Abstract
In recent years, there has been an increasing interest in studying the anemia that occurs after kidney transplantation. Although many of the guidelines for the treatment of kidney transplant patients, including those for anemia, are extrapolated from recommendations for patients with chronic kidney disease, there are important differences in the cause of and response to anemia in kidney transplant recipients. In addition to the correlation of anemia with kidney function as in native renal disease, many other factors are associated with the development of anemia after transplantation, including the use of medications and the inflammation/immune response. Given the lack of large, well-designed, prospective studies, the consequences of anemia, the response to treatment, and the cost-effectiveness of treatment in the posttransplantation setting are also poorly understood.
Although anemia has been investigated with great interest and intensity in patients with chronic kidney disease (CKD), including the dialysis population, studies of the prevalence and clinical relevance of posttransplantation anemia (PTA) were scarce until a few years ago. One could argue that patients with a functioning kidney transplant are similar to patients with chronic native renal insufficiency (not on dialysis) and that evidence from the CKD population could be extrapolated to these kidney transplant recipients (KTR) (1). There are, however, at least three important reasons that such an extrapolation may not be legitimate: The presence of immunosuppressant agents and other transplant-related medications, the altered inflammatory milieu, and the history of prolonged maintenance dialysis therapy in most KTR. The altered inflammatory milieu is due to the systemic immune response to the presence of alloantigen, leading to a proinflammatory state that increases erythropoietin (EPO) resistance. Inadequate dialysis and activation of cytokines by dialysis membranes have also been shown to cause EPO resistance, but whether these factors have long-term consequences that carry over after transplantation are unknown. These and other factors may confound the validity of extrapolating scientific findings and clinical experience from the CKD (not on dialysis) population. Thus, it seems essential to establish evidence directly from the KTR population rather than through inference from patients with CKD. This article provides a critical review of the evidence on PTA.
Course of Anemia after Transplantation
Two major developments have influenced the degree of anemia in patients with renal failure at the time of transplantation. The first is the widespread use of EPO for dialysis patients and patients with CKD. This has had the effect of reducing the degree of anemia in potential transplant recipients and decreased the likelihood of immunologic sensitization in patients as a result of the decreased need for blood transfusions before transplantation. Although decreasing the number of blood transfusions before transplantation has not been shown to affect 1-yr graft survival, 5-yr graft survival in patients who were treated with recombinant human EPO (rh-EPO) was shown to be significantly higher than in dialysis patients who were treated with repeated blood transfusions (2). In addition, the responsiveness of patients to EPO before transplantation seems to have an impact on long-term graft survival, with EPO-hyporesponsive patients having worse graft survival (3).
The second is the increased trend of preemptive transplantation of patients before the need for dialysis. It has been shown that patients who undergo preemptive transplantation fare better in that they have better graft survival, and, as a consequence, these patients are probably less likely to be anemic than patients who receive a transplant after a period on dialysis (4); however, even after transplantation, the majority of patients do not have normal renal function as measured by estimates of GFR, and poorer renal function has been associated with lower hematocrit in the transplant population in a similar manner to native kidney disease (5). The usual time course of anemia after renal transplantation is the development of anemia early after transplantation followed by recovery and then in the longer term an increased risk for anemia (6).
Early after transplantation, blood loss related to the surgical procedure and subsequent inflammation lead to anemia. Also the effects of delayed graft function, induction therapy causing bone marrow suppression, and the abrupt cessation of EPOadministration may play an important role in the development of anemia during this period. At this stage, it is less likely that use of exogenous rh-EPO will be effective as a result of a systemic inflammatory response, characterized by activation of the innate and specific immune response and increased levels of circulating cytokines and acute-phase proteins (7,8).
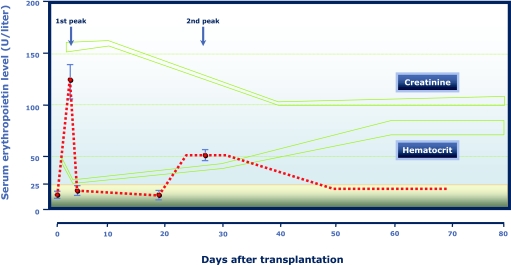
Six to 12 mo after transplantation, most acute disturbances of the peritransplantation and early posttransplantation periods have disappeared and are associated with the lowest rate of anemia during the natural course of life with a kidney transplant (6). The study by Sun et al. (9) even indicated that, as early as approximately 2 mo after transplantation, a normal hematocrit might be expected in patients without graft failure and iron deficiency (Figure 1). With the subsequent decrease in renal function that most patients experience, anemia once again develops associated with decreased transplant function. Although clearly associated with GFR, anemia occurs earlier (i.e., with more preserved renal function) in KTR compared with in patients with CKD (10).
