Summary
Nematodes comprise a large phylum of both free-living and parasitic species that show remarkably diverse lifestyles, ecological niches, and behavioral repertoires. Parasitic species in particular often display highly specialized host-seeking behaviors that reflect their specific host preferences. Many host-seeking behaviors can be triggered by the presence of host odors, yet little is known about either the specific olfactory cues that trigger these behaviors or the neural circuits that underlie them. Heterorhabditis bacteriophora and Steinernema carpocapsae are phylogenetically distant insect-parasitic nematodes whose host-seeking and host-invasion behavior resembles that of some of the most devastating human- and plant-parasitic nematodes. Here we compare the olfactory responses of H. bacteriophora and S. carpocapsae infective juveniles (IJs) to those of Caenorhabditis elegans dauers, which are analogous life stages [1]. We show that the broad host range of these parasites results from their ability to respond to the universally-produced signal carbon dioxide (CO2) as well as a wide array of odors, including host-specific odors that we identified using TD-GC-MS. We show that CO2 is attractive for the parasitic IJs and C. elegans dauers despite being repulsive for C. elegans adults [2–4], and we identify an ancient and conserved sensory neuron that mediates CO2 response in both parasitic and free-living species regardless of whether CO2 is an attractive or a repulsive cue. Finally, we show that the parasites’ odor response profiles are more similar to each other than to that of C. elegans despite their greater phylogenetic distance, likely reflecting evolutionary convergence to insect parasitism. Our results suggest that the olfactory responses of parasitic versus free-living nematodes are highly diverse and that this diversity is critical to the evolution of nematode behavior.
Results and Discussion
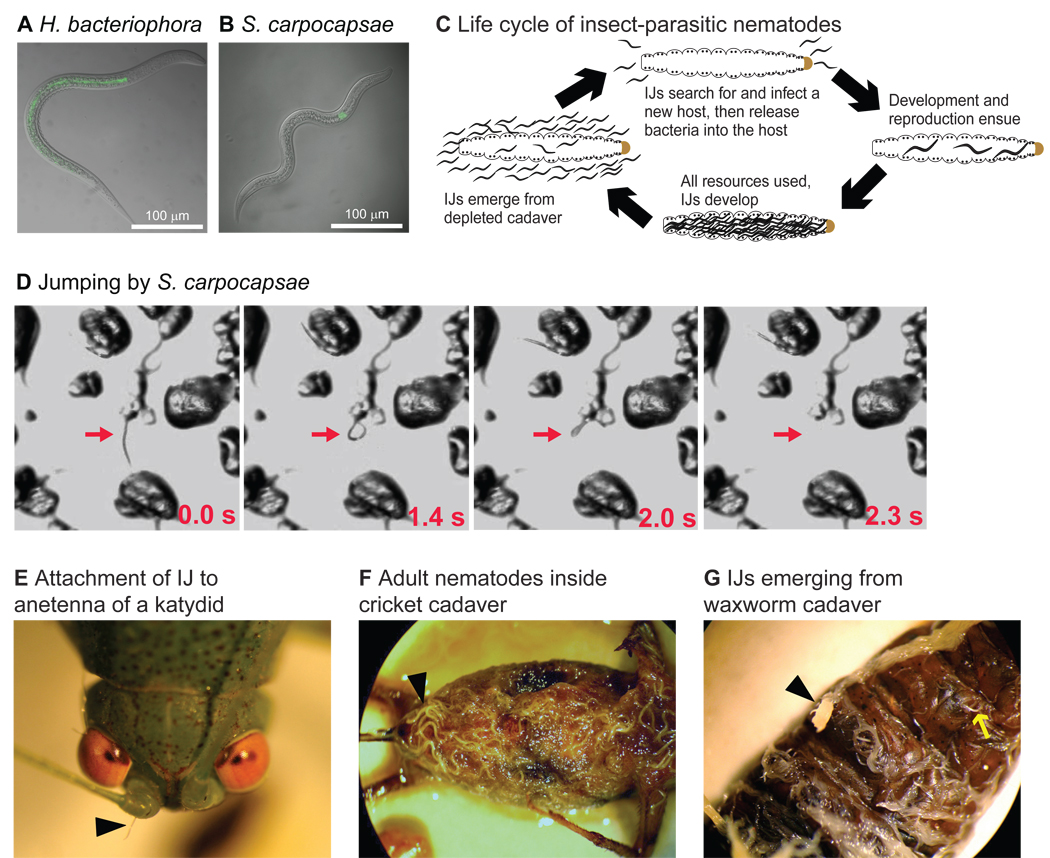
H. bacteriophora and S. carpocapsae are lethal parasites of insect larvae currently used as biocontrol agents for many insect pests. The two species are phylogenetically distant yet share similar lifestyles and ecological niches as a result of convergent evolution to insect parasitism (Figures 1A–C, S1). Both species infect hosts only as infective juveniles (IJs), a developmentally-arrested third larval stage analogous to the dauer stage of C. elegans [1, 5]. Both species are associated with symbiotic bacteria during the IJ stage [6, 7]. IJs live in the soil, where they actively seek out and infect hosts; all other life stages exist exclusively inside the host. IJs infect either by entering through a natural body opening or by penetrating through the insect cuticle. Once inside the hosts, IJs release their symbiotic bacteria, which helps them overcome the host immune system and results in rapid host death [8–11]. The nematodes reproduce inside the insect cadaver for 2–3 generations until resources are depleted, after which new IJs form and disperse into the soil (Figure 1C–G).
