Abstract
Renal osteodystrophy is characterized by abnormalities in bone turnover, mineralization, and bone volume. The effects of treatment modalities for renal osteodystrophy on bone should be analyzed with respect to these abnormalities. The major treatment modalities for renal osteodystrophy include phosphate binders, vitamin D compounds, and calcimimetics. Aluminum-containing phosphate binders have been shown to be toxic to bone secondary to their effects on bone turnover, mineralization, and bone volume. The use of calcium-based phosphate binders has been associated with the development of adynamic bone disease (low bone turnover), bone loss, and worsening of vascular calcifications. New nonaluminum, noncalcium phosphate binders have been developed (sevelamer hydrochloride and lanthanum carbonate). These agents show a potential for improvement in bone turnover and bone volume. Patients with renal osteodystrophy are deficient in calcitriol and often in calcidiol. Calcidiol deficiency has been underappreciated and deserves to be addressed in the treatment of patients with renal osteodystrophy. Calcitriol replacement therapy by daily oral administration is associated with frequent episodes of hypercalcemia and suppression of bone turnover in patients with stages 3 to 5 chronic kidney disease. Pulse oral or intravenous calcitriol administration induces frequent episodes of hypercalcemia or hyperphosphatemia, respectively, and achieves the same degree of correction of bone abnormalities. There are no data on the effects of paricalcitol or doxercalciferol on human bone. Experimental data, however, show that these two analogues and maxacalcitol may control serum parathyroid hormone levels without suppressing bone turnover. Calcimimetics lower parathyroid hormone levels and bone turnover.
Renal osteodystrophy (ROD) starts early with loss of kidney function (approximately 50% loss of glomerular infiltration rates [1]). Virtually all patients with advanced chronic kidney disease (CKD) have ROD, and an association between histologic changes in bone turnover and vascular calcifications has been described (2,3). This association underlines the importance of treatment of ROD. The main abnormalities of ROD encompass changes in turnover, mineralization, and bone volume (4,5); therefore, effects of treatment modalities should be analyzed with respect to these three abnormalities. Three major therapeutic groups are available for the management of ROD: phosphate binders (P-binders), vitamin D or vitamin D analogues, and calcimimetics.
P-Binders
Use of P-binders has evolved in the past 30 yr (6,7). Initially, aluminum-containing P-binders were used during the 1970s to 1980s. They were replaced by calcium-based P-binders in the 1980s because of toxicities associated with aluminum. In the early 1990s, non–calcium-based P-binders were introduced because, again, toxicities were found with high doses of calcium-based binders (2,8–10). Most recently, new calcium- and aluminum-free binders sevelamer hydrochloride and lanthanum carbonate were introduced.
Aluminum-Based Phosphate Binders
The effects of the use of aluminum-based P-binders on bone turnover, mineralization, and bone volume were evaluated in three separate studies (11): (1) A cross-sectional study of 120 patients who were on long-term maintenance dialysis, including patients with and without stainable bone aluminum (SBA); (2) a longitudinal study of eight hemodialysis patients who had progressive accumulation of aluminum in bone (these patients had two bone biopsies 11 to 16 mo apart); and (3) a longitudinal, prospective study of 10 hemodialysis patients who were treated with deferoxamine for 9 to 12 mo for reversal of aluminum accumulation in bone.
In the cross-sectional study, patients with a higher extent of SBA had lower bone turnover and bone volume and various degrees of mineralization defect. In the eight patients who showed development or increase in SBA at the second biopsy, bone turnover and bone volume decreased and mineralization defect developed or worsened. Repeat bone biopsies in patients who were treated with deferoxamine did show less or no SBA. Bone turnover and bone volume increased and mineralization abnormalities improved.
The results of these independent studies demonstrate that SBA is associated not only with disturbed mineralization but also with suppressed bone turnover and loss of bone and that removal of aluminum from bone results in gain in bone, improvement in mineralization, and increase in bone turnover (11). Mechanisms of the bone loss were related to a disproportionately greater negative effect of aluminum on bone formation than bone resorption. This explains why patients with renal failure and SBA not only develop osteomalacia or adynamic bone disease but also bone loss and osteoporosis.
Effects of Calcium-Based versus Non–calcium-Based P-Binders on Bone
Sevelamer Hydrochloride.
