Abstract
Hormone secretion often occurs in a pulsatile manner. In this article we discuss two rhythms of in vivo prolactin release in female rats and the ongoing research that we and others have performed to understand the mechanisms underlying them. The peptide hormone oxytocin appears to play an important role in both rhythms. One rhythm occurs during the first half of pregnancy, but can also be induced in ovariectomized rats. This is characterized by a circadian pattern with two prolactin surges per day. Two methods for triggering this rhythm are discussed, each utilizing a unique physiological pathway that includes oxytocin action, presumably on pituitary lactotrophs. The second rhythm occurs during the estrous cycle and is characterized by a surge of prolactin on the afternoon of proestrus. We discuss recent findings that oxytocin is more effective at stimulating prolactin release from lactotrophs taken from animals on the afternoon of proestrus than from those of animals on the morning of diestrus 1, raising the possibility that this hormone plays a physiological role in the regulation of prolactin secretion during the estrous cycle.
Prolactin is one of the most versatile hormones and its release from pituitary lactotrophs in female rats is stimulated by suckling and mating, and also occurs on the afternoon of proestrus (1). The wide array of factors that contribute to the control of prolactin release are reviewed in (2). Suckling evokes a classic neuroendocrine response, in which prolactin release starts when the suckling begins and ends when the suckling stops. In contrast, mating evokes a prolactin response that lasts for ten days, indicating that some type of “memory” is activated by the stimulus. During pregnancy, this response is rhythmic, consisting of two prolactin surges per day, one in the morning (the nocturnal surge) and one in the afternoon (the diurnal surge). Likewise, prolactin released during the estrous cycle is rhythmic, with a surge occurring every 4–5 days, on the afternoon of proestrus. There is now evidence that the peptide hormone oxytocin is involved in both of these rhythmic behaviors. In this article we provide an overview of recent work done in our lab to determine the role that oxytocin plays in rhythmic prolactin secretion.
Rhythm 1: Circadian prolactin rhythm induced by cervical stimulation
The circadian prolactin rhythm induced by cervical stimulation received during mating occurs during the first half of pregnancy in the female rat and is characterized by two surges per day (3, 4). The released prolactin is necessary to rescue the corpus luteum and maintain its ability to secrete progesterone for ten days (1, 2). After that, progesterone secretion is sustained for the remainder of the 20–22 day pregnancy by placental lactogens (5, 6). A similar prolactin rhythm, lasting up to 12 days, can be induced by artificial cervical stimulaltion in both intact and ovariectomized animals, demonstrating that ovarian steroids are not necessary for triggering or maintaining the prolactin rhythm (7). However, ovarian steroids do play a role in the termination of these surges in intact animals (see (1)). While it has been known for many years that the cervical stimulation-induced prolactin rhythm involves interactions between the hypothalamus and pituitary lactotrophs (8), questions regarding the mechanism for the initiation and maintenance of this rhythm have been hard to answer, and are largely unanswered even today. Three questions immediately come to mind: (1) how does cervical stimulation trigger the memory in ovariectomized rats? (2) what is the memory? (3) what are the elements required for the production of the prolactin rhythm that is maintained by the memory?
We have found that peripheral injection of oxytocin or central injection of ovine prolactin into ovariectomized rats can start the circadian prolactin rhythm (9, 10). Motivated by these findings, we investigated whether cervical stimulation was capable of producing a prolactin rhythm when either an oxytocin receptor antagonist or a prolactin receptor antagonist was applied centrally (via intracerebroventricular infusion) during and/or after the cervical stimulation. Central infusion of the oxytocin receptor antagonist desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT into the lateral cerebral ventricle had little or no effect on the cervical stimulation-induced rhythm (C. Helena, unpublished observation), suggesting that central actions of oxytocin are not involved in the triggering of the memory and are not part of the rhythm mechanism. (In a different strain of rats, however, a direct injection of the oxytocin receptor antagonist into the ventrolateral region of the ventromedial hypothalamus inhibited the prolactin rhythm induced by mating, rather than cervical stimulation (11).) Central infusion of the prolactin receptor antagonist S179D inhibited the rhythm while the antagonist was present, but if the prolactin receptor antagonist was present only on the day of cervical stimulation the prolactin rhythm was still produced (10). This suggests that the central action of prolactin is necessary for the production of the rhythm (the rhythm does not occur when a prolactin receptor antagonist is present), but is not required for triggering the memory (cervical stimulation induced a delayed prolactin rhythm even though central prolactin receptors were blocked at the time of stimulation). Thus, while it remains unclear how cervical stimulation triggers the memory that maintains the prolactin rhythm, these data argue against a role for central oxytocin or prolactin in triggering the memory for the rhythm.
The identity of the memory also remains elusive, although there has been recent progress. Some recent work suggests that the ventrolateral region of the ventromedial hypothalamic nucleus may be a component of the memory (11), and since parvocellular neurons send projections to the ventromedial hypothalamus (12), the paraventricular nucleus may also be involved. Whatever the identity and location of the memory, one may ask whether it acts directly on anterior pituitary lactotrophs or whether it mediates its effect on prolactin secretion through modulation of dopaminergic neuron activities in the arcuate and periventricular nuclei of the hypothalamus. Dopamine released from these neurons is known to have a strong inhibitory action on lactotrophs (13).
