Abstract
We have shown previously that during branching morphogenesis of the mouse prostate gland, Bone morphogenetic protein 7 functions to restrict Notch1-positive progenitor cells to the tips of the prostate buds. Here, we employed prostate-specific murine bi-genic systems to investigate the effects of gain and loss of Notch function during prostate development. We show that Nkx3.1Cre and ProbasinCre alleles drive expression of Cre recombinase to the prostate epithelium and periepithelial stroma. We investigated the effects of gain of Notch function using the RosaNI1C conditional allele, which carries a constitutively active intracellular domain of Notch1 receptor. We carried out the analysis of loss of Notch function in Nkx3.1Cre/+;RBP-Jflox/flox prostates, where RBP-J is a ubiquitous transcriptional mediator of Notch signaling. We found that gain of Notch function resulted in inhibition of the tumor suppressor PTEN, and increase in cell proliferation and progenitor cells in the basal epithelium and smooth muscle compartments. In turn, loss of Notch/RBP-J function resulted in decreased cell proliferation and loss of epithelial and smooth muscle progenitors. Gain of Notch function resulted in an early onset of benign prostate hyperplasia by three months of age. Loss of Notch function also resulted in abnormal differentiation of the prostate epithelium and stroma. In particular, loss of Notch signaling and increase in PTEN promoted a switch from myoblast to fibroblasts lineage, and a loss of smooth muscle. In summary, we show that Notch signaling is necessary for terminal differentiation of the prostate epithelium and smooth muscle, and that during normal prostate development Notch/PTEN pathway functions to maintain patterned progenitors in the epithelial and smooth muscle compartments. In addition, we found that both positive and negative modulation of Notch signaling results in abnormal organization of the prostate tissue, and can contribute to prostate disease in the adult organ.
Keywords: prostate development, Notch, RBP-J, epithelium, smooth muscle, vimentin, PTEN, p63
Introduction
The prostate is a sex-accessory exosecretory gland in mammalian males. Prostate gland develops by branching morphogenesis from the epithelium and mesenchyme of the urogenital sinus (UGS), an enlarged portion of the urethra beneath the bladder. Adult prostate is composed of branched ducts, made of stratified epithelium, separated by the basal lamina from the fibroblast stroma and smooth muscle envelope. The luminal layer of prostate epithelium is characterized by expression of specific intermediate filaments, cytokeratin 8 (CK8) and CK18, contains secretory cells, and is androgen-dependent for cell survival and terminal differentiation (Marker et al., 2003; Wu et al., 2007). In turn, cells in the basal epithelial layer are characterized by expression of CK5 and CK14 (Marker et al., 2003). In addition, the basal epithelium contains rare neuroendocrine cells, expressing neural peptides, such as secretagogin, chromogranin, calcitonin gene-relater peptide and synaptophasin (Abate-Shen et al., 2000; Marker et al., 2003).
The prostate retains the ability to support cell replication, differentiation and morphogenesis throughout adult life, which suggests an abundance of pluripotent stem cells. Abnormal proliferation and transformation of the prostate stem and progenitor cells results in benign prostate hyperplasia and prostate cancers (Abate-Shen et al., 2000; Marker et al., 2003). Recent studies indicate that prostate gland contains at least two different stem cell niches, a “normal” stem cell” niche in the basal compartment (Leong et al., 2008; Xin et al., 2003), and another niche with a capability to generate “cancer stem cells” in the luminal compartment (Wang et al., 2009). Notably, regulation of cell fate choice in both normal and cancer prostate stem cells is susceptible to the evolutionary conserved Notch cell fate selection system (Artavanis et al., 1999; Yoon and Gaiano, 2005; Leong and Gao, 2008; Shahi et al., 2011).
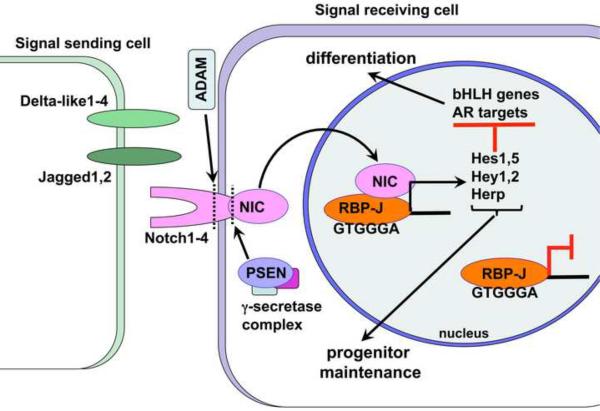
Recent studies by us and others underlined the importance of the Notch1 receptor and Notch ligands, Delta-like1, Jagged1 and Jagged 2, in prostate branching (Wang et al., 2004; Grishina et al., 2005) and epithelial differentiation (Wang et al., 2006). Other studies indicated a role for the Notch2 receptor and Delta-like1 ligand in survival and differentiation of the prostate stroma (Orr et al., 2009). Activation of the Notch receptor results in a γ-secretase dependent proteolytic cleavage of the Notch intracellular domain (NIC) and its nuclear translocation (Figure 1). In the nucleus, NIC forms a transcription complex with its DNA-binding co-factor, RBP-J/Supressor of Hairless/Lag-1/CBF-1, and promotes expression of the Hes/Hey/Herp transcriptional repressors (Artavanis et al., 1999; Belandia et al., 2005; Ohtsuka et al., 1999; Tanigaki et al., 2002; Yoon and Gaiano, 2005). In the absence of Notch signaling, RBP-J forms a complex with transcriptional repressors and prevents opportunistic expression of the Notch target genes (Zhou and Hayward, 2001). The Notch targets, the Hes/Hey/Herp factors, have been shown to repress transcription of the proneural basic helix-loop-helix transcription factors, Mash1, Math1 and Ngn3, which function to promote neural differentiation in the neural tube and forebrain (Yoon et al., 2005), as well as secretory differentiation in the pancreas and intestine (Fre et al., 2005; Murtaugh et al., 2003; Stanger et al., 2005; Yang et al., 2001). Their role in neuroendocrine differentiation in the lungs has been also suggested (Ito et al., 2000). Our studies and studies of other laboratories indicate that during prostate development, Notch signaling is mediated primarily by Hes1 and Hey1 (Grishina et al., 2005; Wang et al., 2004; Belandia et al., 2005). Hey1 has been shown to function as a co-repressor of the androgen receptor targets, indicating that inhibition of Notch signaling may be required for differentiation of the prostate secretory epithelium (Belandia et al., 2005; Wu et al., 2007). Importantly, abnormal regulation of the Notch pathway has been suggested to contribute to benign prostate tumors (Belandia et al., 2005), and may play a role in predisposition to prostate cancer (Whelan et al., 2009). In particular, studies indicate that signaling by the Notch receptor can affect proliferation and cell fate choice in prostate, and other, cancer cell lines by regulating transcription of the phosphatase and tensin homolog gene (PTEN) (Palomero et al., 2007; Salmena et al., 2008; Whelan et al., 2009). PTEN is a lipid and protein phosphatase, and a potent tumor suppressor which functions by antagonizing the phosphoinositide 3-kinase (PI3K)/Akt cell survival pathway. Notably, studies in cell lines pointed that Notch can regulate PTEN both positively and negatively depending on cellular context (Palomero et al., 2007; Salmena et al., 2008; Whelan et al., 2009). These findings make it particularly important to define the interactions between the Notch and PTEN/Akt pathways during normal prostate development, and during the initials stages of benign and malignant prostate tumors.
Figure 1. The Notch pathway.
Notch signaling between adjacent cells functions to diversify cell fate: selecting progenitor cells which will maintain pluripotency, and cells which will terminally differentiate. In mammals, signaling by the Notch 1–4 transmembrane receptors is activated by binding to the Delta-like1–4 and Jagged1,2 ligands, themselves transmembrane receptors on neighboring cells. Ligand-receptor binding promotes a series of cleavages, first, by the intracellular γ-secretase complex, to produce the Notch intracellular domain (NIC), then, by the ADAM metalloproteinase to remove the extracellular domain. Cleaved NIC translocates to the nucleus where it functions as a transcription factor together with its DNA-binding co-factor, RBP-J/Suppressor of Hairless/Lag-1/CBF-1. In the absence of Notch signaling RBP-J remains bound to the consensus sites and functions as a repressor for Notch target genes. NIC induces expression of the Hes/Hey/Herp family of transcriptional repressors which in turn suppress transcription of the patterning basic-loop-helix (bHLH) transcription factors, Mash1, Math1 and Neurogenin. In the prostate, Hey1 functions as a co-factor for the androgen receptor (AR) to repress AR targets.
In this study, we employed three prostate-specific Cre/LoxP systems to investigate the effects of gain and loss of Notch signaling in mouse models, during prostate formation in embryogenesis and during postnatal differentiation of the organ. We used the prostate-specific, Nkx3.1Cre and Probasin(PB)Cre4 drivers to express Cre recombinase in the embryonic and postnatal prostate. Nkx3.1 is a prostate specific homeobox gene which is expressed in the prostate epithelium from the earliest stages of prostate budding at the embryonic (E) day E15.5 (Bhatia-Gaur et al., 1999). PBCre4 drives Cre expression in the prostate epithelium and smooth muscle, postnatally, under a modified promoter for a rat prostate secretory protein (Wu et al., 2001). To investigate the effects of gain of Notch function, we used the conditional strain, RosaNotch1IC (RosaN1IC) (Murtaugh et al., 2003), carrying the constitutively active intracellular domain of the Notch1 receptor, N1IC. To investigate the effect of loss of Notch signaling, we used a floxed allele for the ubiquitous transcriptional mediator of Notch signaling, RBP-Jflox (Tanigaki et al., 2002). Our study uncovered intriguing similarities in Notch function in the prostate epithelium and adjacent stroma. We found that gain of Notch function during prostate development resulted in inhibition of PTEN, activation of the Akt cell survival pathway, and increased proliferation of the basal and myoblast progenitors leading to an early onset of benign prostate hyperplasia by three months of age. In turn, loss of Notch signaling resulted in upregulation of PTEN, loss of basal and myoblast progenitors, and abnormal cell differentiation in both epithelial and stromal compartments. Our studies point to the importance of precise regulation of Notch signaling during prostate development, and the role of Notch in the homeostasis of the adult organ.