Effects of sevelamer hydrochloride on bone were studied in rodent models. Mathew et al. (12) found with sevelamer treatment an increase in bone formation associated with reduction in vascular calcifications in a murine model with metabolic syndrome with renal failure and low bone turnover. This effect was seen in a parathyroid hormone (PTH)-independent manner. Katsumata et al. (13) showed in rats with adenine-induced renal failure and ROD an improvement in bone histology after treatment with sevelamer. The effects of sevelamer hydrochloride and calcium carbonate on bone were compared in a prospective, randomized, open-label 1-yr study in hemodialysis patients (14). Changes in bone turnover, bone structure, and mineralization were assessed by bone biopsies at baseline and 1 yr. Sixty-eight patients completed the study (sevelamer = 33; calcium = 35). Treatment groups were comparable with regard to baseline demographics. Low bone turnover was the most frequent condition at baseline (59%). Serum phosphorus, calcium, and intact PTH (iPTH) were well controlled in both groups, although serum calcium was consistently lower and iPTH higher with sevelamer. There were no significant changes from baseline in mineralization in either group. Bone turnover assessed by activation frequency (Acf) was below normal at baseline in both groups. There were no significant changes from baseline within or between groups in Acf or other parameters used to assess bone turnover, except for a significant increase in bone formation rate/bone surface with sevelamer. Trabecular separation improved in seven of 10 patients who were taking sevelamer and in none who were taking calcium. Serum bone-specific alkaline phosphatase levels increased significantly from baseline with sevelamer but not calcium. These data show that there was histologic and biochemical evidence of improvement in bone formation with sevelamer treatment. These findings are corroborated by a study by Raggi et al. (15), which evaluated bone attenuation of the vertebra by electron-beam tomography. They show that sevelamer-treated patients had greater bone density compared with patients who were treated with calcium-based binders. These findings were associated with fewer calcifications in the sevelamer-treated patients.
Lanthanum Carbonate.
Three independent studies have compared the effects of calcium-based binders with lanthanum carbonate (LC) on bone (16–18). D'Haese et al. (16) studied the effects of LC versus calcium carbonate on ROD in 98 dialysis patients during a 1-yr period. Patients who received calcium carbonate for 1 yr sustained a net decrease in bone turnover as evidenced by a change in adynamic bone disease from 20% at baseline to 30% at follow-up. High bone turnover that is hyperparathyroid bone disease was seen in 20% at baseline and 23% after 1 yr. Mineralization defect that is osteomalacia was seen in 3% at baseline and none of the patients after 1 yr, whereas moderately elevated bone turnover with mineralization defect (mixed uremic osteodystrophy) was seen in 57% at baseline and 44% after 1 yr. In contrast, patients who received LC for 1 yr experienced a reduction in excessively elevated bone turnover from 15 to 9% and in suppressed bone turnover from 36 to 18%. There was an increase in normal bone turnover from 6% at baseline to 15% at follow-up, and severe defective mineralization was no longer observed after 1 yr of treatment with either modality. The authors concluded that bone-related adverse effects that are known to occur with aluminum are not seen with LC.
