Abstract
Bone histomorphometry has been the gold standard in the evaluation and diagnosis of renal osteodystrophy. The recent new definition of renal osteodystrophy as chronic kidney disease–mineral and bone disorder has once again highlighted the use of bone biopsy as a powerful and diagnostic tool to determine skeletal abnormalities in chronic kidney disease. The procedure of iliac crest bone biopsy has been proved safe and associated with very minimal morbidity. In this review, the clinical indications, preparation, instrumentation, and potential complications are discussed. Because current biochemical markers are poor predictors of bone turnover, volume, and mineralization, a wider use of bone biopsy and histomorphometry will lead to a better understanding of the bone and mineral disorders that are associated with chronic kidney disease.
Renal osteodystrophy represents a spectrum of skeletal lesions that range from high-turnover disorders (osteitis fibrosa and mild lesions of secondary hyperparathyroidism) to low-turnover bone diseases (osteomalacia and adynamic lesion) (1). Mixed lesions of renal osteodystrophy have histologic evidence of both osteomalacia and hyperparathyroidism, with the rate of bone formation depending on the predominant lesion. Bone biopsy is the gold standard to establish the precise diagnosis of renal osteodystrophy, determine the severity of the bone disease, and assess the skeletal response to different therapeutic interventions (2,3). The use of this procedure for histomorphometric analysis has provided the basis of our current understanding of the different subtypes of renal bone disease and their pathogenesis in patients with chronic kidney disease (CKD).
In the past few years, various studies have linked the abnormalities of bone and mineral metabolism with the process of vascular calcification (4–6). The magnitude of vascular and skeletal complications that are associated with CKD and the recognition that these complications are integrally linked have led to the recent new definition of renal osteodystrophy as a broader clinical syndrome of abnormalities of mineral and bone metabolism secondary to CKD and/or extraskeletal calcifications. The new consensus on the definition of renal osteodystrophy highlights the use of bone biopsy as a powerful and diagnostic tool to determine precise skeletal abnormalities (7). In describing these abnormalities, it has now been recommended to include three important elements obtained from bone histomorphometric analysis: Bone turnover, mineralization, and volume. These three histologic variables provide clinically relevant information on bone pathology that can be used to define pathophysiology and guide clinicians with therapy (7).
Although bone histomorphometry is the gold standard for diagnosis of bone turnover state in patients with CKD, the use of bone biopsy has been limited by several factors, such as the invasiveness of the procedure, the lack of technical training, and the limited number of specialized centers with expertise in tissue handling and preparation (3). Serum parathyroid hormone (PTH), however, has been used as a noninvasive biomarker of bone turnover. Indeed, in cross-sectional studies, the introduction of first-generation immunometric PTH assay (1st PTH-IMA) has allowed reasonable prediction of the different subtypes of bone lesions in CKD (8–10); however, this predictive value of serum PTH is diminished when patients are treated with intermittent calcitriol therapy such that the development of adynamic bone has been observed in patients with PTH levels that are consistent with secondary hyperparathyroidism (11). In addition, it has been shown that 1st PTH-IMA detect not only the full-length and biologically active PTH (1-84) but also PTH fragments that accumulate in renal failure (12,13). Cross-reaction with PTH amino-truncated fragments [ntPTH(1-84)] have resulted in the overestimation of the true concentration of PTH (1-84). For addressing this shortcoming, second-generation PTH-IMA (2nd PTH-IMA) have been developed. These assays use detection antibody that is raised against the first four amino acids at the amino-terminal end and recognizes exclusively the biologically active full-length PTH(1-84) with no cross-reaction with ntPTH(1-84) fragments (14,15); however, its value in predicting the types of bone lesions is yet to be fully established. Likewise, other serum biomarkers and imaging techniques that are used to assess bone health have not been carefully studied in the context of CKD. Thus, bone biopsy remains the most accurate diagnostic tool in the evaluation of renal osteodystrophy. The current clinical indications for performing the procedure are summarized in Table 1 (16). The applications of this procedure are not limited to clinical evaluation and diagnosis but extend to basic and clinical research. Future studies have been recommended to evaluate how bone biopsy can be best used in clinical practice and how more clinicians can be trained to perform such a procedure (7).