The elements involved in the production of the prolactin rhythm have largely been identified. Hypothalamic dopamine is known to inhibit lactotroph activity via binding to D2-type receptors (14). In contrast, prolactin from lactotrophs stimulates hypothalamic dopamine neurons (15) through specific receptors (16). Thus, prolactin indirectly inhibits its own release through activation of the dopamine neurons. In vitro studies in rat hypothalamic slices (17) and cell cultures from the mediobasal hypothalamus of the embryonic rat pup (18) have shown that prolactin application increases dopamine synthesis through phosphorylation-mediated upregulation of tyrosine hydroxylase activity. This enzyme, which converts tyrosine into L-DOPA, is the rate-limiting enzyme in dopamine production, and these studies showed that the increase in catecholamine levels occurs within 1–2 hr of prolactin application. Prolactin also increases the mRNA level for tyrosine hydroxylase, but this occurs on a slower time scale of 4 hr (18). This slower time scale is similar to that observed in an in vivo study (19). Another in vivo study showed that dopamine neuron activity was elevated and serum prolactin levels were inhibited within an hour of peripheral injection of ovine prolactin (15). These findings suggest an increase in dopamine secretion in response to prolactin. (There was an additional effect, with increased dopamine neuron activity and decreased prolactin levels, three hours after ovine prolactin injection.) Using mathematical modeling, we have demonstrated that the mutual feedback loop between lactotrophs and dopamine neurons, with a time delay, is capable of generating a two-pulse per day prolactin rhythm (20). A key prediction of the model is that dopamine neuron activity should peak at times between the prolactin surges. Because the ratio of DOPAC (3,4-dihydroxyphenylacetic acid, a metabolite of dopamine) to dopamine is an index of dopamine neuronal activity at the synaptic terminals (21, 22), we measured this ratio in the median eminence, the intermediate lobe, and the neural lobe of cervically-stimulated and ovariectomized rats to investigate this prediction. We found that the ratio peaked at noon in both the median eminence and the neural lobe, which is between the two prolactin surges and is consistent with the model prediction (23).
Although interactions between lactotrophs and dopamine neurons appear to be crucial for the prolactin rhythm, additional factors must be involved. Modeling suggests that vasoactive intestinal polypeptide-ergic neurons in the suprachiasmatic nucleus, which innervate (24) and inhibit dopamine neurons of the arcuate nucleus early in the morning in a circadian fashion (25, 26), are responsible for setting the time of the nocturnal surge and for making the nocturnal prolactin surge larger than the diurnal surge (20). Moreover, the fact that administration of vasoactive intestinal polypeptide antisense oligonucleotides in the suprachiasmatic nucleus altered the prolactin (and oxytocin) rhythms in cervically stimulated rats (27) demonstrates an important role for this input from the suprachiasmatic nucleus. Earlier work from our lab suggested a role for oxytocin in the regulation of prolactin surges in cervically stimulated rats (28). More recently, we showed that peripheral infusion of an oxytocin receptor antagonist blocked the cervical stimulation-induced prolactin rhythm as long as this antagonist was present. However, once the oxytocin antagonist had cleared from the system two days after the end of the infusion, the prolactin rhythm appeared (23). Since the oxytocin antagonist does not cross the blood-brain barrier, this suggests that oxytocin action on the lactotrophs is a necessary element of the prolactin rhythm. These findings also indicate that peripheral oxytocin actions are not essential for the triggering of the memory, since cervical stimulation was able to trigger the memory even in the presence of a peripheral oxytocin receptor antagonist (although the prolactin rhythm was not expressed until after the oxytocin antagonist was cleared from the system).
