Abstract
A recent Kidney Disease: Improving Global Outcomes report suggested that bone biopsies in patients with chronic kidney disease should be characterized by determining bone turnover, mineralization, and volume. This article focuses on the calculations and interpretation of these measurements. In most cases of renal osteodystrophy, the bone formation rate is roughly similar to the bone resorption rate; therefore, the bone formation indices can be used to describe turnover. It is important to remember that these conventions will not apply in some situations. Activation frequency should not be confused with bone formation rate or bone metabolic unit birth rate. Abnormal mineralization can be described using the osteoid volume, increased osteoid maturation time, or increased mineralization lag time. The concept of bone volume is the easiest to understand, but there is a large error from one biopsy to the next in the same person. There are some difficulties with each of the measurements, and further research in patients with chronic kidney must be done to enable a consensus to be reached about cut points to define categories within the spectrum of renal osteodystrophy and how to evaluate treatment responses.
Patients with chronic kidney disease (CKD) have complex abnormalities in their bones (1–6). Some of the pathologic factors and the resulting histologic changes are shown in Figure 1. Many more factors affect bone metabolism in patients with CKD than in patients with other types of metabolic bone disease, in which one major pathogenic disturbance usually predominates. In addition to abnormalities shown here, patients may have had bone disease before developing renal failure (e.g., osteoporosis, vitamin D deficiency, steroid-bone disease), which will contribute to the pathologic findings of the bones. Bone biopsies are performed to understand the pathophysiology and course of bone disease, to relate histologic findings to clinical symptoms of pain and fracture, and to determine whether treatments are effective. A recent Kidney Disease: Improving Global Outcomes (KDIGO) report (7) suggested that bone biopsies in patients with CKD should be characterized by determining bone turnover, mineralization, and volume (TMV). The exact methods of measuring these parameters, however, were not specified. This article focuses on the calculations and interpretation of these measurements.
Figure 1.
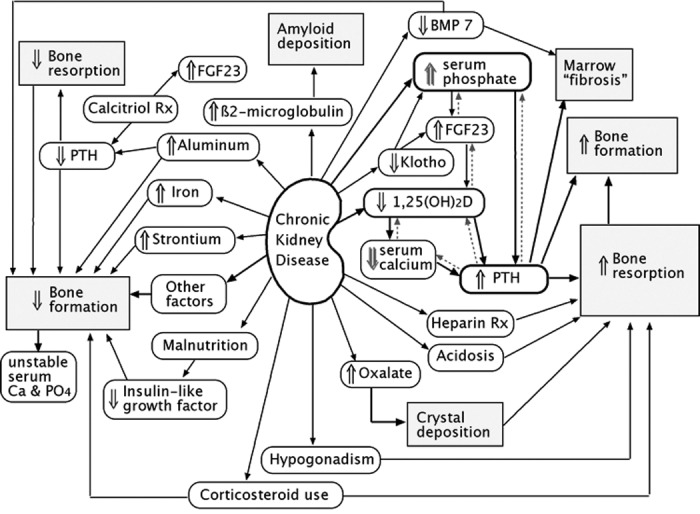
Pathophysiologic mechanisms of renal osteodystrophy. The dashed arrows show feedback loops that are “frustrated” by the renal dysfunction.
The three parameters (TMV) for defining categories of renal osteodystrophy were selected by the KDIGO committee on the basis of their experience with examining bone biopsies from patients with CKD and our current understanding of bone physiology. It has been recognized that these patients display a spectrum of bone formation rates (BFR) from abnormally low to very high. Other measurements that help to define low or high turnover (e.g., eroded surfaces, number of osteoclasts, fibrosis, woven bone) tend to be associated with the BFR as measured by tetracycline labeling. This is the most definite dynamic measurement, so it was chosen to represent bone turnover. Note that a simple change in the BFR will not reveal whether a patient has improved, because restoration of normal physiology may require either increase or decrease in bone turnover, depending on the starting point. The second parameter was mineralization, to distinguish those with osteomalacia (who can form matrix but not form mineralized bone) from those with adynamic disease (who do not even form the matrix). The final parameter was bone volume, which was not usually included in previous schemes of describing renal osteodystrophy. The committee believed that bone volume would be likely to contribute to bone fragility and is separate from the other parameters. The bone volume is the end result of changes in bone formation and resorption rates: If overall BFR is greater than overall bone resorption rate, then the bone is in positive balance and the bone volume will increase.