Table 1.
| 1. Fractures with minimal or no trauma (pathologic fractures) |
| 2. Suspected aluminum bone disease on the basis of clinical symptoms or history of aluminum exposure |
| 3. Intact plasma PTH levels between 100 and 500 pg/ml (11.0 to 55.0 pmol/L; in stage 5 CKD) with coexisting conditions such as unexplained hypercalcemia, severe bone pain, or unexplained increases in bone alkaline phosphatase activity |
| 4. Severe progressive vascular calcification |
| 5. Before parathyroidectomy if there has been significant exposure to aluminum in the past or if the results of biochemical determinations are not consistent with secondary or tertiary hyperparathyroidism |
| 6. Before beginning treatment with bisphosphonates |
CKD, chronic kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes; KDOQI, Kidney Disease Outcomes Quality Initiative; PTH, parathyroid hormone.
Clinical Procedure
Tetracycline Labeling
The value of bone biopsy in the diagnosis of renal osteodystrophy is enhanced when dynamic aspects of bone turnover are characterized (17). For determination of the level of bone turnover, bone formation rates, and mineralization defects, double labeling of the bone with tetracycline compounds is done before performance of the bone biopsy. Tetracycline compounds chelate calcium on bone surfaces and are deposited within the bone at sites of active mineralization. The “labels” can then be visualized in fluorescent light during histomorphometric evaluation (Figure 1). Double bands of tetracycline fluorescence can be seen circumscribing the amount of new bone formed during the labeling interval. Tetracycline and demeclocycline HCl are the most commonly used compounds. The intensity of the label would depend on the drug concentration and dosage. In children, dosage for oral tetracycline is 10 to 15 mg/kg per d and for demeclocycline HCl is 15 to 20 mg/kg per d divided three times a day. Adult dosage for tetracycline HCl is 500 mg by mouth two times a day. Demeclocycline HCl can be given on a similar schedule as tetracycline but at a lower dosage of 300 mg. There are different protocols for double-tetracycline labeling, but the basic and optimal setup would involve allowing a certain amount of time to elapse between two courses of antibiotics. In our center, antibiotics are given during two 2-d periods with each period separated by 10 to 12 d free of tetracycline. Iliac crest bone biopsy will take place 2 to 5 d after double labeling with tetracycline. The drug is usually administered after meals to avoid gastrointestinal discomfort. The patient should avoid milk and dairy products, which contain calcium, as well as calcium-containing binders because they can bind to tetracycline and prevent adequate absorption of the drug. It is imperative that the patient adhere strictly to tetracycline dosing and schedule so as not to encounter any problems in interpretation and computation of the dynamic histomorphometric parameters.
Figure 1.
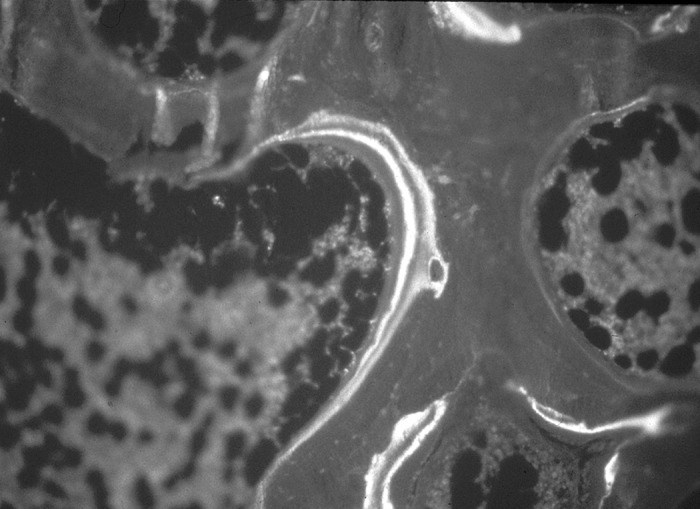
Tetracycline double labeling on bone-osteoid surface as seen in fluorescent light.