Materials and Methods
Mouse lines and X-gal staining
All mouse studies were conducted in accordance with an animal protocol approved by the New York University School of Medicine Institutional Animal Care and Use Committee. All mice strains were maintained in C3H/HeJ background (Taconic, NY) unless otherwise indicated. Nkx3.1Cre strain is a gift from M. Shen (Columbia University). The Cre sequence is a knock-in resulting in a loss of Nkx3.1 function (Wang et al., 2006). Genotyping for Nkx3.1Cre allele was carried out by PCR with a forward, Cre-F: GCG CGG TCT GGC AGT AAA AAC, and a reverse Cre-R: CAG ATG GCG CGG CAA CAC C, primers, under following conditions: 94° C, 5 min; 38 cycles of 94° C for 15 sec, 58° C for 30 sec, 72° C for 1 min; then 72° C for 10 min. PBCre4 is a transgenic strain which drives expression of Cre-recombinase in postnatal prostate epithelium and in smooth muscle (Wu et al., 2001). Rosa26 is a Cre-reporter strain which carries a LacZ gene preceded by stop codon flanked by LoxP sites (Soriano, 1999). RBP-Jflox (Tanigaki et al., 2002) is a conditional loss of function allele of RBP-J, a ubiquitous transcriptional mediator of canonical Notch signaling. RBP-Jflox strain is maintained as homozygous in mixed SW/B16 background. RosaN1IC (Murtaugh et al., 2003) is a conditional transgene, containing a sequence for the Notch1 intracellular domain, N1IC. Embryonic (E) day 0.5 (E0.5) was designated as the day when vaginal plug was first observed, and day of birth was marked as postnatal (P) day 1 (P1). Sex of embryos, starting from E15.5, was determined by the characteristic positions of the sexually committed gonads. X-gal staining of the Nkx3.1Cre/+;Rosa26 and PBCre4/+;Rosa26 tissues has been performed as described previously (Grishina et al., 2005; Wu et al., 2009).
Tissue collection and immunofluorescent analysis
Urogenital sinuses including prostate tissues were dissected from male mice and embryos using Zeiss Stemi SV11 dissection microscope, rinsed in ice cold PBS and fixed in 4 % parafolmaldehyde for 1 hour, dehydrated and embedded in paraffin, and sectioned at 5 μm. For immunofluorescent labeling, sections were processed as described previously (Grishina et al., 2005), and incubated with primary antibodies over night at 4°C in the Tyromide Signal Amplification (Invitrogen, Carlsbad, CA) or Mouse On Mouse (Vector Laboratories, Burlingame, CA) blocking solutions, followed by incubation with Alexa Fluor secondary antibodies (Invitrogen, Carlsbad, CA), or processed with the TSA kit #23 kit (Invitrogen, Carlsbad, CA) according to manufactures instructions. Primary antibodies for Ki-67 (catalogue number15580, Abcam, Cambridge, MA) and phospho-Histone H3 (pHH3) (catalogue number 6570, Upstate Biotechnology, Billerica, MA) were used at 1:100 dilution, and antibodies for smooth muscle α-actin (α-sma) (Clone 1A4, DAKO, Glostrup, Denmark) and myosin (BT-562, BTI, Stoughton, MA) were used at 1:100 dilution, followed by Alexa Fluor secondary antibodies. Primary antibodies for PTEN (catalogue number 9559, Cell Signaling, Danvers, MA) and N1IC (catalogue number 2421, Cell Signaling, Danvers, MA) were used at 1: 100 dilution, and antibodies for CK8 (catalogue number14053, Abcam, Cambridge, MA), CK14 (catalogue number PRB-155P, Covance, Princeton, NJ), Bcl-2 (catalogue number 18210, Abcam, Cambridge, MA), p63 (catalogue number 3239, Abcam, Cambridge, MA), and phospho-Akt (pAkt) (catalogue number 4060, Cell Signaling, Danvers, MA) were used at 1:50 dilution with the Tyromide Signal Amplification kit # 23 (Invitrogen, Carlsbad, CA) as described (Grishina et al., 2005). Fluorescent imaging was performed using Zeiss Axioplan3.1 fluorescent microscope at the Urology Research Laboratories, and the LSW 510 confocal scope at the New York University School of Medicine Microscopy Core Facility.
Analysis of programmed cell death
Programmed cell death was evaluated on tissue sections by terminal deoxynucleotidyl transferase dUTP nick end labeling reaction (TUNEL) following the manufacturer's instructions (Roche Applied Science, Indianapolis, IN).
Quantitations and statistics
In embryonic tissues, calculations of the percentage ratios for the cells positive for TUNEL and immunohistochemical markers were carried out in comparison to the total cell number in the particular epithelial or stromal compartment identified by 4',6-diamidino-2-phenylindole (DAPI) staining of the nuclei. Cell counts were performed on twenty tissue sections, five each from five nonlittermate embryos of each genotype, unless otherwise indicated. In the postnatal prostate, cell counts for specific markers were carried out in five 106 μm2 quadrants on each of twenty sections from five non-littermate animals of each genotype, unless otherwise indicated. Measurements and cell counts were performed using Axiovision 2 (Carl Zeiss MicroImaging, Thornwood, NY) and Volocity 5.3.1 Software (Perkin Elmer, Waltham, MA). Statistical significance of the calculations was determined by the Student's t-test. P< 0.05 was considered significant.
Results
Nkx3.1Cre is expressed in epithelial and mesecnhymal prostate cells
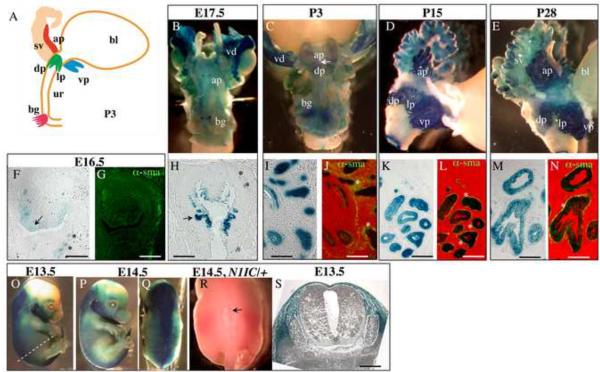
Nkx3.1 is a prostate-specific homeobox gene and tumor-suppressor (Bhatia-Gaur et al., 1999; Kim et al., 2002). In embryogenesis, transient Nkx3.1 expression has been also reported in the lungs, somites, duodenum and elements of the skeleton (Stanfel et al., 2006). First, we determined the domains of Cre recombinase expression in our Nkx3.1Cre strain (gift of M. Shen, Columbia). Nkx3.1Cre/+ males were mated with the Rosa26 females (Sorriano, 1999), and the resulting Nkx3.1Cre/+;Rosa26 embryos and postnatal prostates were analyzed for expression of the LacZ reporter (Fig. 2). LacZ expression was first detected in the UGS epithelium at E16.5 (Fig. 2F, arrow), and at E17.5, in the emerging buds of the prostate (Fig. 2B, H) and bulbourethral glands (Fig. 2B). In embryogenesis, LacZ expression was also present in a few cells in the periepithelial mesenchyme in between the urethra and smooth muscle (* in Fig. 2F–H, and in Supplemental Material Fig. S1A, B). At the postnatal stages, LacZ was expressed in all prostate lobes (Fig. 2C–E), in the luminal and basal epithelial layers (Fig. 1I–N), and also in the smooth muscle compartment (* in Fig. 2J, L, N and Supplemental Material Fig. S1C–F). This shows that the Nkx3.1Cre allele drives expression of Cre recombinase in the prostate epithelium and periepithelial mesenchyme starting from the earliest stages of prostate development (Fig. 2B, F–H). Thus, Nkx3.1Cre is suitable to manipulate gene function in the prostate epithelium and adjacent stroma. In this study, we employed the Nkx3.1Cre driver to analyze the effects of gain and loss of Notch function during embryonic prostate development using the Nkx3.1Cre/+; RosaN1IC/+ (Fig. 3) and Nkx3.1Cre/+;RBP-Jflox/flox systems (Fig. 6–10). We also found that Nkx3.1Cre/+;Rosa26 embryos show LacZ expression in the axial mesoderm (Fig. 2O–Q, and S), similar as described for an independently made Nkx3.1Cre strain (Stanfel et al., 2006). This is consistent with a transient expression of Nkx3.1 in the developing somites (Kos et al., 1998). In our experiments, ectopic activation of Notch1 signaling in the sclerotome in the Nkx3.1Cre/+;RosaN1IC/+ embryos resulted in lethality at birth due to incomplete closure of the neural tube (Fig. 2R). Thus, to analyze the effect of gain of Notch function in the postnatal prostate (Fig. 4–5), we employed the PBCre4 strain, which has been reported to drive expression of Cre in the prostate epithelium and smooth muscle, postnatally (Wu et al., 2001). PBCre4/+;RosaN1IC/+ and Nkx3.1Cre/+;RBP-Jflox/flox males were viable, fertile, and showed no gross morphological defects which allowed us to analyze the effects of gain (Fig. 4–5) and loss of Notch function (Fig. 6–10) in the postnatal and adult prostates.
