Abstract
Green tea and its major polyphenols constituents, tea catechins, have been shown to have many health benefits including cancer prevention. Tea catechins and tea catechin metabolites/catabolites are bioavailable in the systemic circulation after oral intake of green tea or green tea catechins. The metabolites/catabolites identified in humans include glucuronide/sulfate conjugates, methylated tea catechin conjugates, and microflora-mediated ring fission products and phenolic acid catabolites. Plasma levels of unchanged tea catechins in humans are mostly in the sub-μM or nM concentration range, which is much lower than the effective concentrations determined in most in vitro studies. However, some of the catechin metabolites/catabolites are present in the systemic circulation at levels much higher than those of the parent catechins. The contribution of catechin derived metabolites/catabolites to the biological effects associated with green tea is yet to be defined. A limited number of chemoprevention trials of green tea or green tea catechins have been conducted to date and have observed potential preventive activity for oral, prostate, and colorectal cancer. Emerging data from multiple ongoing intervention trials will further contribute to defining the cancer preventive activity of green tea or green tea catechins.
1. Introduction
Tea is a beverage made from the leaves of the Camellia sinensis species of the Theaceae family. This beverage is one of the most ancient and, next to water, the most widely consumed liquid in the world. Tea leaves contain specific polyphenols and polyphenol oxidase. Following harvesting, fresh tea leaves are subjected to a series of treatment steps that result in the manufacturing of different tea products: black tea, green tea, or oolong tea.
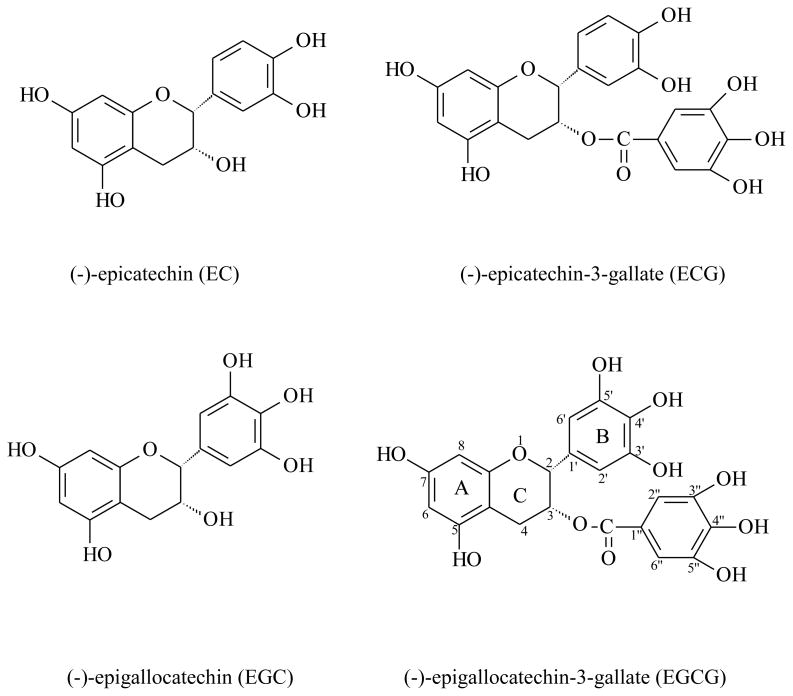
Green tea is made by steaming or frying fresh tea leaves at elevated temperatures to prevent polyphenol oxidation. The chemical composition of green tea is similar to that of the fresh leaves regarding the major components. It contains polyphenols, which include flavanols, flavandiols, flavonoids, and phenolic acids. Flavanols are the most abundant constituents and are commonly known as catechins. The major catechins present in green tea are (−)-epicatechin (EC), (−)-epicatechin-3-gallate (ECG), (−)-epigallocatechin (EGC), and (-)-epigallocatechin-3-gallate (EGCG) (Figure 1). In addition, caffeine, theobromine, theophylline, and phenolic acids such as gallic acids are also present in green tea (Table 1). Black tea is made by promoting enzymatic oxidation of fresh leaves. Most flavanols are converted to the oxidized form known as theaflavins and thearubigins. The total flavanol level is reduced from 35–50% in green tea to 10% in black tea. Theaflavins and thearubigins are present in black tea at a level of 3–6% and 12–18%, respectively [1]. All other components are virtually unchanged. Oolong tea is a half-fermented product. It contains monomeric catechins, theaflavins, and thearubigins, with a catechin level of 8–20% of the total dry matter.
Figure 1.
Chemical structures of major green tea catechins.
Table 1.
Principal components of green and black tea beverage (measured in weight % of extract solids)
| Components | Green tea | Black tea |
|---|---|---|
| Catechins | 30–42 | 3–10 |
| Flavanols | 5–10 | 6–8 |
| Other flavonoids | 2–4 | - |
| Theogallin | 2–3 | - |
| Ascorbic acid | 1–2 | - |
| Gallic Acid | 0.5 | - |
| Quinic acid | 2 | - |
| Other organic acids | 4–5 | - |
| Theanine | 4–6 | - |
| Other amino acids | 4–6 | 13–15 |
| Methyxanthines | 7–9 | 8–11 |
| Carbohydrates | 10–15 | 15 |
| Minerals | 6–8 | 10 |
| Volatiles | 0.02 | <0.1 |
| Theaflavins | - | 3–6 |
| Thearubigins | - | 12–18 |
| Caffeine | 3–4 | 3–4 |
Adapted from Katiyar, S.K. and Mukhtar, H., World Rev. Nutr. Diet., 79, 154, 1996.
Tea consumption is not uniform throughout the world. Large segments of the world's population virtually consume no tea. Not only does tea consumption vary from country to country, but there is also enormous variation in any given population. Extensive laboratory research and epidemiological findings of the past 20 years have suggested that tea or tea components may reduce the risk of a variety of illnesses, including cancer and coronary heart disease. A number of prior reviews [2–6] and reviews in this special issue have summarized the findings.
The epidemiological evidence of the protective effect of tea consumption against the development of human cancers is not always conclusive. As reviewed in this special issue, some studies have shown a protective effect of tea consumption against certain types of cancers, while others found no association between tea consumption and cancer risk. Controlled intervention trials are clearly necessary to define the role of tea or its constituents in cancer prevention. The following sections summarize the clinical pharmacokinetics of tea constituents and results from limited chemoprevention trials.