Some histologic findings in bone document causes of bone disease, such as aluminum, amyloid, or iron deposition; these are independent of the TMV classification. There are many other measurements that are not considered here because either they are too new, there is too little information about their meaning, or they seem to be secondary. It should be stressed that renal osteodystrophy is complicated and further research may indicate that some of these measurements will provide necessary information about treatment of patients: The amount of woven bone, the number and condition of osteocytes, the number of osteoblasts and osteoclasts, the number of apoptotic cells, the structural microarchitecture, the cortical porosity, the mineralization density, the mechanical stiffness of the bone material, the volume of fibrosis, the resorption depth, the volume of the fat cells, the thickness of the lamella, and the characteristics of the tetracycline labels. Immunohistochemical measurements of bone proteins may be used in the future but are beyond the scope of this discussion.
In 1987, the American Society for Bone and Mineral Research Histomorphometry Nomenclature Committee published an article on standardization of nomenclature, symbols, and units (8). This was widely accepted in the scientific community, and the KDIGO bone histomorphometry committee unanimously agreed that these conventions should also be used for renal osteodystrophy. The American Society for Bone and Mineral Research Histomorphometry Nomenclature Committee article provided valuable rationale for the measurements, the importance of the referents, the descriptions of the core samples (whether transiliac or vertical), the methods of measuring the primary measurements, and the equations for deriving the common indices of bone physiology. Although the bone journals all require articles to use this standard nomenclature, some of the recommendations are not always followed. Common omissions are the referent for BFR, specification of the minimum width of osteoid that is measured, and reporting derived indices without the primary data.
The bone biopsies are taken from the anterior iliac crest because that site has normative data and because it is safe to take a biopsy; there are no major nerves, organs, or blood vessels, and the bone is large enough that the biopsy will not compromise bone strength. The size of the biopsy should be at least 4 mm in diameter; in many research studies, the size is 8 mm to allow more accurate measurements. It is critical that the sample not be decalcified. The vast majority of bone biopsies are done for possible cancer, which requires decalcification and very thin sections, so pathology laboratories automatically place the bone samples into decalcifying solution and it is then impossible to tell anything about mineralization. A bone biopsy that is done for diagnosis of any metabolic bone disease requires double tetracycline labeling.
Turnover
Healthy bone is a dynamic tissue, continually resorbing bone and replacing it with new bone in discrete areas known as bone metabolic units (BMU). The BMU in solid cortical bone drills a tunnel and then refills it. On the cancellous bone surface, the BMU can channel like a river flowing or can spread over the surface like fudge flowing over ice cream; therefore, the turnover of bone is different from the turnover on the skin, where the entire surface is continuously forming and shedding. At each BMU, the volume of new bone could completely fill the space that was resorbed, in which case there would be a neutral bone balance at that BMU. More common, the new bone does not quite fill in the resorbed space and the bone balance is negative at that location. On cancellous surfaces, the newly formed bone could occupy a larger volume than the bone resorbed, which would lead to a positive bone balance. The overall bone balance for the skeleton represents the sum of balances at all of the BMU. If each BMU yields a slight loss of bone, then the overall bone loss will depend on the number of BMU. The overall bone balance is also the difference between the average bone resorption rate and the average BFR. A dynamic review of the bone remodeling is available at http://courses.washington.edu/bonephys.
If the bone is in balance, then “turnover” is either the rate of bone formation or the rate of bone resorption, because they are the same. The term “turnover” can be ambiguous if the bone is not in balance. Some people equate turnover to bone resorption, others with bone formation. A condition such as steroid-bone disease, with high resorption and low formation rates, will then be designated “high turnover” by some and “low turnover” by others. The opposite happens during growth, recovery from lactation, or treatment with anabolic drugs, when the bone formation is higher than resorption. When bone is not in balance, both bone formation and bone resorption rates are needed to define the physiologic status.
Postmenopausal osteoporosis is a bone disease in which the bone resorption rate is increased as a result of estrogen deficiency and the BFR are also increased to compensate for the high bone resorption (by a process called “coupling,” whose mechanisms are still uncertain), but the formation rates do not quite match the resorption rates and the net effect is loss of bone. This common form of high turnover is deleterious to the skeleton, because there is net loss of bone. Also, more subtle effects include increased chance of perforation of the trabecular walls, more cement lines that may be weak areas, and a greater proportion of new bone that is not as mineralized and lacks stiffness of mature bone. Nevertheless, the high BFR itself is not the problem, and anabolic medications hold more promise for reversing osteoporosis than antiresorbing ones, which merely prevent the disease from getting worse.
