Abstract
Objective
In preclinical models of peripheral arterial disease the angiogenic response is typically robust, though it can be impaired in conditions such as hypercholesterolemia and diabetes where the endothelium is dysfunctional. Myoglobin (Mb) is expressed exclusively in striated muscle cells. We hypothesized that myocyte specific overexpression of myoglobin attenuates ischemia-induced angiogenesis even in the presence of normal endothelium.
Methods and Results
Mb overexpressing transgenic (MbTg, n=59) and wild-type (WT, n=56) C57Bl/6 mice underwent unilateral femoral artery ligation/excision. Perfusion recovery was monitored using Laser Doppler. Ischemia-induced changes in muscle were assessed by protein and immunohistochemistry assays. Nitrite/nitrate and protein-bound NO, and vasoreactivity was measured. Vasoreactivity was similar between MbTg and WT. In ischemic muscle, at d14 postligation, MbTg increased VEGF-A, and activated eNOS the same as WT mice but nitrate/nitrite were reduced whereas protein-bound NO was higher. MbTg had attenuated perfusion recovery at d21 (0.37±0.03 versus 0.47±0.02, P<0.05), d28 (0.40±0.03 versus 0.50±0.04, P<0.05), greater limb necrosis (65.2% versus 15%, P<0.001), a lower capillary density, and greater apoptosis versus WT.
Conclusion
Increased Mb expression in myocytes attenuates angiogenesis after hind-limb ischemia by binding NO and reducing its bioavailability. Myoglobin can modulate the angiogenic response to ischemia even in the setting of normal endothelium.
Keywords: angiogenesis, animal models of human disease, genetically altered mice, endothelium/vascular type/nitric oxide
Angiogenesis, the process of formation of new blood vessels, can be therapeutic, as in endogenous response to arterial occlusions or when one seeks to deliver growth factors to increase blood flow and treat disorders of inadequate tissue perfusion.1–3 Nitric oxide (NO) plays a crucial role in both the endogenous angiogenic response to ischemia and in the “therapeutic response” sought after administration of growth factors.4–6 It has been established that imbalance in NO production5,7 or availability8 leads to impaired angiogenesis. The bioavailabilty of NO in tissue depends on its production and stability. Most studies focusing on NO derangements in tissue have used models of severe endothelial dysfunction, where there is reduced NO production attributable to dysfunctional endothelial NO synthase (eNOS),9 or reduced half-life of NO attributable to oxidative stress.9,10
Recent evidences indicate that factors outside the endothelium can also regulate NO bioavailability.11,12 Myoglobin is a protein of oxidative metabolism expressed almost exclusively in myocytes of cardiac and striated skeletal muscle, and has been shown to interact with NO.11 Human myoglobin has been shown to react with NO to yield heme-NO (ferrous nitrosyl-Mb) in the absence of dioxygen and to form S-nitrosylated myoglobin (SNO-Mb) in presence or absence of oxygen.13 The interactions of myoglobin with NO can result in scavenging NO11,14 or in yielding more biologically stable NO at sites away from the site of synthesis.15,16 Hearts from Mb−/− mice showed an increased sensitivity (vasodilatation and cardiodepression) in response to NO and bradykinin, indicating a negative role of myoglobin in NO availability.11 On the contrary, deoxymyoglobin can generate NO by reduction of nitrite and thereby regulate mitochondrial respiration,17 and can be cytoprotective during ischemia-reperfusion by decreasing cellular energy consumption.18 Thus, data from published literature on NO-Mb interaction could be predictive of a beneficial or an adverse outcome on NO-dependent pathways. This study was designed to test the hypothesis that manipulating myoglobin expression in myocytes can alter NO availability and thus modulate angiogenesis after hind-limb ischemia in the setting of normal endothelial function.
Materials and Methods
Experiment Design and Groups
In total, 56 wild-type (WT) and 59 myoglobin transgenic (MbTg) mice were studied. Ten mice per group were used for measurement of protein expressions and capillary density at baseline. Twenty mice per group were used for measurement of perfusion recovery after hind-limb ischemia and followed for 28 days. After 28 days, the gastrocnemius muscle was harvested and used for assessment of capillary density and apoptosis. Another 20 mice from each group were euthanized at 14 days after hind-limb ischemia for protein expressions (n=10 per group) or NO measurements (n=10 per group). Aortas from a separate subset of mice (n=6 per group) were used for assessment of vascular reactivity. Animal study protocols were approved by Duke University’s Institutional Animal Care and Use Committee.
