
Specimen preparation and solid phase extraction
One mL blank plasma was added to 13 mm × 100 mm glass test tubes and fortified with appropriate methanolic calibrator or control solution. 25 μL internal standard was added to 1 mL of each unknown plasma specimen, calibrator and control. Three mL acetonitrile was added in 0.5 mL increments while vortexing, followed by centrifugation at 1800 × g for 10 min at 2°C. Supernatants were decanted into clean glass test tubes containing 5 mL water and mixed. SPE columns were preconditioned with 1 mL hexane/ethyl acetate/acetic acid (49:49:2), 1 mL methanol and 1 mL water. Samples were poured directly onto preconditioned polymeric SPE columns, washed with 2 mL water/acetonitrile/ammonium hydroxide (84:15:1 v/v) and dried under vacuum for 20 min. Analytes were eluted with 3 mL hexane/ethyl acetate/glacial acetic acid (49:49:2 v/v) into conical 10 mL centrifuge tubes. Eluates were evaporated under nitrogen at 38°C, reconstituted with 25 μL BSTFA + 1%TMCS and derivatized at 70°C for 30 min. Following derivatization, extracts were centrifuged at 1800 × g for 3 min at 22°C, and transferred to autosampler vials for analysis by 2D-GCMS.
Clinical specimens
Plasma was collected from a subject with a history of cannabis smoking who received Sativex® delivered by oromucosal spray. The Institutional Review Board-approved protocol was conducted at the National Institute on Drug Abuse and the participant provided written informed consent. The plasma specimen was stored at −20°C prior to analysis.
Two-dimensional gas chromatography mass spectrometry
Two capillary fused silica chromatographic columns were employed for 2D-GCMS. Chromatographic separation of analyte from matrix was accomplished with a primary ZB-50 capillary column (30 m × 0.25 mm × 0.25 μm film thickness; Phenomenex, Torrance, CA) and a secondary DB-1ms capillary column (15 m × 0.25 mm × 0.25 μm film thickness; Agilent Technologies, Wilmington, DE) connected by a microfluidic Deans switch in an Agilent 6890 gas chromatograph. Derivatized extracts (4 μL) were injected in splitless injection mode. Analyte retention times on the primary column were determined by injecting a neat derivatized high concentration cannabinoid standard containing CBD, THC, 11-OH-THC and THCCOOH with column effluent directed to a flame ionization detector (FID). Heart cuts (0.4–0.6 min) containing each analyte peak were made, diverting flow to the secondary column. An air-cooled cryogenic focusing trap (Joint Analytical Systems, Marlton, NJ) controlled by back inlet electronics was positioned at the head of the secondary column and condensed analyte cuts at 100°C. Oven temperature was held at 185°C initially for 0.5 min and increased at 45°C/min to 225°C and held for 3.0 min. A second ramp, 15°C/min to 275°C, was held at 275°C for 1.58 min until CBD and THC had been condensed or “trapped” at the beginning of the secondary column. Oven temperature decreased at 80°C/min to 195°C and held for 2.3 min. During this hold time, cryotrap temperature increased at 700°C/min to 225°C to re-vaporize analytes for resolution on the second column of alternate polarity. Oven temperature was ramped slowly 10°C/min to 230°C and then immediately 25°C/min to 275°C. During the last oven ramp, 11-OH-THC and THCCOOH analyte cuts were re-vaporized at 700°C/min to 275°C and resolved on the secondary column. Final detection was accomplished with an Agilent 5973 mass selective detector operated in electron impact-selective ion monitoring (SIM) mode. At the conclusion of each analytical run, post run temperature increased to 300°C, column 1 pressure decreased to 1.0 psi, secondary column pressure increased to 100 psi and the Deans switch implemented carrier gas flow reversal. Flow reversal or back flushing, forced extraneous material on the columns out the split vent and decreased the need for column maintenance. Instrumental method parameters are listed in Table 1.
Table 1.