Biopsy Sites, Instruments, and Technique
Quantitative bone histomorphometry requires a good-quality bone specimen. Obtaining good samples would depend on the instrument used, biopsy technique, and a skillful and experienced operator (3,18). Iliac crest has been the preferred site when doing bone biopsy, because it is easily accessible and associated with fewer complications. Either the right or left iliac crest can be biopsied. Iliac crest biopsies can be obtained in a horizontal and vertical direction with associated advantages and disadvantages (3,19–21). The vertical approach would have specimen that is mostly trabecular bone. In the horizontal approach, intact two cortices separated by trabecular bone can be obtained (19). In either approach, a manual trochar or electric drill can be used (Figures 2 and 3). In our center, modified Bordier trephine (Lepine à Lyon, Lyon, France) with trochar core diameter of 5 to 7 mm is used. This needle consists of four parts: A pointed trochar; guide sleeve with sharp, serrated edges; trephine biopsy needle; and blunt extractor (Figure 3) (17). Regardless of the instrument and technique used, the expertise of the operator plays a significant role in obtaining an intact bone core. Care should be taken in performing the procedure so that the bone sample is not fractured or crushed. It should contain two cortices separated by trabecular bone if the transiliac approach is used. In children, the vertical approach has not been widely used because the presence of the growth plate at the top of the iliac crest may be a confounding factor (22). Bone specimen below the growth plate is normally characterized with high bone turnover and has very low cortical thickness.
Figure 2.
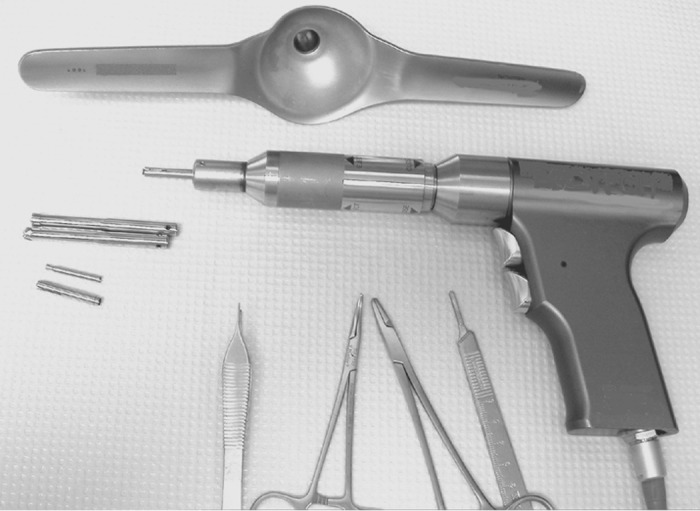
Electric drill for horizontal and vertical iliac crest biopsies.
Figure 3.
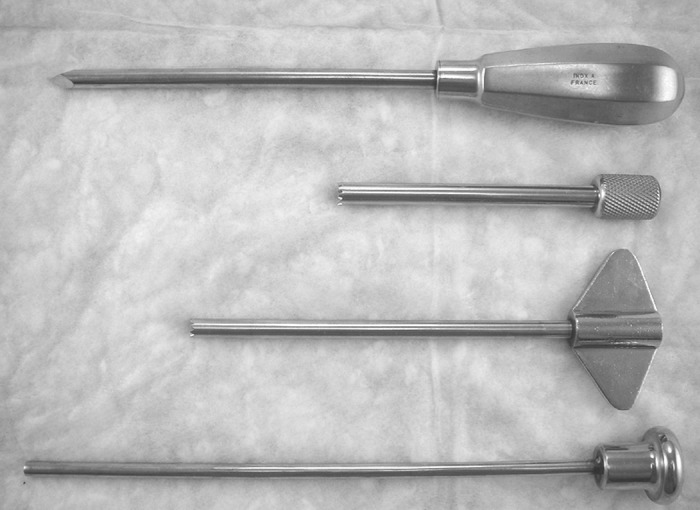
Modified Bordier trephine, from top to bottom, includes a pointed trochar, an outer guide or sleeve, a trephine, and a blunt extractor.
Biopsy Procedure
The biopsy should be performed in a procedural area that is equipped to monitor a sedated patient and provide for a monitored recovery. In hemodialysis patients, we avoid doing the procedure during dialysis day to avoid hematoma and bleeding from heparin exposure. Peritoneal dialysis (PD) patients are instructed not to have a day dwell of PD fluid on the day of the biopsy. The patient should be instructed to fast before the procedure. Moderate to deep sedation is required and should be administered by personnel who are trained and credentialed to do so. In our center, a combination of fentanyl for analgesia combined with propofol infusion for sedation has resulted in excellent biopsy conditions and rapid recovery. Hypotension might be problematic, especially in the relatively dehydrated postdialysis patient, and PD patients should be advised to decrease the dialysate dextrose concentration the night before biopsy. Combinations of narcotic, typically fentanyl, and benzodiazepine, often midazolam, have been used successfully as well. Local anesthetic should be used to decrease procedural stimulation and provide some postprocedure anesthesia.