Figure 1. Life cycles of insect-parasitic nematodes.
A–B. Photomicrographs of an H. bacteriophora (A) and an S. carpocapsae (B) infective juvenile (IJ). Both species harbor a bacterial symbiont – H. bacteriophora harbors Photorhabdus luminescens and S. carpocapsae harbors Xenorhabdus nematophila – in the gut during the IJ stage. Nomarski images are overlaid with epifluorescence images; bacterial symbiont is labeled with GFP. In both cases, the anterior end of the worm is at the top. C. The life cycle of insect-parasitic nematodes. The IJ stage is a developmentally-arrested third larval stage, and is the only free-living stage. IJs infect insect larvae by entering through a natural body opening, although H. bacteriophora can also penetrate directly through the larval cuticle. Following infection, IJs expel their symbiotic bacteria into the host, where it plays a critical role in overcoming the host immune system [6, 7]. The nematodes develop and reproduce inside the insect cadaver until the food is depleted, at which point new IJs form and disperse into the soil in search of new hosts [46]. D. Jumping by S. carpocapsae. Still images of a jumping IJ. A standing IJ (0.0 s) curls (1.4 s) into a lariat structure (2.0 s) and propels itself into the air (2.3 s). Jumping was observed on an agar surface sprinkled with sand. Red arrows indicate the jumping IJ; time is recorded in the lower right. A single jump can propel the nematode nine body lengths in distance and seven body lengths in height, and can be elicited by chemosensory and mechanical stimuli [47]. E–G. Representative photomicrographs illustrating the insect-parasitic lifestyle. E. A Steinernematid IJ jumped onto and attached to a katydid antenna. Arrowhead indicates attached IJ. F. A cricket (Acheta domesticus) cadaver infected with Steinernematids. Adult nematodes are visible beneath the cuticle throughout the cadaver; some of the most prominent nematodes are indicated by the arrowhead. G. IJs emerging from a depleted waxworm (Galleria mellonella) cadaver. Arrowhead indicates a clump of IJs; arrow indicates a single IJ. See also Figure S1 and Move S1.
Despite their similar lifestyles, H. bacteriophora and S. carpocapsae are thought to use different strategies for host location: H. bacteriophora IJs are “cruisers” that move through the soil actively chemotaxing toward potential hosts, while S. carpocapsae IJs are “ambushers” that remain relatively stationary and stand on their tails, a behavior known as nictation, to facilitate attachment to passing hosts [12, 13]. Ambush foraging in S. carpocapsae also consists of an unusual jumping behavior in which the IJ nictates, curls into a loop, and propels itself into the air (Figure 1D and Movie S1). Jumping in nematodes is unique to the genus Steinernema and is considered a specialized evolutionary adaptation that facilitates attachment to passing hosts as well as dispersal to new niches (Figure 1E) [14]. For both H. bacteriophora and S. carpocapsae, exposure to host volatiles can stimulate host-seeking behavior [15–18]. However, our understanding of how these parasites respond to specific olfactory cues is incomplete and nothing is known about the neural basis of these responses.
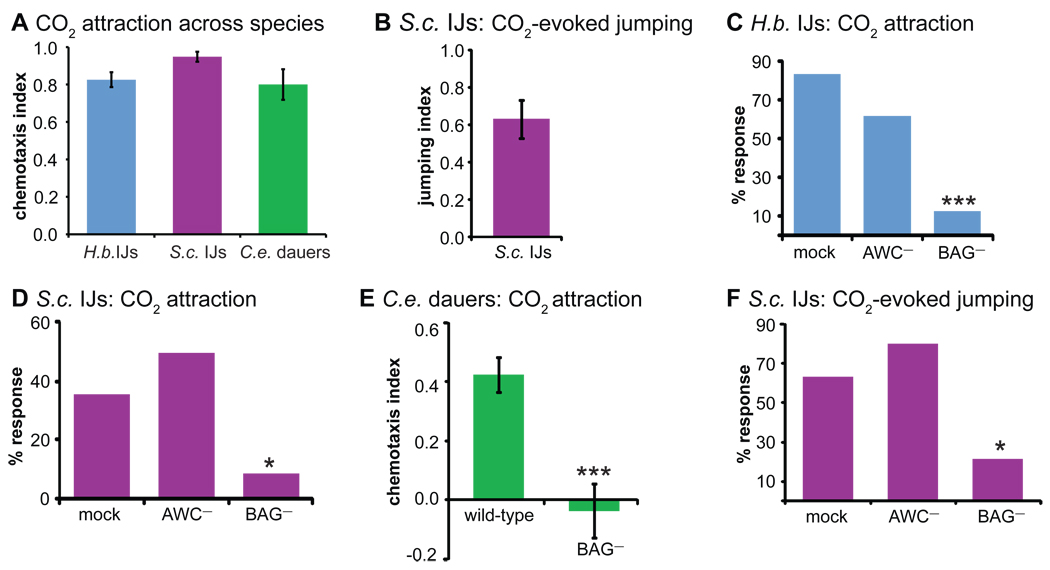
Parasitic IJs and C. elegans dauers are attracted to CO2
To investigate how H. bacteriophora and S. carpocapsae IJs respond to host odors, we first examined responses to carbon dioxide (CO2). CO2 is emitted by all animals as a byproduct of respiration and is a host cue for a wide range of parasites and disease vectors, including many parasitic nematodes [19–21]. We used a chemotaxis assay in which worms were allowed to distribute on a plate in a CO2 concentration gradient (Figure S2A). Parasitic IJs were strongly attracted to CO2 across concentrations (Figures 2A, S2C–D). To assay CO2-evoked jumping, we developed a jumping assay in which standing IJs were exposed to a small puff of CO2 from a syringe and given 8 seconds to jump in response to the puff (Figure S2B, Movie S2). We found that CO2 stimulates jumping by S. carpocapsae (Figures 2B, S2E), demonstrating that CO2 can evoke multiple host-seeking behaviors. CO2 stimulated jumping at concentrations as low as 0.08%, which is ~2-fold higher than atmospheric levels, indicating that jumping is highly sensitive to proximal levels of environmental CO2 (Figure S2E).