Two-dimensional gas chromatography/mass spectrometry (2D-GCMS) method parameters for analysis of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC), 11-hydroxy-Δ9-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) in plasma
| Front inlet | Back inlet | ||
|---|---|---|---|
| Mode | Constant pressure | Initial temp | 100°C |
| Inlet temp | 275°C | Initial time | 10.40 min |
| Injection mode | Splitless | Ramp #1: 700°C/min | Final temp: 225°C |
| Purge flow | 25.0 mL/min | Final time: 4.30min | |
| Purge time | 0.8 min | Ramp #2: 700°C/min | Final temp: 100°C |
| Pressure | 35.0 psi | Final time: 5.50 min | |
| Total flow | 30.2 mL/min | Ramp #3: 700°C/min | Final temp: 275°C |
| Final time: 0.00 min | |||
|
| |||
| Oven | Deans Switch | ||
|
| |||
| Initial oven temp | 185°C | FID restrictor length | 3.0 m |
| Initial oven hold | 0.5 min | FID restrictor i.d. | 0.10 mm |
| Ramp #1: 45°C/min | Final temp: 225°C | Aux 3 pressure | 18.7 psi |
| Final time: 3.00 min | |||
| Ramp #2: 15°C/min | Final temp: 275°C | ||
| Final time: 1.58 min | |||
| Ramp #3: 80°C/min | Final temp: 195°C | ||
| Final time: 2.30 min | |||
| Ramp #4: 10°C/min | Final temp: 230°C | ||
| Final time: 0.00 min | |||
| Ramp #5: 25°C/min | Final temp: 275°C | ||
| Final time: 5.10 min | |||
|
| |||
| Post-run | Flame ionization detector | ||
|
| |||
| Post temp | 300°C | FID temp | 250°C |
| Post time | 1.0 min | Hydrogen flow | 40.0 mL/min |
| Column 1 pressure | 1.0 psi | Air flow | 450 mL/min |
| Column 2 pressure | 100.0 psi | Nitrogen makeup flow | 45.0 mL/min |
Three ions for each analyte and two ions for each deuterated internal standard were monitored (target ion underlined): CBD-d3 461, 393; CBD 458, 390, 301; THC-d3 389, 374; THC 386, 371, 303; 11-OH-THC-d3 374, 477; 11-OH-THC 371, 474, 459; THCCOOH-d3 374, 491; and THCCOOH 371, 488, 473. Dwell times were 50 ms for CBD, 10 ms for THC, 15 ms for 11-OH-THC and 25 ms for THCCOOH. The mass selective detector (MSD) electron multiplier was operated +200 V relative to the daily autotune, and the quadrupole, source and MSD interface were maintained at 150, 250, and 280°C, respectively.
Data analysis
Data were analyzed with Agilent Chemstation software version D.01.00. Analytes were identified by comparing retention times (± 0.15 minutes) and qualifier ion ratios (± 20%) to average calibrator values obtained in the same run. Quantification was determined by the ratio of target analyte peak area to corresponding internal standard peak area. Calibration curves were constructed with linear least squares regression with 1/x weighting to adjust for heteroscedasticity across the calibration range. Calibrator concentrations were required to be within 15% of target, except 20% at LOQ. In each analytical batch, two calibration curves were prepared to achieve adequate sensitivity and linearity for expected specimen concentrations, and extend quantifiable concentrations through three orders of magnitude.
Method Validation
Method validation parameters included sensitivity, specificity, linearity, imprecision, bias, extraction efficiency, dilution integrity, carryover, and stability.
Limit of detection (LOD) was evaluated in triplicate and defined as the analyte concentration with target ion signal-to-noise ratio of three or greater, Gaussian peak shape, retention time within ± 0.15 min of the average calibrator retention time, and qualifier ion ratios within ± 20% of average calibrator qualifier ion ratios assayed in the same run. Limit of quantification (LOQ) was also assessed in triplicate and was the lowest concentration that met LOD criteria and quantified within 20% of target. LOD and LOQ were reported as equal if the extracted calibrator was unable to meet LOD criteria at a concentration less than the LOQ.
Method specificity was investigated by adding 30 structurally similar or commonly co-administered drugs, over-the-counter medications, and illegal drugs of abuse to a low QC (0.35 ng/mL) sample. Low QC samples were fortified with 1,000 ng/mL codeine, norcodeine, 6-monoacetylmorphine, acetylcodeine, morphine, normorphine, morphine-3-glucuronide, morphine-6-glucuronide, cocaine, benzoylecgonine, norcocaine, norbenzoylecgognine, hydrocodone, hydromorphone, oxycodone, diazepam, lorazepam, oxazepam, alprazolam, nitrazepam, temazepam, nordiazepam, nicotine, cotinine, trans-3′-hydroxycotinine, norcotinine, caffeine, aspirin, acetaminophen, or phencyclidine. Cannabinoid analytes were required to quantify within ±20% of target concentration and have acceptable ion ratios (±20% of average calibrator ratio). In addition, ten different plasma pools were assessed for potential endogenous matrix interferences.