The patient is placed in the supine position with the ilium and umbilicus exposed; the anterior ilium is cleaned with chlorhexidine or povidone-iodine solution and draped. The biopsy site is located 2 cm posterior to the anterior-superior iliac spine (Figure 4) (20). This site is easily accessible and associated with minimal complications (23). Approximately 10 ml of 1% lidocaine is used to anesthetize the skin, subcutaneous tissue, and periosteum of the iliac crest (Figure 5). The skin and the subcutaneous tissue are anesthetized with lidocaine using a 25-G needle. The periosteum is anesthetized with lidocaine using a 1.5-inch 20-G needle and injecting while moving over the surface of the lateral ilium on an area of 1 to 2 cm. A vertical skin incision of 0.5 to 1.0 cm is then made at the previously identified site using a scalpel with a No. 11 blade. The underlying muscle and fascia are separated by blunt dissection until the lateral iliac periosteum is exposed (Figure 6). Before doing the biopsy, the trephine biopsy needle is filled with bone wax (using a 1- to 2-cm block of wax), which will help to secure the bone specimen within the cutting trephine while it is being withdrawn. The pointed trochar is inserted through the outer guide sleeve and then inserted through the skin incision. The outer guide and pointed trochar are then applied firmly to the exposed bone, pointing toward the umbilicus. The outer guide is then rotated until it is firmly implanted and anchored to the lateral ilium (Figure 7). Making sure that the outer guide is firmly seated on the bone surface prevents slippage of the biopsy trephine along the surface of the bone when it is advancing through the sleeve during the biopsy. At this time, the pointed trochar is then withdrawn and the trephine is inserted through the outer guide. An assistant stands on the other side of the patient and holds the hips down. The trephine is rotated clockwise with a steady moderate pressure and gradually increased until the cutting action on the bone is felt and the trephine has advanced through the full depth of the iliac crest (Figures 8 and 9). For obtaining an intact bone core, it is essential to use a gentle, steady pressure to advance the trephine, particularly in patients with fragile osteoporotic or soft bones (20). Heavy pressure should be avoided. After the inner cortical bone is penetrated, the trephine is rotated 360°, first in a clockwise direction and then counterclockwise. This step would help to free the biopsy specimen from the connective tissue at the inner periosteal surface. The trephine is then removed using a slow, rotating, counterclockwise motion, and once free, the outer guide is removed while gauze is placed over the incision site. The blunt extractor is inserted through the top of the trephine, gently pushing out the bone core specimen. If the specimen does not come out easily, then a few light taps with the handle of the large trochar would suffice to expel the bone core.
Figure 4.
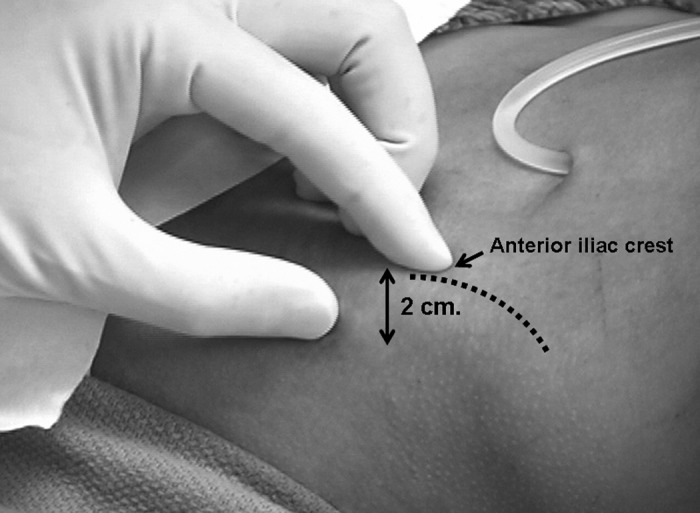
The biopsy site is identified 2 cm posterior to anterior iliac crest (dotted line outlines the iliac crest).
Figure 5.