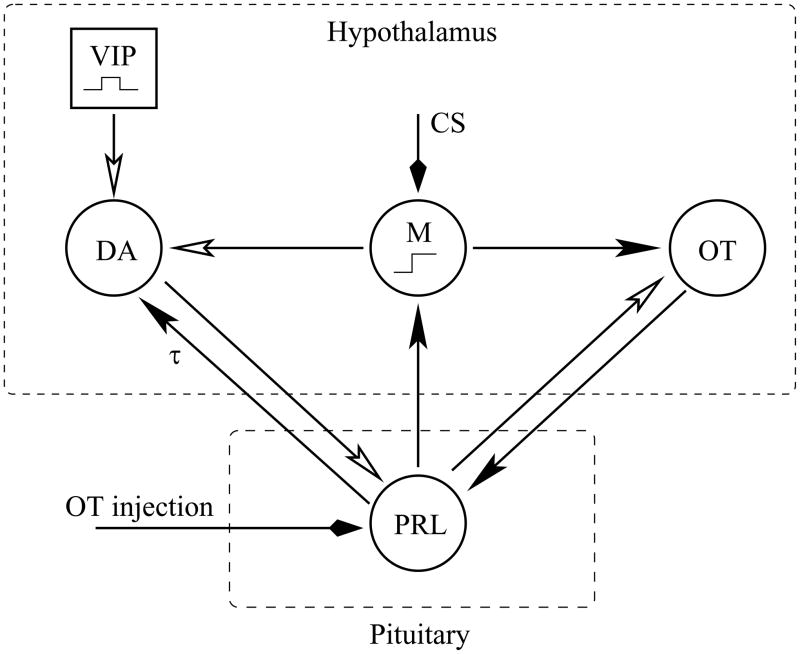
Figure 1 illustrates our proposed model for the cervical stimulation-induced prolactin rhythm. The prolactin rhythm is mediated primarily by the mutual interactions between lactotrophs and hypothalamic dopamine neurons (solid arrowhead indicates stimulatory action, in this case with a delay of τ = 2 hrs, and open arrowhead indicates an inhibitory action). Lactotroph and dopamine neuron activity oscillate out of phase, so that the prolactin level is high when dopamine neuron activity is low and vice versa. The rhythm is facilitated by the stimulatory actions of oxytocin released from neurons of the paraventricular nucleus into the neural lobe of the pituitary (29) and into the median eminence (30). This oxytocin has been shown to increase Ca2+ levels (27) in lactotrophs and to stimulate prolactin secretion both in vivo (9, 31, 32) and in vitro (27, 33–35). The prolactin, in turn, has a rapid inhibitory action on oxytocin neurons of the supraoptic nucleus (36, 37) and the paraventricular nucleus (Sirzen-Zelenskaya et al., in preparation). Both of these actions are included in the model. The circadian inhibitory action of vasoactive intestinal polypeptide neurons of the suprachiasmatic nucleus onto dopamine neurons is also included. Finally, cervical stimulation activates a memory that maintains the prolactin rhythm. It does this by partially inhibiting the dopamine neurons and by stimulating oxytocin neurons. Evidence for the latter comes from experimental data, showing that peripheral oxytocin levels are elevated following cervical stimulation (9, 38). Evidence for an inhibitory effect of the memory on secretion from dopamine neurons comes from a mathematical modeling study (20). Here we demonstrated that a direct stimulatory input to lactotrophs would lead to an elevated prolactin level, but would not initiate a rhythm. In contrast, partial inhibition of dopamine neurons both increased the mean prolactin level and, most importantly, induced a circadian prolactin rhythm. Thus, while we are still far from identifying the central location of “the memory”, we believe that progress has been made in understanding the mechanism through which it works.
Figure 1.
Proposed model for the prolactin rhythm initiated by cervical stimulation (CS) or by oxytocin (OT) injection. Closed arrowheads indicate stimulatory actions, open arrowheads indicate inhibitory actions. The stimulation of dopamine (DA) neurons by prolactin (PRL) has a delay of τ hours. A pulse of vasoactive intestinal polypeptide (VIP) from suprachiasmatic neurons occurs every morning, setting the phase of the prolactin oscillation. The memory, M, is switched on by CS or by PRL.
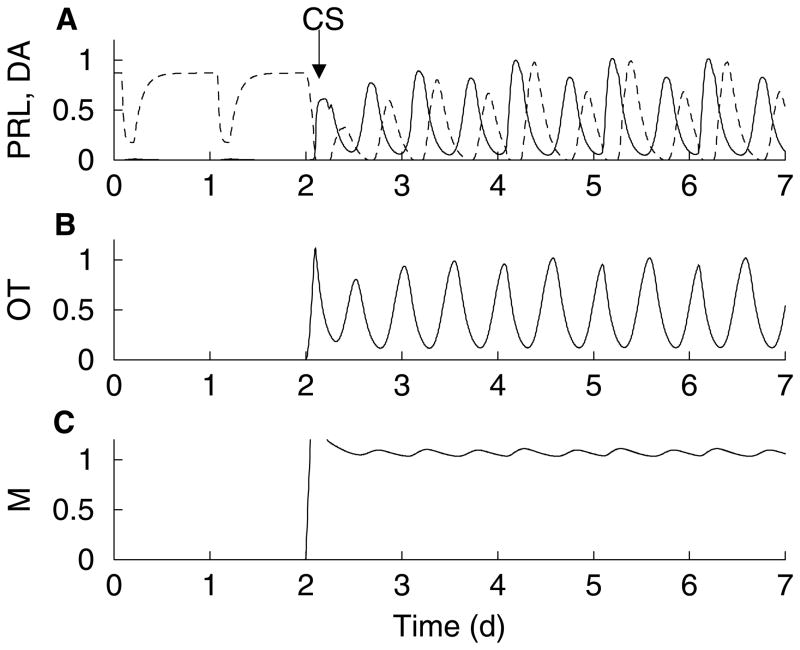
When the descriptive model shown in Fig. 1 is translated into mathematical equations (20, 39) a simulated prolactin rhythm is produced (Fig. 2A, solid). In this simulation, the prolactin level is low prior to cervical stimulation. A rhythm is started by the stimulus, with two prolactin surges per day. The second, (diurnal) surge is smaller than the first, as is typically observed experimentally (23, 27). Along with the prolactin rhythm, there are rhythms in the activities of dopamine neurons (Fig. 2A, dashed) and oxytocin neurons (Fig. 2B). These variables oscillate out of phase with the prolactin rhythm, peaking when the prolactin level is declining (in the case of dopamine) or at its nadir (in the case of oxytocin). This rhythm persists as long as the memory is on (Fig. 2C).
Figure 2.
Numerical simulation of the mathematical model (see (39) for equations). (A) A prolactin rhythm (solid) is initiated by cervical stimulation, as described in Fig. 1. The dopamine neuron activity (dashed) oscillates out of phase with prolactin levels. (B) The cervical stimulation activates oxytocin neurons of the paraventricular nucleus, which oscillate out of phase with prolactin levels due to the inhibitory influence of prolactin on the neurons. (C) The memory is switched on by the cervical stimulation and stays on throughout the simulation. The variables PRL, DA, and OT have all been normalized to facilitate comparison.