Linearity was assessed with least squares regression and a 1/x weighting factor expressed as the coefficient of determination (r2). Linearity was determined with a minimum of five calibrators for all analytes. Each calibrator was required to quantify within 15% of target, except at the LOQ, where imprecision within 20% was acceptable.
Imprecision and bias were evaluated throughout the linear dynamic range with low, medium, and high QCs for all analytes on both calibration curves. Inter-assay imprecision and bias were assessed with 2 replicates at each QC level over ten days (n = 20). Intra-assay imprecision was determined from 5 replicates of each QC within a single analytical run. Imprecision was expressed as percent coefficient of variation (%CV) of the individual concentrations. Within-run, between-run and total imprecision was calculated by the Krouwer and Rabinowitz method [21–22]. Bias was determined by comparing mean concentrations to target and expressed as a percentage.
Extraction efficiency for each analyte was assessed in fortified blank plasma (n = 4) at each QC concentration (0.35, 7.5, 20, and 75 ng/mL). Extraction efficiency was calculated by comparing mean target ion peak areas in samples fortified prior to extraction with samples fortified after extraction, but before evaporation.
Sample dilution integrity was determined by diluting a 100 ng/mL fortified sample 1:5 (n = 5) with blank plasma verified negative for cannabinoids. 200 μL of a 100 ng/mL plasma sample was diluted with 800 μL blank plasma and mixed. Protein was precipitated and SPE performed. Concentrations in the diluted sample were required to be within ± 20% of target. Instrumental carryover was assessed by injecting 4 μL of a 150 ng/mL high calibrator prior to a negative plasma extract containing internal standard.
Analyte stability in plasma was determined by evaluating triplicate QC samples at four concentrations under different storage conditions. Samples were analyzed fresh and following three freeze/thaw cycles at −20°C, refrigerated at 4°C for 48 h or room temperature (22°C) for 16 h. Mean concentrations (n = 3) for each storage condition were compared to mean concentrations of freshly prepared QC samples (n = 3). Autosampler extract stability at room temperature was evaluated by re-injecting low, mid and high QC extracts (n = 4) 48h post-extraction and quantified against the initial calibration curve. High QC (75 ng/mL) extracts (n=4) at 48h were quantified against the high calibration curve. Analyte concentrations from stability extracts injected at 48h were compared to corresponding initial injections immediately after extraction.
RESULTS
Method Development
We initially evaluated a non-polar primary column (DB-1ms, 15 m × 0.25 mm × 0.25 μm) and a secondary intermediate polarity (ZB-50, 30 m × 0.25 mm × 0.25 um) column, as previously reported for the analysis of THC, 11-OH-THC and THCOOH in plasma [13]. However, this column configuration did not provide adequate resolution of CBD ions. The inverse configuration, consisting of a 30 m intermediate polarity primary column and a secondary 15 m non-polar column, successfully resolved CBD ions from matrix, likely due to increased separation in the longer initial column. Unfortunately, we encountered shifting analyte retention times on the 30 m primary column. Although this is not a problem in typical one-dimensional chromatography, it can be a drawback in 2D-GC. Cut windows are set approximately 0.4–0.6 min around the analytes’ retention times on the primary column, and, if a retention time shift is occurs, only a fraction of the analyte or internal standard may be sent to the secondary column for separation and mass spectrometric detection. An extracted calibrator consisting of equal concentrations of analyte and internal standard was injected prior to each validation run to verify that cut windows were properly set. Retention time shifts may be exacerbated by pulsed splitless injection, likely inducing pressure changes, thus, splitless injection specifications were implemented.
Although CBD and THC elute from the secondary column less than one minute apart, a single MS acquisition window was created for CBD and THC ions to manage potential retention time shifts. Also, a new oven temperature ramp was developed for extended retention of analytes on the secondary column to minimize retention time drift (Table 1). Complex oven temperature parameters were required for CBD and THC resolution. THC Q2 m/z 303 was resolved by implementation of two oven ramps from 185°C to 225°C and then to 275°C. The subsequent decrease in temperature to 195°C and ramp to 230°C was needed for 90% baseline resolution of CBD Q2 m/z 390. Prior to instituting this decrease, m/z 390 was co-eluting with another matrix component. 11-OH-THC and THCCOOH did not present chromatographic difficulties.