Figure 1.
Kidney function, erythropoietin production, and hematocrit after kidney transplantation. Adapted from reference (9), with permission. Copyright © 1989 Massachusetts Medical Society. All rights reserved.
Epidemiology of PTA
Several recent studies have provided detailed accounts of the epidemiology of PTA, but this body of literature is difficult to synthesize, because the definitions of anemia used in these studies vary greatly. The “official” definition of anemia as set by the World Health Organization (WHO) in 1964 regards women to have anemia when their hemoglobin concentrations are <12 g/dl and men to have anemia when their hemoglobin concentrations are <13 g/dl. The American Society of Transplantation endorsed this definition in 2001. Few investigations of PTA, however, have used this definition, with most studies using a single threshold for both men and women (e.g., at hemoglobin concentrations of 11 or 12 g/dl [Typically, hemoglobin concentration and hematocrit are being converted following the formula hematocrit (%) = hemoglobin (g/dl) * 3 (i.e., a hematocrit of 33% corresponds to a hemoglobin concentration of 11 g/dl)]). Thus, the estimated prevalence of PTA will depend greatly on the definition used. Furthermore and as described previously, two important—and related—determinants of PTA are transplant function and time since receipt of the current transplant. Because most studies of PTA were cross-sectional in nature, information on the transplant vintage of the investigated population needs to be taken into account.
The overall picture that can be gleaned from the available evidence allows the general conclusion that anemia is, indeed, prevalent in KTR. Yorgin et al. (11) studied 128 adult patients at two US centers (Stanford, University of North Carolina) who received their transplant in 1995 and followed these patients for 5 yr. Whereas 43% of patients were anemic with a hematocrit <33% at the time of the transplant surgery, the prevalence of PTA decreased to 12% at 1 yr after transplantation. By 5 yr after transplantation, however, the prevalence of PTA again increased to 26%. Using the same anemia definition, Mix et al. (6) found that at another US center (New England Medical Center, Boston, MA), 48% were anemic at time of transplantation, but only 7% were at 6 mo after the procedure. Again, 4 yr after receipt of the transplant, 20% had a hematocrit <33%. Using an anemia threshold at a hematocrit <36%, the prevalence of PTA was 76% at transplantation, 21% at 1 yr, and 36% at 4 yr after transplantation. At Brigham and Women's Hospital, of 374 prevalent KTR who had carried their transplant for a mean of 7.7 yr, 48% had a hematocrit of ≤36% and 29% had a hematocrit of ≤33% (5). Four European cross-sectional studies used the WHO definition of anemia, allowing for differences between men and women (hemoglobin concentrations of <13 and <12 g/dl, respectively). In a large European survey and a single-center study at the University of Vienna, approximately 39% of KTR were found to be anemic (12,13), whereas 34% of prevalent KTR were anemic at Semmelweis University in Budapest (14). It is interesting that in the most recent assessment, a study of KTR who were prevalent on December 31, 2004, at several London-area hospitals, as many as 46% were found to be anemic using the WHO definition (15).
Causes and Correlates
There are multiple potential causes and correlates of anemia in the renal transplant population. Factors that have been implicated include poor renal function (6,11,12), bone marrow suppression (5,12), blood loss, autoimmune hemolytic anemia (16), chronic inflammatory state, use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (5). hyperparathyroidism, viral infections, and iron deficiency (Table 1) (17).
Table 1.
Common causes and correlates of anemia in kidney transplant recipientsa
| Age | Infection |
| Donor age | Inflammation |
| Gender | Malignancy |
| Graft dysfunction | Medications |
| acute rejection | RAAS inhibitors |
| chronic allograft | ACE inhibitors |
| nephropathy | ARB |
| Blood loss | immunosuppressants |
| acute | mycophenolate |
| chronic | azathioprine |
| Hemolysis | tacrolimus |
| Iron deficiency | sirolimus |
| absolute | trimethoprim- |
| functional | sulfametoxazole |
| Vitamin deficiency | ganciclovir |
| Hyperparathyroidism | Metabolic acidosis |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; RAAS, renin-angiotensin-aldosterone system.