Rhythm 1 (continued): Circadian prolactin rhythm induced by peripheral oxytocin injection
It has been shown that oxytocin is released into the circulation following cervical stimulation in sheep (40), rats (38), and humans (41, 42). This motivated us to inject oxytocin peripherally into female ovariectomized rats to attempt to start the circadian prolactin rhythm. We found that oxytocin injection starts a prolactin rhythm that is very similar to that induced by cervical stimulation (9). That is, there are two prolactin surges per day, one in the morning and another in the afternoon, which continue for at least 7 days. Do cervical stimulation and peripheral oxytocin injection trigger the prolactin rhythm in the same way? To investigate this, we performed a series of experiments with oxytocin and prolactin receptor antagonists. The results of these experiments are illustrated here (Fig. 3) with the mathematical model (for equations, see Helena et al., in preparation).
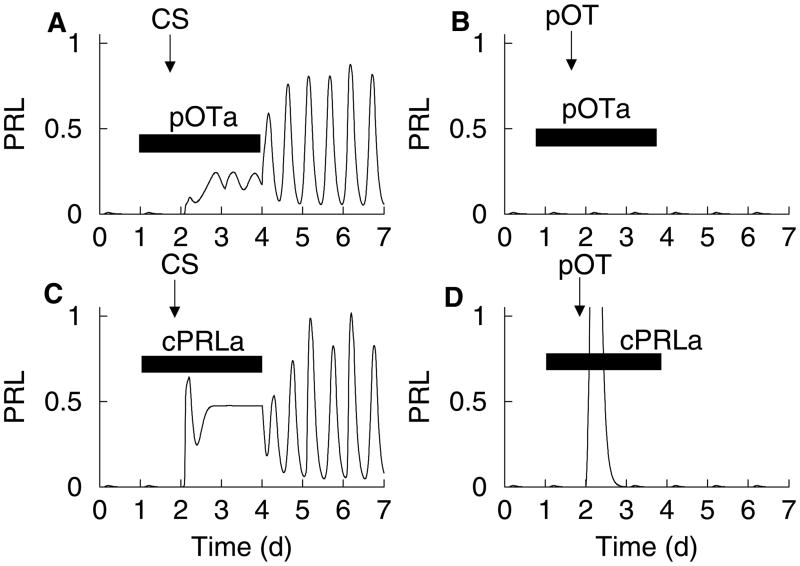
Figure 3.
Numerical simulation of the mathematical model illustrating the effects of two antagonists on the triggering of the memory for the biphasic circadian prolactin rhythm. (A), (C) Both peripheral oxytocin receptor antagonist (pOTa) and central prolactin antagonist (cPRLa) prevent the rhythm induced by cervical stimulation while the antagonist is present. However, the rhythm emerges once the antagonist has cleared from the system. (B), (D) The prolactin rhythm induced by peripheral oxytocin injection (pOT) is not triggered when the same antagonists are present during the injection.
When the oxytocin receptor antagonist is administered peripherally during the cervical stimulation, the prolactin rhythm is initially inhibited. However, once the oxytocin receptor antagonist clears the system a prolactin rhythm emerges (Fig. 3A). This was demonstrated in (23); the prolactin rhythm emerged two days after cessation of the oxytocin receptor antagonist infusion. The explanation for this is that oxytocin stimulatory action on the lactotrophs is necessary for the rhythm to occur, but the trigger for the memory does not require peripheral actions of oxytocin. In contrast, when oxytocin is injected peripherally in the presence of a peripheral oxytocin receptor antagonist there is little or no effect on prolactin levels (Fig. 3B), even after the oxytocin receptor antagonist has cleared the system (Helena et al., in preparation). This shows that the peripheral injection of oxytocin triggers the prolactin rhythm by acting peripherally, most likely on lactotrophs. This is in contrast to the way that cervical stimulation triggers the rhythm, which does not require peripheral oxytocin actions.
How can peripheral oxytocin actions trigger the memory? One possibility is that the peripheral oxytocin injection stimulates lactotrophs sufficiently so that the dopamine inhibition is overcome and prolactin is released into the blood. The prolactin then crosses the blood-brain barrier, possibly through receptors in the choroid plexus (43, 44), and triggers the memory. (In fact, peripheral injection of ovine prolactin at high concentration initiates the prolactin rhythm, bypassing oxytocin altogether (10).) A model simulation illustrating the critical role played by central prolactin is shown in Fig. 3D. In this figure, a central prolactin receptor antagonist (cPRLa) is simulated. When peripheral oxytocin injection is simulated in the model there is a single large surge of prolactin, due to the direct effect of oxytocin on lactotrophs. However, when a central prolactin receptor antagonist is present at the time of the oxytocin injection no rhythm is initiated, even after the prolactin receptor antagonist is removed. This reflects the model hypothesis, supported by experimental data (10), that central prolactin can trigger the memory (Fig. 1). When this link is impaired, by central administration of a prolactin receptor antagonist, the prolactin released in response to the peripheral oxytocin injection cannot trigger the memory, so even after the prolactin receptor antagonist is cleared there will be no prolactin rhythm. This model prediction, that peripheral oxytocin injection fails to initiate the prolactin rhythm when the prolactin receptor antagonist is infused centrally on the day of the oxytocin injection, has been experimentally confirmed in our lab (Helena et al., in preparation).