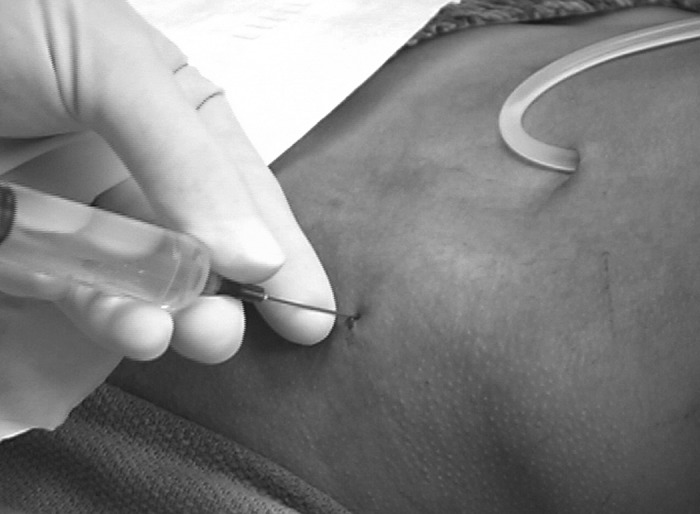
Lidocaine (10%) is used to anesthetize skin, subcutaneous tissue, and periosteum.
Figure 6.
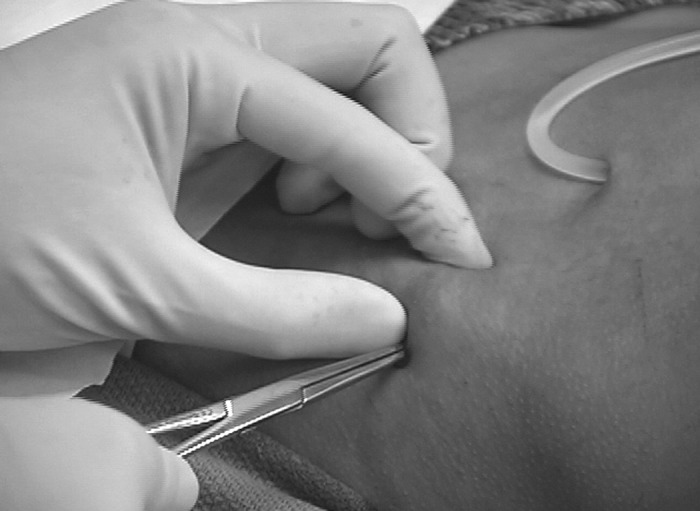
After an incision is made, the muscle and fascia are separated by blunt dissection until the periosteum is exposed.
Figure 7.
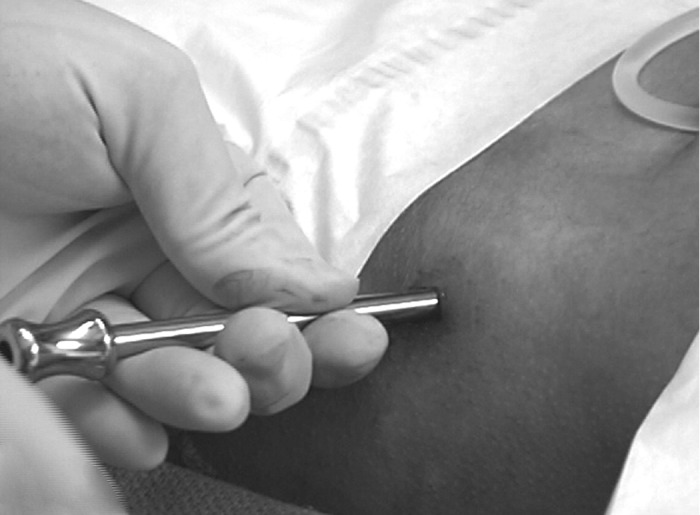
The pointed obturator together with the outer guide is inserted and applied firmly to the exposed bone. The guide is rotated and implanted on the lateral ilium.
Figure 8.

The trephine is inserted into the outer guide and rotated counterclockwise with steady pressure until the cutting action of the trephine on the bone is felt.
Figure 9.
