Abstract
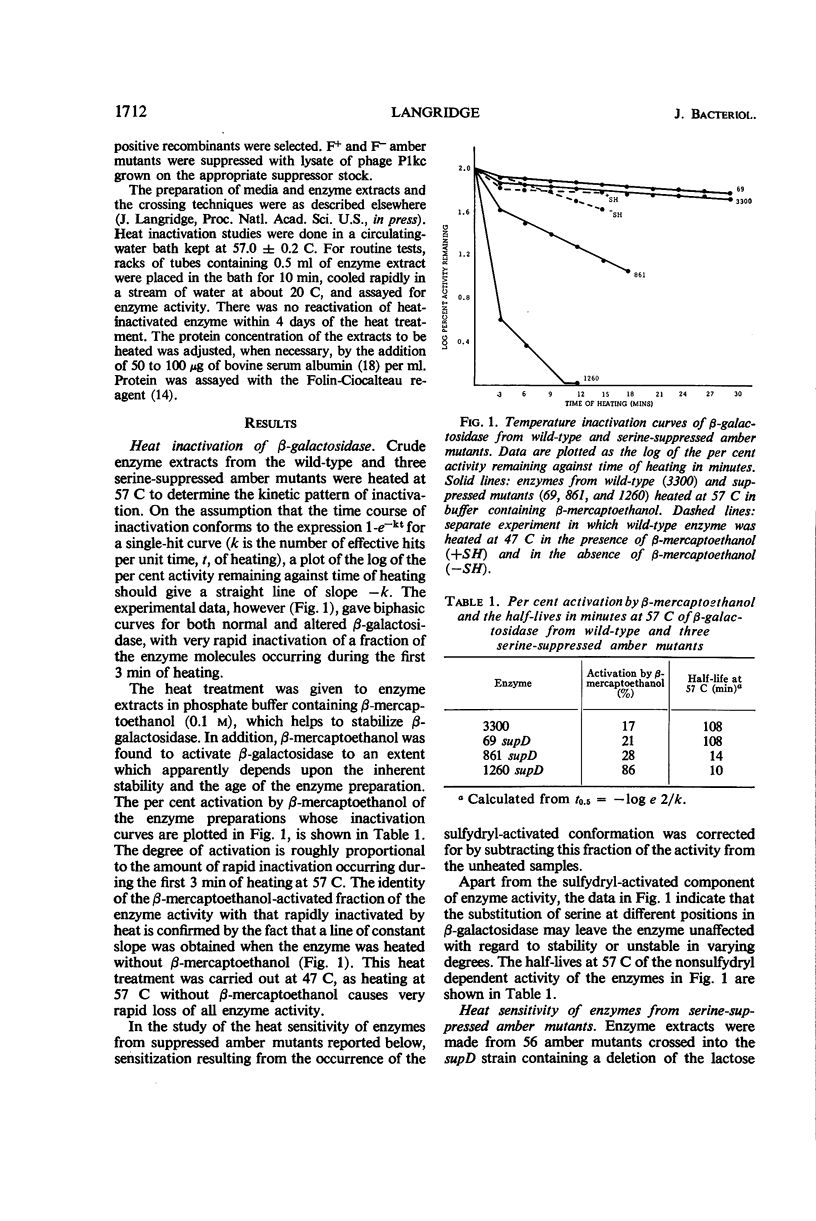
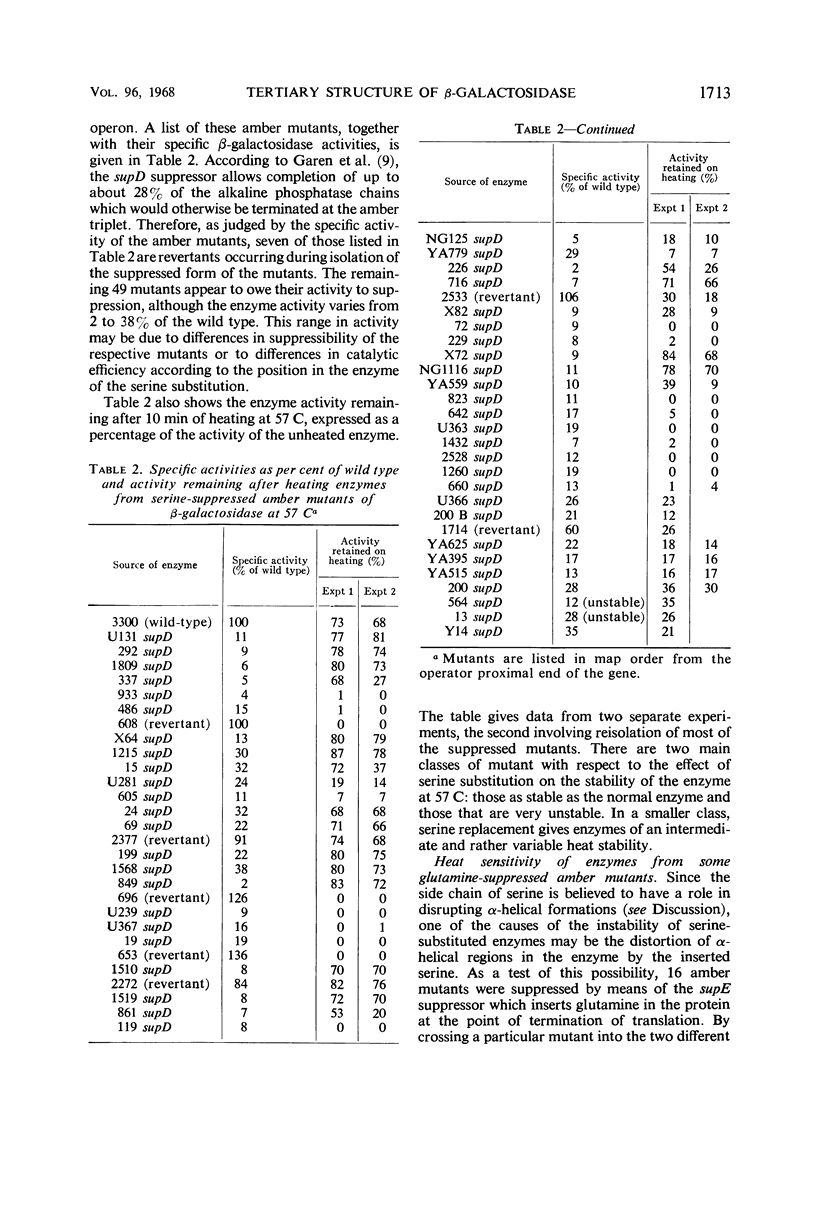
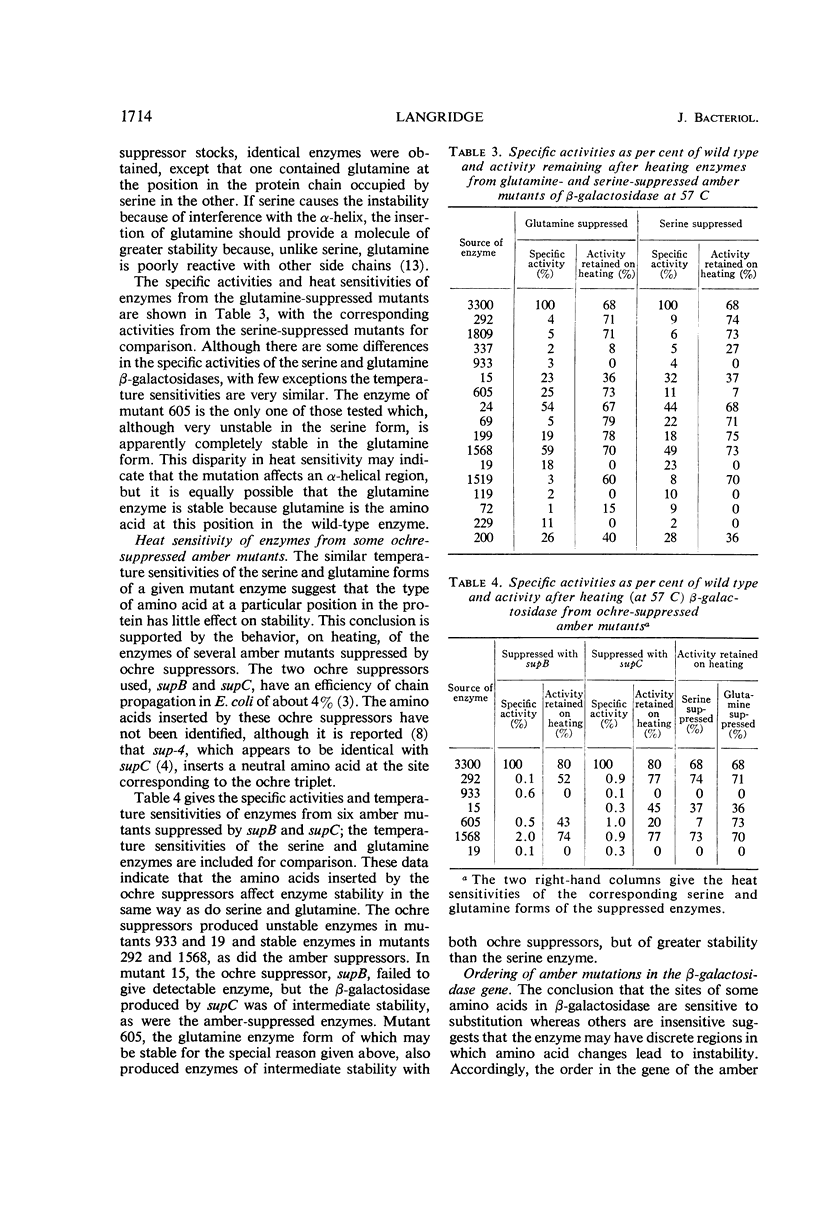
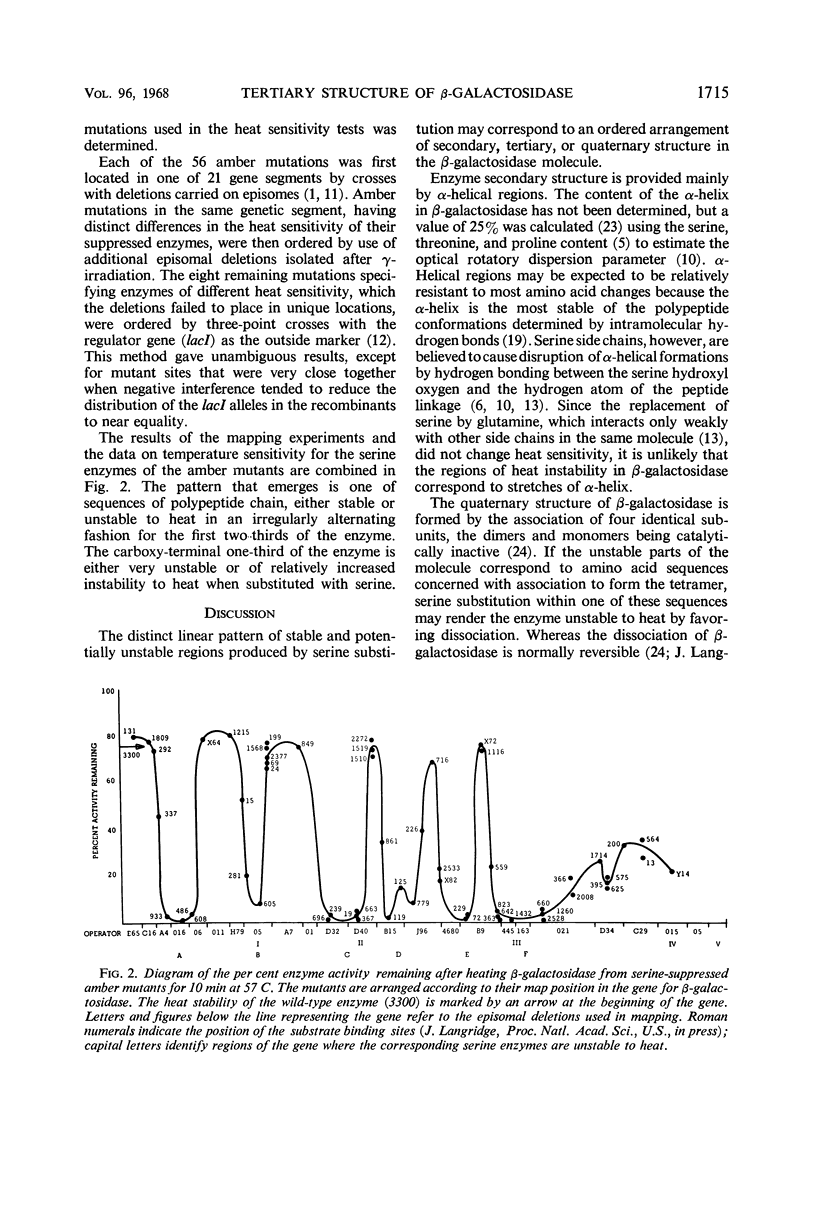
Fifty-six amber mutations of the β-galactosidase gene of Escherichia coli were suppressed by crossing into a stock containing the supD suppressor gene. The resultant enzymes, differing only in the position of the inserted serine, were tested for stability at 57 C. Most of the suppressed enzymes were either as stable to heat as the normal enzyme or very unstable. Tests of enzymes produced by the action of other suppressors showed that the degree of stability was characteristic of a particular position in the polypeptide chain of the amino acid substitution and independent of the amino acid inserted. The mutations were placed in linear order in the gene by deletion mapping and three-point linkage tests. The consequent order of the serine substitutions disclosed an alternating pattern of stable and unstable regions over the amino-terminal two-thirds of the enzyme; the carboxy-terminal third of the enzyme was generally unstable. Considerations of coding relations and