Abstract
Objectives
Widespread implementation of HRQOL measurement in prostate cancer practice and research requires concise instruments. Having 50 questions, the full-length Expanded Prostate cancer Index Composite (EPIC) is cumbersome to administer outside of studies focusing exclusively on HRQOL. To facilitate HRQOL measurement in a broad range of prostate cancer research and practice settings, we developed and validated an abbreviated version of EPIC.
Methods
50 questions that comprise the full-length EPIC-50 were evaluated to identify items suitable for elimination while retaining ability to measure the 5 prostate cancer-specific HRQOL domains of EPIC-50. The resulting abbreviated version (EPIC-26) was validated using question responses from 252 subjects who had brachytherapy, external radiotherapy or prostatectomy for prostate cancer. EPIC-26 internal consistency was measured by Cronbach's alpha coefficient and reliability by test-retest correlation.
Results
Based on high item-scale correlations, clinically relevant content, and preservation of domain psychometrics, 26 items were retained in EPIC-26 from 50 questions in the full length EPIC-50. High correlation was observed between EPIC-50 and EPIC-26 versions of urinary incontinence, urinary irritation/obstruction, bowel, sexual and vitality/hormonal domain scores (all r ≥0.96). Correlations between different domains were low, confirming that EPIC-26 retains the ability to discern 5 distinct HRQOL domains. Internal consistency and test-retest reliability for EPIC-26 (Cronbach's alpha ≥0.70 and r ≥ 0.69, respectively for all 5 HRQOL domains) support its validity.
Conclusions
EPIC-26 is a brief, valid and reliable subjective measure of health quality among prostate cancer patients and suitable for measuring HRQOL among patients undergoing treatment for early stage prostate cancer.
Keywords: prostate cancer, health related quality of life, outcomes research, questionnaires
Introduction
With increased early detection of prostate cancer and high survival rates, health-related quality of life (HRQOL) has been playing an ever more important role in patient care. An ideal HRQOL instrument is brief to administer and comprehensively covers multiple areas related to HRQOL. Indeed, instruments measuring illness-specific domains reflect HRQOL among prostate cancer patients more accurately, since urinary incontinence, bowel function and sexual activity are particularly important 1,2. Abbreviated forms of longer instruments that maintain their breadth without significantly sacrificing reliability have been developed for the general population (e.g., SF-12) 3. At the same time, item reduction has played a critical part of developing the often-used Functional Assessment of Cancer Therapy (FACT) scale for oncology patients 4 and the American Urological Association Symptom Index (AUA-SI) for patients with obstructive voiding 5.
The 50-item Expanded Prostate Cancer Index Composite (EPIC) instrument 6 was developed and validated to expand the scope of the 20-item University of California, Los Angeles Prostate Cancer Index (UCLA-PCI) by adding items on irritative symptoms and to assess the impact of hormonal therapy 7. The EPIC-50 instrument includes urinary incontinence and irritation/obstruction items, along with bowel, sexual and vitality/hormonal domains, each with function and bother sub-domains. Although comprehensive, its initial 50-item version is a lengthy tool to administer and its length can be even more problematic when combined with other patient-report questionnaires. Therefore, we sought to develop a reduced length version of EPIC tool to facilitate its use in research as well as routine prostate cancer care.
Material and Methods
Study population
EPIC-26 was validated in a group of 252 subjects who have been previously described 6. The original, longer EPIC-50 was developed and validated in the same population. Briefly, the validation sub-group of 252 subjects was randomly selected from a larger cross-sectional cohort of 902 men treated for early stage prostate cancer to give equal representation of patients undergoing brachytherapy, external-beam radiation and radical prostatectomy who had provided informed consent to participate in an IRB-approved mail-based questionnaire regarding prostate cancer outcomes.
Item Reduction
In order to create a shorter version of the EPIC (Figure 1), all items were assessed for elimination using an iterative process. Item-scale correlations were assessed for each item; items with weaker correlations were dropped. When two items, such as a bother and function, focused on the same concept, the one with the higher item-scale correlation was retained. Items deemed to be measuring constructs that were particularly clinically relevant were more likely to be retained. After the initial set of drops, new domain scores were calculated. A full battery of psychometric tests was completed (Cronbach's alpha, item-scale correlations and test-retest reliability coefficients) and each domain was correlated with the corresponding scores from the full EPIC-50. This process was repeated until all psychometric properties reached acceptable levels. Just as in the original EPIC, all domains for EPIC-26 are reported on a 0 to 100 score, with higher scores representing favourable HRQOL 6.
Figure 1.