After kidney transplantation, there is a large and immediate increase in EPO production (Figure 1) (9) that is similar to the increase in EPO production associated with renal hypoperfusion and ischemia (18). This precedes graft recovery and is followed by a smaller but more sustained increase in EPO production associated with an improvement in graft function and erythropoiesis. This observation is in keeping with a report that showed that the early use of darbepoetin was not effective at increasing the hematocrit until approximately 3 mo after transplantation (19). EPO production has been shown to return to normal levels after a hematocrit of 32 is reached (14). A more recent study concurred with these findings, showing an increased EPO concentration (in the absence of exogenous administration) in anemic patients compared with nonanemic patients after transplantation (20). It is interesting that it has been documented that anti-EPO antibodies that occasionally are responsible for severe anemia before transplantation tend to disappear after initiation of immunosuppression at the time of transplantation (21).
Immunosuppressive drugs contribute to the development of anemia through a variety of mechanisms. The antiproliferative agents azathioprine and mycophenolate mofetil have been associated with the development of PTA in most but not all studies that have looked for an association (5,22). In addition, although these drugs are known to cause anemia through direct suppression of the bone marrow, azathioprine can cause a macrocytic anemia (23). Other immunosuppressive drugs that are also toxic to the bone marrow are OKT3 and anti-thymocyte globulin. Of the calcineurin inhibitors, tacrolimus is more often associated with leukopenia but in one series was also associated with the development of anemia after transplantation (5). Tacrolimus has also rarely been reported to be responsible for an autoimmune hemolytic anemia that responds to substituting cyclosporine for tacrolimus (24). Sirolimus, the target of rapamycin inhibitor, is also commonly associated with the development of anemia in the posttransplantation setting. The mechanism involved seems to be unique to this drug. A single-center study that directly compared the effects of sirolimus and mycophenolate found that in the patients who were treated with sirolimus, the associated anemia was more common, more severe, and harder to treat (25). As with anemia related to other immunosuppressive drugs, it does respond to exogenous administration of rh-EPO. The mechanism of the anemia associated with sirolimus has been suggested to be related to decreased EPO production or to be an inflammatory-related anemia (25). In a recent randomized study in which patients were either switched from cyclosporine to sirolimus or maintained on cyclosporine, however, hematocrit concentrations were lower in the sirolimus group compared with patients who were maintained on cyclosporine (26). In patients who were treated with sirolimus, both the serum iron and ferritin levels markedly dropped after conversion. Despite the low transferrin saturation (<20%), patients did not respond to iron supplementation and hepcidin levels were not elevated, arguing against an inflammatory-related anemia and suggesting a mechanism related to interference with iron utilization.
Other drugs that have been associated with anemia in the posttransplantation setting are inhibitors of the renin-angiotensin-aldosterone system (RAAS) (5). The RAAS has been shown to be an important regulator of erythropoiesis. Chemical suppression of this system by angiotensin-converting enzyme inhibitors or angiotensin receptor blockers has been shown to depress hematocrit levels in nontransplant patients (27). Patients with a transplant seem to be more susceptible to this effect, and this may represent a higher degree of RAAS activation in patients who receive a transplant. In addition, a number of other drugs that are given in the posttransplantation period, such as trimethoprim-sulfamethoxazole and ganciclovir, are associated with the development of anemia.
An uncommon but sometimes prolonged cause of anemia after transplantation occurs as a result of an anti-rhesus hemolytic anemia. Rhesus-positive recipients of organs from rhesus-negative female donors who have been pregnant with a rhesus-positive fetus and developed an anti-rhesus immune response can sometimes transfer plasma cells within the allograft at the time of transplantation, causing an anti-rhesus immune response and subsequent hemolytic anemia in the recipient (28). This response is usually self-limiting and is treated by blood transfusion and decreasing immunosuppressive medications to allow the host immune response to eliminate the allo-plasma cells within the transplanted organ.