Figure 2. LacZ expression in the Nkx3.1Cre/+; Rosa26 prostates and embryonic axial mesoderm.
(A) Schematic representation of the male urogenital system at P3: bl, bladder; ur, urethra; sv, seminal vesicles; bg, bulbourethral gland; ap, anterior prostate; dp, dorsal prostate lobes; lp, lateral prostate lobes; vp, ventral prostate. (B–N) Nkx3.1Cre;Rosa26 UGS and prostate tissues were stained with X-gal (B–E), sectioned (F–N), and immunolabeled with α-sma (G, J, L, N). Analysis in whole mount at E17.5, P3, P15 and P28 showed LacZ expression in all prostate lobes (C–E) and in the bulbourethral gland (B–C). (F–H, dorsal side is up) In embryogenesis, at E16.5 and E17.5, LacZ expression was detected in the UGS epithelium (arrow in F), in the prostate buds (arrow in H), and in the mesenchyme in between the UGS epithelium and smooth muscle (* in F and H; and in Supplemental Material Fig. S1A, B). (I–N) Sections of Nkx3.1Cre;Rosa26 prostates at P3 (I, J), P15 (K, L) and P28 (M, N) show LacZ expression in the basal and luminal prostate epithelium, and also in the periductal smooth muscle (* in J, L, N, and in Supplemental Material Fig. S1C–D). (O–Q, and S) Analysis of Nkx3.1Cre/+;Rosa26 embryos at E13.5 and E14.5, also showed activity of the Nkx3.1 promoter in the axial mesoderm. Nkx3.1Cre/+;Rosa26 embryos were stained with X-gal in whole mount (O–Q), and sectioned (S) at a position indicated with dashed line in (O). (R) Dorsal view of a Nkx3.1Cre/+;RosaN1IC/+ (N1IC/+) embryo at E14.5 showing incomplete closure of the neural tube (arrow). Scale bars: 50 μm.
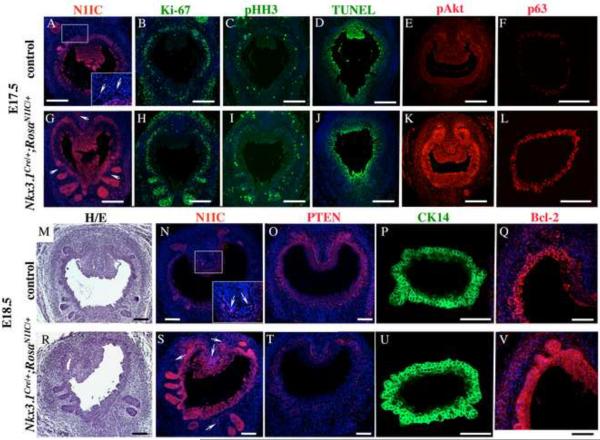
Figure 3. Gain of Notch function in embryogenesis causes downregualtion of PTEN, and increase in cell proliferation and basal progenitors.
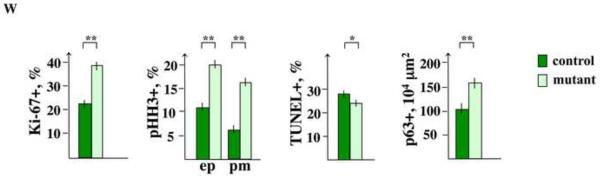
(A–V) Nkx3.1Cre/+;RosaN1IC/+ (N1IC/+) and control, Nkx3.1+/+;RosaN1IC/+, prostate sections at E17.5 (A–L) and E18.5 (M–V) were stained with hematoxylin and eosin (M, R), and labeled for N1IC (A, G, N, S), Ki-67 (B, H), pHH3 (C, I), pAkt (E, K), p63 (F, L), PTEN (O, T); CK14 (P, U), and Bcl-2 (Q, V). Scale bars, 100 μm. (A, G, N, S) Insets in (A) and (N) show high magnification images of the UGS mesenchyme in the framed areas. Arrows indicate N1IC-positive cells in the mesenchyme. (X) Graphs show percentage ratios of cells in the wild-type (dark bar) and N1IC/+ (light bar) UGS epithelium at E17.5 positive for Ki-67, TUNEL assay. Percentage ratio of cells in epithelium (ep) and periepithelial mesenchyme (pm) positive for pHH3. Number of p63+ cells per 104 μm2. For each experiment, positive cells were counted on twenty UGS sections five sections from four non-littermate embryos of each genotype using Volocity 5.3.1 software. Student's t-test; horizontal bars, standard mean; horizontal lines, standard errors; *P < 0.05; ** P < 0.01.
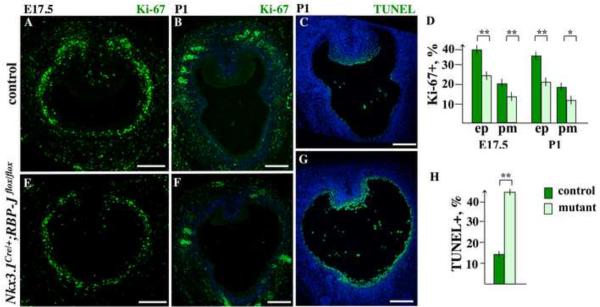
Figure 6. Loss of RBP-J results in decreased cell proliferation and survival in the embryonic and P1 UGS.
(A–C, E–F) Nkx3.1Cre/+;RBP-Jflox/flox and control, Nkx3.1+/+;RBP-Jflox/flox, UGS sections at E17.5 and P1 were labeled for Ki-67 (A and E, and B and F), and processed for TUNEL assay (C, G). Scale bars: 100 μm. (D) Percentage ratios for Ki-67+ (D) cells were calculated separately in the UGS epithelium (ep) and periepithelial mesenchyme (pm), on sixteen sections, four each from four embryos of mutant and control genotype, at E17.5 and P1. (H) TUNEL+ cells in the UGS epithelium were calculated same as described above. Student's t-test; horizontal bars, standard mean; horizontal lines, standard errors; *P < 0.05; ** P < 0.01.
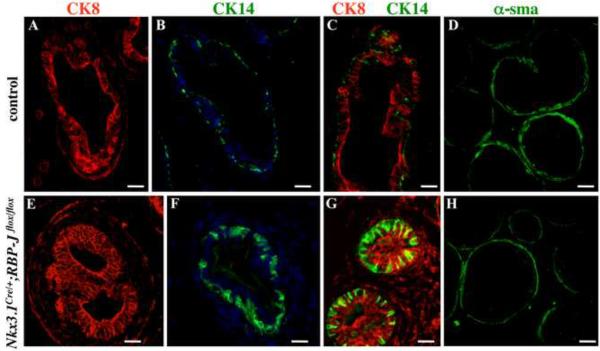
Figure 10. Conditional RBP-J null prostates at P21 show abnormal basal epithelium and loss of smooth muscle.
Sections of P21 control (A–D) and Nkx3.1Cre/+;RBP-Jflox/flox anterior prostates (E–H) were labeled for CK8 (A, C and E, G), CK14 (B, C and F, G) and α-sma (D, H). Scale bars, 12 μm.
Figure 4. Gain of Notch function results in overproliferation of basal epithelium and myoblasts in P7–P14 prostates.
(A) P14 ventral PBCre4/+;Rosa26 prostate stained with X-gal and immunolabeled for α-sma shown in bright light (upper panel), and with fluorescent and bright light (lower panel). LacZ expression is detected in the prostate epithelium and periductal smooth muscle. (B, H) Sections of P14 control, PB+/+;RosaN1IC/+, (B) and PBCre4/+;RosaN1IC/+ (H) ventral prostate lobes, labeled for N1IC. (H) N1IC is upregulated in the PBCre4/+;RosaN1IC/+ prostate epithelium (arrowhead) and smooth muscle (arrow). (C, I) Sections of P7 wild-type (C) and PBCre4/+;RosaN1IC/+ (I) ventral prostates, labeled for p63, show increase in p63+ cells in the N1IC/+ basal epithelium. (D, J) Sections of P14 control (D) and PBCre4/+;RosaN1IC/+ (J) ventral prostates, labeled for CK14, show increase in CK14+ cells. (E, K) Sections of P14 control (E) and PBCre4/+;RosaN1IC/+ (K) ventral prostates, labeled for Ki-67. (F–G, L–M) Sections of P14 control (F–G) and PBCre4/+;RosaN1IC/+ (L–M) ventral prostates, labeled for α-sma. (G) and (M) show higher resolution images of the framed areas in (F) and (L), respectively. Scale bars, 25 μm. (N) Percentage ratios of p63+ cells in control (dark bar) and PBCre4/+;RosaN1IC/+ (light bar) ventral prostate ducts, counted at P7. Percentage ratios of CK14+ cells in PBCre4/+;RosaN1IC/+ and control ventral prostates ducts, counted at P14. Percentage ratios of Ki-67+ cells calculated in the epithelium (ep) and periepithelial mesenchyme (pm). The number (N, sml) and thickness (μm, sml) of periductal smooth muscle layers measured in the control and mutant prostates. Cell counting and measurements were carried out on 20 transverse prostate sections, 4 each from 5 prostates of each genotype. Student's t-test: *P < 0.05, ** P < 0.01.