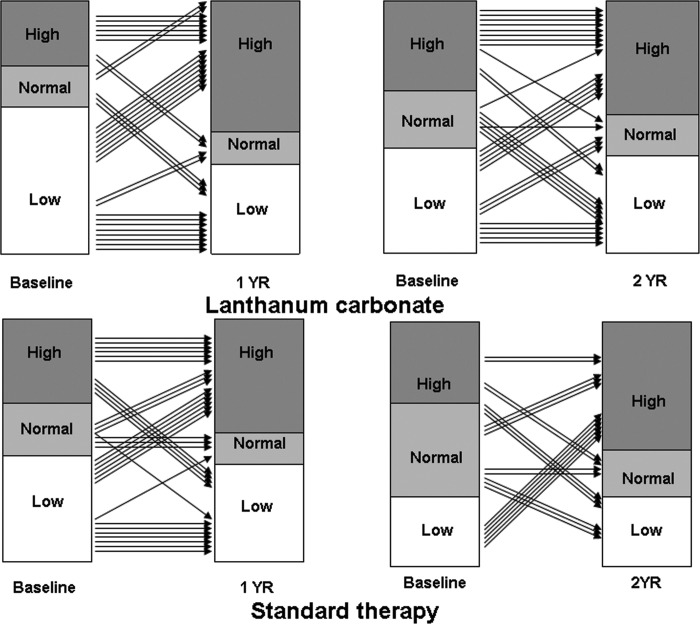
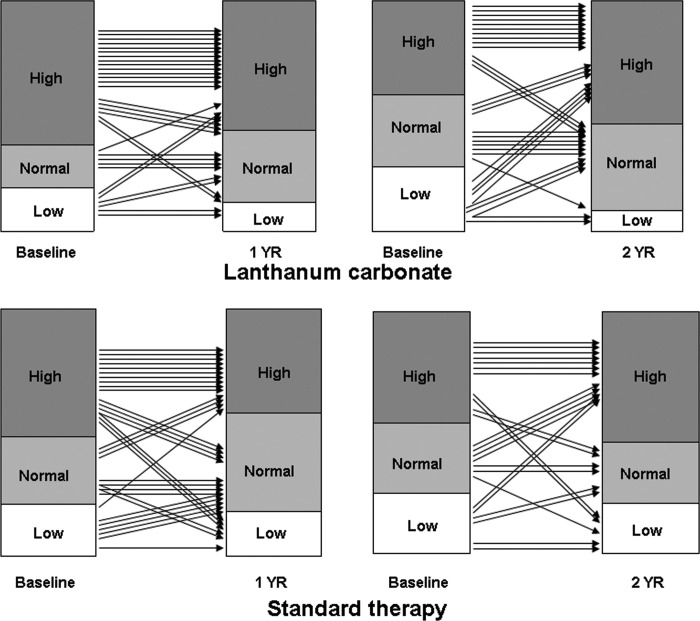
We conducted a randomized, prospective, open-label study to investigate the evolution of ROD over 1 and 2 yr in patients who were on maintenance dialysis and treated with LC versus standard P-binders (98% calcium-based binders). Paired biopsies at baseline and 1 yr were available from 65 patients and at baseline and 2 yr from 57 patients. During maintenance therapy, serum phosphorus levels were similar in both groups, whereas serum calcium levels were higher and serum iPTH levels were lower with standard therapy. Bone turnover (Acf) was low in 58% of patients at baseline in the LC group and in 35.5% of patients after 1 yr (Figure 1). Bone volume was low at baseline in 28% of patients who were treated with LC for 2 yr and in 9.4% after completion of 2 yr of LC therapy (Figure 2). No major changes in bone turnover or bone volume were observed in the other groups, including the standard therapy groups (Figures 1 and 2). One patient in the 2-yr LC group had severe mineralization defect (osteomalacia) at baseline and at the end of therapy. In two other patients, a mineralization defect developed after 2 yr of LC therapy. In one of these patients could the cause for the mineralization defect be identified. No mineralization defects were observed in the standard therapy group, and no correlation was found between bone lanthanum content and parameters of bone mineralization. In both the 1- and the 2-yr studies, a slight but clear increase in concentrations of LC in bone was found; that is, no steady state could be established in bone lanthanum over 1 and 2 yr.
Figure 1.
Changes in activation frequency in patients who were treated with lanthanum carbonate or standard therapy for 1 or 2 yr.
Figure 2.
Changes in bone volume/tissue volume in patients who were treated with lanthanum carbonate or standard therapy for 1 or 2 yr.
A cross-sectional study was performed in 11 patients who had been receiving LC for 4 to 5 yr (18). No baseline bone biopsies were available. Bone lanthanum concentrations were higher compared with the patients who received lanthanum for 1 or 2 yr only, but no direct comparison can be made because bone biopsies were not performed at baseline and patients came from different geographic areas. Bone histology showed a distribution of bone abnormalities similar to what would be expected in a random sample of patients who are on long-term dialysis. Four patients had low bone turnover, no patient had mineralization defect, and seven patients had mild to moderately elevated bone turnover. If lanthanum has an aluminum-like effect on bone, then one would expect to find it after 4 to 5 yr, based on our results of toxic effects of aluminum on bone identifiable after 11 to 16 mo of aluminum exposure (11). These data provide evidence that lanthanum therapy up to 5 yr is not associated with an aluminum-like effect on bone.