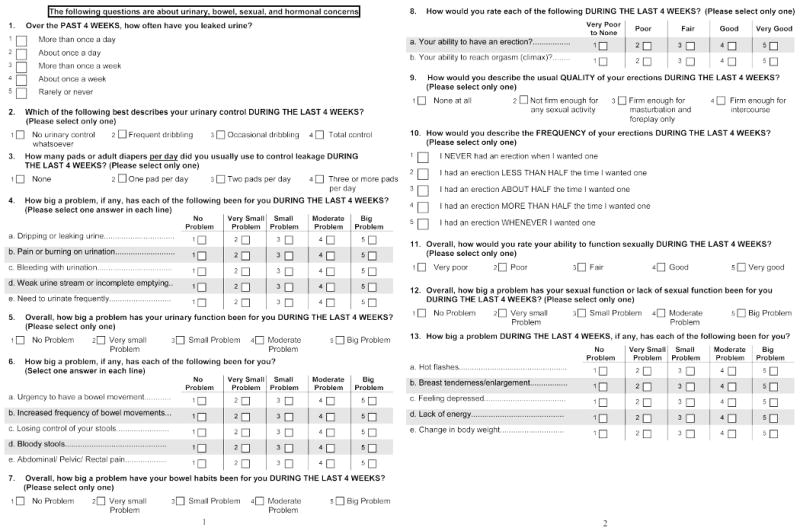
EPIC-26: The 26-item Extended Prostate Index Composite questionnaire (50% reduction in size of usable hardcopy format).
Analyses
Interscale correlation between EPIC-26 and EPIC-50 domains was calculated using the Pearson correlation coefficient. We used the Cronbach's alpha coefficient to evaluate internal consistency of the EPIC-26. Reliability was assessed by re-administering the questionnaire to the validation cohort 2-4 weeks after the initial questionnaire and test-retest reliability coefficients were calculated 8. All statistical analyses were performed using SAS software (v 9.2, SAS Institute, Cary, NC).
Results
The complete, ready to use EPIC-26 is depicted in Figure 1, with 26 of the 50 items from the original 50-item EPIC retained for the abbreviated instrument (Table 1). Since the urinary incontinence domain contained only 4 items in the full length EPIC-50 (item-scale correlation r ≥0.66 for each) each of these 4 items were retained in EPIC-26. Of the 7 items in the EPIC-50 urinary irritation/obstruction domain, dysuria and weak stream bother items were retained in EPIC-26 based on having the highest correlation with the domain score, while hematuria frequency items were kept for content. Of the 14 items in the EPIC-50 bowel domain, 3 bother items (urgency, frequency, pain) and the overall problem item were retained in EPIC-26 based on having the highest item-scale correlation, while fecal incontinence and hematochezia items were retained for content. Of the 13 items in the EPIC-50 sexual domain, 5 function items (poor erections, difficulty with orgasm, erection not firm, erection not reliable and poor sexual function) were retained in EPIC-26 based on having the highest item-scale correlation, while the overall sexuality problem item was retained for content. Of the 11 items in the EPIC-50 vitality/hormonal domain, 2 items (depression, lack of energy) were retained in EPIC-26 based on having the highest correlation with the domain score and 3 items (hot flashes, breast problems and weight change) were kept for content. Function and bother scales of the EPIC-50 were collapsed into single domains to reduce the number of items and because their correlation within a domain was high (r =0.64-0.87) 6. The final EPIC-26 instrument contains 5 multi-item domains: urinary incontinence (4 items), urinary irritation/obstruction (4 items), bowel (6 items), sexual (6 items) and vitality/hormonal function (5 items); in addition, the EPIC-26 retains the single item measure of overall urinary bother from the UCLA-PCI. This item is retained as a distinct measure from the urinary incontinence and urinary irritative subscales because it has overlapping conceptual and biometric correlation with both of these distinct subscales. Missing data were minimal, with a median of 7 (2.8%) missing responses for the 26 items (range 2 [0.8%] to 14 [5.6%]).
Table 1.