This result differs significantly from a similar experiment conducted on cervically stimulated rats (without oxytocin injection). Here, the cervical stimulation-induced prolactin rhythm was prevented when the central prolactin receptor antagonist infusion was maintained continuously. However, when it was infused only during the day of cervical stimulation, the prolactin rhythm started two days later (10). This suggests that cervical stimulation triggers the memory through a mechanism that does not utilize central actions of prolactin. This is illustrated with the model in Fig. 3C. The cervical stimulation triggers the memory, thereby partially inhibiting dopamine neurons and stimulating oxytocin neurons. This results in an elevation of peripheral prolactin. However, while the central prolactin receptor antagonist is present, the positive feedback of prolactin onto the dopamine neurons is blocked, so the rhythm mechanism is dysfunctional. Once the prolactin receptor antagonist is cleared and the prolactin feedback onto dopamine neurons is restored, a prolactin rhythm is produced, since now all the required elements (triggered memory and a functional rhythm generating mechanism) have been reestablished.
From these experiments, it appears that although both cervical stimulation and oxytocin injection can induce a biphasic circadian prolactin rhythm, the pathways through which they operate are different. The model illustrated in Fig. 1 is our current view of the interaction pathways whereby cervical stimulation and peripheral oxytocin (or prolactin) injection produce the prolactin rhythm. Although our understanding of the “memory” and its location in the brain is yet very limited, the structure of the model is consistent with all of our recent data on cervically stimulated, ovariectomized rats (10, 23) and ovariectomized rats in which either oxytocin or prolactin is injected peripherally (9, 10)(see also Helena et al., in preparation). Furthermore, it clearly predicts that feedback interactions between pituitary lactotrophs and dopamine neurons are insufficient to account for mating-induced rhythmic prolactin release and establishes that additional interactions are required.
Rhythm 2: Prolactin rhythm induced by the ovarian cycle
While the first rhythm involving oxytocin does not require hormone steroids, the proestrous afternoon surge is likely due to the rhythmic variations in estradiol levels during the estrous cycle. In fact, in response to a brief exposure to estradiol, ovariectomized rats secrete daily surges of prolactin at times corresponding to that on proestrus (45). This is due in large part to the decrease in the inhibitory influence of dopamine on lactotrophs mediated by the rise in estradiol during the cycle (1, 2). Besides this reduction in inhibition, the proestrous prolactin surge may be facilitated by a stage-specific increase in either the plasma level of a stimulating hormone, or in the responsiveness of lactotrophs to such a hormone, or both. It is well established that plasma oxytocin concentrations increase in response to estradiol administration (46) and it has been demonstrated previously that the concentration of oxytocin in pituitary portal blood reaches a peak on the afternoon of proestrus (47), following the peak estradiol concentrations observed in the morning. Moreover, passive immunoneutralization of endogenous oxytocin or peripheral administration of an oxytocin receptor antagonist has been both shown to inhibit the prolactin surge in the afternoon of proestrus (32, 48) as well as suckling-induced prolactin secretion (33, 49). Complementary to this, we have recently found that proestrous lactotrophs are significantly more responsive to oxytocin than those obtained from diestrus 1 (50). This constitutes a second rhythm, linked to the 4–5 day long estrous cycle of female rats.
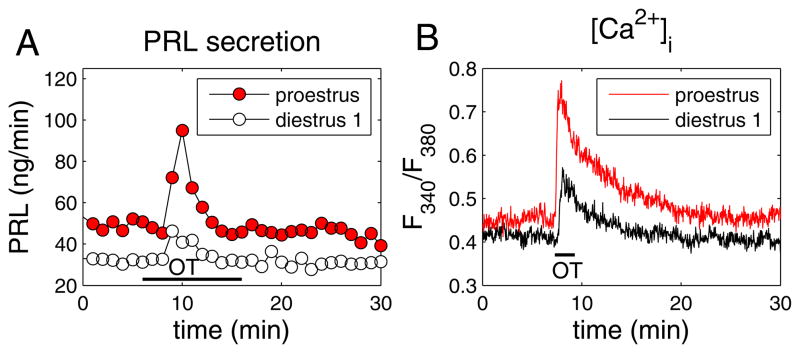
In our experiments, anterior pituitaries were obtained from cycling rats on the morning of diestrus 1 or the afternoon of proestrus. Oxytocin was then applied to the anterior pituitary cells (100 nM for 10 min) and the prolactin response measured using radioimmunoassay. As shown in Fig. 4A, the prolactin response to oxytocin was much greater in cells harvested during proestrus than in cells harvested during diestrus 1. This effect was shown to be statistically significant when data were analyzed from 7 experiments from diestrous cells, and 10 experiments from proestrous cells (50). The enhanced prolactin secretion on proestrus corresponded to an enhanced intracellular Ca2+ response from lactotrophs (Fig. 4B). Indeed, both the number of lactotroph cells showing a Ca2+ response to oxytocin and the single-cell response were greater on proestrus than on diestrus 1 (50). This agrees with the observation that oxytocin receptors are up-regulated by estradiol in the anterior pituitary as shown by our lab (49) and others (51). In addition to receptor upregulation, it is likely that ovarian steroids have additional effects, since the dose-response curve of the prolactin-releasing effect of oxytocin was left-shifted in lactotrophs obtained from proestrous versus diestrus rats. Receptor upregulation alone would have no effect on the half-maximal location of the curve. Thus, it is possible that the enhanced oxytocin response on proestrus is due to an increase of anterior pituitary oxytocin receptor expression and signaling produced by the elevated estradiol levels present in the preovulatory phase of the estrous cycle.
Figure 4.