2. Pharmacokinetic Studies
Several human pharmacokinetic studies of tea catechins have been performed by us and other investigators. An early study by Yang et al. [7] determined the blood and urine levels of tea catechins after ingestion of different amounts of decaffeinated green tea extract (DGT) by human volunteers. In this study, one gram of the DGT contained 73 mg EGCG, 68 mg EGC, 22 mg ECG, and 25 mg EC. After consumption of 1.5 g of DGT, the catechins in human plasma reached peak levels in 1.5 to 2.5 hours. The average peak plasma concentration (Cmax) of EGCG, EGC, and EC was 0.71, 1.8, 0.65 μM, respectively. When the dosage was increased from 1.5 to 3.0 g, the Cmax values increased 2.7 to 3.4 fold. However, increasing the dose to 4.5 g didn't increase the Cmax values significantly. It is likely that constituents in the green tea extract were not completely dissolved in the gastrointestinal fluid when the dose was increased to 4.5 g. Terminal half-lives of EGCG, EGC, and EC after oral administration of 1.5 to 4.5g of DGT were 4.9 to 5.5, 2.5 to 2.8, and 3.2 to 5.7 h, respectively. It should be noted that the tea catechin levels reported in this study were expressed as the sum of unchanged and conjugated catechins and the pharmacokinetic parameters were derived based on these hybrid values.
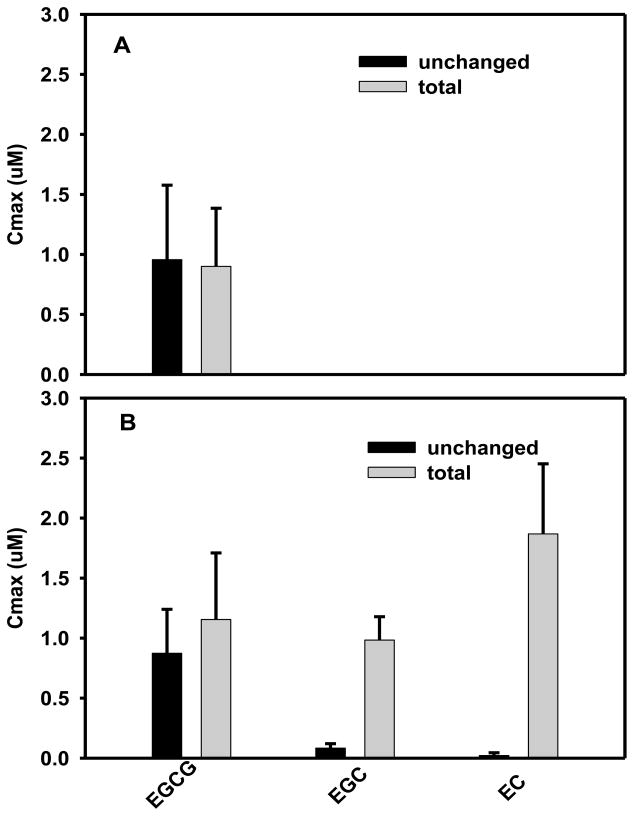
We conducted a clinical pharmacokinetic study of tea catechins following single-dose administration of EGCG or Polyphenon E [8]. Polyphenon E is a defined and decaffeinated green tea catechin extract that contains 80–98% total catechins with EGCG as the main component accounting for 50–75% of the material. Each Polyphenon E capsule used for this study contained 200 mg EGCG, 37 mg EGC, 31 mg EC, and other green tea constituents. We found that, in equivalent EGCG doses, both formulations resulted in similar pharmacokinetics of EGCG. The average Cmax of unchanged EGCG were 0.16, 0.24, 0.37, 0.96 μM after 200, 400, 600, and 800 mg dose of EGCG, respectively. The average Cmax of unchanged EGCG were 0.16, 0.27, 0.36, 0.82 μM after Polyphenon E administration at doses that contained 200, 400, 600, and 800 mg EGCG, respectively. Figure 2 illustrates the peak plasma tea catechin concentration following a single dose administration of 800 mg EGCG or Polyphenon E that contained 800 mg EGCG. Following EGCG administration, only EGCG was detected in human plasma. EGCG levels did not change significantly after the plasma samples were treated with deconjugating enzymes (β-glucuronidase/sulfatase), suggesting that EGCG is present in plasma mostly as the unchanged form. After Polyphenon E administration, EGCG levels were detected in human plasma, while unchanged EGC and EC levels were low or undetectable. After the samples have been treated with deconjugating enzymes, EGCG levels did not change much, whereas EGC and EC levels increased substantially. Similar observation was also reported in the study performed by Lee et al. [9]. In their study, healthy human subjects were instructed to drink green tea in warm water containing 200 mg EGCG, 154 mg EGC, and 45 mg EC. Large percent of EGCG (77%) was present in the unchanged form, whereas 31% of EGC and 21% of EC were in the unchanged form at one hour after tea consumption.
Figure 2.
Peak plasma tea catechin concentrations following a single dose administration of 800 mg EGCG (A) or Polyphenon E containing 800 mg EGCG, 148 mg EGC, 128 mg EC, and other green tea polyphenols (B) [8]. Black bars are data obtained from plasma samples without subjecting to glucuronidase/sulfatase treatment, representing unchanged catechins. Grey bars are data obtained from plasma samples treated with glucuronidase/sulfatase, representing total catechins (sum of unchanged catechins and catechin glucuronides/sulfates).
In our follow up study, we determined the pharmacokinetics of green tea catechins following 4 weeks of daily EGCG or Polyphenon E administration [10]. The study participants received 800 mg EGCG once/day, 400 mg EGCG twice/day, 800 mg EGCG as Polyphenon E once/day, or 400 mg EGCG as Polyphenon E twice/day. We found that there was a greater than 60% increase in the area under the plasma EGCG concentration-time curve (a measurement of systemic availability) after 4 weeks of EGCG or Polyphenon E treatment at a dosing schedule of 800 mg once daily. The observed increase in the systemic exposure of EGCG is not related to drug accumulation after repeated dosing, because the AUC calculation has corrected for this factor. No significant changes were observed in the pharmacokinetics of EGCG after repeated green tea catechin treatment at a regimen of 400 mg twice daily. The mechanism(s) responsible for the observed increase in the systemic exposure of EGCG after repeating dosing at a high daily bolus dose remain(s) to be studied. Reduction in non-enzymatic degradation, saturation of pre-systemic Phase II metabolism, and/or inhibition in intestinal flora metabolism are plausible contributing mechanisms. It is not known whether ingestion of green tea catechins at a high daily bolus dose for greater than 4 weeks will result in further enhancement in the systemic bioavailability of EGCG.