In most cases of renal osteodystrophy, the bone is roughly in balance, so those with high BFR also have high resorption rates and those with adynamic bone disease have low bone resorption rates. The bone formation indices are used to describe turnover, because they can be measured with much more accuracy than the bone resorption rates. It is important to remember that these conventions will not apply in some situations.
BFR can be measured directly. The length of the tetracycline labels (mineralizing surface per bone surface [MS/BS]) multiplied by the distance between labels (mineral apposition rate [MAR]) is the area of new bone formed during the label interval. In other words, the BFR depends both on the rate of apposition at the surface and the total surface involved in forming bone:
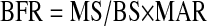 |
This can be expressed in reference to the bone surface (as in this equation), bone volume, or tissue volume (after adjustment for bone surface per bone or tissue volume). The appropriate referent depends on the situation. The most easily understood concept of turnover is the BFR as a percentage per year of the bone volume. The “absolute” amount of bone formed (BFR/TV) would theoretically provide the best correlation with a serum marker of bone formation.
The tetracycline labels usually are seen clearly, and measurements are straightforward. Sometimes the edges tapir gradually into the inactive bone surface, making the exact end of the label indefinite, but this causes only minor measurement difficulties. More common, when there is very rapid bone formation, the labels become blurry and diffuse, making them difficult to measure. Conversely, when the bone is forming very slowly, the labels do not show separation and it is difficult to tell whether a label is a double label or a single label. Use of two different kinds of tetracycline (demeclocycline and tetracycline) is helpful in these cases because they have different colors when viewed using fluorescence microscopy.
Because bone formation occurs in discrete BMU, an area of bone surface is not continuously active. There are intervals when a spot on the surface is actively forming bone; the duration of one of these intervals is called the formation period (FP). The rest of the time, that bone surface is either resorbing or quiescent. The total period (TP) is the duration between the beginning of one FP and the beginning of the next FP. The number of times per year that this spot begins the FP is the activation frequency (Acf). The Acf is related to the BFR, but it is not a BFR, because the Acf also depends on the amount of bone formed during each remodeling cycle. This amount of bone formed during an average remodeling cycle is represented by the wall thickness (WTh), which is the thickness of new bone made in one cycle. The FP is calculated as the WTh divided by the MAR:
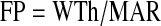 |
For example, if the WTh is 40 μm and the apposition rate is 0.5 μm/d, then that spot on the surface was forming bone for 80 d.
The WTh is a straightforward concept that is easy to demonstrate on an ideal trabecula that has clear lamella with distinct orientations (Figure 2). In practice, however, this can be a difficult and subjective measurement. Often the orientation of lamellae on the old bone is the same as on the new bone, and it is difficult to tell where the cement line is. On thin trabecula, there may be only one cement line, and it is hard to tell which BMU was most recent. Measurements must be strictly randomized to avoid bias of measuring only the thickest and clearest parts of the wall. Walls cannot be measured if the surface is eroded or covered with osteoid, because the calculations assume complete walls. In patients with CKD, this can be a problem because so much of the surface is actively forming or resorbing bone. When the bone is undergoing changes, such as with therapy or worsening disease, some of the existing BMU will be old and the WTh will not represent the current dynamic state of the bone. There is an alternative method of estimating the WTh on the basis of extrapolation of walls on forming surfaces, but that requires many tedious measurements on multiple sections (9).
Figure 2.
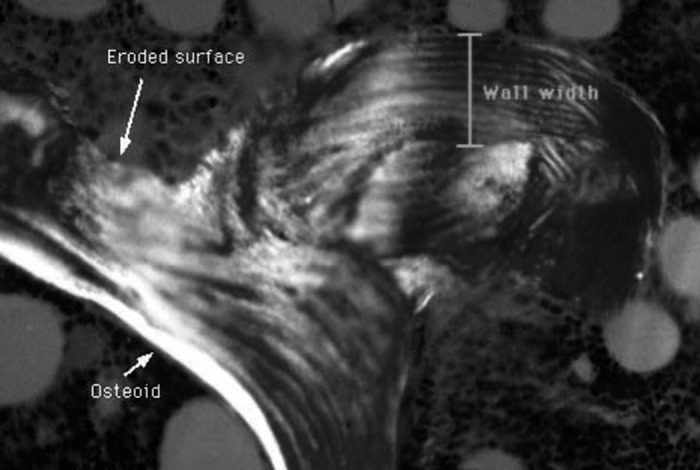
Trabecular surface showing an osteoid surface, an eroded surface, and the wall width. The section is viewed under polarized light to show the lamellar structure.