Comparison of the oxytocin-induced responses in perifused lactotrophs obtained from female rats on the morning of diestrus 1 (open symbols) and the afternoon of proestrus (closed red symbols). (A) Oxytocin application (100 nM for 10 min) evoked a larger prolactin-releasing effect from proestrus cells. Samples were collected every minute. (B) Oxytocin application (100 nM for 2 min) elicited a larger increase in the free intracellular Ca2+ concentration, as measured by the fura-2 fluorescence ratio. See (50) for details and a full description of experimental procedures.
These data show a rhythmic variation in the responsiveness of lactotrophs to oxytocin during the estrous cycle. The increased responsiveness on proestrus follows a common theme in oxytocin signaling, where the actions of oxytocin are typically preceded by an increase in responsiveness of the target tissue. For example, myometrial cells of the uterus are sensitized to the contractile effect of oxytocin immediately prior to parturition (52, 53). In the mammary gland, there is an increase in the number of oxytocin binding sites throughout gestation that remains during the period of lactation (54). Thus, the finding that the oxytocin action on lactotrophs is magnified at the time point when such enhancement is needed is consistent with a physiological role for oxytocin in the regulation of prolactin secretion.
Summary
As is the case with many hormones, prolactin secretion is often rhythmic. The particular rhythm depends on the physiological state of the animal, and the factors mediating one rhythm could be different from those mediating others. However, oxytocin and dopamine appear to be factors involved in most (if not all) of the rhythms. Much work remains to be done in the identification of the “memory” involved in the circadian prolactin rhythms initiated by mating, cervical stimulation or peripheral oxytocin/prolactin injection. This memory is also likely responsible for the phenomenon of “delayed pseudopregnancy” in the rat, in which mating (55), electrical stimulation of the cervix (56), or electrical stimulation of the hypothalamus (57) early in the estrous cycle leads to a circadian prolactin rhythm only after ovulation occurs at the end of the estrous cycle. In addition to the systems-level question of the memory, important cellular questions remain. For example, the intracellular signaling pathway involved in the oxytocin stimulation of prolactin release is largely unknown, as is the mechanism through which this is magnified on proestrus. We hope that the many questions that remain will be answered in the near future.
Acknowledgments
The authors thank past and present lab members Ruth Cristancho-Gordo, Marcel Egli, Cheryl Fitch, Jessica Kennett, De’Nise McKee, Maristela Poletini, Mike Sellix, and Natalia Toporikova for their many contributions to this research. Supported by National Institutes of Health grants DK43200 and DA19356.
References
- 1.Freeman ME. Neuroendocrine control of the ovarian cycle of the rat. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. Elsevier; 2006. pp. 2327–88. [Google Scholar]
- 2.Freeman ME, Kanyicska B, Lerant A, Nagy GM. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 3.Butcher RL, Fugo NW, Collins WC. Semicircadian rhythm in plasma levels of prolactin during early gestation in the rat. Endocrinology. 1972;90:1125–7. doi: 10.1210/endo-90-4-1125. [DOI] [PubMed] [Google Scholar]
- 4.Smith MS, Neill JD. Termination at midpregnancy of the two daily surges of plasma prolactin initiated by mating in the rat. Endocrinology. 1976;98:696–701. doi: 10.1210/endo-98-3-696. [DOI] [PubMed] [Google Scholar]
- 5.Soares MJ, Faria TN, Roby KF, Deb S. Pregnancy and the prolactin family of hormones: Coordination of anterior pituitary, uterine, and placental expression. Endocr Rev. 1991;12:402–23. doi: 10.1210/edrv-12-4-402. [DOI] [PubMed] [Google Scholar]
- 6.Soares MJ, Müller H, Orwig KE, Peters TJ, Dai GL. The uteroplacental prolactin family and pregnancy. Biol Reprod. 1998;58:273–84. doi: 10.1095/biolreprod58.2.273. [DOI] [PubMed] [Google Scholar]
- 7.Freeman ME, Neill JD. The pattern of prolactin secretion during pseudopregnancy in the rat: a daily nocturnal surge. Endocrinology. 1972;90:1292–4. doi: 10.1210/endo-90-5-1292. [DOI] [PubMed] [Google Scholar]
- 8.Freeman ME, Smith MS, Nazian SJ, Neill JD. Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology. 1974;94:875–82. doi: 10.1210/endo-94-3-875. [DOI] [PubMed] [Google Scholar]