We further conducted a study to determine the effect of dose and dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in humans [11]. The study evaluated the pharmacokinetics of tea catechins at three different Polyphenon E doses (400, 800, and 1,200 mg based on the EGCG content) and under both fed and fasting conditions. Table 2 summarizes the effect of dose and dosing condition on the peak plasma tea catechin concentrations. The area under the plasma concentration time curve exhibited changes by the dose and dosing condition similar to that of the peak plasma level. Consistent with previous findings [8, 9], gallated catechins, EGCG and ECG, were present in plasma mostly as the unchanged form, whereas nongallated catechins, EGC and EC, were mostly present as the glucuronide and sulfate conjugates. There was a 3–5 fold increase in plasma levels of EGCG and ECG when Polyphenon E was taken on an empty stomach after an overnight fast than when taken with food. Taking Polyphenon E on an empty stomach after an overnight fast did not have a significant effect on the plasma levels of total (unchanged plus glucuronide and sulfate conjugates) EGC, but resulted in lower plasma levels of total EC. The data also showed that there was a more than proportional increase in the peak plasma levels of EGCG and ECG as the dose level was increased. In addition, the dose normalized plasma levels of unchanged EGCG and ECG were higher than that of unchanged EGC and EC under both dosing conditions. The dose normalized plasma levels of total EGC and EC were higher than that of total EGCG and ECG under fed condition, but were at similar levels under fasting condition. The study suggested that the systemic bioavailability of tea catechins are affected by dose and dosing condition. The mechanism(s) responsible for the observed changes remain(s) to be studied. Saturation of pre-systemic metabolism at higher doses, depletion of Phase II enzymes/cofactors after an overnight fast, and/or disparity in gastric stability, dissolution rate, and food interaction under different dosing conditions are potential contributing factors. It is worth noting that taking Polyphenon E on an empty stomach at higher doses is associated with a higher incidence of gastric discomfort.
Table 2.
Average peak plasma concentrations of green tea catechins after single-dose administration of three different Polyphenon E doses (2, 4, and 6 capsules; each capsule contained 200 mg EGCG, 48.5 mg EGC, 34.2 mg EC, and 20 mg ECG) under both fed and fasting conditions [11].
| Fed | Fasting | |||||||
|---|---|---|---|---|---|---|---|---|
| Unchanged catechin | Total catechin1 | Unchanged catechin | Total catechin | |||||
| Dose (mg) | Cmax (μM) | Cmax/ Dose (nM/mg) | Cmax (μM) | Cmax/ Dose (nM/mg) | Cmax (μM) | Cmax/ Dose (nM/mg) | Cmax (μM) | Cmax/ Dose (nM/mg) |
| EGCG | ||||||||
| 400 | 0.309 | 0.77 | 0.380 | 0.95 | 1.742 | 4.35 | 1.667 | 4.17 |
| 800 | 0.641 | 0.80 | 0.785 | 0.98 | 3.321 | 4.15 | 3.538 | 4.42 |
| 1,200 | 2.015 | 1.68 | 1.902 | 1.59 | 7.355 | 6.13 | 8.700 | 7.25 |
| ECG | ||||||||
| 40 | 0.035 | 0.89 | 0.048 | 1.20 | 0.198 | 4.95 | 0.196 | 4.91 |
| 80 | 0.075 | 0.93 | 0.110 | 1.37 | 0.394 | 4.93 | 0.447 | 5.58 |
| 120 | 0.255 | 2.12 | 0.266 | 2.21 | 0.865 | 7.21 | 1.019 | 8.49 |
| EGC | ||||||||
| 97 | 0.052 | 0.53 | 0.420 | 4.33 | 0.130 | 1.33 | 0.267 | 2.75 |
| 194 | 0.111 | 0.57 | 0.578 | 2.97 | 0.242 | 1.24 | 0.442 | 2.28 |
| 291 | 0.140 | 0.48 | 0.654 | 2.25 | 0.428 | 1.47 | 1.055 | 3.62 |
| EC | ||||||||
| 68.4 | 0.006 | 0.08 | 0.538 | 7.87 | 0.019 | 0.28 | 0.324 | 4.73 |
| 136.8 | 0.010 | 0.07 | 0.806 | 5.89 | 0.007 | 0.05 | 0.516 | 3.77 |
| 205.2 | 0.035 | 0.17 | 1.192 | 5.80 | 0.057 | 0.28 | 0.938 | 4.57 |
Total catechin concentration reflects the sum of unchanged catechin and catechin glucuronides/sulfates.
Henning et al. compared the pharmacokinetics of green tea catechins after consumption of green tea, black tea, or a decaffeinated green tea extract in humans [12]. Each preparation was administered at levels that provided similar amounts of EGCG. Standardization of the EGCG content in the tea preparations affected the contents of the other tea constituents. They found that peak plasma catechin concentrations were achieved at 1.2–1.5 hrs when consumed as black or green tea beverage. The peak plasma levels were achieved at 2.5–2.8 hrs when administered as a green tea supplement in oral capsules, probably due to the delay in disintegration/dissolution of the oral capsules. Despite of the delay in oral absorption when administered as oral capsules, higher catechin peak levels were observed compared with when consumed as black or green tea beverage. This observation suggests that other components in green or black tea beverage may decrease the extent of tea catechin absorption. It is also plausible that subjects can more rapidly swallow the capsules and thus be getting a bolus dose whereas drinking tea beverage takes more time and thus representing a slower infusion.
Little information is available on the pharmacokinetics of black tea polyphenols. A pilot study showed that the peak plasma theaflavins concentration was only 2–7 nM after consuming 700 mg mixed theaflavins (equivalent to about 30 cups of black tea) [13]. The very low systemic bioavailability of theaflavins after oral ingestion may be attributed to their large molecular structures. The pharmacokinetics of thearubigins has not been studied because this class of compounds is still largely uncharacterized.