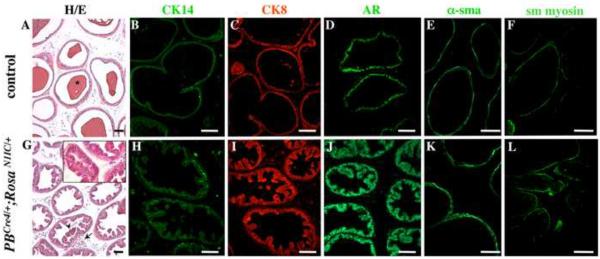
Figure 5. Hyperplastic epithelium and stroma in three month old PBCre4/+;RosaN1IC/+ ventral prostates.
Sections of 3 month old PBCre4/+;RosaN1IC/+ (G– L) and control (A–F) ventral prostates were stained with hematoxilyn and eosin (A, G and a high magnification inset) and immunolabeled for CK14 (B, H), CK8 (C, I), AR (D, J), and smooth muscle (sm) α-actin (E, H) and myosin (F, L). (A) Control prostates show normal tissue histology and accumulation of prostate secretions in the lumen (A, *). (G) PBCre4/+;RosaN1IC/+ ventral prostates lack secretory function, contain detached epithelial aggregates in the lumen (arrowhead), and show fibro-muscular nodules within prostate stroma (arrow). PBCre4/+;RosaN1IC/+ prostate ducts show increased number of CK14+ basal cells (H compare to B); a hyperplastic luminal epithelium (G and inset, I and J, compare to A, C and D); and thicker smooth muscle envelopes (E, K and F, L). Scale bars: 50 γm.
Gain of Notch function in embryonic prostates results in increased cell proliferation and survival
To determine the effect of gain of Notch function during the initial stages of prostate formation and outgrowth of the primary buds, UGS sections were obtained from Nkx3.1Cre/+;RosaN1IC/+ male embryos at E17.5 (Fig. 3A–L) and E18.5 (Fig. 3M–V). To determine the sites of endogenous and transgenic Notch1 activity in the developing prostate, we used antibodies to the intracellular domain of Notch1 (N1IC) cleaved at Valine 1744 (#2421, Cell Signaling, Danvers, MA) which only detect the activated form of Notch1 receptor indicative of Notch signaling. In wild-type E17.5 (Fig. 3A) and E18.5 UGS (Fig. 3N), endogenous Notch1 activity was detected in the UGS epithelium, in the forming prostate buds (Fig. 3A and N), and in a few cells in the urogenital mesenchyme (arrows in insets in Figs. 3A, N). Our previous studies (Grishina et al., 2005) also showed endogenous Notch1 activity, and expression of Hes1, in the embryonic UGS epithelium and mesenchyme. In turn, Nkx3.1Cre/+;RosaN1IC/+ embryos showed significantly higher levels of N1IC in the UGS epithelium, emerging prostate buds, and in the mesenchyme (Fig. 3G compare to A, and S compare to N). This robust increase in the N1IC protein in the Nkx3.1Cre/+;RosaN1IC/+ prostates is a further confirmation that Nkx3.1Cre efficiently drives expression of Cre-recombinase, and the N1IC transgene, in the UGS and prostate epithelium, and in some cell populations in the mesenchyme.
We further examined E17.5 Nkx3.1Cre/+;RosaN1IC/+ prostates for cell proliferation and survival by immunolabeling for Ki-67 (Fig. 3B, H), phospho-histone H3 (pHH3) (Fig. 3C, I), and carrying out a TUNEL assay (Fig. 3D, J). Calculations of percentage ratios for cells positive for Ki-67, pHH3 and TUNEL, were carried out in comparison to the total number of cells, identified by the DAPI staining of the nuclei in the embryonic UGS, or in the mesenchyme within 25 μm of the epithelium using Volocity 5.3.1 Software. We found that E17.5 Nkx3.1Cre/+;RosaN1IC/+ UGS epithelium contained approximately 17 % more Ki-67+ cells (P< 0.01) (Fig. 2X, and B, H), and 9 % (P<0.01) more pHH3+ mitotic cells (Fig. 3W, and C, I) then the control tissues. In addition, we found a 10 % increase in mitotic cells (P<0.01) in the Nkx3.1Cre/+;RosaN1IC/+ periepithelial mesenchyme (Fig. 3W, and C, I). Quantitation of the TUNEL+ epithelial cells in the Nkx3.1Cre/+;RosaN1IC/+ and control UGS epithelium (Fig. 3W and D, J) showed only a modest 4 % (P<0.02) decrease in programmed cell death. These results show that gain of Notch function in the embryonic prostates results in a 10 % increase in cell proliferation in the epithelial and mesenchymal compartments.
Gain of Notch function in the embryonic prostate results in downregulation of PTEN, and increase in phosphorylation of Akt
PTEN is a lipid and protein phosphatase, which functions as a tumor suppressor by antagonizing the PI3K/Akt cell survival pathway and inhibiting the cell divisions cycle (Salmena et al., 2008). Thus, we asked whether Notch signaling functions to favor cell survival and proliferation by limiting levels of PTEN. We detected significant levels of PTEN protein in the normal E18.5 UGS epithelium and adjacent mesenchyme (Fig. 3O). In contrast, in E18.5 Nkx3.1Cre/+;RosaN1IC/+ UGS, PTEN protein was absent in the epithelium, an only present in a few cells in the mesenchyme (Fig. 3T compare to O). Nkx3.1Cre/+;RosaN1IC/+ UGS, analyzed at E17.5 and E18.5, also showed higher levels of phospho-Akt in the epithelium and mesenchyme (Fig. 3K compare to E, and data not shown), and an increase in the phospho-Akt target, the cell survival factor, Bcl-2 (Fig. 3Q, V). These results show that induction of Notch signaling in the embryonic UGS results in downregulation of PTEN and upregulation of the Akt/Bcl2 survival pathway.
Gain of Notch function results in increased number of p63+ progenitors in the embryonic prostate
Several studies indicate that transcription factor p63 marks a basal prostate cell population enriched for stem and progenitor cells (Signoretti et al., 2005; Xin et al., 2007; Shahi et al., 2011). We found that gain of Notch function in embryonic prostate resulted in higher rates of cell divisions in the basal UGS epithelium (Fig. 3H and B, and I and C). Thus, we asked whether this increase in cell proliferation would specifically affect the basal progenitor cells. Indeed, Nkx3.1Cre/+;RosaN1IC/+ E17.5 UGS showed a substantial 52 % increase in the number of p63+ epithelial progenitors (P<0.01) compare to wild-type tissue (Fig. 3W, and L and F). This coincided with increased expression of the basal CK14 in E18.5 Nkx3.1Cre/+;RosaN1IC/+ UGS (Fig. 3U, P), and a prominent epithelial stratification.
Gain of Notch function results in increased rates of cell proliferation in the postnatal prostate
Previous analysis of the PBCre4/+;Rosa26 reporter system (Wu et al., 2001) showed LacZ expression in all prostate lobes from P1 to 8 weeks of age. Wu and colleagues (2001) also performed histological analysis of X-gal stained prostate sections to show Cre expression in the periductal smooth muscle (Wu et al., 2001). Our analysis of sections of P14 PBCre4/+;Rosa26 ventral prostates, X-gal stained and immunolabeled for α-sma, confirmed LacZ expression in the prostate epithelium and in adjacent smooth muscle (Fig. 4A). Further examination of the PBCre4/+;RosaN1IC/+ ventral prostates showed a significant increase in the N1IC signal in the ductal epithelium (Fig. 4H and B), prostate buds (arrowhead in Fig. 4H), and smooth muscle (arrows in Fig. 4H, and in Supplemental Material Fig. S1E, F). We further examined whether gain of Notch function affected cell fate choice in the epithelium or stroma. We first examined whether gain of Notch function in postnatal prostates resulted in increased cell proliferation. We labeled twenty prostate sections from P14 PBCre4/+;RosaN1IC/+ and control, PB+/+;RosaN1IC/+ prostates, for Ki-67, and counted the number of Ki-67+ cells in five 106 μm2 quadrants on each section in the ventral (Fig. 4E, K and O), anterior, dorsal and lateral prostate lobes (not shown). We found a 22 % (P<0.01) increase in Ki-67+ cell in the epithelium of P14 PBCre4/+;RosaN1IC/+ ventral prostates, and approximately 10 % (P<0.05) increase in the proximal mesenchyme (Fig. 4N). Similar increase in the rates of cell proliferation was observed in the anterior, dorsal and lateral lobes (data not shown).