Effects of Vitamin D Therapy on Bone
In interpretation of studies on the effects of vitamin D treatment on bone, calcidiol and calcitriol should separately be considered. We found that patients with normal calcitriol but low calcidiol levels had defective bone mineralization (19). In most studies, no information is provided on calcidiol status. It was assumed that calcidiol deficiency is a rare occurrence in patients with CKD in the absence of nephrotic syndrome. On the basis of the recent observation of a high prevalence of calcidiol deficiency in dialysis patients (20), it is unclear how much this deficiency may play a role in the spectrum of bone changes that occur with ROD.
Calcitriol Effects on Bone in Patients with Stages 3 and 4 CKD
A randomized, prospective, double-blind, placebo-controlled trial on the effects of calcitriol on bone histology was conducted in 16 patients with mild to moderate renal failure (creatinine clearance 20 to 59 ml/min) (21). They were treated either with calcitriol at a dosage of 0.25 to 0.5 μg/d or with placebo (eight patients per group). Bone biopsies were performed at baseline and after 12 mo of treatment. Of the 13 patients who completed the study, initial calcitriol levels were low in seven patients and PTH levels were elevated in seven patients. Bone turnover was elevated in all patients. Calcitriol treatment induced a significant fall in serum phosphorus and alkaline phosphatase as well as decrease in bone turnover; however, 80% of the patients developed oversuppression of bone turnover (adynamic bone disease). This is of concern, and further studies are needed to determine whether the use of smaller dosages of calcitriol or intermittent therapy or an analogue with less suppressive effect of bone turnover may ultimately avoid this potential for oversuppression of bone turnover.
The effect of alfacalcidol on ROD was studied in a double-blind, prospective, randomized, placebo-controlled study conducted in 17 centers from Europe (22). A total of 176 patients with mild to moderate CKD (creatinine clearance 15 to 50 ml/min) and no clinical, biochemical, or radiographic evidence of bone disease were studied. Alfacalcidol was given at dosages ranging from 0.25 to 1.0 μg/d for up to 2 yr. A placebo group was studied for comparison. Bone biopsies revealed that 132 patients had histologic evidence of ROD at baseline. Histologic characteristics were similar for the 89 patients who were given alfacalcidol and the 87 placebo control subjects. After treatment, serum alkaline phosphatase and iPTH concentrations increased by 13 and 126%, respectively, in control subjects but did not change in patients who were given alfacalcidol (P < 0.01). Hypercalcemic episodes occurred in 10 patients who were given alfacalcidol (but responded to decreases in drug dosage) and in three control subjects. On the second biopsy, bone turnover significantly improved in patients who were given alfacalcidol and deteriorated in control subjects. With alfacalcidol treatment, 42% of the patients with abnormal bone turnover at baseline had normal bone turnover at the end of treatment, and 22% developed adynamic bone disease. The authors stated that early administration of alfacalcidol can beneficially alter the natural course of renal bone disease in patients with mild to moderate renal failure; that is, development of progressive elevation in turnover secondary to PTH overactivity could be prevented by alfacalcidol treatment.
It is of note that development of adynamic bone disease was less prominent in the study of Hamdy et al. (22) than in ours (21). The reasons for this apparent discrepancy may be multiple, including that, in Hamdy's study, bone turnover was appreciated qualitatively, whereas, in our study, histomorphometry was used to classify bone turnover with comparison with normal control subjects. Another explanation is that the potency of alfacalcidol is less than that of calcitriol. Indeed, a recent study showed that serum PTH levels rose significantly when calcitriol therapy was changed to alfacalcidol given at similar dosages to patients who were on long-term maintenance dialysis (23).
Calcitriol Effects on Bone in Patients with Stage 5 CKD
The effects of 6 mo of calcitriol therapy on bone was evaluated in 12 patients who were on long-term maintenance dialysis (24,25). Bone biopsies were performed at months 0 and 6. At baseline, all biopsies showed high bone turnover and defective mineralization. Dosages of calcitriol ranged from 0.5 to 3.5 μg/d. Serum alkaline phosphatase was the best parameter for prediction of development of hypercalcemia. When serum alkaline phosphatase levels approached the normal range, dosages of calcitriol had to be reduced to prevent hypercalcemic episodes. Calcitriol treatment resulted in decrease in bone turnover, bone resorption, and formation and reduction of woven osteoid and fibrosis. Mineralization and parameters of bone architecture improved.