Figure 2. BAG neurons are required for CO2 response in free-living and parasitic nematodes.
A. Parasitic IJs and C. elegans dauers are attracted to CO2 in a chemotaxis assay (Figure S2A). n = 5–6 trials for each species. B. CO2 induces jumping by S. carpocapsae in a jumping assay (Figure S2B). n = 4–11 trials. C–E. BAG neurons are required for CO2 attraction in H. bacteriophora and S. carpocapsae IJs, and C. elegans dauers. n = 12–34 worms for each treatment (C–D) or n = 18–29 trials (E). The assay in E was a 10 min. assay, since the difference between wild-type and BAG- animals was apparent after only 10 min. F. BAG neurons are required for CO2-evoked jumping by S. carpocapsae IJs. n = 10–18 worms for each treatment. ***, P<0.001; *, P<0.05, Fisher’s exact test (C, D, F) or unpaired t test (E). Error bars represent SEM. For C, D, and F, y-axis values represent the percentage of worms that yielded a positive behavioral response; error bars are not present because each worm was scored once individually. AWC chemosensory neurons were ablated as a control. 10% CO2 was used for all experiments. See also Figure S2 and Movie S2.
The IJ stage of parasitic worms is analogous to the dauer stage of free-living worms: both are long-lived, non-feeding, developmentally-arrested third larval stages [1], and conserved neurons and signaling pathways mediate exit from the dauer/IJ stage [22, 23]. C. elegans arrests development at the dauer stage when environmental conditions are unfavorable and develops to adulthood only after conditions improve; in nature, C. elegans is found primarily in the dauer stage [24]. We found that C. elegans dauers, like parasitic IJs, are attracted to CO2 (Figures 2A, S2F). By contrast, C. elegans adults are repelled by CO2 [2, 3]. These results demonstrate that both dauers and IJs respond similarly to CO2, and that C. elegans undergoes a developmental change in CO2 response valence from the dauer to the adult stage. Why are dauers attracted to CO2? Although the ecology of C. elegans is poorly understood, C. elegans dauers have been found in association with invertebrates such as slugs, snails, and isopods. CO2 attraction may enable dauers to migrate toward invertebrate carriers, thereby facilitating dispersal to new niches. CO2 attraction may also serve as a means of locating bacterial food [25].
BAG sensory neurons are required for CO2 attraction
To gain insight into the neural circuitry underlying host seeking, we leveraged the fact that neural anatomy and function are highly conserved across nematode species and life stages [22, 26–31]. In C. elegans adults, CO2 repulsion requires a pair of sensory neurons called the BAG neurons [2, 4]. We found that BAG neurons are easily identifiable in the parasitic IJs using the neuroanatomical map of C. elegans [32] (Figure S2G; also see Methods). To investigate the role of BAG neurons in mediating CO2 attraction, we ablated these neurons and examined CO2 response. We found that parasitic IJs and C. elegans dauers that lack BAG neurons are not attracted to CO2 (Figure 2C–E). In addition, S. carpocapsae IJs that lack BAG neurons do not exhibit CO2-induced jumping (Figure 2F). Thus, BAG neurons are required for CO2 attraction in both free-living and parasitic nematodes and contribute to both chemotaxis and jumping.
To further investigate the extent to which BAG neuron function is conserved throughout the phylum Nematoda, we examined a different nematode, Pristionchus pacificus. P. pacificus is a necromenic nematode that opportunistically feeds off insect cadavers and that is thought to represent an evolutionary intermediate between free-living and parasitic lifestyles [33]. Adult P. pacificus were previously shown to avoid CO2 [2]. BAG-ablated P. pacificus adults do not avoid CO2, indicating that BAG neurons are required for CO2 repulsion by P. pacificus (Figure S2H). The four species we have tested ─ H. bacteriophora, S. carpocapsae, C. elegans, and P. pacificus ─ display more molecular sequence divergence from each other than sea squirts do from humans [34]. Thus, BAG neurons play an ancient and conserved role in mediating CO2 response in free-living and parasitic nematodes regardless of whether CO2 is attractive or repulsive.