Gain of Notch function results in increase in the basal and smooth muscle progenitor cells in the postnatal prostate
To determine whether gain of Notch activity in the postnatal prostate affects the p63+ cell population we performed immunolabeling of prostate sections from P7 PBCre4/+;RosaN1IC/+ and control, PB+/+;RosaN1IC/+males (Fig. 4C, I, ventral lobe is shown). We found a 7.6 % (P<0.01) increase in the p63+ population within the ventral prostate lobes (Fig. 4N, and C, I). PBCre4/+;RosaN1IC/+ ventral prostates also showed a 12 % (P < 0.01) increase in CK14+ basal cells at P14 (Fig. 4N, and D, J). The anterior, lateral and dorsal PBCre4/+;RosaN1IC/+ prostate lobes showed a similar increase in p63+ and CK14+ cells (data not shown). Interestingly, we found that P14 PBCre4/+;RosaN1IC/+ ventral ducts were surrounded by an abnormally thick layer of smooth muscle (Fig. 4L, M, compare to F and G). Wild-type P14 ventral ducts have a 1.6 - 2.0 layers of smooth muscle (Fig. 4N). In contrast, P14 PBCre4/+;RosaN1IC/+ ducts had 2.9 - 3.1 (P<0.01) layers of smooth muscle cells (Fig. 4N). Moreover, PBCre4/+;RosaN1IC/+ smooth muscle envelopes were on average 60% thicker then in the control prostates (Fig. 4N, and G compare to M). Measurements conducted using Axioplan 2 (Carl Zeiss MicroImaging) software showed that control P14 ventral ducts were surrounded by a 3.0 - 4.5 μm of smooth muscle (Fig. 4N, and F, G). In contrast, PBCre4/+;RosaN1IC/+ smooth muscle envelopes were 5.5 - 6.7 μm thick (P<0.01) in the ventral lobe (Fig. 4N, and L, M). The anterior, lateral and dorsal lobes showed a similar increase in smooth muscle mass (not shown). Furthermore, high resolution imaging of α-actin filaments and chromatin labeling with DAPI showed that at P14, PBCre4/+;RosaN1IC/+ smooth muscle layer contained cells with rounded morphology and nuclei shape (Fig. 4M), suggesting that these cells are immature myoblasts. In contrast, wild-type prostate ducts were surrounded by tightly packed myocytes with condensed elongated nuclei (Fig. 4G and F). PBCre4/+;RosaN1IC/+ myoblasts also showed a looser arrangement of the actin filaments (Fig. 4M) compared to the tightly packed actin bundles in wild-type smooth muscle (Fig. 4G). Thus, gain of Notch function resulted in a delay in myoblasts to myocyte differentiation. In summary, our studies show that gain of Notch function in the postnatal prostate results in increase in progenitor cells in the basal and smooth muscle compartments.
Gain of Notch function results in an early onset of prostate hyperplasia
We next asked whether gain of Notch activity in postnatal prostates may lead to pathological conditions, such as benign hyperplasia or intraepithelial neoplasia, in the adult. To examine the effect of gain of Notch function on cell differentiation and tissue integrity in the adult gland, we obtained prostate tissues from six three month old PBCre4/+;RosaN1IC/+ and control males, and analyzed tissue histology and differentiation of the epithelium and smooth muscle in all prostate lobes (Fig. 5, ventral prostate is shown). Control prostates showed normal histology and accumulation of prostate secretions in the lumen (Fig. 5A, *). In contrast, ventral and lateral PBCre4/+;RosaN1IC/+ ducts lacked prostate secretions (Fig. 5G, and data not shown), and showed extensive hyperplasia in the epithelial and stromal compartments (Fig. 5G–L compare to A–F) in all mutant animals studied (6:0). Immunolabeling for CK14, showed hyperproliferation of the basal epithelium in PBCre4/+;RosaN1IC/+ prostates (Fig. 5H compare to B). Examination of histological sections (Fig. 5A, G and inset) and immunolabeling for the luminal markers, CK8 (Fig. 5C, I) and androgen receptor (Fig. 5D, J), revealed that PBCre4/+;RosaN1IC/+ prostates also developed hyperplastic luminal epithelium which folded into internal crypts (Fig. 5G and inset, IJ). We noted numerous cell aggregation and occasional bridging in the PBCre4/+;RosaN1IC/+ luminal crypts (Fig. 5G and inset, I, J), and detached epithelial aggregates in the lumen (Fig. 5G, arrowhead). PBCre4/+;RosaN1IC/+ luminal compartment showed normal levels of CK8 (Fig. 5C, I) and androgen receptor (Fig. 5J compare to D). Thus, gain of Notch function resulted in overproliferation of the basal and luminal prostate epithelium. Gain of Notch function did not affect specification of the luminal epithelium, but inhibited secretory differentiation in the ventral and lateral prostate lobes. Next, we examined the effect of gain of Notch function on the organization and differentiation of the stromal compartment in the adult prostate. Sections of three month old PBCre4/+/+;RosaN1IC/+ and control prostates were analyzed for histology (Fig. 5A, G), and by immunolabeling for the myoblast marker,α-sma (Fig. 5K compare to E), and smooth muscle myosin (Fig. 5L compare to F), which is expressed in mature myocytes. Three month old PBCre4/+;RosaN1IC/+ prostates showed thick smooth muscle envelopes with excess of α-sma+ myoblasts (Fig 5K, E). PBCre4/+;RosaN1IC/+ prostates also contained numerous fibro-muscular nodules in the stroma (Fig. 5G, arrow). These stromal conditions are also typical for benign prostate hyperplasia in men. PBCre4/+;RosaN1IC/+ ventral (Fig. 5) and lateral prostate lobes (not shown) showed essentially identical characteristics of benign prostate hyperplasia in the organization of the epithelial and stromal compartments. The anterior and dorsal lobes showed similar levels of excess basal and myoblast cell proliferation, but less extensive cell proliferation in the luminal compartment, and retained secretory activity (not shown). In summary, our results show that gain of Notch function in postnatal prostates resulted in an early onset of benign prostate hyperplasia by three months of age. In particular, we show overproliferation of the basal, luminal and smooth muscle lineages in the PBCre4/+;RosaN1IC/+ model, consistent with human disease.
Loss of Notch/RBP-J function results in decreased cell proliferation and survival
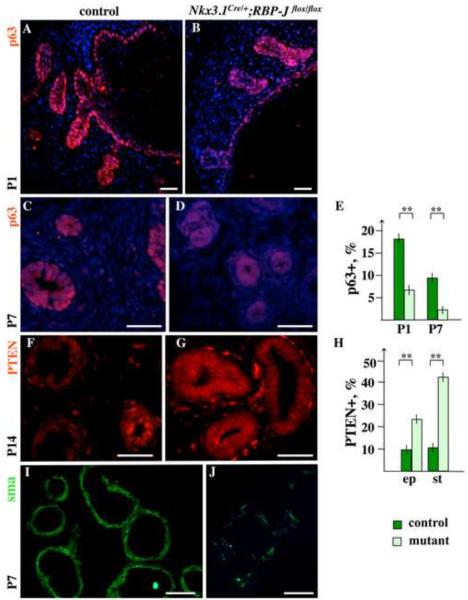
To investigate the effect of loss of Notch signaling during prostate development, we employed the Nkx3.1Cre/+;RBP-Jflox/flox bigenic system, where the ubiquitous transcriptional mediator of Notch, RBP-J, is inactivated in the epithelial and mesenchymal prostate cells where Nkx3.1 is expressed (Fig. 2 and Supplemental Material Fig. S1). Nkx3.1Cre/+;RBP-Jflox/flox males showed no apparent morphological abnormalities, developed to adulthood, and were fertile. Thus, Nkx3.1Cre/+;RBP-Jflox/flox mice and embryos for this study were obtained by mating Nkx3.1Cre/+;RBP-Jflox/flox males to RBP-Jflox/flox females, and progeny were genotyped for the Nkx3.1Cre allele by PCR. We first determined whether loss of RBP-J resulted in altered cell proliferation at the early stages of prostate development, at E17.5 and P1 (Fig. 6A–B, D–F). UGS sections from E17.5 (Fig. 6A, E) and P1 (Fig. 6B, F) Nkx3.1Cre/+;RBP-Jflox/flox and control, Nkx3.1+/+;RBP-Jflox/flox, littermates were labeled for Ki-67' and. Ki-67+ cells quantified separately in the UGS epithelium (Fig. 6D, ep) and within 25 μm of the periepithelial mesenchyme (Fig. 6D, pm). We found that loss of Notch/RBP-J function resulted in lower levels of cell proliferation in embryonic and P1 UGS epithelium and mesenchyme (Fig. 6D, and A compare to E, and B compare to F). At E17.5, Nkx3.1Cre/+;RBP-Jflox/flox UGS epithelium showed 14.6 % (P<0.01) fewer cycling cells then control tissue (Fig. 6D, and A, E). At P1, 15.6 % (P<0.01) fewer dividing cells were found in the mutant prostate tips compared to controls (Fig. 6D, and B, F). In the Nkx3.1Cre/+;RBP-Jflox/flox periepithelial mesenchyme, cell proliferation was 6.5 % (P<0.01) lower at E17.5 (Fig. 6D, and A, E), and 6.2 % (P<0.05) lower at P1 (Fig. 6D, and B, F). We then determined whether loss of RBP-J resulted in altered cell survival by carrying out TUNEL analysis at P1 (Fig. 6C, G, H). We found that loss of Notch/RBP-J function resulted in a significant decrease in cell survival in the UGS epithelium. Specifically, Nkx3.1Cre/+;RBP-Jflox/flox P1 prostates contained 30 % (P<0.01) more apoptotic nuclei in the UGS epithelium then control tissues (Fig. 6H, and C, G).