Myoglobin Transgenic Mice
Myoglobin-overexpressing transgenic mice in an ICR background were obtained from the laboratory of R. Sanders Williams, MD (Duke University) and were then backcrossed 9 generations into C57Bl/6 mice. The transgene contains the intact myoglobin gene including the promoter regions, so that it is expressed only in cardiac and skeletal muscle, as shown previously.19 The ninth generation C57Bl/6 MbTg mice and WT litter mates were used for the study. MbTg mice were identified using markers previously described.19
Surgical Induction of Hind-Limb Ischemia and Hemodynamic Assessment
Unilateral hind-limb ischemia was induced and hind-limb perfusion was measured as described previously.20 At the indicated time points, postligation perfusion was assessed and the data were expressed as the ratio of perfusion in the ischemic limb to that in the contralateral nonischemic limb. The extent of limb necrosis was scored using the following convention: Stage I: necrosis of toes; Stage II: necrosis extending to dorsum pedis; Stage III: extending to crus; and stage IV: Extending to thigh or complete limb. Mice with grade II necrosis and above (3/23 mice from the MbTg group) were excluded from the perfusion analysis.
Protein Analysis, Measures of Capillary Density and Apoptosis
See online supplement of Materials and Methods (available online at http://atvb.ahajournals.org).
Measurement of Nitrate/Nitrite
NO has a short half-life and is oxidized within seconds of its release to various nitrogen oxides including nitrite (NO−2) and nitrates (NO−3).15,21 The levels of nitrate and nitrite are commonly used as measures of endogenous NO production.22,23 We used the Correlate Assay (Assay Designs) to measure the levels of nitrate/nitrite in tissue as indicators of total NO and in mouse food and water to determine the dietary contribution to NO. The assay involves the enzymatic reduction of nitrate to nitrite, followed by the colorimetric detection of nitrite as a colored azo dye product of the Greiss reaction. Tissue homogenates were filtered through biomax 5k NMW membranes (Millipore) to filter proteins above 5 kDa, and the filtrate was used for the assay. Each sample was done in duplicates and the values were normalized to the initial total protein concentration in each sample. We also measured cGMP levels in tissue (supplemental methods) as an alternative measure of NO.
Measurement of protein bound nitric oxide was done using mercury-coupled photolysis chemiluminescence method as described.24 Briefly, total protein extracts from skeletal muscle were ultracentrifuged at 45 000g for 30 minutes at 4°C. The supernatants were introduced as a chromatographic effluent from an attached high-performance liquid chromatography (HPLC) system into the photolysis cell of a chemiluminescence apparatus (model 543 thermal energy analyzer, Thermedix). The sample was introduced with a stream of helium and then irradiated with a 200-W mercury-vapor lamp. Flow rates and illumination levels in the photolysis cell were adjusted to result in complete photolysis of the S-N bond of S-nitrosothiols or Fe-NO bonds of iron-nitrosylmyoglobin. The effluent from the photolysis coil was passed through a series of cold traps, where liquid and gaseous fractions less volatile than NO (such as nitrite and nitrate) were trapped. NO is carried by the helium stream into the chemiluminescence spectrometer, where it is detected by reaction with ozone. Signals are recorded on a digital integrator. Standard curves were derived for NO generated from different dilutions of S-nitrosoglutathione, and a known concentration of S-nitrosoglutathione was used as the reference standard to determine the NO concentration in the samples. To determine what fraction of the total NO detected in samples was derived from S-nitrosothiols, samples were pretreated with mercuric chloride to displace NO selectively from the S-nitrosothiols. Incubation of samples in air after mercuric chloride treatment oxidizes the released NO and thereby makes it undetectable. Comparison of NO concentrations from samples alternatively pretreated or untreated with mercuric chloride enabled us to determine whether NO obtained by photolysis was derived specifically from S-nitrosothiols or other forms, principally heme-NO.
Measurement of Nitric Oxide Synthase Activity and Vascular Reactivity
NOS activity was measured in frozen sections from ischemic muscle while both NOS and vasoreactivity were measured in aortas. See online supplement of Materials and Methods.
Assessment of Skeletal Muscle Atrophic Response to Endotoxin Injury
To determine whether skeletal muscle from MbTg mice is more susceptible to direct injury than WT, we determined the muscle atrophy response of mice after injection of endotoxin, as described.25 Twenty-four hours after the endotoxin injection, mice were euthanized and mRNA expression of MAFbx/Atrogin-1 was quantified in gastrocnemius muscle using Taqman Assay for Atrogin-1 (Applied Biosystems). Endogenous 18s was used for normalization and comparison of mRNA copy number was done using comparative δ-Ct method.
Statistics
Statistical analysis was done using the SPSS software (SPSS 14.0). Comparison between the groups was done using independent Student t test for baseline values and paired t test for nonischemic versus ischemic values. Statistical significance was set at a probability value of <0.05. Repeated measures ANOVA was done to assess perfusion improvement over time within groups. Difference in limb necrosis between the groups was assessed using the nonparametric Mann–Whitney U test.
