Abstract
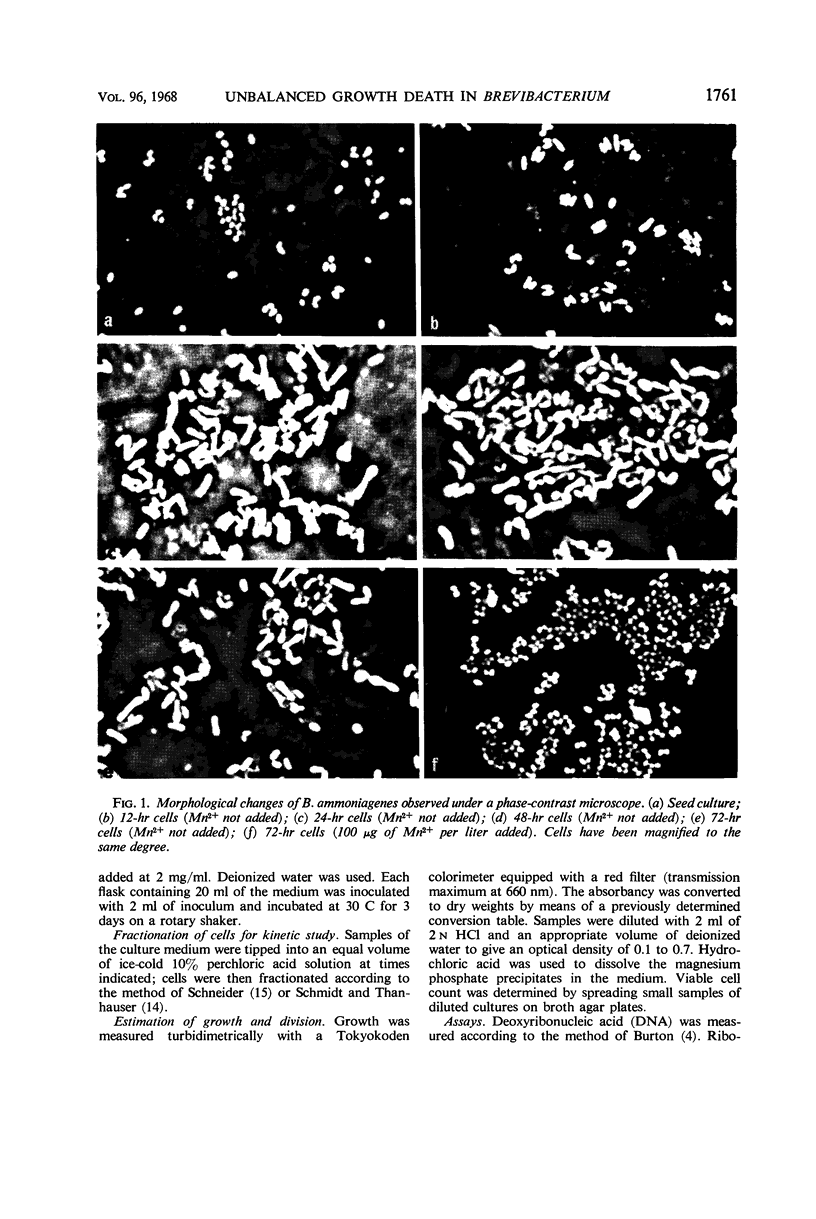
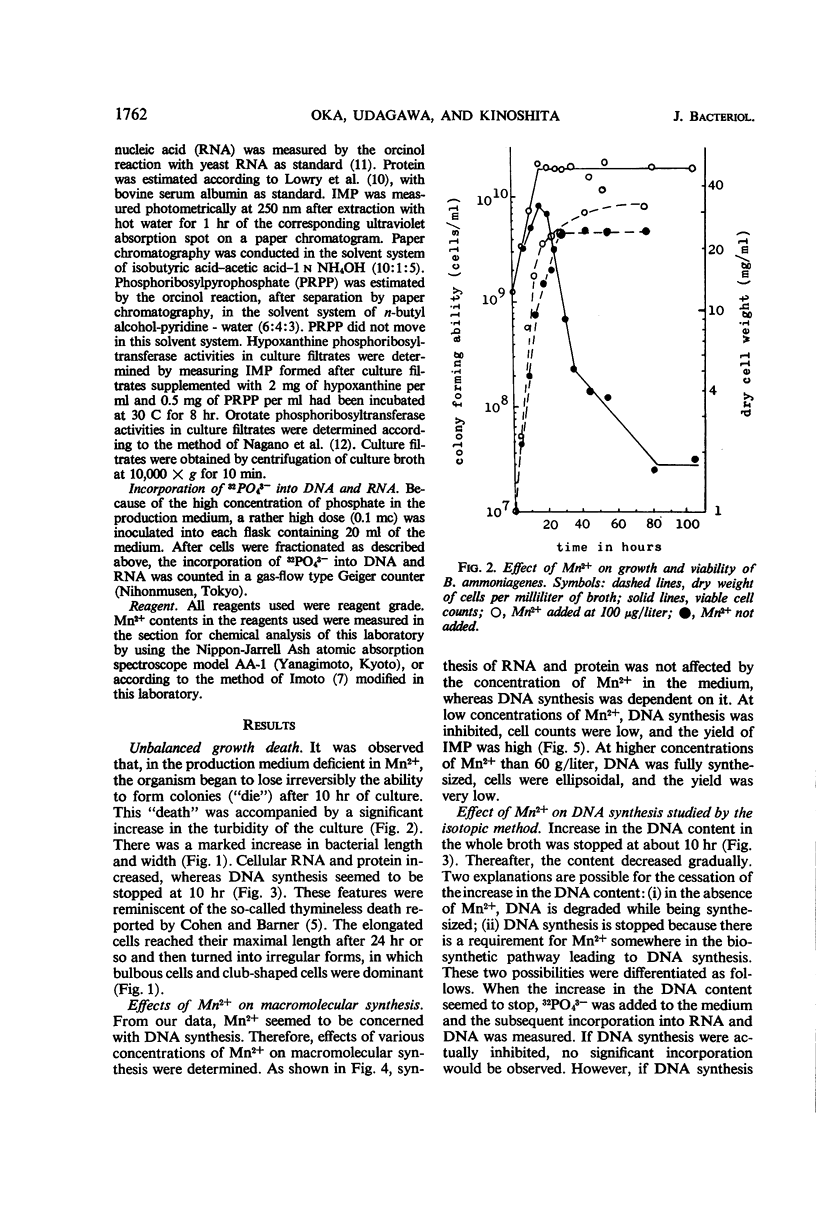
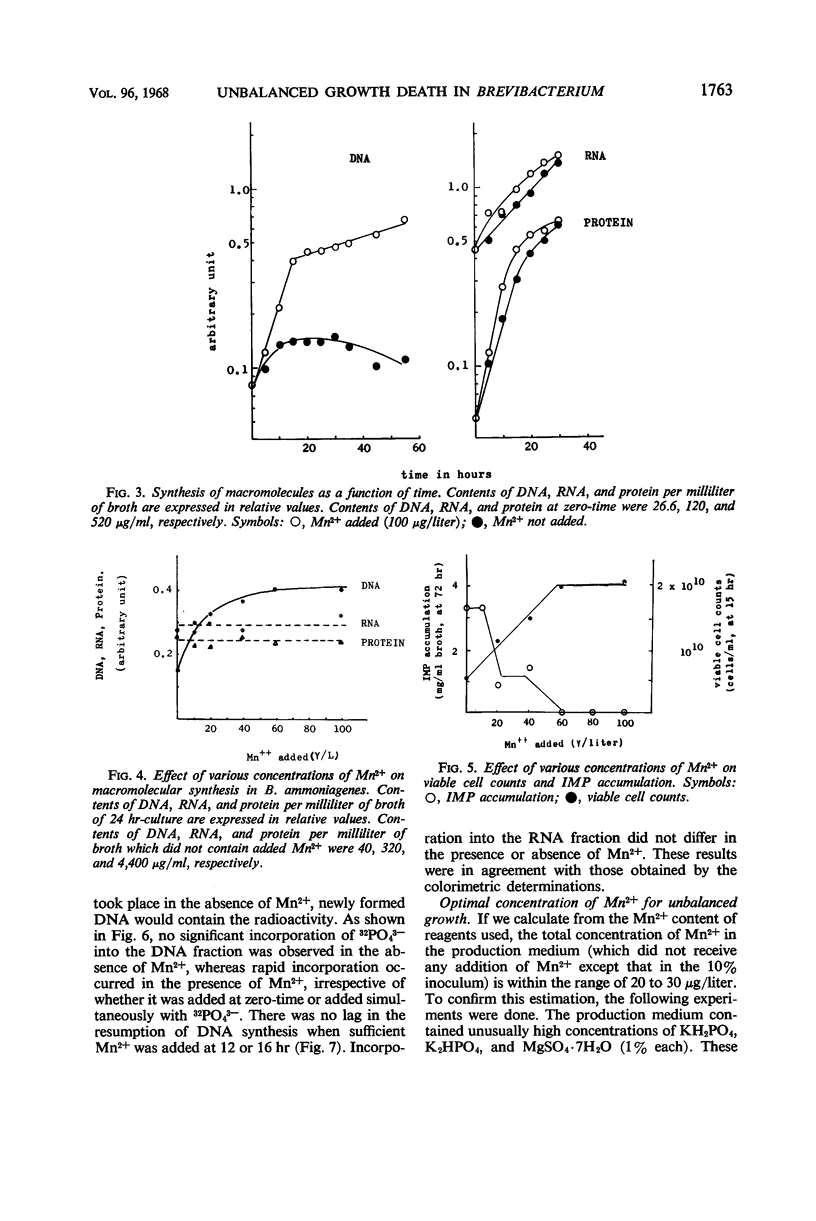
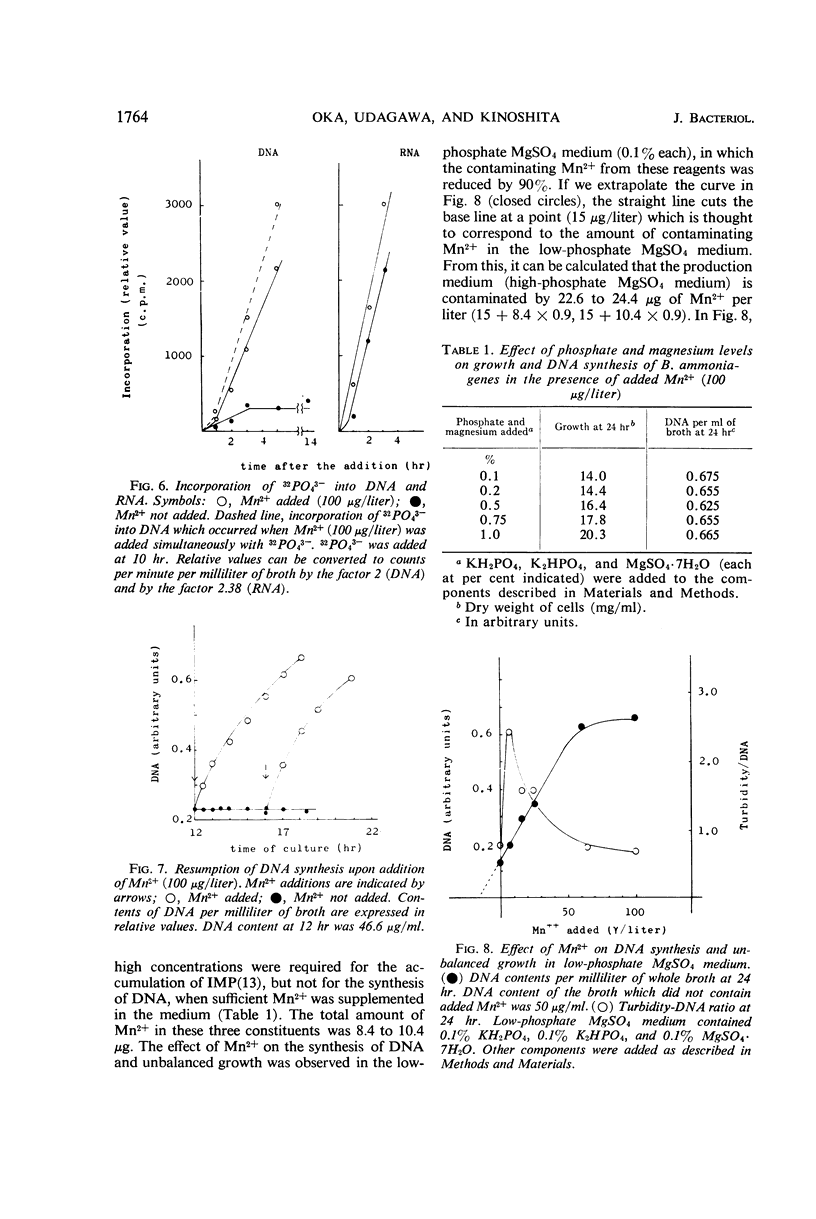
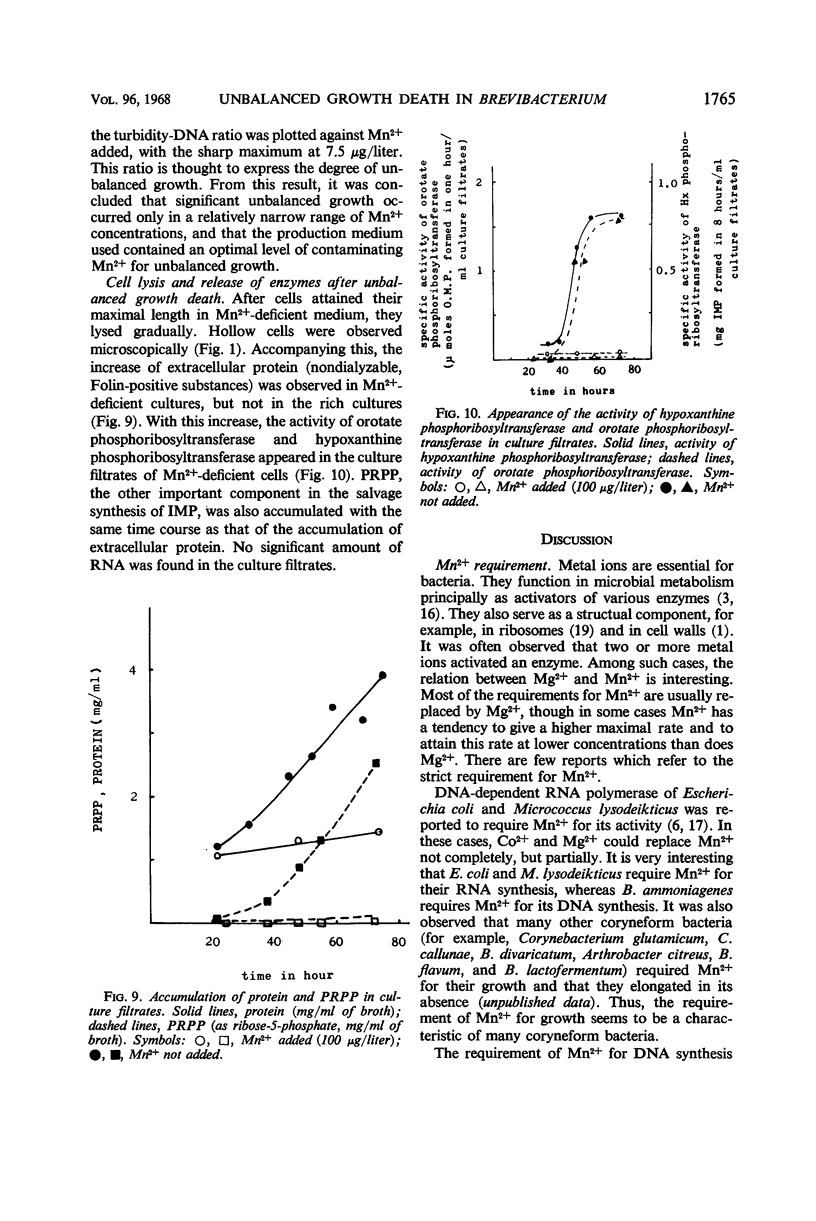
In the microbial conversion of added hypoxanthine to 5′-inosinic acid, Mn2+ concentration in the growth medium is known to have a profound effect both on the yield of 5′-inosinic acid and the morphology of cells of Brevibacterium ammoniagenes. To elucidate the mechanism in which Mn2+ was concerned with cell morphology and 5′-inosinic acid production, effects of Mn2+ on the