- 9.Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W, Freeman ME. Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am J Physiol. 2006;290:E566–E72. doi: 10.1152/ajpendo.00427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helena CV, McKee DT, Bertram R, Walker AM, Freeman ME. The rhythmic secretion of mating-induced prolactin secretion is controlled by prolactin acting centrally. Endocrinology. 2009;150:3245–51. doi: 10.1210/en.2009-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Northrop LE, Erskine MS. Selective oxytocin receptor activation in the ventrolateral portion of the ventromedial hypothalamus is required for mating-induced pseudopregnancy in the female rat. Endocrinology. 2008;149:836–42. doi: 10.1210/en.2007-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:260–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–63. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- 14.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 15.DeMaria JE, Lerant AA, Freeman ME. Prolactin activates all three populations of hypothalamic neuroendocrine dopaminergic neurons in ovariectomized rats. Brain Res. 1999;837:236–41. doi: 10.1016/s0006-8993(99)01667-4. [DOI] [PubMed] [Google Scholar]
- 16.Kokay IC, Grattan DR. Expression of mRNA for prolactin receptor (long form) in dopamine and pro-opiomelanocortin neurons in the arcuate nucleus of non-pregnant and lactating rats. J Neuroendocrinol. 2005;17:827–35. doi: 10.1111/j.1365-2826.2005.01374.x. [DOI] [PubMed] [Google Scholar]
- 17.Pasqualini C, Guibert B, Frain O, Leviel V. Evidence for protein kinase C involvement in the short-term activation by prolactin of tyrosine hydroxylase in tuberoinfundibular dopaminergic neurons. J Neurochem. 1994;62:967–77. doi: 10.1046/j.1471-4159.1994.62030967.x. [DOI] [PubMed] [Google Scholar]
- 18.Ma FY, Grattan DR, Goffin V, Bunn SJ. Prolactin-regulated tyrosine hydroxylase activity and messenger ribonucleic acid expression in mediobasal hypothalamic cultures: The differential role of specific protein kinases. Endocrinology. 2005;146:93–102. doi: 10.1210/en.2004-0800. [DOI] [PubMed] [Google Scholar]
- 19.Demarest KT, Riegle GD, Moore KE. Prolactin-induced activation of tuberoinfundibular dopaminergic neurons: evidence for both a rapid 'tonic' and a delayed 'induction' component. Neuroendocrinology. 1984;38:467–75. doi: 10.1159/000123935. [DOI] [PubMed] [Google Scholar]
- 20.Bertram R, Egli M, Toporikova N, Freeman ME. A mathematical model for the mating-induced prolactin rhythm of female rats. Am J Physiol. 2006;290:E573–E82. doi: 10.1152/ajpendo.00428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annunziato L, Weiner RI. Characteristics of dopamine uptake and 3,4-hydroxyphenylacetic acid (DOPAC) formation in the dopaminergic terminals of the neurointermediate lobe of the pituitary gland. Neuroendocrinology. 1980;31:8–12. doi: 10.1159/000123043. [DOI] [PubMed] [Google Scholar]
- 22.Lookingland KJ, Jarry HD, Moore KE. The metabolism of dopamine in the median eminence reflects the activity of tuberoinfundibular neurons. Brain Res. 1987;419:303–10. doi: 10.1016/0006-8993(87)90597-x. [DOI] [PubMed] [Google Scholar]
- 23.McKee DT, Poletini MO, Bertram R, Freeman ME. Oxytocin action at the lactotroph is required for prolactin surges in cervically stimulated ovariectomized rats. Endocrinology. 2007;148:4649–57. doi: 10.1210/en.2007-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers from the suprachiasmatic nucleus innervate neuroendocrine dopaminergic neurons. Brain Res. 2001;919:48–56. doi: 10.1016/s0006-8993(01)02993-6. [DOI] [PubMed] [Google Scholar]
- 25.Gerhold LM, Sellix MT, Freeman ME. Antagonism of vasoactive intestinal peptide mRNA in the suprachiasmatic nucleus disrupts the rhythm of FRAs expression in neuroendocrine dopaminergic neurons. J Comp Neurol. 2002;450:135–43. doi: 10.1002/cne.10307. [DOI] [PubMed] [Google Scholar]
- 26.Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology. 2004;145:3386–94. doi: 10.1210/en.2003-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arey BJ, Freeman ME. Oxytocin, vasoactive intestinal peptide and serotonin regulate the mating-induced surges of prolactin secretion in the rat. Endocrinology. 1990;126:279–84. doi: 10.1210/endo-126-1-279. [DOI] [PubMed] [Google Scholar]
- 29.Vandesande F, Dierickx K. Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretory system of the rat. Cell Tissue Res. 1975;164:153–62. doi: 10.1007/BF00218970. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar DK, Frautschy SA, Mitsugi N. Pituitary portal plasma levels of oxytocin during the estrous cycle, lactation, and hyperprolactimenia. Ann NY Acad Sci. 1992;652:397–410. doi: 10.1111/j.1749-6632.1992.tb34370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumpkin MD, Samson WK, McCann SM. Hypothalamic and pituitary sites of action of oxytocin to alter prolactin secretion in the rat. Endocrinology. 1983;112:1711–7. doi: 10.1210/endo-112-5-1711. [DOI] [PubMed] [Google Scholar]
- 32.Johnston CA, Negro-Vilar A. Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology. 1988;122:341–50. doi: 10.1210/endo-122-1-341. [DOI] [PubMed] [Google Scholar]
- 33.Samson WK, Lumpkin MD, McCann SM. Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology. 1986;119:554–60. doi: 10.1210/endo-119-2-554. [DOI] [PubMed] [Google Scholar]
- 34.Liu JW, Ben Jonathan N. Prolactin-releasing activity of neurohypophysial hormones: structure-function relationships. Endocrinology. 1994;134:114–8. doi: 10.1210/endo.134.1.8275925. [DOI] [PubMed] [Google Scholar]