Results
MbTg Versus Wild Type: Skeletal Muscle Vasculature and Aortic Vasoreactivity
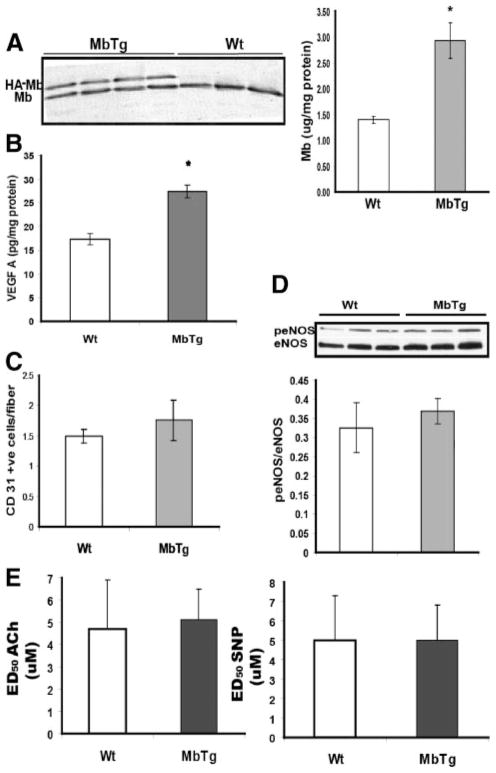
Protein expression of transgene in gastrocnemius muscle of MbTg mice was identified by immunoblotting (Figure 1A) because the HA-tagged myoglobin (HA-Mb) migrates slower in SDS-PAGE than the endogenous myoglobin protein (Mb). Total muscle myoglobin was approximately 2-fold higher (P<0.001) by ELISA in the transgenic compared to WT mice (Figure 1B). Skeletal muscle from MbTg mice had significantly higher expression of VEGF-A compared to WT mice (Figure 1C). However, capillary density (Figure 1D) and the expression of total eNOS and activated/total (phoshorylated/total) eNOS (p-eNOS/eNOS) were similar between the groups (Figure 1F). The contractile responses of the isolated aortic rings to 100 mmol/L K+ and phenylephrine (PE) were comparable between the two groups (maximal active stress normalized to cross sectional area of ring: 100 mmol/L KPSS, WT versus MbTg 5.2±0.8 versus 6.7±2.5 mg/mm2 and 30 μmol/L PE, WT versus MbTg 6.7±1.4 versus 5.5±1.3 mg/mm2, both P=NS). Also, ACh and SNP induced relaxation responses showed that the ED50 for ACh and SNP were comparable between the groups (Figure 1F). NOS activity in aorta, as detected in frozen sections and cGMP levels in homogenates (supplemental Figure IA and IB) were not different between the groups. As expected, vascular smooth muscle did not stain positive for myoglobin (cross section of aorta shown in supplemental Figure II), thus confirming that the transgene was not ectopically expressed in vascular smooth muscle.19 These findings indicate that MbTg and WT mice have similar (normal) endothelial function and have no differences in NOS activity and levels of NO within the vasculature.
Figure 1.
A, Myoglobin(Mb) and Mb-transgene (hemagluttinin-tagged, HA-Mb) expression in Western blots of gastrocnemus muscle from transgenic (MbTg) and wild-type (wt) mice. Total myoglobin is higher in MbTg vs WT mice by ELISA (n=8 per group *P<0.001), as was (B) VEGF-A (n=8 per group; *P<0.05). There was no difference in (C) capillary density or (D) phophorylated endothelial nitric oxide synthase (peNOS) to total eNOS. E, In aortic rings, there was no difference in endothelium-dependent (ACh-mediated) and endothelium-independent (SNP-mediated) relaxation between groups.