An alternative method of calculating the FP is based on the phenomenon of label escape (10). Between the first and the last tetracycline labels, some BMU have stopped forming bone and others have started to form bone. These BMU will have only a single label. The longer the interval between labels, the more labels will be only single labels; therefore, the ratio of single to double labels is related to the FP and the label intervals. By extension, three labels can be used to give more precise measurements, and the FP is calculated as the label interval divided by the ratio of double labels to double + triple labels. This method of calculating FP is not generally used because the labels can be infrequent enough to make the ratio unreliable unless a very large surface is measured.
Once the FP has been calculated, the TP can be calculated by using a surface-interval transformation. The ratio of mineralizing surface (MS) to bone surface (BS) corresponds to the ratio of FP to TP.
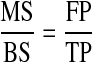 |
Therefore, TP is the FP divided by the mineralizing surface per bone surface.
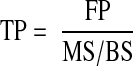 |
For example, if the FP is 80 d and 10% of the surface is forming, then the TP is 800 d. Frequencies are the inverse of periods, so the Acf is once every 800 d, or 0.45 per year:
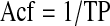 |
Note that the mineralizing surface is used to calculate both the Acf and the BFR, which is why the two are related. By combining the previous equations, it can be shown that the Acf is the BFR divided by the WTh:
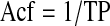 |
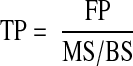 |
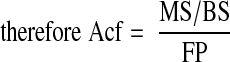 |
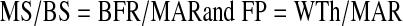 |
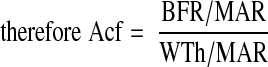 |
The MAR cancel each other out, so the final equation is
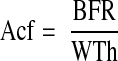 |
It is important to understand that Acf can increase even though the BFR is unchanged. As seen from the previous equation, the Acf can increase merely from a decrease in WTh. With aging, the Acf increases; this is due to a combination of increased bone formation and decreased WTh.
Some people think that an increased Acf causes bone loss. They confuse bone balance with bone turnover. It is the imbalance between resorption and formation that causes osteoporosis, not the increased Acf. If the BFR is greater than the bone resorption rate, then an increased Acf is associated with increasing bone volume (e.g., after therapy with intermittent teriparatide). In a subset of patients with CKD, the cancellous bone volume is increased, probably resulting from increased parathyroid hormone that has stimulated bone formation even more than bone resorption.
The Acf is also confused with the frequency of originating new BMU (BMU birth rate). One must remember that Acf refers to just one spot on the bone surface. When a traveling BMU reaches a spot, that surface becomes active. The birth rate of new BMU depends on how long they live. A bone with many short-lived BMU can have the same Acf, WTh, and BFR as a bone with a few long-lived BMU. The concept of BMU lifespan is simple to understand but cannot be measured on a two-dimensional section of bone, because BMU wander in and out of the plane of the section.
Other measurements that relate to the formation aspects of bone turnover include the osteoblastic surface, the number of active osteoblasts, and the osteoid surface. None of these gives the same accurate information as the tetracycline labeling. Bone resorption is related to the number of osteoclasts and the depth of resorption cavities, but there is no direct way to calculate the bone resorption rate. Some formulas that have been proposed require an assumption that the bone is in balance.
Acf and BFR have been used as indices of bone turnover in patients with CKD. There is definitely a spectrum from very low to very high. Further research is necessary to determine the best place for cut points that will place patients into categories that make sense in terms of pathophysiology or response to therapy. Serum biochemical markers of bone formation and resorption could potentially be very helpful in determining these rates (11–13). They have not yet been validated in patients with CKD. In patients with osteoporosis, the markers relate to BFR determined by tetracycline in some situations but not in others, and there is considerable variability. This is where more research is needed.