Effects of Intravenous versus and Pulse Oral Calcitriol Therapy on Bone
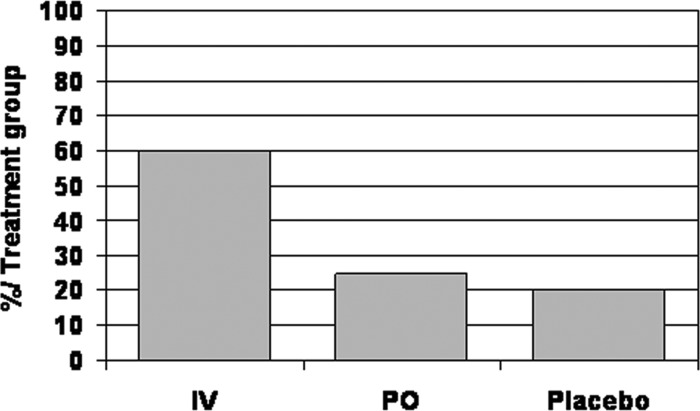
Daily oral administration of calcitriol is efficient in improving hyperparathyroidism and mineralization defect as described previously; however, frequent occurrence of hypercalcemia has hampered its use. The value of pulse oral (PO) versus intravenous calcitriol therapy was studied in 44 patients who were on long-term maintenance dialysis (26). Fifteen patients received intravenous calcitriol, 19 patients received PO calcitriol, and 10 patients received placebo. The cumulative dosage of intravenous and PO calcitriol was similar. Dosages ranged from 1.5 to 4 μg three times per week. Serum PTH levels fell faster in the PO group than in the intravenous group. During the last 3 mo of observation, serum PTH levels were equally controlled with both modalities; however, more patients developed hypercalcemic episodes with the PO administration (Figure 3), whereas more patients had high serum phosphorus levels and high calcium-phosphorus product with the intravenous administration (Figure 4). It is likely that the better control of PTH levels during the first 9 mo of PO therapy resulted in less release of phosphorus from bone but better absorption of calcium from the gut, whereas the slower decline in PTH levels in the intravenous group could have resulted in more phosphorus release from bone. In four of 15 patients who were on intravenous and five of nine patients who were on PO therapy, there was an improvement in bone histology with significant decrease in osteoid volume, number of osteoblasts and osteoclasts, fibrosis, and Acf. In the other patients, no or minor changes (<10%) were observed. No significant changes were seen in the placebo group. The higher incidence of hypercalcemia led to greater rates of interruption of therapy in the group that was treated with PO calcitriol. The data confirm that intravenous and pulse PO calcitriol can be efficient in treating bone disease in patients with stage 5 CKD; however, the improvements could be obtained only in <25% of the patients studied, and careful monitoring was needed because episodes of hypercalcemia and increases in calcium-phosphorus product were frequently observed. Goodman et al. (27) studied the effects of intermittent oral or intraperitoneal calcitriol therapy on bone histology in 14 children with stage 5 CKD and biopsy-proven severe (n = 11) or mild (n = 3) secondary hyperparathyroidism. Bone biopsies were repeated after 1 yr, and histologic improvement was seen in 12 of 14 patients. Severe hyperparathyroid bone disease resolved in 10 of 11 cases; however, bone formation decreased in all patients to normal (n = 6) or below normal (n = 6). Although the decrease in serum PTH and alkaline phosphatase levels suggests the development of adynamic bone disease, bone formation decreased in some patients despite persistently high serum PTH levels. The authors proposed that calcitriol may directly suppress osteoblastic activity in patients with secondary hyperparathyroidism when given in large dosages; however, it is also possible that the circulating serum PTH levels include a preponderance of PTH fragments, such as PTH-(7-84), that have been shown to antagonize the effects of PTH-(1-84) on osteoblast activity (28,29).
Figure 3.
Percentage of patients who exhibited episodes of hypercalcemia during treatment with pulse intravenous (IV) calcitriol, pulse oral (PO) calcitriol, or placebo.
Figure 4.
Percentage of patients who exhibited episodes of increased calcium-phosphate product during treatment with pulse IV calcitriol, PO calcitriol, or placebo.