Solid phase extraction columns and extraction procedures (UCT Clean Screen ZSTHC020) from our previously published method [13] were initially investigated for specimen preparation. Previous research suggested CBD conversion to its structural isomer THC under acidic conditions [23]. Interestingly, when CBD was extracted from plasma using our previous SPE conditions, approximately 2% conversion of CBD to THC was observed. There was no measurable conversion in neat methanolic, non-extracted, derivatized samples, nor when HCl was eliminated and polymeric extraction columns (UCT SSTHC) were employed.
Method validation
No exogenous interferences were noted when low plasma control samples (0.35 ng/mL) were fortified with over-the-counter medications, other illicit drugs, and drug metabolites at 1,000 ng/mL. Low QCs quantified within 20% of target in all cases. Additionally, ten blank plasma pools evaluated for endogenous interferences demonstrated no quantifiable analyte peaks at method LOQ.
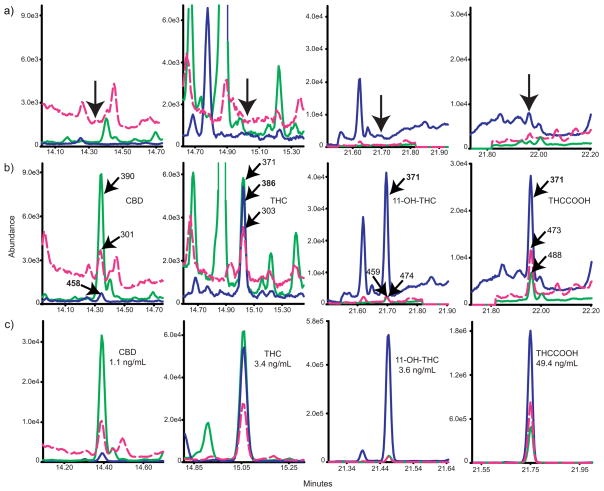
Low calibration curve linear dynamic ranges were 0.25–25 ng/mL for CBD and THC, 0.125–25 ng/mL for 11-OH-THC and 0.25–50 ng/mL for THCCOOH (Table 2). A second curve (5–100 ng/mL) was constructed following a smaller 2 μL injection volume, permitting quantification of THC and 11-OH-THC >25 ng/mL and THCCOOH >50 ng/mL. Samples were quantified on the low calibration curve unless concentrations were above the upper limit of linearity. All calibrators were within 15% of target except the LOQ, which quantified within 20% of target when calculated against the full calibration curve. Coefficients of determination were >0.990 for all calibration curves (n=5). LOD ranged from 0.125–0.25 and LOQ were 0.25 for CBD, THC and THCCOOH and 0.125 ng/mL for 11-OH-THC (Table 2). Analyte extracted ion chromatograms at LOQ are presented in Figure 1.
Table 2.
Limits of detection (LOD) and quantification (LOQ) and linear range characteristics of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) for low and high calibration curves (n = 5). Samples were quantified on the low calibration curve unless concentrations were above the upper limit of linearity.
| Analyte | LOD (ng/mL) | LOQ (ng/mL) | Slope (mean ± SD) | Intercept (mean ± SD) | Linear range (ng/mL) |
|---|---|---|---|---|---|
| CBD | |||||
| Low Curve | 0.25 | 0.25 | 0.1682 ± 0.01 | 0.0147 ± 0.00 | 0.25–25 |
| THC | |||||
| Low Curve | 0.25 | 0.25 | 0.1691 ± 0.00 | 0.0142 ± 0.00 | 0.25–25 |
| High Curve | -- | -- | 0.1351 ± 0.00 | 0.2679 ± 0.06 | 5–100 |
| 11-OH-THC | |||||
| Low Curve | 0.125 | 0.125 | 0.1760 ± 0.01 | 0.0072 ± 0.00 | 0.125–25 |
| High Curve | -- | -- | 0.1394 ± 0.00 | 0.2819 ± 0.05 | 5–100 |
| THCCOOH | |||||
| Low Curve | 0.125 | 0.25 | 0.1686 ± 0.01 | 0.0142 ± 0.00 | 0.25–50 |
| High Curve | -- | -- | 0.1333 ± 0.01 | 0.2494 ± 0.04 | 5–100 |
Figure 1.