Loss of RBP-J resulted in defects in stratification in the UGS epithelium
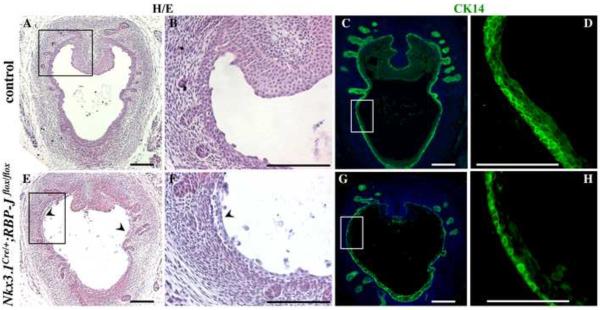
Histological analysis of prostate sections at P1 showed no significant difference in the number of the initial prostate buds in the Nkx3.1Cre/+;RBP-Jflox/flox and control prostates (Fig. 7A, C and E, G). Thus, Notch/RBP-J function is not required for specification of the prostate lobes. However, we found severe defects in organization of the Nkx3.1Cre/+;RBP-Jflox/flox UGS epithelium (Fig. 7). Normally, at the embryonic and early postnatal stages, urethral epithelium is multilayered and pseudostratified (Marker et al., 2003). This was confirmed by histological analysis (Fig. 7A–B), and labeling of the wild-type P1 UGS for CK14 (Fig. 7C,D). In contrast, Nkx3.1Cre/+;RBP-Jflox/flox UGS epithelium consisted of a single layer of cuboidally shaped cells (Fig. 7H). Nkx3.1Cre/+;RBP-Jflox/flox UGS epithelium also showed significant shedding of cells into the lumen (Fig. 7E, F arrowheads) consistent with increased levels of cell death (Fig. 6G, H). This data indicates that loss of Notch/RBP-J function resulted in significant defects in differentiation and organization of the UGS epithelium.
Figure 7. Loss of RBP-J results in defects in organization of the UGS epithelium.
(A–H) Nkx3.1Cre/+;RBP-Jflox/flox and control littermate UGS sections at P1 were stained with eosin and hematoxylin (A, E, and higher magnification images of the framed areas in B and F), and immunolabeled for CK14 (C, G, and higher magnification images of the framed areas in D and H). (E–H) Nkx3.1Cre/+;RBP-Jflox/flox UGS showed simple cuboidal epithelium (H), and delamination of cells into the lumen (arrowheads). Scale bars: 100 μm.
Loss of Notch/RBP-J function results in increased levels of PTEN, and defects in maintenance of the basal and myoblast progenitors
We further determined whether loss of RBP-J affected maintenance of epithelial and smooth muscle progenitors in the postnatal prostate. Immunolabeling for p63 showed that Nkx3.1Cre/+;RBP-Jflox/flox prostates rapidly loose p63+ basal cells postnatally (Fig. 8A–E). At P1, Nkx3.1Cre/+;RBP-Jflox/flox prostate ducts showed about 59 % (P<0.01) lower content of p63+ epithelium when compared to control tissues (Fig. 8A, B, E). At P7, Nkx3.1Cre/+;RBP-Jflox/flox epithelium contained only 28 % (P<0.01) of the normal p63+ cells (Fig. 8C–E). Further, we found that Nkx3.1Cre/+;RBP-Jflox/flox prostates, analyzed from P1 to P14 consistently showed about 14 % (P<0.01) more PTEN+ cells in the prostate epithelium, and about 32 % (P<0.01) more PTEN+ cells in the periductal mesenchyme (Fig. 8F–H, and data not shown). The major function of PTEN is to negatively regulate the PI3K/Akt cell survival pathway (Salmena et al., 2008). Thus, the increase in PTEN in the Nkx3.1Cre/+;RBP-Jflox/flox prostate epithelium is likely to account for the decreased survival and proliferation of the p63+ cells. Further examination of Nkx3.1Cre/+;RBP-Jflox/flox periductal stroma at P7 showed significantly lower content of α-sma+ cells, as well as discontinuous smooth muscle envelopes in the distal regions of the gland (Fig. 8J compare to I). We also found that within prostate stroma, high levels of PTEN are present only in vimentin+ cells, namely, in vimentin+ α-sma− fibrobasts and vimentin+ α-sma+ myofibroblasts (Supplemental Material Fig. S2). In contrast, vimentin−α-sma+ myoblasts and myocytes were negative for PTEN (Fig. S2B, D). Thus, loss of RBP-J, and a resulting increase in PTEN in the prostate stroma results in a shift from the myoblast to fibroblast lineage.
Figure 8. Loss of RBP-J results in increased levels of PTEN, and loss of basal and smooth muscle cells in postnatal prostates.
(A–E) Immunolabeling shows a reduction of p63-positive basal epithelium in Nkx3.1Cre/+;RBP-Jflox/flox ventral prostates (B and D) compare to the control Nkx3.1+/+;RBP-Jflox/flox littermates at P1 (A) and P7 (C). (E) Percentage ratios of p63+ cells in the control (dark bar) and Nkx3.1Cre/+;RBP-Jflox/flox (light bar) prostate epithelium at P1 and P7. Student's t-test; horizontal bars, standard mean; horizontal lines, standard errors; ** P < 0.01. (F–H) Immunolabeling of control (F) and Nkx3.1Cre/+;RBP-Jflox/flox(G) sections at P14 shows increased levels of PTEN in Nkx3.Cre/+;RBP-Jflox/flox epithelial ducts and periductal stroma. (H) Percentage ratios of PTEN+ cells in P14 wild-type (dark bar) and Nkx3.1+/+;RBP-Jflox/flox (light bar) prostate epithelium (ep) and periductal stroma (st). (I, J) Labeling for α-sma shows reduced number of myoblasts surrounding distal Nkx3.1Cre/+;RBP-Jflox/flox prostate ducts (J) compared to the control littermate tissue (G). Scale bars: 25 μm. See also Supplemental Material Fig. S2 for double-label analysis of Nkx3.1Cre/+;RBP-Jflox/flox prostate for PTEN/α-sma and PTEN/vimentin.
Loss of Notch/RBP-J function results in abnormal organization of the ductal epithelium and smooth muscle
We next examined young adult Nkx3.1Cre/+;RBP-Jflox/flox prostates at P21 and P28 for abnormalities in tissue organization and cellular composition (Fig. 9–10). Histological analysis of Nkx3.1Cre/+;RBP-Jflox/flox prostate sections at P28 (Fig. 9B) revealed distended ductal lumens and abnormally thin epithelium in 80 % (P<0.01) of ventral prostate ducts, and in 67 % (P<0.01) of the lateral ducts. In addition, Nkx3.1Cre/+;RBP-Jflox/flox ventral and lateral prostate ducts showed thin and discontinuous smooth muscle envelopes (Fig. 9B, arrowhead; and Fig. 10H). Analysis of epithelial differentiation, conducted at P21, showed that Nkx3.1Cre/+;RBP-Jflox/flox prostate ducts failed to compartmentalize the CK8+, basal, and CK14+, luminal, epithelium in all prostate lobes (Fig. 10A–C, E–G, anterior prostate is shown). Nkx3.1Cre/+;RBP-Jflox/flox prostates also showed significantly reduced epithelial secretions in all prostate lobes (Fig. 9, and data not shown). These findings are consistent with defects in epithelial differentiation in cNotch1 null prostates (Wang et al., 2006). In the normal differentiated prostate ducts, CK14 is expressed in the basal epithelium, and is absent from the luminal compartment (Fig. 10B, C). In turn, CK8 is expressed exclusively in the luminal epithelium (Fig. 10A, C). In contrast, in the Nkx3.1Cre/+;RBP-Jflox/flox ducts most CK8+ cells co-express CK14 (Fig. 10E–G). In wild-type prostate ducts, CK14+ basal cells have a characteristic elongated morphology and attach along the basal membrane (Fig. 10B). In contrast, Nkx3.1Cre/+;RBP-Jflox/flox ducts contained large CK14+ cells that extended towards the lumen (Fig. 10F). Thus, loss of Notch/RBP-J function in the developing prostate resulted in defects in specification of the basal and luminal prostate epithelium, and the smooth muscle cells.
Figure 9. Conditional RBP-J null prostates show distended ductal lumens, and thin epithelium and smooth muscle at P28.
Histological analysis of P28 control (A) and Nkx3.1Cre/+;RBP-Jflox/flox (B) prostate sections shows larger ductal lumens in the mutant ventral (VP) and lateral (LP) prostate ducts. P28 Nkx3.1Cre/+;RBP-Jflox/flox prostate ducts (B) have thinner ductal epithelium (arrows) and smooth muscle envelopes (arrowheads), and lower stromal content (*) then control tissues (A). Transverse P28 UGS sections were stained with eosin and hematoxylin. Dorsal side is up. Scale bars: 100 μm.
Discussion
We and other laboratories have shown the importance of the Notch cell fate selection system in regulating prostate growth, regeneration and epithelial differentiation (Grishina et al., 2005; Wang et al., 2004; 2006). Notch signaling is also required for the maintenance of epithelial progenitors in the pancreas, intestine and mammary gland (Buono et al., 2006; Fre et al., 2005; Murtaugh et al., 2003; Stanger et al., 2005). In this study, we employed conditional murine models to systematically investigate the role of Notch signaling in development of the prostate epithelium and stroma.
Our analysis of gain and loss of Notch function indicates that during normal development, Notch signaling inhibits accumulation of the tumor suppressor PTEN in the UGS and prostate epithelium, and in the adjacent stroma. Notch-dependent inhibition of PTEN has been reported to regulate cell survival and proliferation in T-cell leukemias (Palomero et al., 2007). In contrast, Notch has been suggested to upregulate PTEN in Du145 and 22Rv1 prostate cancer cell lines (Whelan et al., 2009). Our studies in conditional murine models strongly indicate that during normal prostate development Notch signaling inhibits accumulation of PTEN, and supports maintenance of the patterned progenitor cells in the epithelial and smooth muscle compartments. We show that gain of Notch function in the embryonic prostates results in a significant decrease in the levels of PTEN, and increase in the active phosphorylated form of Akt, and its target, Bcl2, both in the prostate epithelium and mesenchyme. Consistent with the effects of gain of Notch function, loss of Notch/RBP-J resulted in upregulation of PTEN, and decreased rates of cell division and survival in the prostate epithelium and smooth muscle.