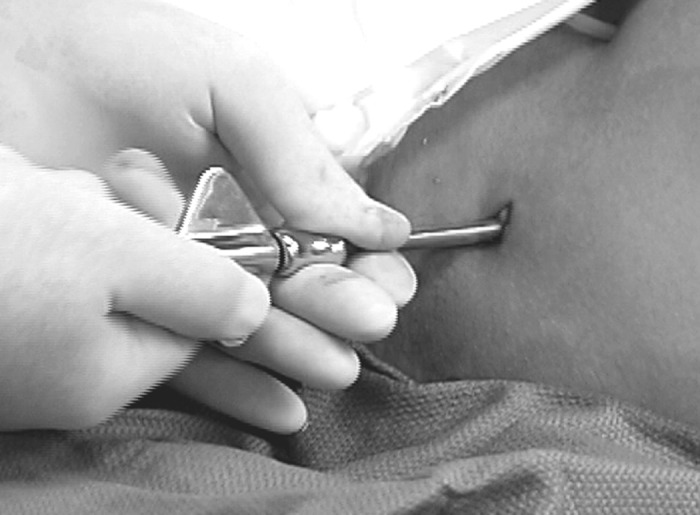
Trephine advancing through the full length of the iliac crest.
Bone biopsy can also be done using a new electric drill (Straumann, Cambridge, MA; Figure 2) with a one-step drilling and extraction procedure. This drill is now widely used and has the advantage of providing easier and shorter surgical time for performing bone biopsies. The use of disposable drill bits in this new instrument decreases infectious complications, avoids problems with dull drill bits, and eliminates the need for frequent resharpening (21). After incision of the skin is made and blunt dissection of the subcutaneous tissue is done, a funnel-shaped winged positioner is introduced through the incision and placed over the iliac crest surface. The axis of the hand-held funnel is aligned by an assistant with the axis of the underlying bone to stabilize and prevent the trephine from exiting through the pelvic bone during drilling. The trephine consists of a precutter drill bit, which can be placed first to remove subcutaneous tissue and then cut through the periosteum, and a core drill or actual trephine, which drills and separates the bone biopsy core from the surrounding bone. The precutter on the drill is placed in the center of the funnel, and the electric drill is engaged by pressing the first trigger or button (21). The actual trephine then replaces the precutter and is inserted into the funnel in the same manner. Drilling is done with minimal pressure and continues until the base of the drill just reaches the bottom of the funnel without touching it. Within the trephine, internal forceps fracture the core of bone graft away from the iliac crest. As the sleeve of the drill is pulled slowly upward while continuing to drill, extraction forceps move over the bone core and capture the sample. The entire trephine is now removed slowly upward while the drill is still rotating. An internal plunger is used to push out the core of bone from the trephine. The operator ejects the bone core sample while pressing the second button on the drill. Sterile medical wax is then used to plug the cavity created by the trephine. It is important that care and prevention of exerting undue pressure while using the drill be observed because “bone powder” can accumulate in the periphery of the trabecular bone. Avoidance of using high speed in drilling can prevent creation of heat artifacts on bone cells (3).
The bone specimen after the biopsy is placed in a container filled with 10% phosphate-buffered formalin. The incision is approximated and closed with 3-0 nylon sutures. Povidone-iodine ointment is applied, and the site is covered with a small adhesive pad (Band Aid; Johnson & Johnson, New Brunswick, NJ) and elastic pressure dressing. The procedure takes approximately 15 to 20 min, and blood loss is less than 1 ml. When fully awake after sedation wears off, the patient can get out of bed in 3 h and go home the same day. The pressure dressing is removed after 24 h and replaced with a Band Aid. The patient can shower after 48 h, cleaning the incision site with soap and water then covering it with a Band Aid. The sutures are then removed 7 to 10 d after bone biopsy.
Specimen Handling and Processing
In our center, biopsy specimens are fixed in commercially prepared 10% phosphate-buffered formalin and kept at room temperature for 24 h. The specimen is then transferred into 70% ethanol alcohol and sent at room temperature to the laboratory. In other centers, however, the bone specimen is immediately placed in 70% ethanol after the bone biopsy procedure. Regardless of the initial fixative used, specimen should be placed right away into the solution to preserve the bone tissue. Duration should not exceed 48 h because tetracycline labeling can be washed out with prolonged fixation. Concentrated formalin should not be used because it has the tendency to leach out calcium, aluminum, and tetracycline from bone (3). After the initial placement into the fixative solution, the specimen is dehydrated in alcohol and later embedded in methylmethacrylate. Cutting of the specimen is done using a special microtome. The sections are later stained with either toluidine blue or Masson-Goldner trichome. Two computer-based software applications are commercially available to facilitate bone histomorphometric measurements: OsteoMeasure (Osteometrics, Decatur, GA) and Bioquant Osteo II (Bioquant Image Analysis Corp., Nashville, TN). The software applications are user-friendly and automatically calculate more than 100 bone parameters based on American Society for Bone and Mineral Research nomenclature.