Mineralization
After the osteoblasts lay down new collagen, they direct mineralization of the matrix. This is normally a regulated and orderly process, but in patients with CKD, the mineralization can be delayed or disorganized. This results in thickened osteoid. Rapid bone formation also can result in thick osteoid, but in that case, the tetracycline labels are also more widely separated. The osteoid maturation time is the osteoid width divided by the distance between labels per day. The mineralization lag time is the osteoid maturation time adjusted for the percentage of osteoid surface that has a tetracycline label. This adjustment, however, assumes that the osteoid surface without a label is in a resting phase, but there is no evidence to support a resting phase. Osteomalacia has been defined by various investigators as increased osteoid volume, increased osteoid maturation time, or increased mineralization lag time. Parfitt (14) defined osteomalacia in patients with malabsorption when they had a combination of osteoid volume/bone volume >10%, osteoid thickness >12.5 μm (note that the thickness is a measurement that requires a correction for section obliqueness, which is the width/1.2), and mineralization lag time >100 d. There is no consensus about the exact definition of abnormal mineralization in patients with CKD.
Volume
The concept of bone volume is the easiest to understand. It is direct and reproducible to measure this within a sample. There is a large error, however, from one biopsy to the next in the same person. From right to left iliac crest, there is an average of 29% difference (15).
Bone volume is related to bone strength, but the same volume can have different microarchitecture. The trabecular thickness can be calculated from measurements of the bone surface per volume relationship, and this is an index that relates to bone strength. Other methods of measuring the architecture include the strut length, the star volume, and the number of nodes connecting trabeculae. These two-dimensional measurements are inferior to newer, three-dimensional measurements of connectivity that are made with microcomputed tomography.
There is consensus that bone volume is expressed as bone volume per tissue volume, which is the same as bone area per tissue area when expressed in two dimensions. This can be measured in cortical or in cancellous bone. Although there is agreement about the measurement, it has not been related to other categories of renal osteodystrophy. Each category of renal osteodystrophy may have patients with high or low bone volume (16). Some investigators have noted that the average bone volume is lower in those with adynamic bone disease, but there is still wide overlap. With the new TMV system of classification, the bone volume will be included in the descriptions of renal osteodystrophy. It is expected that the patients with low bone volume will be at higher risk for clinical disease and fractures, but more research is required to prove the theory.
Bone volume is related to the bone density measured by dual-energy x-ray absorptiometry (DEXA). The radiographic methods, however, cannot tell how dense bone material is, so a bone with high volume and low mineralization will have the same DEXA value as one with lower volume and higher mineralization density. This property is important, because DEXA cannot tell whether a bone has osteomalacia. Also, bisphosphonates do not increase bone volume, but they increase the DEXA values because the bone becomes more densely mineralized (harder, as in a piece of petrified wood). Despite these difficulties, DEXA has the advantage of measuring a larger and more representative area of bone, is noninvasive, and can be done repeatedly on the same location. In the general population, each SD decrease in DEXA predicts approximately a two-fold increase in osteoporotic fractures.
Quantitative computed tomography (QCT) is another noninvasive method of measuring bone mass. QCT also cannot differentiate between osteomalacia and other kinds of bone disease or between bones with dense mineralization. It has the advantage of providing measurements in three dimensions and of separating the cortical from the cancellous bone. In the general population, QCT and DEXA give similar predictions for fractures, but neither of these techniques seems to be as predictive in patients with severe CKD (10).
The emerging technique of micromagnetic resonance imaging can determine bone volume in a “virtual biopsy” that also can be analyzed for trabecular structure and connectivity. This noninvasive technique can image the same small area in bone over time and can document trabecular perforations that increase (in women going through menopause) or fill in (in men who are treated with androgens [17]). In the future, this technique may become the method of choice for determining bone volume.
Conclusions
The results of bone biopsies for patients with CKD should include description of the turnover (using BFR/TV or Acf), mineralization (using osteoid width or osteoid maturation time), and volume (bone volume/tissue volume). These are calculated using standardized methods, but there are some difficulties with each of the measurements, and further research in patients with CKD must be done to enable us to reach a consensus about cut points to define categories of these diseases and how to evaluate treatment responses.
Disclosures
None.