The fact that BAG neurons can mediate both attractive and repulsive responses is unusual for nematode sensory neurons, most of which are hard-wired for either attraction or repulsion. For example, the ASH sensory neurons play a conserved role in mediating repulsion to chemical and mechanical stimuli in free-living and parasitic nematodes [26, 28, 29], while the ADL neurons play a conserved role in mediating chemical avoidance [28]. The mechanism by which the BAG neuron can mediate either attraction or repulsion to the same stimulus is not yet understood.
BAG neurons are required for some but not all host-seeking behaviors
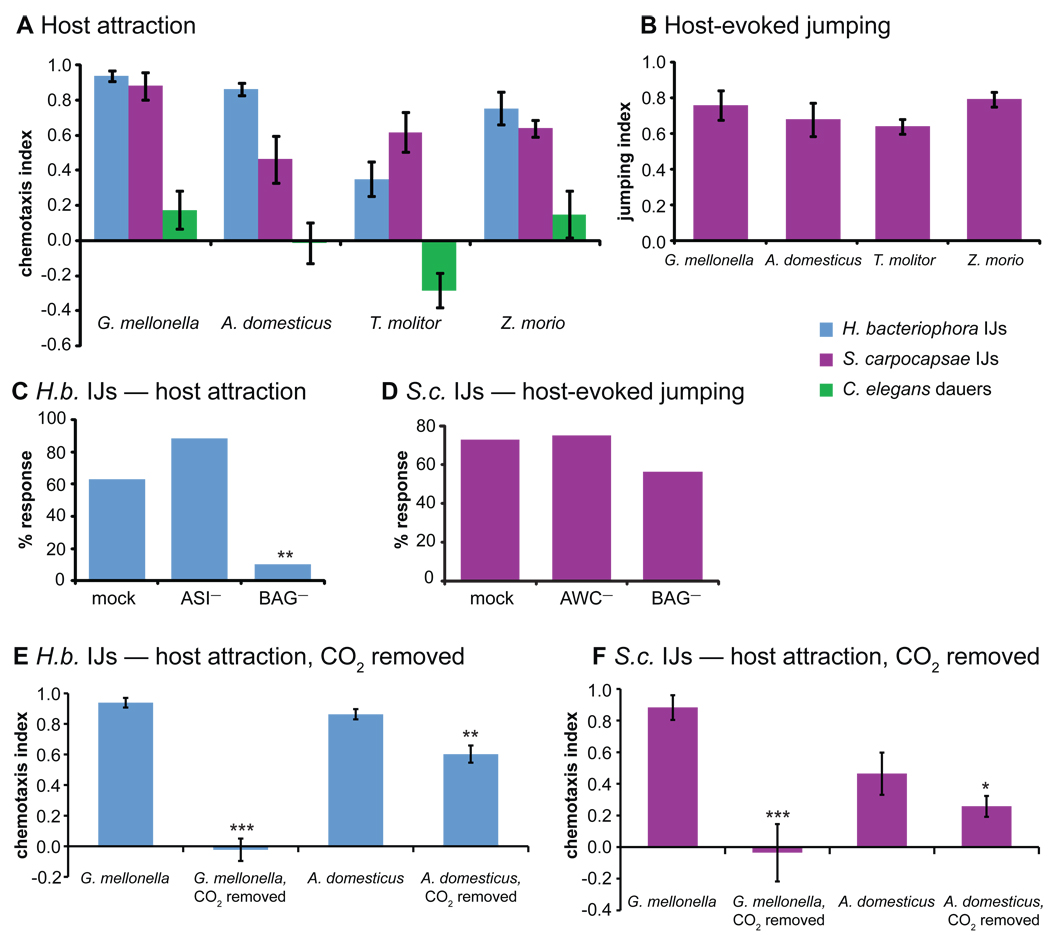
To test whether BAG neurons are required for host finding, we developed an assay in which headspace from a syringe containing insect larvae is used to establish a gradient of host odors. We examined responses to odors emitted by four insects that IJs are capable of using as hosts: waxworms (Galleria mellonella), superworms (Zophobas morio), mealworms (Tenebrio molitor), and crickets (Acheta domesticus). We found that H. bacteriophora and S. carpocapsae were attracted to all four insects (Figure 3A). Odors emitted by all four insects also stimulated jumping by S. carpocapsae (Figure 3B). The fact that S. carpocapsae chemotaxed toward host volatiles suggests that although these worms are generally considered ambushers, they are capable of utilizing a cruising strategy for host location. In contrast to the parasitic worms, C. elegans dauers were not attracted to these insects and in fact were repelled by mealworm odors (Figure 3A).
Figure 3. BAG neurons are required for some but not all host-seeking behaviors.