Effects of Vitamin D Analogues on Bone
Even though the majority of patients with stage 5 CKD are currently treated with either paricalcitol or doxercalciferol, there are no prospective human studies on the effects of these analogues on bone. In a study in rats with experimentally induced renal insufficiency, it was shown that paricalcitol controlled serum PTH levels without oversuppression of bone turnover (30). The effects of maxacalcitol were evaluated in a dog model with reduced kidney function (31). Sixty dogs underwent either nephrectomy or sham operation. The dogs received supplemental phosphate to enhance PTH secretion. Fourteen weeks after the start of phosphate supplementation, half of the nephrectomized and sham-operated dogs received maxacalcitol (three times per week); the other half were given vehicle for 60 wk. In nephrectomized dogs, maxacalcitol significantly decreased serum PTH levels soon after the induction of renal insufficiency. There were, however, frequent episodes of hypercalcemia and hyperphosphatemia. Maxacalcitol reversed abnormal bone formation (woven osteoid, fibrosis) but did not significantly alter bone turnover. In addition, maxacalcitol improved mineralization in both nephrectomized and sham dogs. These results indicate that even though maxacalcitol does not completely prevent the occurrence of hypercalcemia, it may be useful in the management of secondary hyperparathyroidism. Most interesting is that it did not induce low bone turnover and thus should have lower risk for development of adynamic bone disease.
Effects of the Calcimimetic Cinacalcet HCl on Renal Osteodystrophy
We conducted a prospective, double-blind, placebo-controlled trial to assess the effect of cinacalcet on bone histology and serum markers of bone metabolism in dialysis patients with ROD (32). Patients with intact PTH ≥300 pg/ml were selected to be assigned 2 to 1 to receive cinacalcet or placebo with concurrent vitamin D and/or phosphate binder therapy. Cinacalcet (30 to 180 pg/d) was used to achieve iPTH levels of ≤200 pg/ml. Bone biopsies were performed before and after 1 yr of treatment. Baseline and end-of-study data were available from 32 patients (19 cinacalcet; 13 placebo). Overall cinacalcet treatment decreased iPTH and reduced Acf, bone formation rate/bone surface, and fibrosis/bone surface. At baseline in the cinacalcet group, bone turnover was elevated (Acf >0.72/yr) in 16 of 19 patients, normal in two, and low in one patient who presented with adynamic bone disease (Acf <0.49/yr). After 1 yr of cinacalcet treatment, the patient with adynamic bone disease still presented with this histologic abnormality, and one patient who had normal bone turnover and two patients who had elevated bone turnover developed adynamic bone disease; two of these patients had serum iPTH <100 pg/ml at the end of the study. The other patients showed either a decrease in Acf to levels above normal (n = 6) or normal levels (n = 2) or an increase in Acf (n = 6). In the placebo group, at baseline, Acf was low (adynamic bone disease) in one patient and normal (n = 1) and elevated in the other 11 patients. At the end of the study, Acf in the patient with adynamic bone disease became normal. Acf fell in five patients (to normal levels in four of five patients), was unchanged in two, and increased in the other five patients. Bone mineralization parameters remained normal in both groups. The changes in bone parameters correlated with PTH, bone-specific alkaline phosphatase, and N-telopeptide reductions. Thus, treatment with cinacalcet lowered PTH, improved bone histology, and reduced bone turnover in most of the studied patients with secondary hyperparathyroidism.
Conclusions
The therapeutic intervention that was done during the past few decades were mainly aimed at decreasing bone turnover. The risk for oversuppression with resultant vascular calcifications has only recently been appreciated. Non–calcium-containing P-binders have less risk for oversuppression (Table 1), may improve bone balance, and thus may lower the risk for progression in calcifications. Vitamin D compounds improve mineralization but decrease bone turnover at various degrees (Table 1). Bone volume falls with aluminum-based P-binders and may increase with use of non–calcium-based P-binders (Table 1). There is a need for further development and testing of therapeutic modalities to treat low bone turnover. Moreover, appreciation of calcidiol deficiency in the management of ROD should receive more consideration. Calcidiol supplementation seems indicated before administration of vitamin D analogues.
Table 1.
Effects of therapeutic modalities for renal osteodystrophy on bone histologya
| Parameter | Bone Turnover | Bone Mineralization | Bone Volume |
|---|---|---|---|
| Aluminum based P-binders | ↓↓↓ | ↓↓↓ | ↓↓ |
| Calcium based P-binders | ↓ | ↔ | ↔ |
| Sevelamer hydrochloride | ↔ | ↑ | |
| Lanthanum carbonate | ↑ | ↔ | ↑ |
| Calcitriol, intravenous | ↓↓ | ↑ | |
| Calcitriol PO | ↓↓ | ↑ | |
| Paricalcitol | ? | ? | ? |
| Doxercalciferol | ? | ? | ? |
| Maxacalcitol | ↔ | ↑ | |
| Cinacalcet | ↓ | ↔ | ↔ |
P-binder, phosphate binder; PO, pulse oral.