Complications
Complications as a result of bone biopsies can include pain, hematoma, wound infection, and, rarely, neuropathy. Studies have shown that horizontal and transiliac bone biopsies are associated with very small morbidity and no mortality (24). The operator's experience is an important factor in minimizing morbidity and ensuring specimen adequacy. Our group has performed more than 700 bone biopsies in pediatric patients in the past several years with excellent results and with minimal morbidity.
Allergic reactions, gastrointestinal disturbances, and photosensitivity secondary to tetracycline intake can occur. Giving the drugs after meals can diminish gastrointestinal upset, and avoiding sun exposure while taking tetracycline prevents skin photosensitivity. The bone procedure described here is generally well tolerated with minimal pain and discomfort. Pain is the most feared complication, which can be easily prevented by use of generous amounts of local anesthetic over the skin and periosteum (20). Osteomyelitis and skin incision site infection can be avoided by adherence to strict aseptic techniques during the procedure (19). Duncan et al. (25) surveyed complications in 9131 transiliac biopsies as reported by physicians who performed the procedure. The total incidence of complications was 0.6%: 22 (0.24%) patients had hematoma, 17 (0.20%) had pain, 11 (0.12%) had transient neuropathy, and six (0.07%) had wound infection. Two (0.02%) patients had hip fractures, and one (0.01%) had osteomyelitis. No deaths or permanent disability was reported.
Conclusions
Bone biopsy procedure is safe and associated with minimal morbidity. Tissue analysis using bone histomorphometry, however, would require laboratory expertise in specialized centers. Because current biochemical markers are poor predictors of bone turnover, bone volume, and mineralization, a wider use of bone histomorphometry will lead to a better understanding of the pathogenesis and the effects of various therapeutic interventions on bone and mineral disorders that are associated with CKD.
Disclosures
None.
Acknowledgments
This work was supported in part by US Public Health Service grants DK675632, DK35423, and RR000865 and by funds from the Casey Lee Ball Foundation.
References
- 1.Salusky IB, Coburn JW, Brill J, Foley J, Slatopolsky E, Fine RN, Goodman WG: Bone disease in pediatric patients undergoing dialysis with CAPD or CCPD. Kidney Int 33 :975 –982,1988 [DOI] [PubMed] [Google Scholar]
- 2.Weinstein RS: Clinical use of bone biopsy. In: Disorders of Bone and Mineral Metabolism, 2nd Ed., edited by Coe FL, Favus MJ, Philadelphia, Lippincott Williams & Wilkins,2002. , pp448 –468
- 3.Monier-Faugere M-C, Langub MC, Malluche HH: Bone biopsies: A modern approach. In: Metabolic Bone Diseases and Clinically Related Disorders, 3rd Ed., edited by Avioli LV, Krane SM, San Diego, Academic Press,1998. , pp237 –273
- 4.Ketteler M, Gross ML, Ritz E: Calcification and cardiovascular problems in renal failure. Kidney Int Suppl S120 –S127,2005 [DOI] [PubMed]
- 5.Ketteler M, Westenfeld R, Schlieper G, Brandenburg V: Pathogenesis of vascular calcification in dialysis patients. Clin Exp Nephrol 9 :265 –270,2005 [DOI] [PubMed] [Google Scholar]
- 6.London GM, Marchais SJ, Guerin AP, Metivier F: Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens 14 :525 –531,2005 [DOI] [PubMed] [Google Scholar]
- 7.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G: Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69 :1945 –1953,2006 [DOI] [PubMed] [Google Scholar]