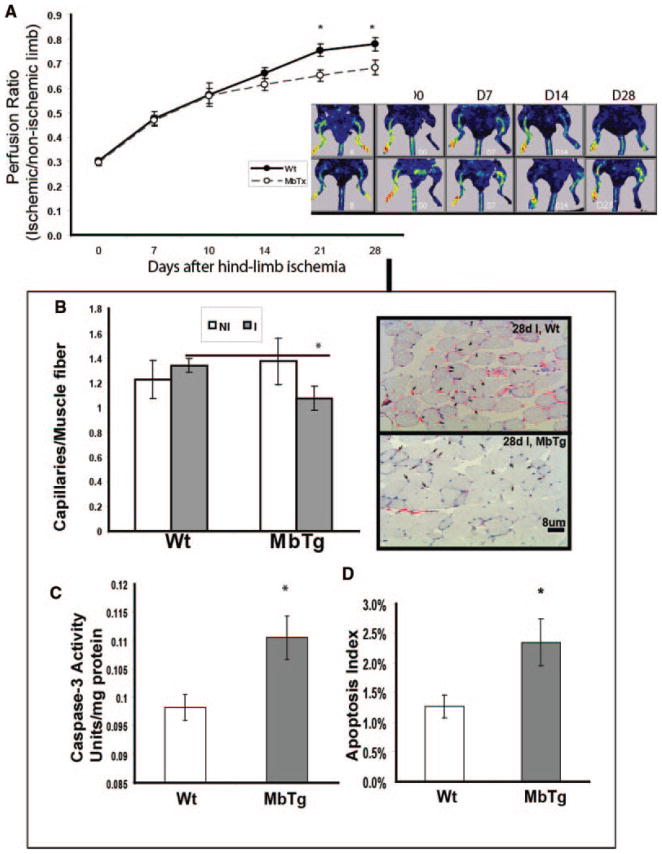
MbTg Versus Wild-Type: Attenuated Perfusion Recovery, Reduced Capillary Density, Increased Apoptosis and Increased Necrosis After Hind-Limb Ischemia
Immediately after induction of hind-limb ischemia, there was no difference in the perfusion ratio between the two groups (d0, perfusion ischemic/nonischemic limb; WT versus MbTg 0.30±0.01 versus 0.29±0.01; P=NS). The extent of perfusion recovery between the MbTg and WT groups was not significantly different at 7-, 10-, and 14-day postligation time points. However, the perfusion ratio was significantly lower in MbTg versus WT at 21 (0.65±0.02 versus 0.75±0.03, P<0.05) and 28 (0.68±0.03 versus 0.78±0.03, P<0.05) days after ligation (Figure 2A). In the 28-day postischemic gastrocnemius muscle, capillary density was significantly less in MbTg mice compared to WT (Figure 2B, WT 0.31±0.04 versus MbTg 0.18±0.03 capillaries/fiber; n=8; P<0.05). There was no difference in macrophage infiltration or number of smooth muscle positive blood vessels in the ischemic muscle between the two groups (supplemental Figure IIIA and IIIB).
Figure 2.
A, Time course (left) and image (right) of laser Doppler perfusion imaging (LDPI) shows myoglobin-transgenic (MbTx) had impaired perfusion recovery compared to wild-type (WT) mice, day 21 and 28 after hind-limb ischemia (n=20 per group, *P<0.05). B, Ischemic muscle from MbTg mice (I, 28 days after ischemia) had lower capillary density compared to WT, indicating an attenuated angiogenic response (n=8 per group, *P<0.05) with more apoptosis by (C) caspase-3 activity (n=8 per group, *P<0.01) and (D) Tunel staining (n=8 per group, *P<0.05).
There was increased apoptosis in the ischemic muscle of MbTg compared to that in WT as determined by caspase-3 activity (Figure 2C) and tunel staining (Figure 2D). In addition, the incidence of limb necrosis was significantly higher in MbTg (65.2%) compared to wild-type (15%) and when present, the degree of necrosis was greater in MbTg mice (Table).
Table.
Incidence and Grades of Limb Necrosis in WT vs MbTg Mice After Hind-Limb Ischemia
| Total No. | No. of Necrosis | Incidence of Necrosis | 0 | I | II | III | IV | |
|---|---|---|---|---|---|---|---|---|
| Grades of necrosis | ||||||||
| WT | 20 | 3 | 15% | 17 | 3 (15%) | 0 | 0 | 0 |
| MbTg | 23 | 15 | 65.2%* | 8 | 12 (52%) | 2 (9%) | 1 (4%) | 0 |
P<0.001 vs WT.
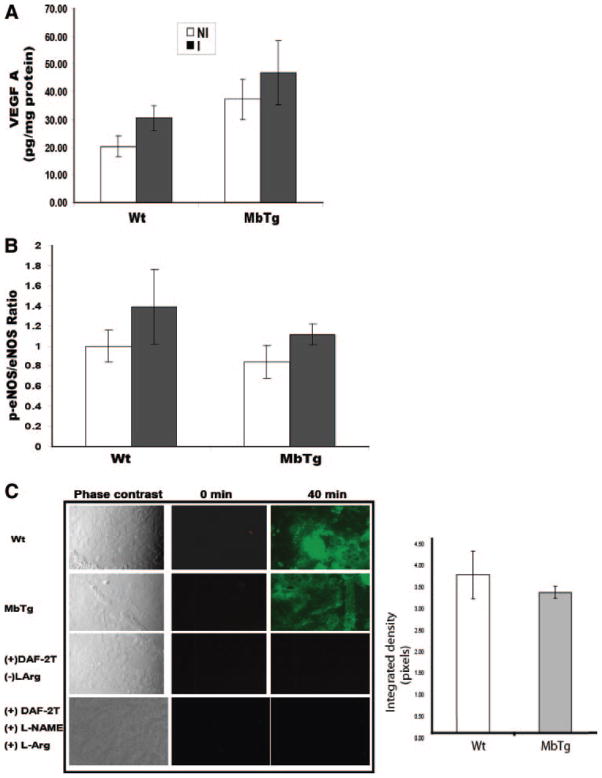
Both MbTg and Wild Type Increase VEGF-A Expression, Activate eNOS, and Generate NO to Similar Degrees
As shown in Figure 1, differences in perfusion recovery between groups could not be accounted for by findings in the muscle preligation. Indeed, the higher levels of VEGF-A in nonischemic muscle in the MbTg mice (Figure 1B) might have been expected to improve perfusion recovery. We next sought to determine whether the “angiogenic” response to ischemia was similar between the transgenic and wild-type mice. VEGF-A and the ratio of p-eNOS/total eNOS was similar between MbTg and WT mice at the time point (14-day) when perfusion recovery was similar between the groups. In both groups, there was a comparable increase in VEGF-A in the ischemic compared to the nonischemic limb (Figure 3A). After ischemia, both MbTg and WT mice had upregulation of p-eNOS (Figure 3B). There was no difference between the groups. In addition, in 14-day postischemic tissue, there was no difference in total NOS activity between MbTg and WT mice (Figure 3C), thereby cumulatively indicating that both groups had comparable production of NO.