Next, our findings strongly indicate a positive role for Notch signaling in maintenance of the p63+ basal progenitors (Kurita et al., 2004; Signoretti et al., 2005). Gain of Notch function resulted in formation and maintenance of a 50 % larger population of basal progenitors in the urogenital sinus. Gain of Notch function also resulted in a higher rates of epithelial cell divisions throughout development, and an increased mass of basal and luminal epithelial cells in the adult prostates. This resulted in the early onset of prostate hyperplasia at three months of age manifested in increased mass of the basal and luminal epithelium. Interestingly, our results indicate that gain of Notch signaling does not prevent specification of the luminal epithelium. Gain of Notch activity also resulted in hyperproliferation of the patterned smooth muscle progenitor cells, the α-actin-positive myoblasts. As a result, PBCre4/+;RosaN1IC/+ prostates form abnormally thick smooth muscle envelopes and develop fibromuscular nodules in the glandular stroma. These epithelial and stromal pathologies are consistent with benign prostate hyperplasia in men (Lee and Peehl, 2004), and in murine models (Kim et al., 2002).
Consistent with the effects of gain of Notch function, loss of Notch/RBP-J resulted in upregulation of PTEN, decreased rates of cell division and survival in the prostate epithelium, and a rapid loss of basal prostate progenitors, postnatally. Loss of Notch function also resulted in defects in differentiation of the basal and luminal epithelium. Previous studies employed pharmacological γ-secretase inhibitors, and performed a conditional deletion of the Notch1 receptor in the postnatal prostate, to show the importance of Notch signaling for compartmentalization and secretory differentiation of the prostate epithelium (Wang et al., 2006). Our studies show that loss of Notch function resulted in a significant decrease in cell survival and proliferation, as well as severe defects in epithelial differentiation in the embryonic, postnatal and adult prostate. Embryonic and newborn UGS epithelium is normally multilayered and pseudostratified. In contrast, loss of Notch signaling manifested in a severe reduction of the UGS epithelium to a single layer of cuboidal cells at the basal membrane. Defects in epithelial differentiation also manifested in the prostate ducts. Normal differentiated prostate ducts contains small elongated basal cells stretched along the lamina, and a single layer of luminal epithelium. Loss of Notch signaling resulted in severe defects in compartmentalization of the basal and luminal epithelial layers. As result, Nkx3.1Cre/+;RBP-Jflox/flox ducts contained abnormally large CK14+ basal cells which extended into the luminal compartment, and co-expressed the luminal marker, CK8. In summary, our studies show that Notch signaling controls survival and proliferation of the pluripotent basal progenitors, and that precise level of Notch signaling is necessary for specification of the basal and luminal lineages.
Our studies also strongly indicate the importance of Notch signaling for maintenance and differentiation of smooth muscle progenitors. Gain of Notch function resulted in increased proliferation of myoblast progenitors, a delay in terminal differentiation, and formation of an additional 3rd layer of smooth muscle in the adult gland. Persistent Notch signaling also caused a delay in condensation of smooth muscle. At P14, adolescent prostates were surrounded by thick but loosely packed smooth muscle envelopes, which retained partially-differentiated myoblasts. Consistently, loss of Notch function manifested in loss of myoblasts, and abnormally thin and discontinuous smooth muscle envelopes. Interestingly, we found that loss of Notch function and increase in PTEN+ cells in the periductal mesenchyme favored the PTEN+, vimentin+ fibroblast lineage over the PTEN− vimentin− α-sma+ myoblasts and myocytes. This is a strong evidence that Notch signaling is necessary for maintenance and differentiation of the smooth muscle lineage. The role for Notch in differentiation of the smooth muscle is a relatively novel concept. However, recent report pointed to the significance of the Notch2 signaling in differentiation and survival of the prostate stroma (Orr et al., 2009). Other studies implicated Notch1 in differentiation and proliferation of the arterial smooth muscle (Feng et al., 2010; Tang et al., 2010; Morrow et al., 2010). In addition, ectopic Notch1 signaling has been implicated in the pathological differentiation of myofibroblasts in pulmonary fibrosis (Liu et al., 2009), and in osteogenic mineralization of vascular smooth muscle in arteosclerotic plaques (Shimizu et al., 2009).
Our analysis of the Nkx3.1Cre;Rosa26 reporter system revealed that Nkx3.1Cre drives Cre expression in the prostate epithelium and smooth muscle compartments. This suggests that the Nkx3.1 prostate-specific homeobox factor (Bhatia-Gaur et al., 1999) functions to pattern both prostate-specific epithelium and adjacent mesenchyme. In addition, we show that both prostate-specific drivers used in this study, the Nkx3.1Cre and PBCre4, target Cre expression to the same domains in the postnatal prostate epithelium and smooth muscle. In further support, we experimentally confirmed the increase Notch1 signaling in the embryonic Nkx3.1Cre;RosaN1IC urogenital mesenchyme, and in PBCre4;RosaN1IC smooth muscle. Thus, we provide evidence that in our systems, Notch signaling is modulated cell-autonomously in the epithelium and stroma. Yet, considering the importance of signaling interactions between the epithelium and mesenchyme for prostate development and homeostasis, it is necessary to evaluate other signaling pathways which may contribute to the observed phenotypes. For instance, signaling by the epithelial Sonic hedgehog (Shh) (Podlasek et al., 1999), Bone morphogenetic proteins 4 and 7 (Lamm et al., 2001; Grishina et al., 2005) and Wnt molecules (Ontiveros et al., 2008; Huang et al., 2009; Yu et al., 2009) can affect differentiation of the myoblast progenitors and formation of the peridictal smooth muscle (Shaw et al., 2006; Perez et al., 2011). Our unpublished studies in embryonic Nkx3.1Cre/+;RosaN1IC/+ and Nkx3.1Cre/+;RBP-Jflox/flox prostates indicate that Notch signaling does not affect expression of the Shh ligand. Although, we did not examine expression of the Shh reporters and mediators. Signaling by the Bmp and Wnt ligands have been shown to inhibit PTEN in the normal and cancerous prostate epithelium (Jerde et al., 2010; Ohigashi et al., 2005). Our unpublished studies indicate that gain and loss of Notch signaling in the embryonic prostates does not affect the Bmp/phospho-Smad1/5/8 pathway. However, our previous studies show that Notch and Wny pathways function in the same cell populations in the prostate epithelium and in the adjacent mesenchyme (Grishina et al., 2005; Wu et al., 2010). We also found that gain of Notch function the embryonic prostates resulted in upregulation of Lef1, a mediator and reporter in the canonical Wnt pathway (Wu et al., 2010, and our unpublished data). Other reports confirm the complex interactions between the Notch and Wnt pathways in maintenance of the progenitor cells and their niche (Radtke et al., 2006; Buske et al., 2011; Shahi et al., 2011). Thus, further studies should clarify the roles and interactions between Notch and Wnt pathways in maintenance and differentiation of the prostate progenitor cells in the epithelial and stromal compartments.
In the last decade, studies in multiple species and organs systems pointed to the importance of the Notch cell fate selection system in maintenance and proliferation of pluripotent progenitors, and the importance of timely inhibition of Notch signaling for differentiation of the secretory and neural lineages. Our analysis of the effects of gain and loss of Notch signaling during prostate development demonstrates the importance of fine regulation of Notch signaling for proper development of the prostate epithelium and smooth muscle. The peculiarity of prostate homeostasis is that prostate retains a high number of progenitor/stem cells in the adult tissue which allows for prostate regeneration, and also makes the gland vulnerable to the hormonal and developmental signals, such as by steroid hormones, and Notch and Wnt ligands. Importantly, our studies demonstrate that both loss and gain of Notch function have significant consequences for prostate differentiation and tissue integrity, and can lead to abnormal tissue homeostasis and predisposition to disease in the adult organ.
Supplementary Material
Highlights
-
>
Gain and loss of Notch function in prostate development.
-
>
Nkx3.1Cre and PBCre4 drive Cre expression to prostate epithelium and smooth muscle.
-
>
N1IC inhibits PTEN.
-
>
N1IC promotes maintenance of progenitor cells in the epithelium and smooth muscle.
-
>
Notch/RBP-J is required for differentiation of prostate epithelium and smooth muscle.