Collectively, the available data showed that tea catechins are absorbed and eliminated rapidly in humans. Peak plasma concentrations were achieved between 1–3 hrs after oral administration and reached total catechin concentrations in the sub- or low μM range. With a half-life of 2–4 hrs, the parent catechins do not appear to accumulate in the systemic circulation.
2.1 Oral Absorption and Bioavailability
Most of the absorption and oral bioavailability studies of green tea catechins were performed in laboratory animals. Chen et al. [14] compared the plasma pharmacokinetics of EGCG in rats after intravenous (10 mg/kg) and oral (75 mg/kg) dosing and found that the oral bioavailability of EGCG was 1.6%. In this study, DGT containing 73, 68, and 27 mg/g of EGCG, EGC, and EC, respectively, was also administered to rats via intravenous (25 mg/kg) and oral (200 mg/kg) routes. The oral bioavailability was found to be 0.1%, 13.7%, and 31.2% for EGCG, EGC, and EC, respectively, following DGT administration. The authors suggested that the difference in oral bioavailability of EGCG after pure EGCG and DGT administration is due to the effect of other components in DGT on the oral absorption of EGCG. However, the dose normalized systemic exposure of EGCG was different between intravenous dosing of pure EGCG and DGT. This study determined the oral bioavailability based on the combined unchanged and conjugated catechin concentrations which could have complicated the interpretation of the bioavailability data because more conjugated metabolites could be formed during pre-systemic first-pass metabolism after oral catechin administration. Lambert et al. determined the pharmacokinetics of EGCG in mice after oral and intravenous dosing [15]. The oral bioavailability of unchanged EGCG was found to be 15.8%. We compared the systemic exposure of EGCG in rats after intravenous and intraportal administration and found that the area under the plasma concentration-time curve of EGCG was similar between the two routes of administration, suggesting that tea catechins do not undergo significant pre-systemic hepatic metabolism [16]. Consistently, when EGCG was administered at a dose of 100 mg/kg to rats by intraperitoneal injection, much higher dose normalized plasma concentrations of free EGCG were observed [17]. These studies suggest that pre-systemic loss/metabolism within the GI tract may contribute more significantly to the low oral bioavailability of green tea catechins.
Recent clinical studies have examined tea catechin absorption in the small intestine in individuals with an ileostomy. Auger et al. [18] determined the tea catechin levels in ileal fluid collected for 24 hrs after ingestion of 200 mg of Polyphenon E. They showed that the average recovery was 27% for the nongallated catechins, EC and EGC, and was 59% for the gallated catechins, EGCG and ECG, in the ileal fluid. Stalmach et al. [19] found similar levels of recovery for the parent catechins in the ileal fluid after green tea consumption. In vitro studies in gastric juice and ileal fluid indicated that tea catechins are stable in these fluid over a 4 h period at 37°C [18]. Therefore, the recoveries in ileal fluid imply that nongallated catechins are absorbed from the small intestine more efficiently than their gallated analogs. The data also indicate that in healthy humans with an intact colon, substantial quantities of the green tea catechins would pass from the small intestine to the large intestine, where they will subject to breakdown by colonic bacteria.
2.2 Metabolism
Tea catechins have been shown to undergo extensive biotransformation via methylation, sulfation, and glucuronidation reactions. Multiple tea catechin metabolites were detected in human plasma and urine after oral green tea or green tea extract administration. Glucuronides/sulfates of EGCG, EGC, and EC were detected in plasma and that of EGC and EC were present in urine after oral ingestion of DGT or green tea catechins in humans [8, 20]. After oral administration of DGT, the major metabolites appeared in human urine included not only the glucuronides and sulfates of EGC and EC but also the O-methyl-EGC-O-glucuronides/sulfates and O-methyl-EC-O-sulfates [21]. The methylated EGC conjugates were also detected in human plasma after oral DGT administration [22]. However, no methylated EGC was detected either in plasma or urine in another clinical study [23].
In addition to Phase II metabolites, metabolites that derive from microflora mediated catabolic processes have been identified in human plasma and urine. Earlier studies have identified 5-(3′, 4′, 5′-trihydroxyphenyl)-γ-valerolactone (M4) and 5-(3', 4'-dihydroxyphenyl)-γ-valerolactone (M6) as catechin-derived microflora metabolites [24]. These were found to be present in the conjugated form. M4 and small amounts of M6 were detected and only M6 was detected in human urine after pure EGC or EC oral administration, respectively. The study results suggest that these metabolites are produced by intestinal microorganisms, with EGC and EC as the precursors of M4 and M6, respectively, and subsequently absorbed into the systemic blood and excreted in the urine. The urinary recovery of these two metabolites accounted for 6–39% of the ingested EGC and EC. After oral administration in rats, EGCG has been shown to be metabolized by intestinal microflora to form EGC and 5-(3',5'-dihydroxyphenyl)-γ-valerolactone [25]. However, when EGCG was administered orally in humans, EGC was not found in blood or urine [8].
Recently, HPLC with multi-stage mass spectrometry detection has been utilized to facilitate the identification of a range of additional metabolites. Stalmach et al. [26] analyzed the catechin metabolite levels in plasma and urine collected over 24 hours after consuming 500 ml Choladi green tea (consisting of 78.6 mg EGC, 105.3 mg EGCG, 16.8 mg EC, 21.7 mg ECG). A total 10 metabolites, in the form of O-methylated, sulfated, and glucuronide conjugates of EC and EGC, with 29-126 nM peak plasma concentrations, were identified in human plasma. Unchanged EGCG and ECG were also detected with respective peak plasma values of 55 and 25 nM. Fifteen metabolites of EC and EGC were detected in urine, in the form of O-methylated, sulfated, and glucuronide conjugates, but EGCG and ECG were not detected in urine. The overall urinary excretion of the Phase II catechin metabolites accounted for about 8% of the total catechin intake. A follow up green tea feeding study by the same research group was carried out using human volunteers with an ileostomy [19]. The ileal fluid contained around 70% of the ingested catechins in the form of the parent compounds (33%) and 23 metabolites (37%). The main metabolites effluxed back into the lumen of the small intestine were sulfates and methyl sulfates of EC and EGC. This indicates that in subjects with a functioning colon, substantial quantities of catechins pass from the small intestine to the large intestine. Once the catechins enter into the colon, the microflora are capable of hydrolyzing and removing conjugated moieties, such as glucuronides and sulfates. The released aglycones can be catabolized to ring fission products and low molecular weight phenolic acids. Roowi et al. determined the urinary excretion of phenolic acid catabolites after green tea consumption [27]. Two low molecular weight phenolic acids, pyrocatechol and pyrogallol, believed to be derived from the gallic acid moiety of the gallated catechins, were detected in human urine after green tea consumption. The combined amount of pyrocatechol and pyrogallol detected in urine is equivalent to 47% of the gallated catechins detected in ileal fluid [19, 27]. Other urinary catabolites, excreted in significantly higher amounts after green tea consumption are 5-(3,4,5-trihydroxyphenyl)-γvaleric acid, 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, and 4-hydroxybenzoic acid. Additional potential catabolites include 3-methoxy-4-hydroxyphenylacetic acid, 4-hydroxyphenylacetic acid, and hippuric acid. Quantitatively, the phenolic acid catabolites may account for 40% of the catechin intake, pointing to the importance of the colonic absorption in the overall bioavailability. In addition, these catabolites could contribute to the chemopreventive activity.