Correlation between individual items and total scores for the EPIC-50 and EPIC-26 among prostate cancer patients treated with brachytherapy, external-beam radiation and radical prostatectomy (n=252).
| Quality-of-life domain and EPIC questionnaire item | Item number | Item-scale correlation | ||
|---|---|---|---|---|
| EPIC-50** | EPIC-26 | EPIC-50 | EPIC-26 | |
| Urinary domains | ||||
| Incontinence subscale (4) | ||||
| Leaking >1 time per day* | 1 | 1 | 0.75 | 0.75 |
| Frequent dribbling* | 4 | 2 | 0.77 | 0.77 |
| Any pad use* | 5 | 3 | 0.66 | 0.66 |
| Leaking problem* | 6 | 4.a | 0.83 | 0.83 |
| Irritation/obstruction subscale (4) | ||||
| Dysuria | 7 | 4.b | 0.77 | 0.65 |
| Hematuria | 8 | 4.c | 0.36 | 0.32 |
| Weak stream | 9 | 4.d | 0.69 | 0.67 |
| Frequency | 11 | 4.e | 0.66 | 0.61 |
| Overall urinary problem (1)* | 12 | 5 | n/a | n/a |
| Bowel domain (6) | ||||
| Urgency | 20 | 6.a | 0.74 | 0.77 |
| Frequency | 21 | 6.b | 0.80 | 0.81 |
| Fecal incontinence | 23 | 6.c | 0.68 | 0.65 |
| Bloody stools | 24 | 6.d | 0.59 | 0.55 |
| Rectal pain | 25 | 6.e | 0.74 | 0.65 |
| Overall bowel problem* | 26 | 7 | 0.83 | 0.83 |
| Sexual domain (6) | ||||
| Poor erections* | 28 | 8.a | 0.83 | 0.86 |
| Difficulty with orgasm* | 29 | 8.b | 0.73 | 0.68 |
| Erections not firm* | 30 | 9 | 0.77 | 0.79 |
| Erections not reliable* | 31 | 10 | 0.79 | 0.81 |
| Poor sexual function* | 35 | 11 | 0.82 | 0.80 |
| Overall sexuality problem* | 39 | 12 | 0.58 | 0.50 |
| Vitality or hormonal domain (5) | ||||
| Hot flashes | 45 | 13.a | 0.50 | 0.38 |
| Breast problems | 46 | 13.b | 0.39 | 0.31 |
| Depression | 48 | 13.c | 0.70 | 0.62 |
| Lack of energy | 49 | 13.d | 0.65 | 0.58 |
| Weight change | 50 | 13.e | 0.49 | 0.42 |
These 12 items (1, 2, 3, 4.a, 5, 7, 8.a, 8.b, 9, 10, 11, 12) are the original UCLA-PCI items 7, retained for generalizability and clinically significant assessment. Item 5 in EPIC-50 and item 12 in EPIC-26 are the UCLA Urinary bother item, and because this item related to both the incontinence and irritative/obstructive scale in factor analyses and conceptual content, the item is not included in either the irritative/obstructive nor in the urinary incontinence scale, but is retained to enable assessment of overall urinary bother as might be influenced by either or both of these domains.
Item numbers are taken from the published version of EPIC-50 6.
Summary scores for the 26-item EPIC tool correlate strongly with the corresponding summary scores for the original EPIC-50 (r ≥0.96 for all summary domains; Table 2), whereas each EPIC-26 domain score is conceptually distinct from the other domains and merits distinct measure, for example that the urinary irritative/obstructive domain is distinct from urinary incontinence, with only moderate correlation between these 2 conceptually distinct domains (r = 0.36-0.41; Table 2). We have previously shown that EPIC-50 HRQOL domain scores have low correlations with other instruments that were not specific to prostate cancer 6. The one exception was a strong correlation between the AUA-SI and the EPIC-50 irritation/obstruction urinary scale (r =0.77); the Pearson correlation coefficient between the EPIC-26 urinary irritative/obstructive domain and the AUA-SI is 0.79.
Table 2.
Correlation between EPIC-26 and EPIC-50 domain scores.
| EPIC-26 domain | |||||
|---|---|---|---|---|---|
| EPIC-50 HRQOL domain | Urinary | ||||
| Incontinence | Irritation or obstruction | Bowel | Sexual | Vitality or hormonal | |
| Urinary summary | |||||
| Incontinence | 1.00 | 0.41 | 0.20 | 0.23 | 0.27 |
| Irritation or obstruction | 0.36 | 0.97 | 0.40 | 0.24 | 0.38 |
| Bowel summary | 0.19 | 0.39 | 0.97 | 0.21 | 0.48 |
| Sexual summary | 0.21 | 0.29 | 0.25 | 0.96 | 0.28 |
| Hormonal summary | 0.27 | 0.40 | 0.44 | 0.27 | 0.96 |
Correlation r for convergent EPIC-26 and EPIC-50 domains are highlighted in bold and underlined typeface.
Finally, we determined the characteristics of each EPIC-26 domain summary score (Table 3). Modest ceiling effects are evident in the urinary, bowel, and vitality/hormonal scores, and not in the sexual HRQOL score, with 31-46% of subjects scoring the maximum possible score in these domains. Nevertheless, each of the five domain summary scores had strong internal consistency (Cronbach's alpha=0.70-0.90) and reliability (test-retest reliability coefficient=0.69-0.90).