Acknowledgements
We are greatfull to Michael Shen for the gift of the Nkx3.1Cre strain, Douglas Melton for the RosaN1IC strain, and Tasuku Honjo for the RBPJflox strain. We also thank Lynn Wilson and Susan Logan for reading the manuscript prior to publication and useful discussions of this study. This work was supported by the NIH NIDDK grant DK-068007, the DOD PCRP Hypothesis Exploration Award, and the Kidney and Urology Foundation Young Investigator Award to I.G.; the Chinese pre-doctoral Fellowship to X.K.; the NIH NIAMS grant R01 AR053163, the NIH NEI grant R21 EY021292, and the Neurosciences Research Foundation to H.P.M; and by NCRR S10 RR023704 grant to the Microscopy Core at the New York University Langone Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- Abate-Shen C, Banach-Petrosky WA, Sun X, Economides KD, Desai N, Gregg JP, Borowsky AD, Cardiff RD, Shen MM. Nkx3.1;Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 2003;63:3886–3890. [PubMed] [Google Scholar]
- Alimirah F, Panchanathan R, Davis FJ, Chen J, Choubey D. Restoration of p53 expression in human cancer cell lines upregulates the expression of Notch1: implications for cancer cell fate determination after genotoxic stress. Neoplasia. 2007;9:427–434. doi: 10.1593/neo.07211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis -Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Belandia B, Powell SM, Garcia-Pedrero JM, Walker MM, Bevan CL, Parker MG. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol Cell Biol. 2005;25:1425–1436. doi: 10.1128/MCB.25.4.1425-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, Honjo T, Hennighausen L. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006;293:565–580. doi: 10.1016/j.ydbio.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Buske P, Galle J, Barker N, Aust G, Clevers H, Loeffler M. A comprehensive model of the spatio-temporal stem cell and tissue organisation in the intestinal crypt. PLoS Comput. Biol. 2011;7 doi: 10.1371/journal.pcbi.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Grishina IB, Kim SY, Ferrara C, Makarenkova HP, Walden PD. BMP7 Inhibits Branching Morphogenesis in the Prostate Gland and Interferes with Notch Signaling. Dev. Biol. 2005;288:334–347. doi: 10.1016/j.ydbio.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin RE, Takaesu NT, Robertson EJ, Dudley AT. Regulation of BMP7 expression during kidney development. Development. 1998;125:3473–3482. doi: 10.1242/dev.125.17.3473. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Baskin LS, Haughney PC, Foster BA, Cunha AR, Dahiya R, Prins GS, Cunha GR. Stromal development in the ventral prostate, anterior prostate and seminal vesicle of the rat. Acta Anat (Basel) 1996;155:94–103. doi: 10.1159/000147794. [DOI] [PubMed] [Google Scholar]
- Huang L, Pu Y, Hu WY, Birch L, Luccio-Camelo D, Yamaguchi T, Prins GS. The role of Wnt5a in prostate gland development. Dev Biol. 2009;328:188–199. doi: 10.1016/j.ydbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development. 2000;127:3913–3921. doi: 10.1242/dev.127.18.3913. [DOI] [PubMed] [Google Scholar]
- Jerde TJ, Wu Z, Theodorescu D, Bushman W. Regulation of phosphatase homologue of tensin protein expression by bone morphogenetic proteins in prostate epithelial cells. Prostate. 2010 doi: 10.1002/pros.21295. http://www.ncbi.nlm.nih.gov/pubmed/21053204. [DOI] [PMC free article] [PubMed]
- Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, Desai N, Wang Y, Hayward SW, Cunha GR, Cardiff RD, Shen MM, Abate-Shen C. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 2002;62:2999–3004. [PubMed] [Google Scholar]
- Kos L, Chiang C, Mahon KA. Mediolateral patterning of somites: multiple axial signals, including Sonic hedgehog, regulate Nkx-3.1 expression. Mech Dev. 1998;70:25–34. doi: 10.1016/s0925-4773(97)00168-8. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina R,T, Mills A,A, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–4964. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- Lee KL, Peehl DM. Molecular and cellular pathogenesis of benign prostatic hyperplasia. J Urol. 2004;172:1784–1791. doi: 10.1097/01.ju.0000133655.71782.14. [DOI] [PubMed] [Google Scholar]
- Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- Leong KG, Gao WQ. The Notch pathway in prostate development and cancer. Differentiation. 2008;76:699–716. doi: 10.1111/j.1432-0436.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- Loikkanen I, Toljamo K, Hirvikoski P, Väisänen T, Paavonen TK, Vaarala MH. Myosin VI is a modulator of androgen-dependent gene expression. Oncol Rep. 2009;22:991–995. doi: 10.3892/or_00000526. [DOI] [PubMed] [Google Scholar]
- Lopes ES, Foster BA, Donjacour AA, Cunha GR. Initiation of secretory activity of rat prostatic epithelium in organ culture. Endocrinology. 1996;137:4225–4234. doi: 10.1210/endo.137.10.8828481. [DOI] [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev. Biol. 2003;253:165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohigashi T, Mizuno R, Nakashima J, Marumo K, Murai M. Inhibition of Wnt signaling downregulates Akt activity and induces chemosensitivity in PTEN-mutated prostate cancer cells. Prostate. 2005;62:61–68. doi: 10.1002/pros.20117. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as Notch effectors in mammalian neuronal differentiation. The EMBO Journal. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontiveros CS, Salm SN, Wilson EL. Axin2 expression identifies progenitor cells in the murine prostate. Prostate. 2008;68:1263–1272. doi: 10.1002/pros.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr B, Grace OC, Vanpoucke G, Ashley GR, Thomson AA. A role for notch signaling in stromal survival and differentiation during prostate development. Endocrinology. 2009;150:463–472. doi: 10.1210/en.2008-0383. [DOI] [PubMed] [Google Scholar]
- Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL, Bhagat G, Agarwal AM, Basso G, Castillo M, Nagase S, Cordon-Cardo C, Parsons R, Zúñiga-Pflücker JC, Dominguez M, Ferrando AA. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, Shen MM, Cardiff RD. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol. 2002;161:727–735. doi: 10.1016/S0002-9440(10)64228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VA, Ali Z, Alastalo TP, Ikeno F, Sawada H, Lai YJ, Kleisli T, Spiekerkoetter E, Qu X, Rubinos LH, Ashley E, Amieva M, Dedhar S, Rabinovitch M. BMP promotes motility and represses growth of smooth muscle cells by activation of tandem Wnt pathways. J. Cell Biol. 2011;192:171–188. doi: 10.1083/jcb.201008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Clevers H, Riccio O. From gut homeostasis to cancer. Curr. Mol. Med. 2006;6:275–289. doi: 10.2174/156652406776894527. Review. [DOI] [PubMed] [Google Scholar]
- Robinson EJ, Neal DE, Collins AT. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 1998;37:149–160. doi: 10.1002/(sici)1097-0045(19981101)37:3<149::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Shahi P, Seethammagari MR, Valdez JM, Xin L, Spencer DM. Wnt and Notch Pathways have Interrelated Opposing Roles on Prostate Progenitor Cell Proliferation and Differentiation. Stem Cells. 2011 doi: 10.1002/stem.606. http://www.ncbi.nlm.nih.gov/pubmed/21308863. [DOI] [PMC free article] [PubMed]
- Shaw A, Papadopoulos J, Johnson C, Bushman W. Isolation and characterization of an immortalized mouse urogenital sinus mesenchyme cell line. Prostate. 2006;66:1347–1358. doi: 10.1002/pros.20357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S, Pires MM, Lindauer M, Horner JW, Grisanzio C, Dhar S, Majumder P, McKeon F, Kantoff PW, Sellers WR, Loda M. p63 regulates commitment to the prostate cell lineage. Proc Natl Acad Sci U S A. 2005;102:11355–11360. doi: 10.1073/pnas.0500165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lac-Z expressio with the ROSA26 Cre reporter strain. Nat genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfel MN, Moses KA, Carson JA, Zimmer DB, DeMayo F, Schwartz RJ, Zimmer WE. Expression of an Nkx3.1-CRE gene using ROSA26 reporter mice. Genesis. 2006;44:550–555. doi: 10.1002/dvg.20250. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat. Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- Van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Wang XD, Shou J, Wong P, French DM, Gao WQ. Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J. Biol. Chem. 2004;279:24733–24744. doi: 10.1074/jbc.M401602200. [DOI] [PubMed] [Google Scholar]
- Wang XD, Leow CC, Zha J, Tang Z, Modrusan Z, Radtke F, Aguet M, de Sauvage FJ, Gao WQ. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol. 2006;290:66–80. doi: 10.1016/j.ydbio.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–279. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- Whelan JT, Kellogg A, Shewchuk BM, Hewan-Lowe K, Bertrand FE. Notch-1 signaling is lost in prostate adenocarcinoma and promotes PTEN gene expression. J. Cell. Biochem. 2009;107:992–1001. doi: 10.1002/jcb.22199. [DOI] [PubMed] [Google Scholar]
- Wu CT, Altuwaijri S, Ricke WA, Huang S, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007;104:12679–12684. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech. Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- Wu X, Ferrara C, Shapiro E, Grishina I. Bmp7 expression and null phenotype in the urogenital system suggest a role in re-organization of the urethral epithelium. Gene Expression Patterns. 2009;9:224–230. doi: 10.1016/j.gep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Daniels G, Shapiro E, Xu K, Li Y, Huang H, Chiriboga L, Gellert LL, Alba M, Greco AM, Grishina I, Lee P. LEF1 Activity Identifies Androgen Independent Pluripotent Epithelium in Fetal Human and Mouse Prostates. Molecular Endocrinology. accepted 03/28/11. [Google Scholar]
- Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc. Natl. Acad. Sci. U S A. 2003;100(Suppl. 1):11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 2007;25:2760–2769. doi: 10.1634/stemcells.2007-0355. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: Insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang Y, Jiang M, Bierie B, Roy-Burman P, Shen MM, Taketo M, Wills M, Matusik RJ. Activation of beta-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate. 2009;69:249–262. doi: 10.1002/pros.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Hayward SD. Nuclear localization of CBF1 is regulated by interactions with the SMRT corepressor complex. Mol. Cell. Biol. 2001;21:6222–62232. doi: 10.1128/MCB.21.18.6222-6232.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.