2.3 Tissue Distribution
Tissue distribution of tea catechins has been mostly studied in laboratory animals. Tissue distribution of EGCG was determined in rats 1 hour after a single oral dose of EGCG at 500 mg/kg. The highest level of unchanged EGCG was found in the small intestine mucosa (565 μM), followed by colon mucosa (69 μM), liver (48 μM), plasma (12 μM), and brain (0.5 μM) [28]. Tea catechin tissue distribution was also determined in rats when 0.6% of GTP was dosed for 8 days [29]. In this study, tea catechin concentrations were presented as the total of unchanged and conjugated tea catechin levels. Substantial amounts of EGC and EC were found in the bladder (2–3 μM), large intestine (1–3 μM), kidney (1–2 μM), lung (0.5–1 μM), and esophagus (0.5–0.7 μM). Levels of EGC and EC were low in the spleen, liver, thyroid, and heart. The amount of EGCG was higher in large intestine (1.1 μM), esophagus (0.61 μM), and bladder (0.44 μM) and lower in kidney, prostate, spleen, liver, and lung. Tea catechin liver and lung distribution was also determined in mice when 0.6% of GTP was dosed for 12 days [29]. The concentration of EGCG was higher than EGC and EC in the lung, whereas EGC was slightly higher than EGCG in the liver. However, at any time, tea catechin concentrations in the lung were higher than those in the liver. In both tissues, tea catechins peaked on Day 4 and then declined to Day 12.
Studies on the tissue distribution of tea catechins in humans are limited. Henning et al. [30] conducted the first clinical study to determine the tea catechin levels in prostate tissue. In this study, men scheduled to undergo prostatectomy were assigned to consume 5 cups of green tea, black tea, or a caffeine-matched soda each day for 5 days prior to surgery. The four major tea catechins were detected in the prostate tissue after green tea or black tea consumption with mean concentrations ranging from 21 to 107 pmol/g tissue. A follow up study by the same group showed that 4”-O-methyl EGCG was present in the prostate tissue in amounts similar to that of EGCG [31]. EGCG has been shown previously to convert to 4”-O-methyl EGCG and 4’, 4”-dimethyl EGCG by liver cytosolic catechol O-methyltransferase [32]. However, only trace amounts of 4”-O-methyl EGCG were detected in human plasma after green tea consumption in a reported study [33] and in our unpublished data, suggesting that 4”-O-methyl EGCG detected in the human prostate may derive from methylation of EGCG in the target tissue. Consistent with this hypothesis, Henning’s group was able to show that LNCaP prostate cancer cells were able to methylate EGCG to 4”-O-methyl EGCG [31]. Nevertheless, 4”-O-methyl EGCG has reduced activity in inhibiting cell proliferation and NF-κB activation and in inducing apoptosis when compared to the parent catechin. Therefore, genetic polymorphisms of catechol O-methyltransferase may affect the methylation status of EGCG and subsequently modulate its preventive effect on prostate cancer and possibly other cancers. Further studies on the tissue disposition of tea catechins and catechin-derived metabolites are warranted to delineate the moieties responsible for target tissue activities.
3. Chemoprevention Trials
Despite mounting preclinical evidence to support the cancer preventive activity of tea catechins, only limited chemoprevention trials have been completed to date. These studies are summarized below by organ site.
3.1 Oral Leukoplakia
Oral cancer is a worldwide health problem with more than 170,000 cases reported annually [34]. It is associated with severe morbidity and the 5-year overall survival rate is less than 50% [35]. Li et al. [36] conducted a double-blind, randomized, placebo-controlled intervention trial in 59 patients with oral leukoplakia using a mixed tea products. The mixed tea is composed of a dried mixture of the whole water extract of green tea, green tea polyphenols (40%), and tea pigments in the ratio of 4:1:1. Tea pigments are the oxidized product of 40% green tea polyphenols and are composed primarily of theaflavins and thearubigins. Participants in the tea-treated group took 3 g/d of mixed tea in oral capsules in 4 divided doses and applied an ointment that contains 10% mixed tea in glycerin t.i.d. Tea extract taken in divided doses is likely to result in nM peak plasma catechin concentrations based on the available pharmacokinetic information. The tissue distribution of tea constituents to the oral mucosa from oral dosing has not been previously studied. Applying the tea extracts directly to the lesions may help improve the local concentrations of the active constituents. Participants in the placebo group received the same amount of starch capsules and applied the same amount of starch containing glycerin as the tea-treated group. After 6 months of intervention, the lesion size was decreased in 37.9 % and increased in 3.4% of the tea-treated patients; whereas the lesion size was decreased in 10.0% and increased in 6.7% of the placebo treated patients. The pathological results showed significant decrease in the number and total volume of the silver-stained nucleolar organizer regions and the proliferation cell nuclear antigen in oral mucosa of the treated group compared to the control group.