A. Volatiles released by live waxworms (Galleria mellonella), crickets (Acheta domesticus), mealworms (Tenebrio molitor), and superworms (Zophobas morio) attract the parasitic IJs but not C. elegans dauers. n = 6–27 trials. B. Insect volatiles also stimulate jumping by S. carpocapsae. n = 3–11 trials. **, P<0.01, one-way ANOVA with Dunnett’s post-test. For A–B, error bars represent SEM. C. BAG neurons are required for chemotaxis toward waxworms in H. bacteriophora. n = 10–38 worms for each treatment. **, P<0.01, Fisher’s exact test. D. BAG neurons are not required for jumping evoked by waxworm odors in S. carpocapsae. n = 20–39 worms for each treatment. No significant differences were observed between treatment groups. For C–D, values shown represent the percentage of worms that yielded a positive behavioral response; error bars are not present because each worm was scored once individually. AWC or ASI chemosensory neurons were ablated as controls. E–F. Attraction of H. bacteriophora (E) and S. carpocapsae (F) to G. mellonella is eliminated and A. domesticus is reduced when CO2 is chemically removed from host headspace using soda lime. n = 6–14 trials for each treatment. ***, P<0.001; **, P<0.01; *, P<0.05, Mann-Whitney or unpaired t test (host vs. host + soda lime). See also Figure S3.
We then examined host attraction in BAG-ablated animals. We focused on attraction to G. mellonella because it is the most commonly used laboratory host and IJs are capable of locating and infecting G. mellonella in complex soil environments [35, 36]. BAG-ablated H. bacteriophora IJs no longer chemotax to G. mellonella (Figure 3C), demonstrating a critical role for BAG neurons in host localization. Because BAG neurons are sensory neurons that detect CO2 [4], our results suggest that CO2 is an essential host cue for attraction of H. bacteriophora to G. mellonella. Insect-parasitic nematodes have a broad host range: they can infect a diverse array of insects and even some non-insect arthropods [37–39]. Our results suggest that H. bacteriophora may achieve this broad host range by relying primarily on CO2 for attraction to some hosts. By contrast, ablation of the BAG neurons did not significantly affect the ability of S. carpocapsae IJs to jump in response to G. mellonella volatiles (Figure 3D), demonstrating that other neurons besides BAG and other host odors besides CO2 are sufficient to mediate host-evoked jumping.
Host attraction involves responses to CO2 as well as other host volatiles
To investigate the contribution of other host odors besides CO2 to host attraction, we modified our host chemotaxis assay such that host volatiles were passed through a column of soda lime to chemically remove CO2 (Figure S3D). We found that removal of CO2 completely eliminated the attractive response to G. mellonella, consistent with our BAG-ablation results (Figure S3E–F). By contrast, CO2 removal reduced but did not eliminate attractive responses to A. domesticus (Figure 3E–F), demonstrating that other host volatiles besides CO2 contribute to the attractiveness of some insect hosts.
Identification of volatiles emitted by insect larval hosts
To investigate the contribution of other odors to host-seeking behaviors, we used thermal desorption-gas chromatography-mass spectroscopy (TD-GC-MS) to identify odorants emitted by the four insects studied above. Overall, we identified eleven odorants released in relatively high abundance by these hosts: hexanal and α-pinene from G. mellonella larvae; 2,3-butanedione and trimethylamine from Z. morio larvae; and acetic acid, 2-butanone, 3-hydroxy-2-butanone, dimethylsulfone, propanol, propionic acid, γ-terpinene, and trimethylamine from A. domesticus (Figure S3). No abundant odorants were identified from T. molitor larvae using this technique (Figure S3), suggesting that IJs may rely primarily on CO2 to locate T. molitor.
Olfactory behavior in free-living versus parasitic nematodes
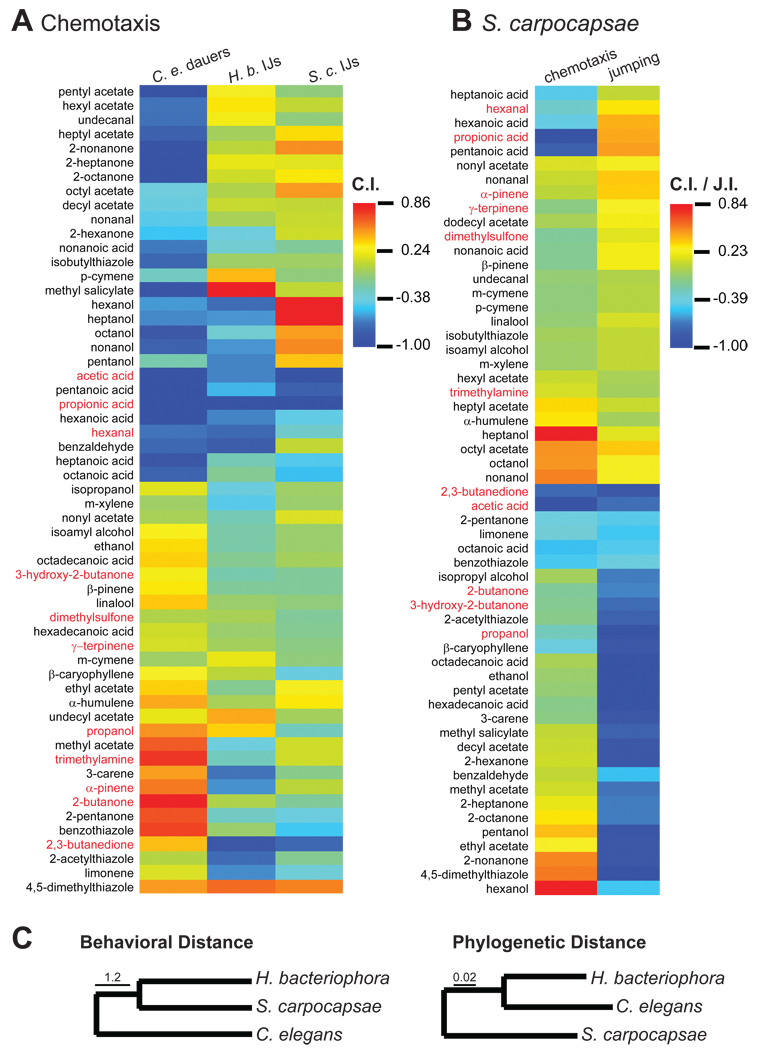