Figure 3.
A, At 14 days postoperatively, both myoglobin-transgenic (MbTg) and wild-type (WT) mice had comparable increases in VEGF-A in ischemic (I) vs nonischemic (NI) limbs (n=10 per group). B, The expression of p-eNOS/eNOS (eNOS activation) was similar (n=6 per group) and (C) there was no difference in total NOS activity (representative picture from n=4 per group, see methods for details).
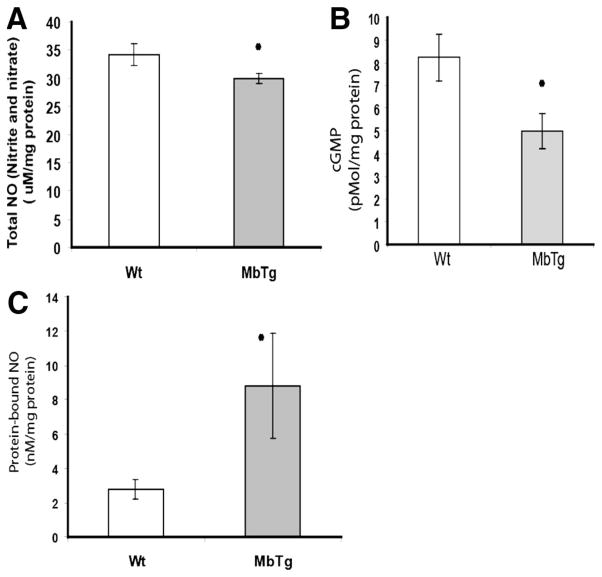
MbTg Versus Wild Type Postischemia: Reduced Tissue Levels of Total NO With Higher Protein-Bound NO
We measured the levels of nitrite and nitrate in skeletal muscle as indicators of NO in the tissue 14 days after ischemia, when perfusion recovery in both groups was comparable. Total NO in the ischemic limb was significantly less in the MbTg mice compared to that in WT (Figure 4A, WT 34.2±1.9 versus MbTg 29.9±0.8 μmol/L/mg protein; n=10; P<0.05). We determined the levels of nitrate and nitrite in the rodent chow and water. Whereas regular laboratory water had nitrate/nitrite levels of 63.7±0.2 μmol/L/mL, these levels were undetectable in the water used in the animal facilities. The rodent chow had nitrate/nitrite levels of only 6.4±5 μmol/L/mg. Because both groups received the same diet and water, dietary source should not account for the difference in tissue nitrate/nitrite. We also measured the tissue levels of cGMP as an alternative measure of tissue NO. Whereas cGMP levels were similar between the groups in the nonischemic limb, ischemic tissue from MbTg mice had significantly lower levels of cGMP (Figure 4B, 5.0±0.8 versus 8.2±1.0 pM/mg protein in WT, n=8, P<0.05). The levels of protein-bound NO were significantly higher in muscles from MbTg mice compared to that from WT (Figure 4C, WT 2.8±0.6 versus MbTg 8.8±3.1 nmol/L/mg protein; n=10; n=9; P<0.05). There was no difference in the values following pretreatment of the samples with mercuric chloride, thereby indicating that the protein-bound NO existed predominantly in the ferrous-nitrosyl-Mb form.
Figure 4.
A, The sum of stable NO products nitrate and nitrite was measured as an index of the available NO. At 14 days postoperatively, ischemic skeletal muscle from myoglobin-transgenic mice (MbTg) had significantly less NO compared to wild-type (WT) mice (n=10 per group; *P<0.05). B, cGMP levels did not differ in the nonischemic limb, but were significantly lower in the ischemic muscle from MbTg vs wt mice (n=8 per group, P<0.05). C, MbTg muscle had significantly higher levels of protein-bound NO compared to WT mice; indicating NO scavenging by myoglobin (n=9 per group; P<0.05).