An increased inflammatory response including rejection seems to downregulate genes that are involved in erythropoiesis (29). It has also been shown that in patients who return to hemodialysis after graft failure and who undergo nephrectomy have a lower high-sensitivity C-reactive protein level and lower requirements for rh-EPO with higher hemoglobin levels than in patients who have returned to hemodialysis but did not undergo nephrectomy (30). This “EPO resistance” mirrors that seen in other hemodialysis patients with elevated C-reactive protein levels (31).
Similar to other herpesviridae, parvovirus B19 infection is not rare after kidney transplantation. Although there is ample evidence that this virus can, rarely, cause aplastic anemia in both the transplant and nontransplant settings, it has also been linked to chronic and EPO-resistant PTA; it is unclear, however, whether this is by cause or association. Parvovirus B19 was identified using PCR in as many as 40% of KTR with resistant anemia compared with only 12% in KTR overall (32,33). The treatment for B19 infection is administration of intravenous Ig with reduction of immunosuppression to allow the patient's own immune response to eliminate the virus (34).
One of the most important causes of PTA is loss of renal allograft function, paralleling the loss of endogenous EPO production in patients with CKD. Nearly all studies that have examined the association of renal function and development of anemia after kidney transplantation have found one. Although this is not surprising in itself, the degree of anemia that occurs seems to be worse for any given degree of renal dysfunction compared with native kidney disease. For any given creatinine clearance, hemoglobin concentrations are lower in KTR compared with patients with native CKD, and the prevalence of anemia is nearly 10-fold greater in KTR compared with patients with CKD in any stratum of creatinine clearance (10). It has further been emphasized recently that renal transplant function alone can explain only part of the observed rate of anemia in these patients and that endogenous EPO levels predict anemia independent of transplant function (20). Most interesting, EPO concentrations were found to be unrelated to transplant function in that study (R = 0.02; P = 0.74) (20). Recently, it was suggested that metabolic acidosis is an important and correctable factor in the development of PTA, even though the exact mechanism remains elusive (35).
Consequences of PTA
Several investigations have studied the consequences of PTA. Specifically, the relevant question is whether anemia is causally related to detrimental outcomes in KTR, especially with erythropoiesis-stimulating agents (ESA) available for treatment of anemia. The outcomes of greatest interest include cardiovascular morbidity and mortality, all-cause mortality, deterioration of allograft function, and allograft loss. As we show, all of the studies of the associations between PTA and outcomes have serious limitations in their design, scope, or analysis. The methodologically soundest analyses come from Rigatto et al. (36), who assembled a retrospective cohort of incident transplant patients at two Canadian centers (Winnipeg, MB, and St. John's, NF). Patients were included in their study only when they were alive with functioning graft and free from clinical heart disease at 1 yr after transplantation. These 638 patients were then followed during a median of 7.2 yr for the incident clinical outcomes of congestive heart failure (n = 63), ischemic heart disease (n = 61), and all-cause (n = 145) and cardiovascular death (n = 67). After (limited) multivariate adjustment, for each 1-g/dl decrease in hemoglobin concentration, the incidence of congestive heart failure increased by 24%, whereas no association was evident with the incidence of ischemic heart disease. Both all-cause and cardiovascular mortality were also associated with hemoglobin concentration at 1 yr after transplantation (16 and 15%, respectively, for each 1-g/dl decrease). Although the obvious strength of this study is the focus on incident rather than prevalent KTR and on incident outcomes among KTR free of cardiovascular disease, the main limitation of this study is the small number of outcomes, which precluded more extensive multivariate adjustment for other potentially important confounders that were NS in this relatively small data set. Another important limitation is the analytical approach to use hemoglobin concentrations as a continuous variable, which assumes a constant relationship between this variable and the outcomes of interest across the entire range of observed hemoglobin concentrations. Thus, it is possible that the results of the study are mainly driven by patients in a certain limited range of hemoglobin concentrations (e.g., the very low end of the range). The results from the study by Rigatto et al. (36) are supported by a similar study from the University of Pennsylvania. In 626 incident patients whose immunosuppressant regimen included mycophenolate mofetil, PTA defined as a hemoglobin concentration <12 g/dl was assessed at 12 mo after transplantation; mortality was the outcome of interest (22). After 5.4 yr of follow-up, 37 deaths were recorded, 19 of which were attributed to cardiovascular causes. From multivariate analyses, the authors estimated that patients with PTA had three times the mortality rate of those without PTA (hazard ratio [HR] 3.0; 95% confidence interval [CI] 1.3 to 6.7), with very similar findings for the association between PTA and cardiovascular mortality. Unfortunately, the authors inappropriately included too many variables in their multivariate model (“overfitting”), which typically yields artificially inflated and often grotesque estimates of effect. As a consequence of this serious methodologic flaw, the evidence from this study must be considered weak.