We constructed a panel of 57 odorants that included the identified host odorants, structurally-related odorants, and other insect, plant, and bacterial odorants that nematodes are likely to encounter in their soil microenvironments. We then examined responses of H. bacteriophora IJs, S. carpocapsae IJs, and C. elegans dauers to these odorants. We found that all three species exhibited robust responses to many of the tested odorants (Figures 4A–B, S4, and Table S1). In the case of S. carpocapsae, we found that many odorants differentially stimulated jumping and chemotaxis (Figure 4B), suggesting that different odorants are sufficient for different host-seeking behaviors. Five of the eleven host odorants that we identified ─ propanoic acid, hexanal, 2,3-butanedione, α-pinene, and γ-terpinene ─ stimulated jumping by S. carpocapsae (Figure 4B). By contrast, only one host odorant ─ 1-propanol ─ was attractive to H. bacteriophora and none were attractive to S. carpocapsae in a chemotaxis assay (Figure 4A). Thus, the identified host odorants may function primarily in short-range host seeking. Two of the five host odorants that stimulated jumping are released by insect-damaged plants [40–42], raising the possibility that these odorants attract beneficial nematodes as a means of combating insect infestation. Such a strategy has already been documented for other species of insect-parasitic nematodes [43–45].
Figure 4. Odor response profiles of free-living and parasitic nematodes.
A. Odor response profiles of C. elegans dauers, H. bacteriophora IJs, and S. carpocapsae IJs. n = 5–33 trials for each odorant. B. A comparison of odorant-evoked chemotaxis and jumping by S. carpocapsae. Both the chemotaxis index (C.I.) and the jumping index (J.I.) range from −1 to +1, with −1 indicating perfect repulsion and +1 indicating perfect attraction (Figures S2B and S4A). n = 5–8 trials for chemotaxis and 3–10 trials for jumping. Data for chemotaxis is from A. For A and B, response magnitudes are color-coded according to the scale shown to the right of each heat map, and odorants are ordered based on hierarchical cluster analysis. Host odorants identified by TD-GC-MS of insect headspace are highlighted in red. C. The odor response profiles of H. bacteriophora and S. carpocapsae are more similar to each other than to that of C. elegans, despite the fact that H. bacteriophora and C. elegans are more closely related phylogenetically. Left, behavioral dendrogram of olfactory responses across species. Behavioral distance is based on the Euclidian distances between species based on their odor response profiles. Right, phylogenetic neighbor-joining tree. Branch lengths in the phylogenetic tree are proportional to genetic distances between taxa; scale bar represents 0.02 nucleotide substitutions per site. See also Figure S4 and Table 1.
Using hierarchical cluster analysis, we found that the odor response profiles of H. bacteriophora and S. carpocapsae are more similar to each other than to that of C. elegans (Figure 4C). This contrasts with the phylogenetic relationship among these species: H. bacteriophora and C. elegans are much more closely related to each other than to S. carpocapsae (Figures 4C and S1). The fact that H. bacteriophora and S. carpocapsae show more similar odor response profiles thus suggests a key role for olfaction in their convergently evolved parasitic lifestyles. Our data also provide insight into the evolution of olfactory behavior in free-living and parasitic nematode lineages. The fact that CO2 attraction at the dauer/IJ stage is conserved in phylogenetically distant nematodes and that conserved neural circuitry mediates these responses suggests that CO2 attraction may be an ancestral feature of nematodes that precedes their divergence into free-living and parasitic lineages. By contrast, responses to other odorants differ among species, suggesting that these responses may be more highly derived features that reflect niche-specific ecological requirements. Our discovery that BAG neurons mediate CO2 response and host-seeking behavior in phylogenetically distant nematode species raises the possibility that compounds that block BAG neuron function may be useful for nematode control.
Experimental Procedures
See supplemental methods.