- 8.Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, Saiphoo C, Fenton SS, Segre GV: The spectrum of bone disease in end-stage renal failure: An evolving disorder. Kidney Int 43 :436 –442,1993 [DOI] [PubMed] [Google Scholar]
- 9.Salusky IB, Ramirez JA, Oppenheim W, Gales B, Segre GV, Goodman WG: Biochemical markers of renal osteodystrophy in pediatric patients undergoing CAPD/CCPD. Kidney Int 45 :253 –258,1994 [DOI] [PubMed] [Google Scholar]
- 10.Torres A, Lorenzo V, Hernandez D, Rodriguez JC, Concepcion MT, Rodriguez AP, Hernandez A, de Bonis E, Darias E, Gonzalez-Posada JM, et al.: Bone disease in predialysis, hemodialysis, and CAPD patients: Evidence of a better bone response to PTH. Kidney Int 47 :1434 –1442,1995 [DOI] [PubMed] [Google Scholar]
- 11.Goodman WG, Ramirez JA, Belin TR, Chon Y, Gales B, Segre GV, Salusky IB: Development of adynamic bone in patients with secondary hyperparathyroidism after intermittent calcitriol therapy. Kidney Int 46 :1160 –1166,1994 [DOI] [PubMed] [Google Scholar]
- 12.Lepage R, Roy L, Brossard JH, Rousseau L, Dorais C, Lazure C, D'Amour P: A non-(1-84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem 44 :805 –809,1998 [PubMed] [Google Scholar]
- 13.Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barre M, D'Amour P: Accumulation of a non-(1-84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: Importance in the interpretation of PTH values. J Clin Endocrinol Metab 81 :3923 –3929,1996 [DOI] [PubMed] [Google Scholar]
- 14.John MR, Goodman WG, Gao P, Cantor TL, Salusky IB, Juppner H: A novel immunoradiometric assay detects full-length human PTH but not amino-terminally truncated fragments: Implications for PTH measurements in renal failure. J Clin Endocrinol Metab 84 :4287 –4290,1999 [DOI] [PubMed] [Google Scholar]
- 15.Gao P, Scheibel S, D'Amour P, John MR, Rao SD, Schmidt-Gayk H, Cantor TL: Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1-84: Implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res 16 :605 –614,2001 [DOI] [PubMed] [Google Scholar]
- 16.National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42 :S1 –S202,2003 [PubMed] [Google Scholar]
- 17.Norris KC, Goodman WG, Howard N, Nugent ME, Coburn JW: Iliac crest bone biopsy for diagnosis of aluminum toxicity and a guide to the use of deferoxamine. Semin Nephrol 6 :27 –34,1986 [PubMed] [Google Scholar]
- 18.Malluche HH, Faugere MC: Bone biopsy. In: Atlas of Mineralized Bone Histology, New York, Karger,1986. , pp18 –25
- 19.Rao DS: Practical approach to bone biopsy. In: Bone Histomorphometry: Techniques and Interpretation, edited by Recker RR, Boca Raton, FL, CRC Press,1983. , pp3 –11
- 20.Recker RR, Barger-Lux MJ: Transilial bone biopsy. In: Principles of Bone Biology, 2nd Ed., edited by Bilezikian JP, Raisz LG, Rodan GA, San Diego, Academic Press,2002. , pp1625 –1634
- 21.Trueba DB, Sawaya BP, Mawad H, Malluche HH: Bone biopsy: Indications, techniques and complications. Semin Dial 16 :341 –345,2003 [DOI] [PubMed] [Google Scholar]
- 22.Rauch F: Watching bone cells at work: What we can see from bone biopsies. Pediatr Nephrol 21 :457 –462,2006 [DOI] [PubMed] [Google Scholar]
- 23.Rauch F: Bone histomorphometry. In: Pediatric Bone: Biology and Diseases, edited by Glorieux FH, Pettifor JM, Juppner H, San Diego, Academic Press,2003. , pp359 –374
- 24.Johnson KA, Kelly PJ, Jowsey J: Percutaneous biopsy of the iliac crest. Clin Orthop Relat Res 34 –36,1977 [PubMed]
- 25.Duncan H, Rao SD, Parfitt AM: Complications of bone biopsy. In: Bone Histomorphometry: Third International Workshop, Sun Valley—May 28/June 2, 1980, edited by Jee WS, Parfitt AM, Paris, Societe Nouvelle de Publications Medicales et Dentaires,1981. , pp483 –486