Lack of Increased Muscle Injury in MbTg Versus WT With Endotoxin Injury
The expression of Atrogin-1 mRNA (Atrogin/18s copy #, mean ± SEM) was not different between the groups (MbTg 0.39±0.2 versus WT 0.56±0.3, n=4/group, P=NS) after endotoxin induced muscle injury.
Discussion
The results from this study demonstrate, for the first time, that processes within myocytes can directly modulate angiogenesis in the setting of normal endothelial function. Our data add to a well established literature that NO plays an important role in angiogenesis, but these other studies have largely focused on the endothelium as the site for altered NO homeostasis.6,19,21,26 Myoglobin is a muscle-specific protein that is present only in striated skeletal muscle. Accumulating evidence indicates that NO interacts with myoglobin to yield different products,11,13,27–33 but the definitive functional role of myoglobin-NO interactions remains to be elucidated.16,30 Using a transgenic model, we show the first evidence that myoglobin can play a significant role in modulating the endogenous angiogenic response to ischemia by reducing NO bioavailablity, in part via sequestering NO as nitrosyl-heme. The impaired angiogenic response occurred despite increased levels of VEGF-A, and activation of eNOS after ischemia. Data from this study that perfusion recovery in the MbTg mice is significantly attenuated only at late time points (day 21 and 28) after ischemia is consistent with an impaired angiogenic response, as opposed to differences in preexisting collaterals.34 We could find no difference in macrophage infiltration between the ischemic tissues of MbTg and WT mice (supplemental Figure III). The aortic ring studies, where myoglobin is not locally expressed, confirm that MbTg mice have similar endothelial function, NOS activity and NO availability in the vasculature, as wild-type mice.
Myoglobin has traditionally been characterized as the oxygen carrier that is abundantly expressed in the skeletal muscle.33 The results of our study clearly support the work of others to suggest that myoglobin also plays a vital role in NO homeostasis.27,30 As shown by Van Weel et al, in a rodent model of hind-limb ischemia, treatment with VEGF-A induced myoglobin gene expression in the ischemic muscle.35 These mice failed to mount a robust response to ischemia despite greater amounts of VEGF-A, and although excess myoglobin expression might improve muscle oxygenation and have beneficial effects, our data clearly show that excess myoglobin can inhibit the extent of the angiogenic response. Data from our current study that nonischemic skeletal muscle from MbTg mice had higher levels of VEGF-A (Figure 1B) compared to the same muscle group in wild-type mice, could be attributable to greater NO-scavenging in the absence of injury. When we previously examined across different striated muscle groups within the same animal, also in the absence of injury, the expression of VEGF-A closely paralleled that of myoglobin, with greater expression in oxidative/red skeletal muscle compared to glycolytic/white skeletal muscle.36
The results of our study could not have been predicted a priori. The role of myoglobin as an NO scavenger is supported by several studies. NO interacts with low molecular weight thiols, protein sulfhydryls,37,38 and heme proteins.32,39 Whereas human myoglobin has a free cysteine residue that can interact with NO to form S-nitrosothiols, mouse myoglobin does not have a free cysteine.13,40 Therefore, it is most likely that mouse myoglobin sequesters NO primarily as nitrosyl-heme myoglobin, as supported by this study. In our study we measured protein bound NO, and a limitation of our study is that we inferred it as myoglobin-bound NO. Although we cannot definitively rule out other protein-bound NO, our data are most consistent with myoglobin bound NO as supported by fact and observations that: (1) myoglobin is the predominant heme protein in the skeletal muscle, (2) myoglobin is overexpresed in the transgenic animals (Figure 1A), and (3) the protein-bound NO existed almost exclusively in the nitrosyl-heme form, thereby indicating that it is bound primarily to heme proteins. Wegener and coworkers41 showed that isolated cardiomyocytes form myoglobin knockout mice had greater reduction in the force generation in response to NO donors compared to myocytes from wild-type mice. This indicates that, under physiological conditions, myoglobin acts as a scavenger preventing NO from reaching its intracellular targets in cardiomyocytes. Using a computational model, Tsoukias and Popel42 showed that NO originating from capillary wall can diffuse toward the parenchymal cells and sustain physiologically significant concentrations. But, the presence of myoglobin in the parenchyma can deplete NO substantially. To the best of our knowledge, there is no evidence that myoglobin has a direct role in NO-mediated signaling pathways in angiogenesis, but this cannot be ruled out completely. Despite the implications of myoglobin–NO interactions being cytoprotective under normoxic conditions29 and in ischemia–reperfusion injury,18 our study demonstrate that in vivo, overexpression of myoglobin in muscle cells leads to increased limb necrosis and impairs angiogenesis and perfusion recovery.