Table 3.
Characteristics of EPIC-26 domain-specific scores.
| HRQOL domain | Mean score (SD) | Scoring minimum (%) | Scoring maximum (%) | Median (Range) | Cronbach's alpha | Test-retest |
|---|---|---|---|---|---|---|
| Urinary | ||||||
| Incontinence | 83.2 (22.9) | 1.3 | 46.4 | 93.8 (0.0-100.0) | 0.86 | 0.87 |
| Irritation or obstruction | 80.5 (20.2) | 0.4 | 24.7 | 87.5 (0.0-100.0) | 0.74 | 0.80 |
| Bowel | 85.0 (19.3) | 0.0 | 34.0 | 91.7 (8.3-100.0) | 0.89 | 0.86 |
| Sexual | 34.4 (28.1) | 13.5 | 0.4 | 30.5 (0.0-100.0) | 0.90 | 0.90 |
| Vitality or hormonal | 87.1 (15.0) | 0.0 | 30.8 | 90.0 (30.0-100.0) | 0.70 | 0.69 |
Comment
The development of HRQOL instruments requires a balance between clinical usefulness and comprehensiveness. Lengthy HRQOL questionnaires can often be shortened, eliminating redundant items, while minimizing sacrificed validity, as has been achieved during the development and validation of the AUA-SI 5 and other tools 3,4. The 8-item AUA-SI was developed from an initial 16 questions, which were reduced to one bother item and 7 function items. Of the function questions, 6 (emptying, frequency, intermittency, urgency, weak stream and nocturia) were retained for their high correlation with the bother item while the hesitancy item was kept for content.
The UCLA-PCI was selected as the foundation for the EPIC because, at the time of EPIC development, the UCLA-PCI had already been broadly used, had robust construct validity and was the first instrument to have been validated for measurement of patient-reported outcomes in early stage prostate cancer. The initial 50-item version of the EPIC instrument retained 17 of the original UCLA-PCI questions; retained and refined the assessment of urinary incontinence, bowel/rectal, and sexual domains that comprised the range of HRQOL queried by UCLA-PCI; and expanded the scope of HRQOL assessment to include urinary irritative/obstructive and vitality/hormonal domains not covered in the UCLA-PCI. The brief format of EPIC-26 retains these 5 domains and 12 of the original UCLA-PCI questions, achieving the goal of being both clinically useful and retaining the comprehensiveness of the original EPIC.
EPIC-26 has been used in a multi-center, prospective study of change in prostate cancer HRQOL after primary treatment for early stage prostate cancer in 1201 men 9. Findings from this study, which are relevant to general use of the EPIC-26, include observed pre-treatment EPIC HRQOL scores that can be considered as reflecting norms among men with early stage prostate cancer who have not yet undergone treatment. The mean pre-treatment scores were 93.5 for urinary incontinence, 87.8 for urinary irritation/obstruction, 95.9 for bowel/rectal, 70.7 for sexual and 92.1 for the vitality/hormonal HRQOL domain scores.
Several other instruments have been developed for measuring prostate cancer-specific HRQOL outcomes. The 29-item instrument developed by Clark and Talcott 10 was validated in patients treated with prostatectomy or external-beam radiation, spanning urinary incontinence and irritation/obstruction, bowel and sexual domains, but did not include questions in the vitality/hormonal domain. The instrument used by Madalinska et al. 2 consists of the UCLA-PCI urinary and bowel domains and a sexual function module previously developed in patients with erectile dysfunction 11, as the UCLA-PCI sexual domain was deemed insufficiently detailed. It does not include a vitality/hormonal or urinary irritation/obstruction domain. The tool published by Giesler et al. 12 consists of urinary, sexual, bowel and cancer worry domains. While it includes a unique anxiety domain, the 52-item tool is lengthy and does not distinguish between urinary incontinence and irritative/obstructive symptoms. The 12-item FACT - Prostate module 13 was developed in patients with more advance prostate cancer than the setting queried by EPIC and does not distinguish between urinary, bowel, sexual and vitality/hormonal domains, providing a single summary score instead of individual domain scores. The 17-item Prostate Cancer Outcomes Study 14 used 5 of the original UCLA-PCI urinary incontinence and bowel questions along with a new sexual function domain, but it did not include urinary irritation/obstruction or vitality/hormonal domains.