Extracted ion chromatograms from a) blank extracted plasma, b) blank plasma fortified with analytes at the limits of quantification- 0.25 ng/mL cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC), 11-nor-9-carboxy-THC (THCCOOH) and 0.125 ng/mL 11-hydroxy-THC (11-OH-THC), and c) extracted participant specimen from Sativex® administrationa. Arrows indicate retention times of analytes.
aPanel C’s retention times do not match panels A and B due to column maintenance performed just prior to this analytical run.
Imprecision and bias were determined at 0.35, 7.5, 20 ng/mL for all analytes and additionally at 75 ng/mL for THC, 11-OH-THC and THCCOOH. Inter- and intra-assay imprecision (%CV) were <7.8 and <6.4% for all analytes, respectively (Table 3). The method was highly reproducible and QC samples quantified within ± 9.2% of target. Within-run, between-run and total imprecision were less than 8.4, 4.5, and 9.5%, respectively, for all analytes. Calculations were made in accordance with Krouwer and Rabinowitz [21–22]. Extraction efficiencies were 72.9–99.6% for all analytes (Table 3).
Table 3.
Inter- and intra-assay imprecision and bias of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) for low and high calibration curves.
| Analyte | Target (ng/mL) | Inter-assay imprecision (%CV; n = 20) | Intra-assay Imprecision (%CV; n = 5) | Bias (% target; n =20) |
|---|---|---|---|---|
| CBD | ||||
| Low Curve | 0.35 | 6.5 | 5.6 | 94.3 |
| 7.5 | 6.1 | 1.9 | 100.6 | |
| 20 | 3.7 | 1.7 | 97.3 | |
| THC | ||||
| Low Curve | 0.35 | 7.7 | 6.4 | 94.9 |
| 7.5 | 6.8 | 1.3 | 97.9 | |
| 20 | 3.8 | 1.7 | 97.5 | |
| High Curve | 7.5 | 7.0 | 2.2 | 92.4 |
| 20 | 4.2 | 1.6 | 105.7 | |
| 75 | 4.0 | 2.0 | 105.8 | |
| 11-OH-THC | ||||
| Low Curve | 0.35 | 5.5 | 1.3 | 98.7 |
| 7.5 | 5.5 | 0.9 | 98.0 | |
| 20 | 4.1 | 2.0 | 96.7 | |
| High Curve | 7.5 | 6.8 | 1.5 | 91.8 |
| 20 | 3.6 | 1.3 | 105.2 | |
| 75 | 5.2 | 4.0 | 103.3 | |
| THCCOOH | ||||
| Low Curve | 0.35 | 7.8 | 3.1 | 90.8 |
| 7.5 | 5.0 | 1.5 | 104.5 | |
| 20 | 3.9 | 1.8 | 99.6 | |
| High Curve | 7.5 | 7.0 | 2.3 | 91.9 |
| 20 | 2.5 | 1.2 | 103.9 | |
| 75 | 3.6 | 2.4 | 101.6 | |
Patients receiving oral THC or Sativex® have lower cannabinoid concentrations than achieved following smoked cannabis [3, 7]. Plasma specimen dilution may be necessary to quantify cannabinoids after the smoked route. Cannabinoid-fortified plasma (n=5) diluted 1:5 with blank plasma quantified within 20% of target for all analytes. No carryover was observed in specimens containing only internal standard following injection of the high 150 ng/mL extract.
CBD, THC, 11-OH-THC and THCCOOH stability under different storage conditions are presented in Table 4. After three freeze-thaw cycles all analytes quantified within −7.7 to 10.4% of target. When stored at room temperature or at 4°C, analytes were within ±11.3% of expected concentrations. Stability of autosampler extracts 48h after initial injection was within ±5.4% of initial assay concentration for all compounds and all control concentrations.
Table 4.