In contrast to these studies of incident KTR, several others ascertained PTA in prevalent patients and then followed these patients over time. A large study from the University of Vienna evaluated 825 prevalent KTR and followed these patients for a median of 8.2 yr; 251 patients died (37). Anemia as defined using the WHO definition was not associated with all-cause mortality in that study (HR 1.08; 95% CI 0.80 to 1.45), but an association with allograft loss was found (HR 1.25; 95% CI 1.02 to 1.59). Although this study was particularly carefully adjusted as a result of available measurement of several relevant laboratory parameters, information on existing cardiovascular disease and smoking status at baseline had not been collected. Another study was conducted at the Semmelweis University in Budapest and included 938 prevalent KTR (14). Although a very similar methodologic approach compared with that of Winkelmayer et al. (37) was applied, the results were very different. After multivariate adjustment, anemia (WHO definition) was associated with a 69% (95% CI 12 to 156%) increased mortality risk and a 97% (95% CI 43 to 171%) increased risk for allograft loss from any cause. It is unclear what contributed to the very different findings from these otherwise similar studies. By contrast to the Austrian study, the Hungarian study had collected information on self-reported comorbid conditions, but these did not differ between anemic and nonanemic patients. Serum albumin concentration was also available and was marginally but significantly higher in nonanemic KTR (42 versus 41 g/L); it is unlikely that the strikingly different results can be attributed to presence versus absence of data on albumin.
Overall, it becomes evident that data supporting an association between anemia and mortality or cardiovascular outcomes are weak and highly conflicting. Similarly conflicting conclusions have been drawn from these studies regarding the recommendations on treatment of PTA. Because medications such as ESA are available to treat PTA, the most important clinical (and health-economic) question is, of course, whether KTR with PTA should be treated and, if so, when treatment should be initiated and which hemoglobin concentrations should be targeted.
Treatment
Just like with any other medical intervention, several basic considerations need to go into the treatment decision of PTA: Efficacy, effectiveness, safety, and cost-effectiveness. Because it is uncertain whether findings from studies of patients with CKD can be applied to the kidney transplant population, it is desirable to have information available from studies conducted in the actual target population, KTR. Such studies are available regarding the efficacy of ESA. Indeed, ESA can increase hemoglobin concentrations in KTR, at least after the immediate posttransplantation period. As mentioned previously, early use of EPO was not effective at increasing the hematocrit until approximately 3 mo after transplantation (19,38). Later, use of EPO leads to higher hematocrit concentrations, and it has been shown that patients who have low endogenous levels of EPO respond to relatively low dosages of exogenous EPO compared with those who have high endogenous levels of EPO and require relatively larger dosages of exogenous EPO (39). Of note, use of EPO rapidly leads to iron deficiency that requires supplementation (19).
Several of the studies of PTA have also investigated patterns of EPO use in anemic KTR. Not surprising, several studies found that EPO use was low, even in KTR with pronounced anemia. In a European survey, 18% of patients with severe anemia (hemoglobin concentration <11 g/dl for men and <10 g/dl for women) received EPO (12). In two Boston-based studies, 25% of patients with hemoglobin concentration <11 g/dl and 40 to 42% of patients whose hemoglobin concentration was <10 g/dl received EPO (5,6). Similar observations were made in several other studies. Most authors of these investigations and several commentators then concluded that such patterns of EPO use indicated underuse of EPO and undertreatment of PTA in these patients. An alternative explanation, however, is that the physicians who treated these patients perceived a lack of evidence supporting EPO treatment in these patients. Indeed, although EPO is efficacious in raising hemoglobin concentrations in KTR, at least after the immediate posttransplantation period, the data on the importance of anemia as a prognostic factor are conflicting, and randomized trials of the effect of higher target hemoglobin concentrations on meaningful clinical outcomes are lacking. Such evidence, however, is sorely needed, especially in light of the large costs of EPO treatment.