Recently, Tsao et al. [37] reported a Phase II double-blind, randomized, placebo-controlled trial of green tea extract in patients with high risk oral premalignant lesions. Forty-one patients were randomized to receive placebo (n = 11), green tea extract at 0.5 g/m2 (n = 11), green tea extract at 0.75 g/m2 (n = 9), and green tea extract at 1 g/m2 (n = 10) three times daily for 12 weeks. The green tea extract contains 26.9% catechins and 6.8% caffeine. The dose levels and dosing schedule employed in this study are likely to result in nM peak plasma catechin concentrations. The intervention was well tolerated, although higher doses increased insomnia/nervousness, most likely due to the caffeine content. The clinical response rate was higher in all green tea arms (n = 28; 50%) versus placebo (n = 11, 18.2%; p = 0.09) but did not reach statistical significance. The two higher-dose green tea arms had higher responses, suggesting a dose-response effect. Green tea treatment also improved histology response, although not statistically significant. Stromal VEGF and cyclin D1 expression were down-regulated in clinically responsive green tea treated patients and up-regulated in nonresponsive patients at 12 weeks, suggesting that green tea may suppress oral premalignant lesion, in part, through inhibition of angiogenesis.
The results from the completed studies support a potential role of tea for oral cancer prevention with nM plasma catechin concentrations. In addition to these completed studies, there is an ongoing trial designed to determine the oral cancer preventive activity of green tea extract in combination with the Erlotinib (NCT01116336).
3.2 Lung Cancer
Tobacco exposure is implicated in almost 90% of lung carcinomas. One of the genotoxic effects of tobacco is oxidative DNA damage induced by reactive oxygen species and 8-hydroxydeoxyguanosine (8OHdG) is probably one of the most abundant DNA lesions formed during oxidative stress [38]. We completed a phase II randomized, controlled tea intervention trial to study the effect of high consumption of decaffeinated green or black tea on oxidative DNA damage, as measured by urinary 8-OHdG, among smokers [39]. In this study, 133 heavy smokers were randomized to drink 4 cups/day of either decaffeinated green or black tea or water for 4 months. The total polyphenols in the decaffeinated green tea and black tea were 146 and 112 mg/cup, respectively. The total catechins in the decaffeinated green tea and black tea were 73 and 8 mg/cup, respectively. The tea consumption level employed in this study is likely to result in nM peak plasma catechin concentrations. We showed that smokers in the green tea group had a highly significant decrease in urinary 8OHdG (−31%) after 4 months of drinking decaffeinated green tea (p = 0.002). No change in urinary 8OHdG was seen among smokers in the black tea group.
In subsequent analyses, we showed that polymorphism in glutathione S-transferase (GST) modulates the intervention effects on 8OHdG [40]. GSTs are involved in the defenses against carcinogen detoxification and oxidative stress. Reduction in 8OHdG was observed in GSTM1-positive (p = 0.006) and GSTT1-positive (p = 0.004) green tea groups, but not in the GSTM1-negative (P = 0.07) or GSTT1-negative (P = 0.9) green tea groups. We also analyzed the effect of hOGG1 polymorphism on the intervention effect [41]. hOGG1 gene is involved in the excision repair of 8OHdG from oxidatively damaged DNA. We showed that green tea intervention resulted in reduction of urinary 8OHdG levels in all GSTM1-positive smokers regardless of their hOGG1 genotype. Collectively, our findings suggest that green tea intervention might be highly effective in decreasing DNA damage in the subgroup of smokers who are GSTM1 positive regardless of their hOGG1 genotype.
There are a number of ongoing trials designed to determine the lung cancer preventive activity of green tea or green tea extract in high risk cohorts (NCT00363805, NCT00573885, NCT00611650).
3.3 Prostate Cancer
According to the American Cancer Society, prostate cancer in the most diagnosed malignancy in men and the second leading cause of cancer mortality in men in the United States. Bettuzzi et al. [42] conducted a randomized, double-blind, placebo-controlled clinical study to evaluate the prostate cancer preventive activity of green tea catechins. Sixty patients with high grade prostate intraepithelial neoplasia were randomly assigned to receive 200 mg of green tea catechins (51.8% EGCG, 5.5% EGC, 12.24% EC, 6.12% ECG with <1% caffeine) or placebo three times a day. This dose regimen is likely to result in nM peak plasma tea catechin concentrations. Following 1 year of intervention, only one prostate cancer case was diagnosed among the green tea catechin-treated men (incidence, 3.3%), whereas nine prostate cancer cases were diagnosed among the placebo-treated men (incidence, 30%). Total prostate-specific antigen (PSA) did not change significantly between the two arms. Although the clinical response is dramatic and highly promising, the data need to be validated in larger randomized trials.
McLarty et al. [43] conducted an open label trial to determine the effects of short-term Polyphenon E supplementation on serum biomarkers in patients with prostate cancer. Twenty-six men with positive prostate biopsies scheduled for radical prostatectomy were given daily doses of Polyphenon E (a total of 1,300 mg tea polyphenols with 800 mg as EGCG) until the time of prostatectomy (median duration of 34.5 days). The pharmacokinetics of Polyphenon E given at this dose level have been well characterized by our research group [8][10]. The dosing regimen employed in this study resulted in peak plasma tea catechin concentrations in the sub- or low μM concentration range. Polyphenon E intervention resulted in favorable changes in serum levels of cytokines and growth factors relevant to prostate cancer progression, including PSA, hepatocyte growth factor, vascular endothelial growth factor, and insulin-like growth factor axis. Although the study is limited by the lack of a placebo arm, the data support a potential role for Polyphenon E in prostate cancer prevention or treatment.
In addition to these completed studies, there are a number of ongoing trials designed to determine the prostate cancer preventive activity of green tea, green tea extract, or black tea (NCT00459407; NCT00685516; NCT00253643; NCT01105338).
3.4 Colorectal Cancer
Experimental studies support the chemopreventive properties of green tea extract on colorectal cancer, although epidemiologic studies did not show consistent association between green tea consumption and colorectal cancer. Shimizu et al. [44] conducted a randomized trial to determine the preventive effect of green tea extract supplements on metachronous colorectal adenomas by raising green tea consumption in the target population from an average of 6 cups daily to ≥10 cups equivalent by supplemental green tea extract tablets. They recruited 136 patients who had undergone endoscopic polypectomy and had a second colonoscopy 1 year later to confirm the absence of polyps. Following the second colonoscopy, these patients were randomized into two groups while maintaining their lifestyle on green tea drinking (average of 6 cups per day). Seventy-one patients were supplemented with 1.5 g green tea extract (3 x 500 mg tablets, each containing 52.5 mg EGCG, 12.3 mg EC, 34.6 mg EGC, 11.1 mg ECG, and 15.7 mg caffeine) per day (to ≥10 cups equivalent) for 12 months and 65 control patients without supplementation. Follow-up colonoscopy was conducted 12 months later in 125 patients (60 in the supplemented group and 65 in the control group). The incidence of metachronous adenomas at the end-point colonoscopy was 31% (20 of 65) in the control group and 15% (9 of 60) in the supplemented group (relative risk, 0.49; 95% confidence interval, 0.24–0.99; p < 0.05). The size of relapsed adenomas was also smaller in the supplemented group. Although the study is limited by the lack of a placebo controlled arm, small sample size, and short duration of intervention for adenoma follow up, the data suggested potential clinical activity of high dose green tea intervention for colorectal cancer prevention.