enzyme structure suggested that serine and glutamine suppression usually result in a change in the hydrophilic nature of the side chains on the outside of the enzyme molecule. It was shown that the potentially unstable regions of the enzyme are probably not indicative of stretches of α-helix or of sites of association. The apparent position of the substrate binding sites was correlated with the location of some of the potentially unstable regions, which may mark the parts of the polypeptide chain in proximity with the substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. R. A DELETION ANALYSIS OF THE LAC OPERATOR REGION IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:427–430. doi: 10.1016/s0022-2836(64)80206-0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Koenig D. F., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. Nature. 1965 May 22;206(4986):757–761. doi: 10.1038/206757a0. [DOI] [PubMed] [Google Scholar]
- Brenner S., Kaplan S., Stretton A. O. Identity of N2 ochre nonsense mutants. J Mol Biol. 1966 Aug;19(2):574–575. doi: 10.1016/s0022-2836(66)80024-4. [DOI] [PubMed] [Google Scholar]
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- DAVIES D. R. A CORRELATION BETWEEN AMINO ACID COMPOSITION AND PROTEIN STRUCTURE. J Mol Biol. 1964 Aug;9:605–609. doi: 10.1016/s0022-2836(64)80232-1. [DOI] [PubMed] [Google Scholar]
- Eisenstark A., Eisenstark R., Van Sickle R. Mutation of Salmonella typhimurium by nitrosoguanidine. Mutat Res. 1965 Feb;2(1):1–10. doi: 10.1016/0027-5107(65)90002-3. [DOI] [PubMed] [Google Scholar]
- Garen A., Garen S., Wilhelm R. C. Suppressor genes for nonsense mutations. I. The Su-1, Su-2 and Su-3 genes of Escherichia coli. J Mol Biol. 1965 Nov;14(1):167–178. doi: 10.1016/s0022-2836(65)80238-8. [DOI] [PubMed] [Google Scholar]
- Havsteen B. H. A study of the correlation between the amino acid composition and the helical content of proteins. J Theor Biol. 1966 Jan;10(1):1–10. doi: 10.1016/0022-5193(66)90174-3. [DOI] [PubMed] [Google Scholar]
- JACOB F., ULLMAN A., MONOD J. LE PROMOTEUR, 'EL'EMENT G'EN'ETIQUE N'ECESSAIRE 'A L'EXPRESSION D'UN OP'ERON. C R Hebd Seances Acad Sci. 1964 Mar 16;258:3125–3128. [PubMed] [Google Scholar]