Analyte stability (n = 3) & mean extraction efficiencies (n = 4) for cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH) in plasma.
| Analyte | Target (ng/mL) | Mean % difference from target
|
Extraction Efficiency | ||
|---|---|---|---|---|---|
| 22°C 16 h | 4°C 16 h | Freeze/thaw (x3 cycles) | |||
| CBD | 0.35 | 9.4 | 0.0 | 10.4 | 86.0 |
| 7.5 | 7.8 | 2.2 | −3.9 | 83.8 | |
| 20 | 4.9 | 5.8 | 1.0 | 90.4 | |
| THC | 0.35 | 4.2 | 4.9 | 6.3 | 82.0 |
| 7.5 | 8.0 | 2.1 | −2.6 | 83.9 | |
| 20 | 1.8 | 4.1 | −7.7 | 81.4 | |
| 75 | 0.9 | −7.3 | −2.7 | 72.9 | |
| 11-OH-THC | 0.35 | 3.2 | −1.0 | −3.2 | 94.2 |
| 7.5 | −8.7 | −1.9 | −1.0 | 99.6 | |
| 20 | 3.9 | −4.8 | −6.6 | 98.4 | |
| 75 | −2.5 | −10.8 | −2.6 | 94.7 | |
| THCCOOH | 0.35 | 7.7 | 1.1 | 0.0 | 87.5 |
| 7.5 | −9.2 | −0.7 | −1.1 | 91.4 | |
| 20 | 5.2 | −3.3 | −0.5 | 89.9 | |
| 75 | −4.1 | −11.3 | −3.5 | 78.8 | |
Proof of method
This validated analytical method was applied to a plasma specimen from a participant enrolled in a controlled CBD and THC administration protocol. The plasma specimen contained 1.1 ng/mL CBD, 3.4 ng/mL THC, 3.6 ng/mL 11-OH-THC and 49.4 ng/mL THCCOOH. Extracted ion chromatograms are shown in Figure 1.
DISCUSSION
A validated 2D-GCMS method for simultaneous quantification of CBD, THC, 11-OH-THC and THCCOOH is presented. Validation of a second higher calibration curve from 5–100 ng/mL extends the dynamic range and enables the same specimen extract to be re-injected, preventing time-consuming re-extraction. Separate calibration ranges permit cannabinoid quantification throughout three orders of magnitude, achieving high sensitivity for low concentrations expected after oral or oromucosal administration or for samples collected hours after smoked cannabis [3]. Jones et al. observed a 1.0 ng/mL median whole blood THC concentration (equivalent to 2.0 ng/mL in plasma) in 8794 individuals detained after driving under the influence of drugs (DUID) in Sweden [24].
As cannabinoids are the most frequently encountered analytes in DUID investigations [25] and workplace drug testing [26], our objective was to validate a selective and sensitive method for cannabinoid quantification appropriate for use in most clinical and forensic laboratories. Modification of GCMS to 2D-GCMS with the addition of a pneumatic Deans switch and a cryofocusing trap were required to achieve lower LOQs than observed with traditional one-dimensional chromatography. Additionally, the current method was validated with a target ion, and two identifying qualifier ions, making it applicable to forensic investigations. Limits of quantification were equal to or lower than previous reports and extraction efficiencies were >72.9%, improving upon our previous plasma cannabinoid extraction efficiencies of >49.7% [13]. All QCs were within ± 9.2% of target concentrations and imprecision was <7.8% for all analytes.
Advantages of a 2D-GCMS system include signal-to-noise enhancement and improved chromatographic resolution, with potential application to multiple complex matrices. A disadvantage was the increased chromatographic run time of 23.0 min that was due to complex oven temperature parameters required to resolve THC m/z 303 and CBD m/z 390 from matrix components. Additionally, retention time shifts on the primary capillary column occasionally moved analytes out of cut windows. Daily injection of a neat derivatized high concentration standard prior to each analytical sequence was used to verify cut windows. An extracted 5 ng/mL calibrator also was injected. If d0 and d3 analyte peak areas had equivalent abundances, heart cuts were considered appropriate and analyses begun.
To our knowledge, this is the first analytical method to measure not only CBD, but also THC, 11-OH-THC and THCCOOH concentrations at or below 0.25 ng/mL in plasma. This robust and sensitive method permits simultaneous quantification of multiple cannabinoid analytes in human plasma and will be applied to clinical specimens from controlled oral THC and Sativex® administration. This complex instrumental method should be applicable to multiple biological matrices, following matrix validation, and should be highly useful for clinical research, forensic toxicology, workplace drug testing, and DUID programs.