Although such considerations were academic and focused on economic costs of higher hemoglobin targets until recently, the publication of new trial evidence on the outcomes of different hemoglobin target strategies in patients with CKD may inform our decision-making. Indeed, one study found an increase in adverse outcomes in the higher target hemoglobin group (13.5 g/dl) compared with the lower target hemoglobin arm (11.3 g/dl) (40), and another trial failed to demonstrate any cardiovascular benefits of more aggressive anemia management (target hemoglobin concentrations 10.5 to 11.5 versus 13 to 15 g/dl) (41). Although these studies were conducted in patients with CKD and similar evidence is lacking in KTR, the available evidence needs to be taken into account when making treatment decisions of PTA.
Conclusions
Anemia in KTR is prevalent and predominantly related to transplant function, so the best prevention of PTA is preservation of transplant function. Several important and established and some lesser known factors cause or contribute to anemia in these patients, many of those being modifiable. Should PTA occur, we conclude from the available evidence that management of PTA ought to be conservative, aimed at patients with hemoglobin concentrations <10 g/dl and patients who seem symptomatic from their anemia. More aggressive treatment may turn out to be costly in terms of both adverse outcomes and increased costs and scarce resources that could be spent better and more evidence based elsewhere. Further prospective studies using hard study end points are required and need to be conducted in KTR to inform and support any modifications of the suggested conservative approach to anemia management in these patients.
Disclosures
None.
Acknowledgments
We thank Dr. Gere Sunder-Plassmann for allowing us to use one of his slides for Figure 1.
References
- 1.Bostom AG, Kronenberg F, Gohh RY, Schwenger V, Kuen E, König P, Kraatz G, Lhotta K, Mann JF, Muller GA, Neyer U, Riegel W, Riegler P, Ritz E, Selhub J: Chronic renal transplantation: A model for the hyperhomocysteinemia of renal insufficiency. Atherosclerosis 156 :227 –230,2001 [DOI] [PubMed] [Google Scholar]
- 2.Lietz K, Lao M, Paczek L, Gorski A, Gaciong Z: The impact of pretransplant erythropoietin therapy on late outcomes of renal transplantation. Ann Transplant 8 :17 –24,2003 [PubMed] [Google Scholar]
- 3.Campise M, Mikhail A, Quaschning T, Snyder J, Collins A: Impact of pre-transplant anaemia correction and erythropoietin resistance on long-term graft survival. Nephrol Dial Transplant 20[Suppl 8] :viii8 –viii12,2005 [DOI] [PubMed] [Google Scholar]
- 4.Cransberg K, Smits JM, Offner G, Nauta J, Persijn GG: Kidney transplantation without prior dialysis in children: The Eurotransplant experience. Am J Transplant 6 :1858 –1864,2006 [DOI] [PubMed] [Google Scholar]
- 5.Winkelmayer WC, Kewalramani R, Rutstein M, Gabardi S, Vonvisger T, Chandraker A: Pharmacoepidemiology of anemia in kidney transplant recipients. J Am Soc Nephrol 15 :1347 –1352,2004 [DOI] [PubMed] [Google Scholar]
- 6.Mix TC, Kazmi W, Khan S, Ruthazer R, Rohrer R, Pereira BJ, Kausz AT: Anemia: A continuing problem following kidney transplantation. Am J Transplant 3 :1426 –1433,2003 [DOI] [PubMed] [Google Scholar]
- 7.Ganz T: Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102 :783 –788,2003 [DOI] [PubMed] [Google Scholar]
- 8.Lee P, Peng H, Gelbart T, Wang L, Beutler E: Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A 102 :1906 –1910,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun CH, Ward HJ, Paul WL, Koyle MA, Yanagawa N, Lee DB: Serum erythropoietin levels after renal transplantation. N Engl J Med 321 :151 –157,1989 [DOI] [PubMed] [Google Scholar]