References
- 1.Elder G: Pathophysiology and recent advances in the management of renal osteodystrophy. J Bone Miner Res 17 :2094 –2105,2002 [DOI] [PubMed] [Google Scholar]
- 2.Ferreira A: Development of renal bone disease. Eur J Clin Invest 36 [Suppl 2]:2 –12,2006 [DOI] [PubMed] [Google Scholar]
- 3.Hruska KA, Teitelbaum SL: Renal osteodystrophy. N Engl J Med 333 :166 –174,1995 [DOI] [PubMed] [Google Scholar]
- 4.Sherrard DJ, Hercz G, Pei Y, Greenwood C, Manuel A, Saiphoo C, Fenton SS, Segre GV: The spectrum of bone disease in end-stage renal failure: An evolving disorder. Kidney Int 43 :436 –442,1993 [DOI] [PubMed] [Google Scholar]
- 5.Cunningham J, Sprague SM, Cannata-Andia J, Coco M, Cohen-Solal M, Fitzpatrick L, Goltzmann D, Lafage-Proust MH, Leonard M, Ott S, Rodriguez M, Stehman-Breen C, Stern P, Weisinger J, Osteoporosis Work Group: Osteoporosis in chronic kidney disease. Am J Kidney Dis 43 :566 –571,2004 [DOI] [PubMed] [Google Scholar]
- 6.Freemont T, Malluche HH: Utilization of bone histomorphometry in renal osteodystrophy: Demonstration of a new approach using data from a prospective study of lanthanum carbonate. Clin Nephrol 63 :138 –145,2005 [DOI] [PubMed] [Google Scholar]
- 7.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G, Kidney Disease: Improving Global Outcomes (KDIGO): Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69 :1945 –1953,2006 [DOI] [PubMed] [Google Scholar]
- 8.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR: Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2 :595 –610,1987 [DOI] [PubMed] [Google Scholar]
- 9.Paddock C, Youngs T, Eriksen E, Boyce R: Validation of wall thickness estimates obtained with polarized light microscopy using multiple fluorochrome labels: Correlation with erosion depth estimates obtained by lamellar counting. Bone 16 :381 –383,1995 [DOI] [PubMed] [Google Scholar]
- 10.Ott SM: Calculation of active formation period using label escape and three labels. Bone 14 :487 –490,1993 [DOI] [PubMed] [Google Scholar]
- 11.Fletcher S, Jones RG, Rayner HC, Harnden P, Hordon LD, Aaron JE, Oldroyd B, Brownjohn AM, Turney JH, Smith MA: Assessment of renal osteodystrophy in dialysis patients: Use of bone alkaline phosphatase, bone mineral density and parathyroid ultrasound in comparison with bone histology. Nephron 75 :412 –419,1997 [DOI] [PubMed] [Google Scholar]
- 12.Haas M, Leko-Mohr Z, Roschger P, Kletzmayr J, Schwarz C, Domenig C, Zsontsich T, Klaushofer K, Delling G, Oberbauer R: Osteoprotegerin and parathyroid hormone as markers of high-turnover osteodystrophy and decreased bone mineralization in hemodialysis patients. Am J Kidney Dis 39 :580 –586,2002 [DOI] [PubMed] [Google Scholar]
- 13.Martin KJ, Olgaard K, Coburn JW, Coen GM, Fukagawa M, Langman C, Malluche HH, McCarthy JT, Massry SG, Mehls O, Salusky IB, Silver JM, Smogorzewski MT, Slatopolsky EM, McCann L, Bone Turnover Work Group: Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy. Am J Kidney Dis 43 :558 –565,2004 [DOI] [PubMed] [Google Scholar]
- 14.Parfitt A: Osteomalacia and related disorders. In: Metabolic Bone Disease, edited by Aviolo LV, Krane SM, Philadelphia, Saunders,1990. , pp329 –396
- 15.Chavassieux PM, Arlot ME, Meunier PJ: Intersample variation in bone histomorphometry: Comparison between parameter values measured on two contiguous transiliac bone biopsies. Calcif Tissue Int 37 :345 –350,1985 [DOI] [PubMed] [Google Scholar]
- 16.Schober HC, Han ZH, Foldes AJ, Shih MS, Rao DS, Balena R, Parfitt AM: Mineralized bone loss at different sites in dialysis patients: Implications for prevention. J Am Soc Nephrol 9 :1225 –1233,1998 [DOI] [PubMed] [Google Scholar]
- 17.Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, Wright AC, Zemel B, Cucchiara A, Snyder PJ: Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res 20 :1785 –1791,2005 [DOI] [PubMed] [Google Scholar]
- 18.Jamal SA, Hayden JA, Beyene J: Low bone mineral density and fractures in long-term hemodialysis patients: A meta-analysis. Am J Kidney Dis 49 :674 –681,2007 [DOI] [PubMed] [Google Scholar]