Disclosures
None.
References
- 1.Malluche H, Ritz E, Lange H, Kutschera K, Hodgson M, Seiffert U, Schoeppe W: Bone histology in incipient and advanced renal failure. Kidney Int 9 :355 –362,1976 [DOI] [PubMed] [Google Scholar]
- 2.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC: Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 15 :1943 –1951,2004 [DOI] [PubMed] [Google Scholar]
- 3.Adragao T, Ferreira A, Frazao J, Gil C, Oliveira C, Sarmento J, Ribero S, Dickson J, Carvahlo B, Rodrigues I, Baldai J, Faugere M, Malluche H: Vascular calcifications and bone turnover in hemodialysis patients. Nephrol Dial Transplant 21 [Suppl iv]:292 ,2006 [Google Scholar]
- 4.Malluche HH, Monier-Faugere MC: Renal osteodystrophy: What's in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin Nephrol 65 :235 –242,2006 [DOI] [PubMed] [Google Scholar]
- 5.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G: Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69 :1945 –1953,2006 [DOI] [PubMed] [Google Scholar]
- 6.Malluche H, Monier-Faugere M: Hyperphosphatemia: Pharmacologic intervention. Yesterday, today, and tomorrow. Clin Nephrol 54 :309 –317,2000 [PubMed] [Google Scholar]
- 7.Malluche HH, Monier-Faugere MC: Understanding and managing hyperphosphatemia in patients with chronic renal disease. Clin Nephrol 52 :267 –277,1999 [PubMed] [Google Scholar]
- 8.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342 :1478 –1483,2000 [DOI] [PubMed] [Google Scholar]
- 9.Malluche HH, Monier-Faugere MC: Risk of adynamic bone disease in dialyzed patients. Kidney Int Suppl 38 :S62 –S67,1992 [PubMed] [Google Scholar]
- 10.Chertow GM, Burke SK, Raggi P: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62 :245 –252,2002 [DOI] [PubMed] [Google Scholar]
- 11.Faugere MC, Arnala IO, Ritz E, Malluche HH: Loss of bone resulting from accumulation of aluminum in bone of patients undergoing dialysis. J Lab Clin Med 107 :481 –487,1986 [PubMed] [Google Scholar]
- 12.Mathew S, Lund RJ, Strebeck F, Tustison KS, Geurs T, Hruska KA: Reversal of the adynamic bone disorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J Am Soc Nephrol 18 :122 –130,2007 [DOI] [PubMed] [Google Scholar]
- 13.Katsumata K, Kusano K, Hirata M, Tsunemi K, Nagano N, Burke SK, Fukushima N: Sevelamer hydrochloride prevents ectopic calcification and renal osteodystrophy in chronic renal failure rats. Kidney Int 64 :441 –450,2003 [DOI] [PubMed] [Google Scholar]
- 14.Ferreira A, Frazao J, Faugere M, Mueller R, Malluche H: Effects of sevelamer hydrochloride and calcium carbonate on bone mineralisation and turnover in haemodialysis patients: A one-year randomised, open-label bone biopsy study [Abstract]. Nephrol Dial Transplant 21 :293 ,2006 [Google Scholar]
- 15.Raggi P, James G, Burke SK, Bommer J, Chasan-Taber S, Holzer H, Braun J, Chertow GM: Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res 20 :764 –772,2005 [DOI] [PubMed] [Google Scholar]
- 16.D'Haese PC, Spasovski GB, Sikole A, Hutchison A, Freemont TJ, Sulkova S, Swanepoel C, Pejanovic S, Djukanovic L, Balducci A, Coen G, Sulowicz W, Ferreira A, Torres A, Curic S, Popovic M, Dimkovic N, De Broe ME: A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int Suppl S73 –S78,2003 [DOI] [PubMed]
- 17.Malluche H, Faugere, M-C, Wang G, Finn WF: Lanthanum carbonate and bone: No adverse effects observed after 1 year of treatment in a randomized, comparator-controlled trial [Abstract]. J Am Soc Nephrol 15 :271A ,2004 [Google Scholar]