- 10.Chadban SJ, Baines L, Polkinghorne K, Jefferys A, Dogra S, Kanganas C, Irish A, Eris J, Walker R: Anemia after kidney transplantation is not completely explained by reduced kidney function. Am J Kidney Dis 49 :301 –309,2007 [DOI] [PubMed] [Google Scholar]
- 11.Yorgin PD, Scandling JD, Belson A, Sanchez J, Alexander SR, Andreoni KA: Late post-transplant anemia in adult renal transplant recipients: An under-recognized problem? Am J Transplant 2 :429 –435,2002 [DOI] [PubMed] [Google Scholar]
- 12.Vanrenterghem Y, Ponticelli C, Morales JM, Abramowicz D, Baboolal K, Eklund B, Kliem V, Legendre C, Morais Sarmento AL, Vincenti F: Prevalence and management of anemia in renal transplant recipients: A European survey. Am J Transplant 3 :835 –845,2003 [DOI] [PubMed] [Google Scholar]
- 13.Winkelmayer WC, Lorenz M, Kramar R, Hörl WH, Sunder-Plassmann G: Percentage of hypochromic red blood cells is an independent risk factor for mortality in kidney transplant recipients. Am J Transplant 4 :2075 –2081,2004 [DOI] [PubMed] [Google Scholar]
- 14.Molnar MZ, Czira M, Ambrus C, Szeifert L, Szentkiralyi A, Beko G, Rosivall L, Remport A, Novak M, Mucsi I: Anemia is associated with mortality in kidney-transplanted patients: A prospective cohort study. Am J Transplant 7 :818 –824,2007 [DOI] [PubMed] [Google Scholar]
- 15.Shah N, Al-Khoury S, Afzali B, Covic A, Roche A, Marsh J, Macdougall IC, Goldsmith DJ: Posttransplantation anemia in adult renal allograft recipients: Prevalence and predictors. Transplantation 81 :1112 –1118,2006 [DOI] [PubMed] [Google Scholar]
- 16.Kim HC, Park SB, Han SY, Whang EA: Anemia following renal transplantation. Transplant Proc 35 :302 –303,2003 [DOI] [PubMed] [Google Scholar]
- 17.Lorenz M, Kletzmayr J, Perschl A, Furrer A, Hörl WH, Sunder-Plassmann G: Anemia and iron deficiencies among long-term renal transplant recipients. J Am Soc Nephrol 13 :794 –797,2002 [DOI] [PubMed] [Google Scholar]
- 18.Fisher JW, Radtke HW, Jubiz W, Nelson PK, Burdowski A: Prostaglandins activation of erythropoietin production and erythroid progenitor cells. Exp Hematol 8[Suppl 8] :65 –89,1980 [PubMed] [Google Scholar]
- 19.El Haggan W, Vallet L, Hurault de Ligny B, Pujo M, Corne B, Lobbedez T, Levaltier B, Ryckelynck JP: Darbepoetin alfa in the treatment of anemia in renal transplant patients: A single-center report. Transplantation 77 :1914 –1915,2004 [DOI] [PubMed] [Google Scholar]
- 20.Sinnamon KT, Courtney AE, Maxwell AP, McNamee PT, Savage G, Fogarty DG: Level of renal function and serum erythropoietin levels independently predict anaemia post-renal transplantation. Nephrol Dial Transplant 22 :1969 –1973,2007 [DOI] [PubMed] [Google Scholar]
- 21.Snanoudj R, Beaudreuil S, Arzouk N, Jacq D, Casadevall N, Charpentier B, Durrbach A: Recovery from pure red cell aplasia caused by anti-erythropoietin antibodies after kidney transplantation. Am J Transplant 4 :274 –277,2004 [DOI] [PubMed] [Google Scholar]
- 22.Imoagene-Oyedeji AE, Rosas SE, Doyle AM, Goral S, Bloom RD: Posttransplantation anemia at 12 months in kidney recipients treated with mycophenolate mofetil: Risk factors and implications for mortality. J Am Soc Nephrol 17 :3240 –3247,2006 [DOI] [PubMed] [Google Scholar]
- 23.Al-Uzri A, Yorgin PD, Kling PJ: Anemia in children after transplantation: Etiology and the effect of immunosuppressive therapy on erythropoiesis. Pediatr Transplant 7 :253 –264,2003 [DOI] [PubMed] [Google Scholar]
- 24.Lin CC, King KL, Chao YW, Yang AH, Chang CF, Yang WC: Tacrolimus-associated hemolytic uremic syndrome: A case analysis. J Nephrol 16 :580 –585,2003 [PubMed] [Google Scholar]