3.5 Other organ sites
A number of clinical studies are ongoing to evaluate the cancer preventive activity of green tea or green tea extract for other organ sites. For breast cancer prevention, the activity of green tea or green tea extract is being studied in various study cohorts (NCT00949923; NCT00917735; NCT00516243; NCT00676793; NCT01060345). For cervical cancer prevention, the activity of Polyphenon E is being studied in patients with human papillomavirus and low grade cervical intraepithelial neoplasia (NCT00303823).
4. Summary
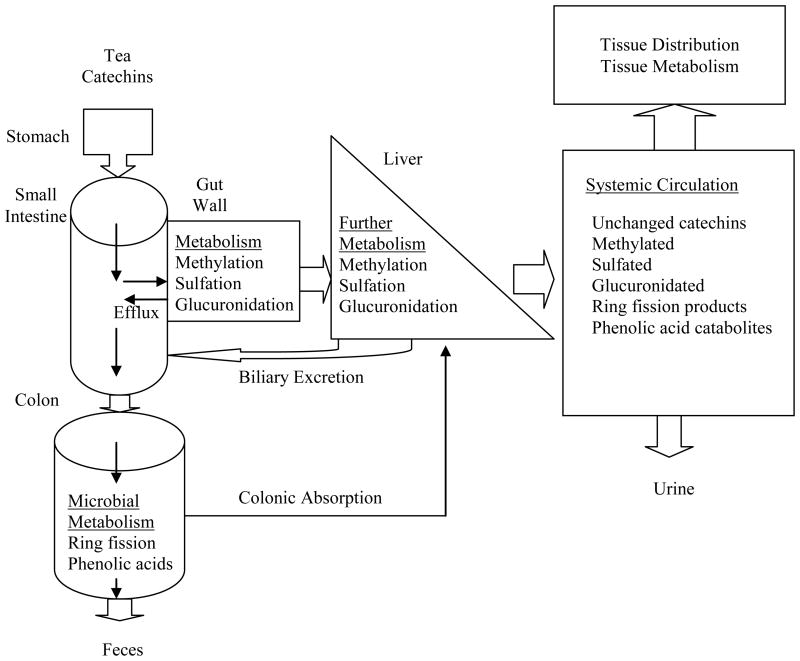
In summary, tea catechins are bioavailable in the systemic circulation after oral administration of green tea or green tea catechins. Plasma concentrations of unchanged tea catechins in humans after oral administration of green tea as a beverage or as an oral product are mostly in the sub-μM or nM concentration range, which is much lower than the effective concentrations determined in most in vitro studies. Multiple metabolites of green tea catechins have been identified after oral administration. These include glucuronide/sulfate conjugates, methylated tea catechin conjugates, and microflora-mediated ring fission products and phenolic acid catabolites. The complex metabolic processes are summarized in Figure 3. Some of the catechin metabolites/catabolites are present in the systemic circulation at levels much higher than those of the parent catechins. The contribution of catechin derived metabolites/catabolites to the biological effects associated with green tea is yet to be defined. Tea catechins are found to distribute widely into various tissues in rodent studies with a distribution pattern coinciding with the site of metabolism and the excretion pathway. Recently, tea catechins and a methylated metabolite are detected in human prostate.
Figure 3.
Metabolism and disposition of tea catechins in humans.
Limited clinical trials of green tea or green tea extract have shown potential preventive activity for oral leukoplakia, prostate cancer, and colorectal cancer, despite achieving only nM peak plasma catechin concentrations with the different green tea products and dosing designs. Emerging data from multiple ongoing intervention trials will further contribute to defining the cancer preventive activity of green tea or green tea catechins. Incorporation of available pharmacokientic information into future clinical trial design could help improve the interpretation and extrapolation of the study outcome data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katiyar SK, Mukhtar H. Tea consumption and cancer. World Review of Nutrition & Dietetics. 1996;79 :154–184. [PubMed] [Google Scholar]
- 2.Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. 2010;62(7):931–937. doi: 10.1080/01635581.2010.509536. [DOI] [PubMed] [Google Scholar]
- 3.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9(6):429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011;22(1):1–7. doi: 10.1016/j.jnutbio.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Cooper R, Morre DJ, Morre DM. Medicinal benefits of green tea: Part I. Review of noncancer health benefits. Journal of Alternative & Complementary Medicine. 2005;11(3):521–528. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- 6.Cooper R, Morre DJ, Morre DM. Medicinal benefits of green tea: part II. review of anticancer properties. Journal of Alternative & Complementary Medicine. 2005;11(4):639–652. doi: 10.1089/acm.2005.11.639. [DOI] [PubMed] [Google Scholar]
- 7.Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiology, Biomarkers & Prevention. 1998;7(4):351–354. [PubMed] [Google Scholar]
- 8.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, Crowell JA, Yang CS, Hara Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiology, Biomarkers & Prevention. 2001;10(1):53–58. [PubMed] [Google Scholar]
- 9.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiology, Biomarkers & Prevention. 2002;11(10 Pt 1):1025–1032. [PubMed] [Google Scholar]
- 10.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clinical Cancer Research. 2003;9 (9):3312–3319. [PubMed] [Google Scholar]
- 11.Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clinical Cancer Research. 2005;11 (12):4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 12.Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, Wang H, Go VL, Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. American Journal of Clinical Nutrition. 2004;80(6):1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 13.Mulder TP, van Platerink CJ, Wijnand Schuyl PJ, van Amelsvoort JM. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. Journal of Chromatography B, Biomedical Sciences & Applications. 2001;760(2):271–279. doi: 10.1016/s0378-4347(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metabolism & Disposition. 1997;25(9):1045–1050. [PubMed] [Google Scholar]
- 15.Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, Sturgill MG, Yang CS. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. Journal of Nutrition. 2003;133(12):4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y, Anavy ND, Chow HH. Contribution of presystemic hepatic extraction to the low oral bioavailability of green tea catechins in rats. Drug Metabolism & Disposition. 2002;30(11):1246–1249. doi: 10.1124/dmd.30.11.1246. [DOI] [PubMed] [Google Scholar]
- 17.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141(3):980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 18.Auger C, Mullen W, Hara Y, Crozier A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J Nutr. 2008;138(8):1535S–1542S. doi: 10.1093/jn/138.8.1535S. [DOI] [PubMed] [Google Scholar]