In summary, data from this study show that increased myoglobin expression in skeletal myocytes attenuates perfusion recovery after hind-limb ischemia, in part, by scavenging NO and reducing its bioavailability. This study provides the first direct evidence that myoglobin levels can serve as significant regulators of bioavailable NO in skeletal muscle and can modulate the angiogenic response in an endothelium independent manner. In addition, this describes a novel model for impaired ischemic angiogenesis for studying NO biology.
Supplementary Material
Acknowledgments
The authors thank: Dr R. Sanders Williams (Duke University) for providing the original MbTg mice for breeding; Dr Mark Davies (University of Rochester) for his expertise in interpretation of the vascular ring experiments, and Jennifer Jackson for her technical assistance.
Sources of Funding
This work was supported by R33-HL088286 and UO1-DK 076136 to B.H.A.
Footnotes
Disclosures
None.
References
- 1.Annex BH, Simons M. Growth factor-induced therapeutic angiogenesis in the heart: protein therapy. Cardiovasc Res. 2005;65:649–655. doi: 10.1016/j.cardiores.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Inoue N, Kondo T, Kobayashi K, Aoki M, Numaguchi Y, Shibuya M, Murohara T. Therapeutic angiogenesis using novel vascular endothelial growth factor-E/human placental growth factor chimera genes. Arterioscler Thromb Vasc Biol. 2007;27:99–105. doi: 10.1161/01.ATV.0000251504.61247.d5. [DOI] [PubMed] [Google Scholar]
- 3.Lee M, Aoki M, Kondo T, Kobayashi K, Okumura K, Komori K, Murohara T. Therapeutic angiogenesis with intramuscular injection of low-dose recombinant granulocyte-colony stimulating factor. Arterioscler Thromb Vasc Biol. 2005;25:2535–2541. doi: 10.1161/01.ATV.0000190609.28293.17. [DOI] [PubMed] [Google Scholar]
- 4.Cooke JP, Losordo DW. Nitric oxide and angiogenesis. Circulation. 2002;105:2133–2135. doi: 10.1161/01.cir.0000014928.45119.73. [DOI] [PubMed] [Google Scholar]
- 5.Lee PC, Salyapongse AN, Bragdon GA, Shears LL, II, Watkins SC, Edington HD, Billiar TR. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol. 1999;277:H1600–H1608. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- 6.Luque Contreras D, Vargas Robles H, Romo E, Rios A, Escalante B. The role of nitric oxide in the post-ischemic revascularization process. Pharmacol Ther. 2006;112:553–563. doi: 10.1016/j.pharmthera.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation. 2000;102:III370–III376. doi: 10.1161/01.cir.102.suppl_3.iii-370. [DOI] [PubMed] [Google Scholar]
- 9.d’Uscio LV, Baker TA, Mantilla CB, Smith L, Weiler D, Sieck GC, Katusic ZS. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- 10.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 11.Flogel U, Merx MW, Godecke A, Decking UK, Schrader J. Myoglobin: A scavenger of bioactive NO. Proc Natl Acad Sci U S A. 2001;98:735–740. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kavdia M, Tsoukias NM, Popel AS. Model of nitric oxide diffusion in an arteriole: impact of hemoglobin-based blood substitutes. Am J Physiol Heart Circ Physiol. 2002;282:H2245–H2253. doi: 10.1152/ajpheart.00972.2001. [DOI] [PubMed] [Google Scholar]
- 13.Witting PK, Douglas DJ, Mauk AG. Reaction of human myoglobin and nitric oxide. Heme iron or protein sulfhydryl(s) nitrosation dependence on the absence or presence of oxygen. J Biol Chem. 2001;276:3991–3998. doi: 10.1074/jbc.M005758200. [DOI] [PubMed] [Google Scholar]
- 14.Blomberg LM, Blomberg MR, Siegbahn PE. A theoretical study of myoglobin working as a nitric oxide scavenger. J Biol Inorg Chem. 2004;9:923–935. doi: 10.1007/s00775-004-0585-5. [DOI] [PubMed] [Google Scholar]
- 15.Lancaster JR., Jr Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rayner BS, Wu BJ, Raftery M, Stocker R, Witting PK. Human S-nitroso oxymyoglobin is a store of vasoactive nitric oxide. J Biol Chem. 2005;280:9985–9993. doi: 10.1074/jbc.M410564200. [DOI] [PubMed] [Google Scholar]
- 17.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 18.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100:1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 19.Yan Z, Serrano AL, Schiaffino S, Bassel-Duby R, Williams RS. Regulatory elements governing transcription in specialized myofiber subtypes. J Biol Chem. 2001;276:17361–17366. doi: 10.1074/jbc.M101251200. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Hazarika S, Xie D, Pippen AM, Kontos CD, Annex BH. In mice with type 2 diabetes, a vascular endothelial growth factor (VEGF)-activating transcription factor modulates VEGF signaling and induces therapeutic angiogenesis after hindlimb ischemia. Diabetes. 2007;56:656–665. doi: 10.2337/db06-0999. [DOI] [PubMed] [Google Scholar]