Acknowledgments
The authors acknowledge G.W. Pharma, Ltd. for generously providing Sativex® and placebo Sativex® and Fred Feyerherm of Agilent Technologies for technical assistance. This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
References
- 1.Pertwee RG. Euphytica. 2004;140:73–82. [Google Scholar]
- 2.Benowitz N. Clinical pharmacology of inhaled drugs of abuse: implications in understanding nicotine dependence. In: Chiang CN, Hawks RL, editors. Research Findings on Smoking of Abused Substances. NIDA Research Monograph; Rockville: 1990. pp. 12–29. [PubMed] [Google Scholar]
- 3.Huestis MA, Henningfield JE, Cone EJ. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 4.Huestis MA, Smith ML. Human cannabinoid pharmacokinetics and interpretation of cannabinoid concentrations in biological fluids and tissues. In: ElSohly MA, editor. Marijuana and the Cannabinoids. Humana Press; Totawa, New Jersey: 2006. pp. 205–236. [Google Scholar]
- 5.Van der Kooy F, Pomahacova B, Verpoorte R. Inhal Toxicol. 2009;21:87–90. doi: 10.1080/08958370802187296. [DOI] [PubMed] [Google Scholar]
- 6.Russo E, Guy GW. Med Hypotheses. 2006;66:234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Guy GW, Robson PJ. Journal of Cannabis Therapeutics. 2004;3:121–152. [Google Scholar]
- 8.Russo E. Therapeutics and Clinical Risk Management. 2008;4:245–259. doi: 10.2147/tcrm.s1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bland TM, Haining RL, Tracy TS, Callery PS. Biochem Pharmacol. 2005;70:1096–1103. doi: 10.1016/j.bcp.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Life Sci. 2007;80:1415–1419. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Gustafson RA, Moolchan ET, Barnes A, Levine B, Huestis MA. J Chromatogr B. 2003;798:145–154. doi: 10.1016/j.jchromb.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Huang W, Moody DE, Andrenyak DM, Smith EK, Foltz RL, Huestis MA, Newton JF. J Anal Toxicol. 2001;25:531–537. doi: 10.1093/jat/25.7.531. [DOI] [PubMed] [Google Scholar]
- 13.Lowe RH, Karschner EL, Schwilke EW, Barnes AJ, Huestis MA. J Chromatogr A. 2007;1163:318–327. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadulski T, Sporkert F, Schnelle M, Stadelmann AM, Roser P, Schefter T, Pragst F. J Anal Toxicol. 2005;29:782–789. doi: 10.1093/jat/29.8.782. [DOI] [PubMed] [Google Scholar]
- 15.Scurlock RD, Ohlson GB, Worthen DK. J Anal Toxicol. 2006;30:262–266. doi: 10.1093/jat/30.4.262. [DOI] [PubMed] [Google Scholar]
- 16.Fritch DF, Quimby BD. Agilent Technologies. 2006. [Google Scholar]
- 17.Moore C, Coulter C, Rana S, Vincent M, Soares J. J Anal Toxicol. 2006;30:409–412. doi: 10.1093/jat/30.7.409. [DOI] [PubMed] [Google Scholar]
- 18.Moore C, Rana S, Coulter C, Day D, Vincent M, Soares J. J Anal Toxicol. 2007;31:187–194. doi: 10.1093/jat/31.4.187. [DOI] [PubMed] [Google Scholar]
- 19.Moore C, Rana S, Coulter C, Feyerherm F, Prest H. J Anal Toxicol. 2006;30:171–177. doi: 10.1093/jat/30.3.171. [DOI] [PubMed] [Google Scholar]
- 20.Marin SJ, Coles R, Urry FM, McMillin GA. J Chromatogr B. 2007;858:59–64. doi: 10.1016/j.jchromb.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Krouwer JS, Rabinowitz R. Clin Chem. 1984;30:290–292. [PubMed] [Google Scholar]
- 22.Krouwer JS. Clin Chem. 1981;27:202. [PubMed] [Google Scholar]
- 23.Gaoni Y, Mechoulam R. Tetrahedron. 1966;22:1481–1488. [Google Scholar]
- 24.Jones AW, Holmgren A, Kugelberg FC. Addiction. 2008;103:452–461. doi: 10.1111/j.1360-0443.2007.02091.x. [DOI] [PubMed] [Google Scholar]
- 25.Farrell LJ, Kerrigan S, Logan BK. J Forensic Sci. 2007;52:1214–1218. doi: 10.1111/j.1556-4029.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 26.Huestis MA. Cannabinoids. In: Ropero-Miller JD, Goldberger BA, editors. Handbook of Workplace Drug Testing, Chapter 13-Cannabinoids. AACC press; Washington DC: 2009. pp. 355–382. [Google Scholar]