- KENDREW J. C. Side-chain interactions in myoglobin. Brookhaven Symp Biol. 1962 Dec;15:216–228. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. Three-dimensional structure of tosyl-alpha-chymotrypsin. Nature. 1967 May 13;214(5089):652–656. doi: 10.1038/214652a0. [DOI] [PubMed] [Google Scholar]
- URNES P., DOTY P. Optical rotation and the conformation of polypeptides and proteins. Adv Protein Chem. 1961;16:401–544. doi: 10.1016/s0065-3233(08)60033-9. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Jacob F., Monod J. Characterization by in vitro complementation of a peptide corresponding to an operator-proximal segment of the beta-galactosidase structural gene of Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):339–343. doi: 10.1016/0022-2836(67)90341-5. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Perrin D., Jacob F., Monod J. Identification par complémentation in vitro et purification d'un segment peptidique de la beta-galatosidase d'escherichia coli. J Mol Biol. 1965 Jul;12(3):918–923. doi: 10.1016/s0022-2836(65)80338-2. [DOI] [PubMed] [Google Scholar]
- WALLENFELS K., SUND H., WEBER K. DIE UNTEREINHEITEN DER BETA-GALAKTOSIDASE AUS E. COLI. Biochem Z. 1963;338:714–727. [PubMed] [Google Scholar]