- 19.Stalmach A, Mullen W, Steiling H, Williamson G, Lean ME, Crozier A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol Nutr Food Res. 2010;54(3):323–334. doi: 10.1002/mnfr.200900194. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Wang ZY, Li H, Chen L, Sun Y, Gobbo S, Balentine DA, Yang CS. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiology, Biomarkers & Prevention. 1995;4 (4):393–399. [PubMed] [Google Scholar]
- 21.Li C, Meng X, Winnik B, Lee MJ, Lu H, Sheng S, Buckley B, Yang CS. Analysis of urinary metabolites of tea catechins by liquid chromatography/electrospray ionization mass spectrometry. Chemical Research in Toxicology. 2001;14(6):702–707. doi: 10.1021/tx0002536. [DOI] [PubMed] [Google Scholar]
- 22.Meng X, Lee MJ, Li C, Sheng S, Zhu N, Sang S, Ho CT, Yang CS. Formation and identification of 4'-O-methyl-(-)-epigallocatechin in humans. Drug Metabolism & Disposition. 2001;29(6):789–793. [PubMed] [Google Scholar]
- 23.Wang JS, Luo H, Wang P, Tang L, Yu J, Huang T, Cox S, Gao W. Validation of green tea polyphenol biomarkers in a phase II human intervention trial. Food Chem Toxicol. 2008;46(1):232–240. doi: 10.1016/j.fct.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, Huang B, Chung JY, Yan S, Ho CT, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chemical Research in Toxicology. 2000;13(3):177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- 25.Kohri T, Matsumoto N, Yamakawa M, Suzuki M, Nanjo F, Hara Y, Oku N. Metabolic fate of (-)-[4-(3)H]epigallocatechin gallate in rats after oral administration. Journal of Agricultural & Food Chemistry. 2001;49(8):4102–4112. doi: 10.1021/jf001491+. [DOI] [PubMed] [Google Scholar]
- 26.Stalmach A, Troufflard S, Serafini M, Crozier A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol Nutr Food Res. 2009;53 (Suppl 1):S44–53. doi: 10.1002/mnfr.200800169. [DOI] [PubMed] [Google Scholar]
- 27.Roowi S, Stalmach A, Mullen W, Lean ME, Edwards CA, Crozier A. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J Agric Food Chem. 2010;58(2):1296–1304. doi: 10.1021/jf9032975. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa K, Miyazawa T. Absorption and distribution of tea catechin, (-)-epigallocatechin-3-gallate, in the rat. Journal of Nutritional Science & Vitaminology. 1997;43(6):679–684. doi: 10.3177/jnsv.43.679. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Lee MJ, Hong J, Li C, Smith TJ, Yang GY, Seril DN, Yang CS. Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutrition & Cancer. 2000;37(1):41–48. doi: 10.1207/S15327914NC3701_5. [DOI] [PubMed] [Google Scholar]
- 30.Henning SM, Aronson W, Niu Y, Conde F, Lee NH, Seeram NP, Lee RP, Lu J, Harris DM, Moro A, et al. Tea polyphenols and theaflavins are present in prostate tissue of humans and mice after green and black tea consumption. J Nutr. 2006;136(7):1839–1843. doi: 10.1093/jn/136.7.1839. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D, Henning SM. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res (Phila) 2010;3 (8):985–993. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metabolism & Disposition. 2003;31(5):572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 33.Meng X, Sang S, Zhu N, Lu H, Sheng S, Lee MJ, Ho CT, Yang CS. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chemical Research in Toxicology. 2002;15(8):1042–1050. doi: 10.1021/tx010184a. [DOI] [PubMed] [Google Scholar]
- 34.Petti S. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol. 2003;39 (8):770–780. doi: 10.1016/s1368-8375(03)00102-7. [DOI] [PubMed] [Google Scholar]
- 35.Fuller CD, Wang SJ, Thomas CR, Jr, Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973–1998. Cancer. 2007;109(7):1331–1343. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Sun Z, Han C, Chen J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proceedings of the Society for Experimental Biology & Medicine. 1999;220(4):218–224. doi: 10.1046/j.1525-1373.1999.d01-37.x. [DOI] [PubMed] [Google Scholar]
- 37.Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El-Naggar AK, Wistuba I, Culotta KS, Mao L, Gillenwater A, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res (Phila) 2009;2(11):931–941. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387(3):147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 39.Hakim IA, Harris RB, Brown S, Chow HH, Wiseman S, Agarwal S, Talbot W. Effect of increased tea consumption on oxidative DNA damage among smokers: a randomized controlled study. Journal of Nutrition. 2003;133(10):3303S–3309S. doi: 10.1093/jn/133.10.3303S. [DOI] [PubMed] [Google Scholar]
- 40.Hakim IA, Harris RB, Chow HH, Dean M, Brown S, Ali IU. Effect of a 4-month tea intervention on oxidative DNA damage among heavy smokers: role of glutathione S-transferase genotypes. Cancer Epidemiology, Biomarkers & Prevention. 2004;13(2):242–249. doi: 10.1158/1055-9965.epi-03-0193. [DOI] [PubMed] [Google Scholar]
- 41.Hakim IA, Chow HH, Harris RB. Green tea consumption is associated with decreased DNA damage among GSTM1-positive smokers regardless of their hOGG1 genotype. J Nutr. 2008;138(8):1567S–1571S. doi: 10.1093/jn/138.8.1567S. [DOI] [PubMed] [Google Scholar]
- 42.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Research. 2006;66(2):1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 43.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila) 2009;2(7):673–682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, Suganuma M, Fujiki H, Moriwaki H. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3020–3025. doi: 10.1158/1055-9965.EPI-08-0528. [DOI] [PubMed] [Google Scholar]