The EPIC-26 is a broadly accepted, user-friendly instrument that measures HRQOL concerns related to early prostate cancer. EPIC has been used to asses the impact of aging on domain-specific HRQOL 15, satisfaction and regret with prostatectomy 16, erectile dysfunction in patient treated with external beam radiation 17 and HRQOL among patients treated with bladder preservation therapy for muscle-invasive bladder cancer 18. Having been successfully used in the field by several investigative teams suggests the EPIC has robust construct validity. Although EPIC-26 improves the ease of administration, the EPIC-50 remains valuable whenever there is a need to evaluate function as distinct from bother.
Our development and validation of EPIC-26 presented herein has limitations. The abbreviated EPIC-26 takes about 10 minutes to complete, and although this length is more practical for widespread research use than are longer versions, it may nevertheless be cumbersone to administer routinely in some clinical practice settings. Accordingly, in the next phase of refinement of the EPIC instrument we plan to explore item reduction and format revision to facilitate ease of administration further; however, excessive item reduction can compromise reliability of a patient-report instrument. 8 Another potential limitation of this study was our use, for item reduction, of cohort data that had been collected some 10 years previously. Nevertheless, the contemporary relevance of EPIC-26 has been ascertained in a subsequent, contemporary, multi-center study, and ultimately a robust HRQOL instrument should retain relevance through an extended period of time, as exemplified by the SF-36 and other tools. 3
The EPIC-26 is a validated short form of EPIC-50 that has been effectively employed to follow long-term domain-specific changes in HRQOL among prostate cancer survivors in single-centre 19 and multi-institutional studies, 9 with the latter having been facilitated by availability of a telephone script for administration of EPIC-26 by phone survey or Computer-Assisted Telephone Interview (CATI). In practice, the EPIC-26 instrument is completed quickly, taking 10-15 minutes to administer 9, making it a comprehensive and practical tool for use in research and making it less onerous than the 50-item EPIC for use either in combination with other patient-report instruments or for efficient administration in routine clinical practice.
Acknowledgments
Funding: NIH R01 CA95662
Abbreviations
- HRQOL
health-related quality of life
- FACT
Functional Assessment of Cancer Therapy
- EPIC
expanded prostate cancer index composite
- AUA-SI
American Urological Association Symptom Index
- UCLA-PCI
University of California, Los Angeles Prostate Cancer Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273(2):129–135. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 2.Madalinska JB, Essink-Bot ML, de Koning HJ, et al. Health-related quality-of-life effects of radical prostatectomy and primary radiotherapy for screen-detected or clinically diagnosed localized prostate cancer. J Clin Oncol. 2001;19(6):1619–1628. doi: 10.1200/JCO.2001.19.6.1619. [DOI] [PubMed] [Google Scholar]
- 3.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 5.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 6.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 7.Litwin MS, Hays RD, Fink A, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36(7):1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Streiner DL, Norman GR. Health measurement scales : a practical guide to their development and use. 3rd. xii. Oxford; New York: Oxford University Press; 2003. p. 283. [Google Scholar]
- 9.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 10.Clark JA, Talcott JA. Symptom indexes to assess outcomes of treatment for early prostate cancer. Med Care. 2001;39(10):1118–1130. doi: 10.1097/00005650-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Slob AK, Blom JH, van der Werff ten Bosch JJ. Erection problems in medical practice: differential diagnosis with relatively simple method. J Urol. 1990;143(1):46–50. doi: 10.1016/s0022-5347(17)39861-0. [DOI] [PubMed] [Google Scholar]
- 12.Giesler RB, Miles BJ, Cowen ME, Kattan MW. Assessing quality of life in men with clinically localized prostate cancer: development of a new instrument for use in multiple settings. Qual Life Res. 2000;9(6):645–665. doi: 10.1023/a:1008931703884. [DOI] [PubMed] [Google Scholar]
- 13.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50(6):920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 14.Potosky AL, Harlan LC, Stanford JL, et al. Prostate cancer practice patterns and quality of life: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 1999;91(20):1719–1724. doi: 10.1093/jnci/91.20.1719. [DOI] [PubMed] [Google Scholar]
- 15.Korfage IJ, Roobol M, de Koning HJ, et al. Does “normal” aging imply urinary, bowel, and erectile dysfunction? A general population survey Urology. 2008;72(1):3–9. doi: 10.1016/j.urology.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 16.Schroeck FR, Krupski TL, Sun L, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54(4):785–793. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 17.Pinkawa M, Gagel B, Piroth MD, et al. Erectile Dysfunction After External Beam Radiotherapy for Prostate Cancer. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Hashine K, Miura N, Numata K, et al. Health-related quality of life after bladder preservation therapy for muscle invasive bladder cancer. Int J Urol. 2008;15(5):403–406. doi: 10.1111/j.1442-2042.2008.02032.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]