- 21.Ignarro LJ. Endothelium-derived nitric oxide: pharmacology and relationship to the actions of organic nitrate esters. Pharm Res. 1989;6:651–659. doi: 10.1023/a:1015926119947. [DOI] [PubMed] [Google Scholar]
- 22.Malinski T, Mesaros S, Tomboulian P. Nitric oxide measurement using electrochemical methods. Methods Enzymol. 1996;268:58–69. doi: 10.1016/s0076-6879(96)68009-4. [DOI] [PubMed] [Google Scholar]
- 23.Nagano T. Practical methods for detection of nitric oxide. Luminescence. 1999;14:283–290. doi: 10.1002/(SICI)1522-7243(199911/12)14:6<283::AID-BIO572>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P, Waters RE, Redfern SI, Zhang M, Mao L, Annex BH, Yan Z. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol. 2007;170:599–608. doi: 10.2353/ajpath.2007.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay D, Ahmed A. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res. 2006;99:715–722. doi: 10.1161/01.RES.0000243989.46006.b9. [DOI] [PubMed] [Google Scholar]
- 27.Copeland DM, Soares AS, West AH, Richter-Addo GB. Crystal structures of the nitrite and nitric oxide complexes of horse heart myoglobin. J Inorg Biochem. 2006;100:1413–1425. doi: 10.1016/j.jinorgbio.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Giuffre A, Forte E, Brunori M, Sarti P. Nitric oxide, cytochrome c oxidase and myoglobin: competition and reaction pathways. FEBS Lett. 2005;579:2528–2532. doi: 10.1016/j.febslet.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 29.Godecke A, Molojavyi A, Heger J, Flogel U, Ding Z, Jacoby C, Schrader J. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J Biol Chem. 2003;278:21761–21766. doi: 10.1074/jbc.M302573200. [DOI] [PubMed] [Google Scholar]
- 30.Kreutzer U, Jue T. Role of myoglobin as a scavenger of cellular NO in myocardium. Am J Physiol Heart Circ Physiol. 2004;286:H985–H991. doi: 10.1152/ajpheart.00115.2003. [DOI] [PubMed] [Google Scholar]
- 31.Kreutzer U, Jue T. Investigation of bioactive NO-scavenging role of myoglobin in myocardium. Pflugers Arch. 2006;452:36–42. doi: 10.1007/s00424-005-0011-z. [DOI] [PubMed] [Google Scholar]
- 32.Meuwly M, Becker OM, Stote R, Karplus M. NO rebinding to myoglobin: a reactive molecular dynamics study. Biophys Chem. 2002;98:183–207. doi: 10.1016/s0301-4622(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 33.Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J Exp Biol. 2003;206:2011–2020. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]
- 34.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 35.van Weel V, Deckers MM, Grimbergen JM, van Leuven KJ, Lardenoye JH, Schlingemann RO, van Nieuw Amerongen GP, van Bockel JH, van Hinsbergh VW, Quax PH. Vascular endothelial growth factor overexpression in ischemic skeletal muscle enhances myoglobin expression in vivo. Circ Res. 2004;95:58–66. doi: 10.1161/01.RES.0000133247.69803.c3. [DOI] [PubMed] [Google Scholar]
- 36.Annex BH, Torgan CE, Lin P, Taylor DA, Thompson MA, Peters KG, Kraus WE. Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. Am J Physiol. 1998;274:H860–H867. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- 37.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 38.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 39.Tiravanti E, Samouilov A, Zweier JL. Nitrosyl-heme complexes are formed in the ischemic heart: evidence of nitrite-derived nitric oxide formation, storage, and signaling in post-ischemic tissues. J Biol Chem. 2004;279:11065–11073. doi: 10.1074/jbc.M311908200. [DOI] [PubMed] [Google Scholar]
- 40.Hubbard SR, Hendrickson WA, Lambright DG, Boxer SG. X-ray crystal structure of a recombinant human myoglobin mutant at 2.8 A resolution. J Mol Biol. 1990;213:215–218. doi: 10.1016/S0022-2836(05)80181-0. [DOI] [PubMed] [Google Scholar]
- 41.Wegener JW, Godecke A, Schrader J, Nawrath H. Effects of nitric oxide donors on cardiac contractility in wild-type and myoglobin-deficient mice. Br J Pharmacol. 2002;136:415–420. doi: 10.1038/sj.bjp.0704740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsoukias NM, Popel AS. A model of nitric oxide capillary exchange. Microcirculation. 2003;10:479–495. doi: 10.1038/sj.mn.7800210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.