- 25.Augustine JJ, Knauss TC, Schulak JA, Bodziak KA, Siegel C, Hricik DE: Comparative effects of sirolimus and mycophenolate mofetil on erythropoiesis in kidney transplant patients. Am J Transplant 4 :2001 –2006,2004 [DOI] [PubMed] [Google Scholar]
- 26.Maiorano A, Stallone G, Schena A, Infante B, Pontrelli P, Schena FP, Grandaliano G: Sirolimus interferes with iron homeostasis in renal transplant recipients. Transplantation 82 :908 –912,2006 [DOI] [PubMed] [Google Scholar]
- 27.Marathias KP, Agroyannis B, Mavromoustakos T, Matsoukas J, Vlahakos DV: Hematocrit-lowering effect following inactivation of renin-angiotensin system with angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Curr Top Med Chem 4 :483 –486,2004 [DOI] [PubMed] [Google Scholar]
- 28.Ramsey G, Israel L, Lindsay GD, Mayer TK, Nusbacher J: Anti-Rho(D) in two Rh-positive patients receiving kidney grafts from an Rh-immunized donor. Transplantation 41 :67 –69,1986 [DOI] [PubMed] [Google Scholar]
- 29.Chua MS, Barry C, Chen X, Salvatierra O, Sarwal MM: Molecular profiling of anemia in acute renal allograft rejection using DNA microarrays. Am J Transplant 3 :17 –22,2003 [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Gomez JM, Perez-Flores I, Jofre R, Carretero D, Rodriguez-Benitez P, Villaverde M, Perez-Garcia R, Nassar GM, Niembro E, Ayus JC: Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol 15 :2494 –2501,2004 [DOI] [PubMed] [Google Scholar]
- 31.Gunnell J, Yeun JY, Depner TA, Kaysen GA: Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 33 :63 –72,1999 [DOI] [PubMed] [Google Scholar]
- 32.Egbuna O, Zand MS, Arbini A, Menegus M, Taylor J: A cluster of parvovirus B19 infections in renal transplant recipients: A prospective case series and review of the literature. Am J Transplant 6 :225 –231,2006 [DOI] [PubMed] [Google Scholar]
- 33.Zolnourian ZR, Curran MD, Rima BK, Coyle PV, O'Neill HJ, Middleton D: Parvovirus B19 in kidney transplant patients. Transplantation 69 :2198 –2202,2000 [DOI] [PubMed] [Google Scholar]
- 34.Liefeldt L, Buhl M, Schweickert B, Engelmann E, Sezer O, Laschinski P, Preuschof L, Neumayer HH: Eradication of parvovirus B19 infection after renal transplantation requires reduction of immunosuppression and high-dose immunoglobulin therapy. Nephrol Dial Transplant 17 :1840 –1842,2002 [DOI] [PubMed] [Google Scholar]
- 35.Ambuhl PM: Posttransplant metabolic acidosis: A neglected factor in renal transplantation? Curr Opin Nephrol Hypertens 16 :379 –387,2007 [DOI] [PubMed] [Google Scholar]
- 36.Rigatto C, Parfrey P, Foley R, Negrijn C, Tribula C, Jeffery J: Congestive heart failure in renal transplant recipients: Risk factors, outcomes, and relationship with ischemic heart disease. J Am Soc Nephrol 13 :1084 –1090,2002 [DOI] [PubMed] [Google Scholar]
- 37.Winkelmayer WC, Chandraker A, Brookhart MA, Kramar R, Sunder-Plassmann G: A prospective study of anaemia and long-term outcomes in kidney transplant recipients. Nephrol Dial Transplant 21 :3559 –3566,2006 [DOI] [PubMed] [Google Scholar]
- 38.Van Biesen W, Vanholder R, Veys N, Verbeke F, Lameire N: Efficacy of erythropoietin administration in the treatment of anemia immediately after renal transplantation. Transplantation 79 :367 –368,2005 [DOI] [PubMed] [Google Scholar]
- 39.Nampoory MR, Johny KV, al-Hilali N, Seshadri MS, Kanagasabhapathy AS: Erythropoietin deficiency and relative resistance cause anaemia in post-renal transplant recipients with normal renal function. Nephrol Dial Transplant 11 :177 –181,1996 [PubMed] [Google Scholar]
- 40.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355 :2085 –2098,2006 [DOI] [PubMed] [Google Scholar]
- 41.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355 :2071 –2084,2006 [DOI] [PubMed] [Google Scholar]