Supplementary Material
Acknowledgements
We thank Todd Ciche, Heidi Goodrich-Blair, Patrick McGrath, and Cori Bargmann for nematode and bacterial stocks; Nathan Dalleska, the Caltech Environmental Analysis Center, and Andrea Choe for help with TD-GC-MS; Scott Peat and Byron Adams for help with phylogenetic analysis; and Jagan Srinivasan, David Prober, Byron Adams, Bruce Hay, Hillel Schwartz, and lab members for critical reading of the manuscript. This work was supported by the Howard Hughes Medical Institute, with which P.W.S. is an investigator, a Helen Hay Whitney postdoctoral fellowship and NIH Pathway to Independence award to E.A.H, an NIH USPHS Training Grant (T32GM07616) to A.R.D., and Summer Undergraduate Research Fellowships (SURFs) to A.V.H., Y.Z., and J.M.Y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Viney ME, Thompson FJ, Crook M. TGF-β and the evolution of nematode parasitism. Int J Parasitol. 2005;35:1473–1475. doi: 10.1016/j.ijpara.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bretscher AJ, Busch KE, de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallem EA, Spencer WC, McWhirter RD, Zeller G, Henz SR, Ratsch G, Miller DM, 3rd, Horvitz HR, Sternberg PW, Ringstad N. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1017354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton FT, Li J, Schad GA. Chemo- and thermosensory neurons: structure and function in animal parasitic nematodes. Vet Parasitol. 1999;84:297–316. doi: 10.1016/s0304-4017(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 6.Ciche TA, Ensign JC. For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl Environ Microbiol. 2003;69:1890–1897. doi: 10.1128/AEM.69.4.1890-1897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martens EC, Heungens K, Goodrich-Blair H. Early colonization events in the mutualistic association between Steinernema carpocapsae nematodes and Xenorhabdus nematophila bacteria. J Bacteriol. 2003;185:3147–3154. doi: 10.1128/JB.185.10.3147-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y, Ji D, Cho S, Park Y. Two groups of entomopathogenic bacteria, Photorhabdus and Xenorhabdus, share an inhibitory action against phospholipase A2 to induce host immunodepression. J Invertebr Pathol. 2005;89:258–264. doi: 10.1016/j.jip.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Au C, Dean P, Reynolds SE, ffrench-Constant RH. Effect of the insect pathogenic bacterium Photorhabdus on insect phagocytes. Cell Microbiol. 2004;6:89–95. doi: 10.1046/j.1462-5822.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 10.Daborn PJ, Waterfield N, Blight MA, Ffrench-Constant RH. Measuring virulence factor expression by the pathogenic bacterium Photorhabdus luminescens in culture and during insect infection. J Bacteriol. 2001;183:5834–5849. doi: 10.1128/JB.183.20.5834-5839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowen D, Rocheleau TA, Blackburn M, Andreev O, Golubeva E, Bhartia R, ffrench-Constant RH. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science. 1998;280:2129–2132. doi: 10.1126/science.280.5372.2129. [DOI] [PubMed] [Google Scholar]
- 12.Lewis EE. Behavioral Ecology. In: Gauger R, editor. Entomopathogenic Nematology. New York: CAB International; 2002. pp. 205–223. [Google Scholar]
- 13.Lewis EE, Campbell J, Griffin C, Kaya H, Peters A. Behavioral ecology of entomopathogenic nematodes. Biol Control. 2006;38:66–79. [Google Scholar]
- 14.Campbell JF, Gauger R. Nictation behaviour and its ecological implications in the host search strategies of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) Behaviour. 1993;126:155–169. [Google Scholar]
- 15.O'Halloran DM, Burnell AM. An investigation of chemotaxis in the insect parasitic nematode Heterorhabditis bacteriophora. Parasitol. 2003;127:375–385. doi: 10.1017/s0031182003003688. [DOI] [PubMed] [Google Scholar]
- 16.Pye AE, Burman M. Neoaplectana carpocapsae: Nematode Accumulations on Chemical and Bacterial Gradients. Exp Parasitol. 1981;51:13–20. doi: 10.1016/0014-4894(81)90037-0. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt J, All JN. Attraction of Neoaplectana carpocapsae (Nematoda: Steinernematidae) to Common Excretory Products of Insects. Environ. Entomol. 1979;8:55–61. [Google Scholar]
- 18.Campbell JF, Kaya HK. Influence of insect-associated cues on the jumping behavior of entomopathogenic nematodes (Steinernema spp.) Behavior. 2000;137:591–609. [Google Scholar]
- 19.Haas W. Parasitic worms: strategies of host finding, recognition and invasion. Zoology (Jena, Germany) 2003;106:349–364. doi: 10.1078/0944-2006-00125. [DOI] [PubMed] [Google Scholar]
- 20.Sciacca J, Forbes WM, Ashton FT, Lombardini E, Gamble HR, Schad GA. Response to carbon dioxide by the infective larvae of three species of parasitic nematodes. Parasitol Int. 2002;51:53–62. doi: 10.1016/s1383-5769(01)00105-2. [DOI] [PubMed] [Google Scholar]
- 21.Klowden MJ. Blood, Sex, and the Mosquito. BioScience. 1995;45:326–331. [Google Scholar]