macromolecular synthesis were measured. It was found that Mn2+ strongly governed deoxyribonucleic acid (DNA) synthesis and that, in the medium lacking Mn2+, DNA synthesis was stopped at the level corresponding to one-fourth to one-third that in the medium supplemented with Mn2+ (100 μg/liter). On the other hand, cellular ribonucleic acid and protein synthesis was quite indifferent to Mn2+ concentration. Consequently, cells showed so-called “unbalanced growth death” after 10 hr of culture, losing the ability to form colonies while cell mass was increasing. The elongated cells turned into irregular forms (bulbous, club-shaped, etc.) which finally lysed. Two main reaction components in the conversion of hypoxanthine to 5′-inosinic acid, phosphoribosylpyrophosphate and hypoxanthine phosphoribosyltransferase, were liberated into the medium during lysis. The role of Mn2+ in the synthesis of DNA and the role of the unbalanced growth death in the conversion of hypoxanthine to 5′-inosinic acid are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazill G. W. Lethal unbalanced growth in bacteria. Nature. 1967 Oct 28;216(5113):346–349. doi: 10.1038/216346a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX C. F., WEISS S. B. ENZYMATIC SYNTHESIS OF RIBONUCLEIC ACID. II. PROPERTIES OF THE DEOXYRIBONUCLEIC ACID-PRIMED REACTION WITH MICROCOCCUS LYSODEIKTICUS RIBONUCLEIC ACID POLYMERASE. J Biol Chem. 1964 Jan;239:175–185. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- STEVENS A., HENRY J. STUDIES ON THE RIBONUCLEIC ACID POLYMERASE FROM ESCHERICHIA COLI. I. PURIFICATION OF THE ENZYME AND STUDIES OF RIBONUCLEIC ACID FORMATION. J Biol Chem. 1964 Jan;239:196–203. [PubMed] [Google Scholar]
- TS'O P. O., BONNER J., VINOGRAD J. Structure and properties of microsomal nucleoprotein particles from pea seedlings. Biochim Biophys Acta. 1958 Dec;30(3):570–582. doi: 10.1016/0006-3002(58)90104-5. [DOI] [PubMed] [Google Scholar]
- WEBLEY D. M., DUFF R. B., ANDERSON G. The metabolism of iron-, zinc- and manganese-deficient Nocardia opaca. J Gen Microbiol. 1962 Sep;29:179–187. doi: 10.1099/00221287-29-1-179. [DOI] [PubMed] [Google Scholar]
- WEBLEY D. M. The effect of deficiency of iron, zinc and manganese on the growth and morphology of Nocardia opaca. J Gen Microbiol. 1960 Aug;23:87–92. doi: 10.1099/00221287-23-1-87. [DOI] [PubMed] [Google Scholar]