- 18.Malluche H, Faugere, M-C, Wang G, Finn WF: No evidence of osteomalacia in dialysis patients treated with lanthanum carbonate up to 5 years [Abstract]. J Am Soc Nephrol 15 :270A ,2004 [Google Scholar]
- 19.Malluche HH, Goldstein DA, Massry SG: Osteomalacia and hyperparathyroid bone disease in patients with nephrotic syndrome. J Clin Invest 63 :494 –500,1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ: Vitamin D insufficiency and deficiency in chronic kidney disease: A single center observational study. Am J Nephrol 24 :503 –510,2004 [DOI] [PubMed] [Google Scholar]
- 21.Baker LR, Abrams L, Roe CJ, Faugere MC, Fanti P, Subayti Y, Malluche HH: 1,25(OH)2D3 administration in moderate renal failure: A prospective double-blind trial. Kidney Int 35 :661 –669,1989 [DOI] [PubMed] [Google Scholar]
- 22.Hamdy NA, Kanis JA, Beneton MN, Brown CB, Juttmann JR, Jordans JG, Josse S, Meyrier A, Lins RL, Fairey IT: Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ 310 :358 –363,1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arenas MD, Muray S, Amoedo ML, Egea JJ, Millan I, Gil MT: A long-term comparative study of calcitriol versus alphacalcidol in patients with secondary hyperparathyroidism on hemodialysis [in Spanish]. Nefrologia 26 :226 –233,2006 [PubMed] [Google Scholar]
- 24.Goldstein DA, Malluche HH, Massry SG: Long-term effects of 1,25(OH)2D3 on clinical and biochemical derangements of divalent ions in dialysis patients. Contrib Nephrol 18 :42 –54,1980 [DOI] [PubMed] [Google Scholar]
- 25.Malluche HH, Goldstein DA, Massry SG: Effects of 6 months therapy with 1,25 (OH)2D3 on bone disease of dialysis patients. Contrib Nephrol 18 :98 –104,1980 [DOI] [PubMed] [Google Scholar]
- 26.Monier-Faugere MC, Malluche HH: Calcitriol pulse therapy in patients with end-stage renal failure. Curr Opin Nephrol Hypertens 3 :615 –619,1994 [DOI] [PubMed] [Google Scholar]
- 27.Goodman WG, Ramirez JA, Belin TR, Chon Y, Gales B, Segre GV, Salusky IB: Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int 46 :1160 –1166,1994 [DOI] [PubMed] [Google Scholar]
- 28.Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, D'Amour P: Synthetic carboxyl-terminal fragments of parathyroid hormone (PTH) decrease ionized calcium concentration in rats by acting on a receptor different from the pth/pth-related peptide receptor. Endocrinology 142 :1386 –1392,2001 [DOI] [PubMed] [Google Scholar]
- 29.Langub MC, Monier-Faugere MC, Wang G, Williams JP, Koszewski NJ, Malluche HH: Administration of PTH-(7-84) antagonizes the effects of PTH-(1-84) on bone in rats with moderate renal failure. Endocrinology 144 :1135 –1138,2003 [DOI] [PubMed] [Google Scholar]
- 30.Slatopolsky E, Cozzolino M, Lu Y, Finch J, Dusso A, Staniforth M, Wein Y, Webster J: Efficacy of 19-Nor-1,25-(OH)2D2 in the prevention and treatment of hyperparathyroid bone disease in experimental uremia. Kidney Int 63 :2020 –2027,2003 [DOI] [PubMed] [Google Scholar]
- 31.Monier-Faugere MC, Geng Z, Friedler RM, Qi Q, Kubodera N, Slatopolsky E, Malluche HH: 22-Oxacalcitriol suppresses secondary hyperparathyroidism without inducing low bone turnover in dogs with renal failure. Kidney Int 55 :821 –832,1999 [DOI] [PubMed] [Google Scholar]
- 32.Malluche H, Monier-Faugere M, Wang G, Frazao J, Charytan C, Coburn J, Coyne D, Kaplan M, Baker N, McCary L, Turner S, Goodman W: Cinacalcet HCl reduces bone turnover and fibrosis in hemodialysis patients with secondary hyperparathyroidism (HPT) [Abstract]. Nephrol Dial Transplant 19 :219 ,2004 [Google Scholar]