- 35.Chadio SE, Antoni FA. Specific oxytocin agonist stimulates prolactin release but has no effect on inositol phosphate accumulation in isolated rat anterior pituitary cells. J Mol Endocrinol. 1993;10:107–14. doi: 10.1677/jme.0.0100107. [DOI] [PubMed] [Google Scholar]
- 36.Townsend J, Cave BJ, Norman MR, Flynn A, Uney JB, Tortonese DJ, Wakerley JB. Effects of prolactin on hypothamic supraoptic neurones: evidence for modulation of STAT5 expression and electrical activity. Neuro Endocrinol Lett. 2005;26:125–30. [PubMed] [Google Scholar]
- 37.Kokay IC, Bull PM, Davis RL, Ludwig M, Grattan DR. Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. Am J Physiol. 2006;290:R1216–R25. doi: 10.1152/ajpregu.00730.2005. [DOI] [PubMed] [Google Scholar]
- 38.Moos F, Richard P. Level of oxytocin release induced by vaginal dilation (Ferguson reflex) and vagal stimulation (vago-pituitary reflex) in lactating rats. J Physiol (Paris) 1975;70:307–14. [PubMed] [Google Scholar]
- 39.Freeman ME, McKee DT, Egli M, Bertram R. Biological and mathematical modeling approaches to defining the role of oxytocin and dopamine in the control of mating-induced PRL secretion. In: Bridges RS, editor. Neurobiology of the Parental Brain. Elsevier; 2008. pp. 235–47. [Google Scholar]
- 40.Kendrick KM, Keverne EB, Hinton MR, Goode JA. Cerebrospinal fluid and plasma concentrations of oxytocin and vasopressin during parturition and vaginocervical stimulation in the sheep. Brain Res Bull. 1991;26:803–7. doi: 10.1016/0361-9230(91)90178-m. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson J. A study of the motility of the intact uterus at term. Surgical Gynecology and Obstetrics. 1941;73:359–66. [Google Scholar]
- 42.Lippert TH, Mueck AO, Seeger H, Pfaff A. Effects of oxytocin outside pregnancy. Horm Res. 2003;60:262–71. doi: 10.1159/000074243. [DOI] [PubMed] [Google Scholar]
- 43.Mangurian LP, Walsh RJ, Posner BI. Prolactin enhancement of its own uptake at the choroid plexus. Endocrinology. 1992;131:698–702. doi: 10.1210/endo.131.2.1639017. [DOI] [PubMed] [Google Scholar]
- 44.Walsh RJ, Slaby FJ, Posner BI. A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology. 1987;120:1846–50. doi: 10.1210/endo-120-5-1846. [DOI] [PubMed] [Google Scholar]
- 45.Neill JD, Smith M. Pituitary-ovarian interrelationships in the rat. In: James VHT, Martini L, editors. Current Topics in Experimental Endocrinology. New York: Academic Press; 1974. pp. 73–106. [PubMed] [Google Scholar]
- 46.Yamaguchi K, Asaishi T, Negoro H. Effect of oestrogen treatment on plasma oxytocin and vasopressin in ovariectomized rats. Endocrinologica Japonicum. 1979;26:197–205. doi: 10.1507/endocrj1954.26.197. [DOI] [PubMed] [Google Scholar]
- 47.Sarkar DK, Gibbs DM. Cyclic variation of oxytocin in the blood of pituitary portal vessels of rats. Neuroendocrinology. 1984;39:481–3. doi: 10.1159/000124024. [DOI] [PubMed] [Google Scholar]
- 48.Sarkar DK. Immunoneutralization of oxytocin attenuates preovulatory prolactin secretion during proestrus in the rat. Neuroendocrinology. 1988;48:214. doi: 10.1159/000125012. [DOI] [PubMed] [Google Scholar]
- 49.Kennett JE, Poletini MO, Fitch CA, Freeman ME. Antagonism of oxytocin prevents suckling- and estradiol-induced, but not progesterone-induced, secretion of prolactin. Endocrinology. 2009;150:2292–9. doi: 10.1210/en.2008-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabak J, Gonzalez-Iglesias AE, Toporikova N, Bertram R, Freeman ME. Variations in the response of pituitary lactotrophs to oxytocin during the rat estrous cycle. Endocrinology. 2010;151:1806–13. doi: 10.1210/en.2009-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breton C, Pechoux C, Morel G, Zingg HH. Oxytocin receptor messenger ribonucleic acid: characterization, regulation, and cellular localization in the rat pituitary gland. Endocrinology. 1995;136:2928–36. doi: 10.1210/endo.136.7.7540544. [DOI] [PubMed] [Google Scholar]
- 52.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 53.Larcher A, Neculcea J, Breton C, Arslan A, Rozen F, Russo C, Zingg HH. Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology. 1995;136:5350–6. doi: 10.1210/endo.136.12.7588281. [DOI] [PubMed] [Google Scholar]
- 54.Breton C, Di Scala-Guenot D, Zingg HH. Oxytocin receptor gene expression in rat mammary gland: structural characterization and regulation. J Mol Endocrinol. 2001;27:175–89. doi: 10.1677/jme.0.0270175. [DOI] [PubMed] [Google Scholar]
- 55.Zeilmaker GH. Normal and delayed pseudopregnancy in the rat. Acta Endocrinologica. 1965;49:558–66. doi: 10.1530/acta.0.0490558. [DOI] [PubMed] [Google Scholar]
- 56.Beach JE, Tyrey L, Everett JW. Serum prolactin and LH in early phases of delayed versus direct pseudopregnancy in the rat. Endocrinology. 1975;96:1241–6. doi: 10.1210/endo-96-5-1241. [DOI] [PubMed] [Google Scholar]
- 57.Quinn DL, Everett JW. Delayed pseudopregnancy induced by selective hypothalamic stimulation. Endocrinology. 1967;80:155–62. doi: 10.1210/endo-80-1-155. [DOI] [PubMed] [Google Scholar]