- 22.Hallem EA, Rengarajan M, Ciche TA, Sternberg PW. Nematodes, bacteria, and flies: a tripartite model for nematode parasitism. Curr Biol. 2007;17:898–904. doi: 10.1016/j.cub.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Tissenbaum HA, Hawdon J, Perregaux M, Hotez P, Guarente L, Ruvkun G. A common muscarinic pathway for diapause recovery in the distantly related nematode species Caenorhabditis elegans and Ancylostoma caninum. Proc Natl Acad Sci USA. 2000;97:460–465. doi: 10.1073/pnas.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barriere A, Felix MA. High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr Biol. 2005;15:1176–1184. doi: 10.1016/j.cub.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Felix MA, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–R969. doi: 10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan J, Durak O, Sternberg PW. Evolution of a polymodal sensory response network. BMC Biol. 2008;6:52. doi: 10.1186/1741-7007-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashton FT, Zhu X, Boston R, Lok JB, Schad GA. Strongyloides stercoralis: Amphidial neuron pair ASJ triggers significant resumption of development by infective larvae under host-mimicking in vitro conditions. Exp Parasitol. 2007;115:92–97. doi: 10.1016/j.exppara.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes WM, Ashton FT, Boston R, Zhu X, Schad GA. Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet Parasitol. 2004;120:189–198. doi: 10.1016/j.vetpar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Ketschek AR, Joseph R, Boston R, Ashton FT, Schad GA. Amphidial neurons ADL and ASH initiate sodium dodecyl sulphate avoidance responses in the infective larva of the dog hookworm Anclyostoma caninum. Int J Parasitol. 2004;34:1333–1336. doi: 10.1016/j.ijpara.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Bumbarger DJ, Crum J, Ellisman MH, Baldwin JG. Three-dimensional fine structural reconstruction of the nose sensory structures of Acrobeles complexus compared to Caenorhabditis elegans (Nematoda: Rhabditida) J Morphol. 2007;268:649–663. doi: 10.1002/jmor.10535. [DOI] [PubMed] [Google Scholar]
- 31.Bumbarger DJ, Wijeratne S, Carter C, Crum J, Ellisman MH, Baldwin JG. Three-dimensional reconstruction of the amphid sensilla in the microbial feeding nematode, Acrobeles complexus (Nematoda: Rhabditida) J Comp Neurol. 2009;512:271–281. doi: 10.1002/cne.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans Royal Soc London B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 33.Dieterich C, Sommer RJ. How to become a parasite - lessons from the genomes of nematodes. Trends Genet. 2009;25:203–209. doi: 10.1016/j.tig.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Kiontke K, Gavin NP, Raynes Y, Roehrig C, Piano F, Fitch DH. Caenorhabditis phylogeny predicts convergence of hermaphroditism and extensive intron loss. Proc Natl Acad Sci USA. 2004;101:9003–9008. doi: 10.1073/pnas.0403094101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hominick WM. Biogeography. In: Gaugler R, editor. Entomopathogenic Nematology. New York: CABI Publishing; 2002. pp. 115–143. [Google Scholar]
- 36.Bedding RA, Akhurst RJ. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 1975;21:109–116. [Google Scholar]
- 37.Poinar GO., Jr. Nematodes for biological control of insects. Boca Raton: CRC Press; 1979. [Google Scholar]
- 38.Samish M, Glazer I. Infectivity of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) to female ticks of Boophilus annulatus (Arachnida: Ixodidae) J Med Entomol. 1992;29:614–618. doi: 10.1093/jmedent/29.4.614. [DOI] [PubMed] [Google Scholar]
- 39.Vasconcelos VO, Furlong J, Marques de Freitas G, Dolinski C, Aguillera MM, Rodrigues RCD, Prata M. Steinernema glaseri Santa Rosa strain (Rhabditida: Steinernematidae) and Heterorhabditis bacteriophora CCA Strain (Rhabditida: Heterorhabditidae) as biological control agents of Boophilus microplus (Acari: Ixodidae) Parasitol Res. 2004;94:201–206. doi: 10.1007/s00436-004-1178-5. [DOI] [PubMed] [Google Scholar]
- 40.Loughrin JH, Manukian A, Heath RR, Turlings TC, Tumlinson JH. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc Natl Acad Sci USA. 1994;91:11836–11840. doi: 10.1073/pnas.91.25.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali JG, Alborn HT, Stelinski LL. Subterranean Herbivore-induced Volatiles Released by Citrus Roots upon Feeding by Diaprepes abbreviatus Recruit Entomopathogenic Nematodes. J Chem Ecol. 2010;36:361–368. doi: 10.1007/s10886-010-9773-7. [DOI] [PubMed] [Google Scholar]
- 42.Sun X-L, Wang G-C, Cai X-M, Jin S, Gao Y, Chen Z-M. The Tea Weevil, Myllocerinus aurolineatus, is Attracted to Volatiles Induced by Conspecifics. J Chem Ecol. 2010;36:388–395. doi: 10.1007/s10886-010-9771-9. [DOI] [PubMed] [Google Scholar]
- 43.Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TC. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- 44.Boff MIC, Zoon FC, Smits PH. Orientation of Heterorhabditis megidis to insect hosts and plant roots in a Y-tube sand olfactometer. Entomol Exp Appl. 2001;98:329–337. [Google Scholar]
- 45.Van Tol RHWM, Van der Sommen ATC, Boff MIC, Van Bezooijen J, Sabelis MW, Smits PH. Plants protect their roots by alerting the enemies of grubs. Ecol. Lett. 2001;4:292–294. [Google Scholar]
- 46.Dowds BCA, Peters A. Virulence Mechanisms. In: Gaugler R, editor. Entomopathogenic nematology. New York: CAB International; 2002. pp. 79–98. [Google Scholar]
- 47.Campbell JF, Kaya HK. How and why a parasitic nematode jumps. Nature. 1999;397:485–486. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.