Abstract
Purpose
To systematically review the evidence for the efficacy and safety of botulinum toxin in the management of OAB.
Materials and Methods
We performed a systematic review of the literature to identify articles published between 1985 and March 2009 on intravesical botulinum toxin A (BTX) injections for the treatment of refractory idiopathic overactive bladder in both men and women. Database searched included MEDLINE, CENTRAL, and EMBASE. Data were tabulated from case series and from randomized controlled trials (RCTs). Data were pooled where appropriate.
Results
Our literature search identified 432 titles. Twenty-three full articles were included in the final review. Three randomized placebo-controlled trials addressing the use of botulinum toxin-A were identified (99 patients total). The pooled random effects estimate of effect across all three studies was 3.88 (95% C.I. -6.15, -1.62), meaning that patients treated with BTX had 3.88 fewer incontinence episodes per day. UDI data revealed significant improvements in quality of life compared with placebo, with a standardized mean difference of -0.62 (CI -1.04, -0.21). Data from case series demonstrated significant improvements in OAB symptoms and quality of life, despite heterogeneity in methodology and case mix. However, based on the randomized controlled trials, there was a nine-fold increased risk of elevated post-void residual after BTX compared with placebo (8.55, 95% CI 3.22-22.71).
Conclusions
Intravesical injection of botulinum toxin resulted in improvement in medication-refractory OAB symptoms. However, the risk of elevated post-void residual and symptomatic urinary retention was significant. Several questions remain concerning the optimal administration of BTX for the OAB patient.
Introduction
Poor outcomes of overactive bladder (OAB) treatments have lead to a search for better therapies. Intravesical injection of botulinum toxin-A (BTX) has shown promise in the treatment of medication-refractory OAB. BTX is a neurotoxin produced by the bacterium Clostridium botulinum that prevents acetylcholine release at the neuromuscular junction, resulting in flaccid muscle paralysis1, 2. Schurch and colleagues first reported the efficacy of Botulinum toxin-A in the treatment of refractory neurogenic detrusor overactivity3. Since then, although its use is off-label, there have been numerous case series and observational studies which have documented its application in the treatment of a wide range of dysfunctional voiding disorders.
A previous systematic review published in 2007 by Duthie et al. included studies that chiefly involved participants with neurogenic detrusor activity4. Of the eight studies analyzed in their systematic review, only one included participants with idiopathic OAB only. Since the publication of their review, there have been several new studies published, including three randomized controlled trials1, 5, 6. This systematic review is an attempt to extend this work and summarize the evidence for the efficacy and safety of Botulinum toxin-A in the management of refractory non-neurogenic (idiopathic) OAB.
Methods
Literature Search and Selection
We performed a systematic review of the literature in order to identify articles published between 1985 and March 2009 on intravesical botulinum toxin A (BTX) injections for the treatment of refractory idiopathic overactive bladder in both men and women. Search terms included botulinum toxin, Botox, overactive bladder, and urge urinary incontinence. We began with an electronic search of MEDLINE on January 30, 2009 followed by a search of the Cochrane Central Register of Controlled Trials (CENTRAL) on February 4, 2009 as well as EMBASE on March 4, 2009 at which point our search ended. Abstracts presented but not yet published were also included. Article titles, abstracts and finally full articles were captured and analyzed from this literature review. Two independent reviewers, (JA and AW), reviewed each study title and abstract. One reviewer abstracted the data from the full articles while the second reviewer verified and modified the corresponding data tables. Disagreements were resolved by consensus7. Data presented in abstract form but not yet published in manuscript form was also included5.
Study Inclusion
The literature search included review articles, randomized controlled trials, prospective observational studies and large case series. To be included, studies had to be an original research article and discuss the use of Botulinum toxin-A in adult men and women with refractory idiopathic OAB. We excluded studies that reported data for pediatric patients and neurogenic detrusor overactivity only, analyses of botulinum toxin-B, as well as basic science articles, case reports, and non-systematic review articles.
Data Abstraction, Quality Assessment and Synthesis of Results
Study results were abstracted separately into randomized controlled trials and case series data tables. Each table summarizes the patient population baseline characteristics, interventions, comparisons, outcomes assessed, and study timeline of follow-up and duration (Table 1 8). We assessed the methodological quality of the three identified randomized studies using the method described by Jadad et al9. To avoid double counting of subjects, multiple analyses of the same patient population were included only once.
Table 1. RCTs of botulinum toxin-A for overactive bladder.
| Author, year | Patients | Treatment | Sample Size | Duration | Outcomes measured | Jadad | Results | |
|---|---|---|---|---|---|---|---|---|
| Findings | Adverse | |||||||
| Brubaker, 200811 | Women with idiopathic OAB Mean age = 66 Mean # prior tx = 4.5 Mean # IE on 3-day void diary = 17 Mean IIQ score =150 |
Intervention: 200U BTX-A injected 15- 20 detrusor sites, trigone-sparing | Intervention = 28 | 12 months duration | Primary: time to failure (PGI-I>4), initiation of new treatment postinjection, increased intensity of previous treatment | Randomized = 1 Double blind = 1 Described/appropri ate randomization = 1 Described/appropri ate blinding = 1 Withdrawals/dropo uts = 0 |
Median time to failure 307 d vs. 62 d., p<0.001 | Increased PVR (12/28) in 43% (PVR>200cc) |
| Control: placebo | Placebo = 15 (2:1 randomization) |
Follow-up at monthly intervals starting at 2 months postinjection | Secondary: changes in frequency of incontinence, changes in symptom and QoL measures | Mean PGI-I score, 2.7 vs 4.0, p=0.003 | CISC for median 62 days | |||
| BTX group experienced >75% reduction in IE's; from mean IE's/3- day diary of 17 to 3, (p<0.0001) | UTI rate: 44% in BTX vs 22% placebo | |||||||
| BTX group had improved “Patient Perception of adequacy of symptom control” score (p<0.0001) | Study placed on hold due to 43% rate of increased PVR and associated UTI in treatment group | |||||||
| Decrease in UDI subscale irritative symptom bother: 54 to 31 for BTX compared to no change for placebo group, p=0.003 | ||||||||
|
| ||||||||
| Sahai, 20076 | Men (15) and women (19) Mean age = 50 Mean OAB sx/day: Frequency = 15 Urgency = 10 Mean IE/day = 5 Mean IIQ-7 = 18 Mean UDI-6 = 11 |
Intervention: 200 U BTX-A at 20 detrusor sites, trigone-sparing Control: placebo |
Intervention = 16 | 12 weeks, followed by unblinding | Primary: change in MCC | Randomized = 1 Double blind = 1 Described/appropriate randomization = 1 Described/appropri ate blinding = 1 Withdrawal/dropo uts described = 1 |
Significant increase in MCC at 12 wks, mean difference 95.7ml, 95% CI 47.5 to 172.5, p = 0.001, BTX 263.88 (+82.06 from baseline) vs. placebo 168.17 (-29.89) | In BTX group, PVR increased +52.1ml, p=0.02 at 4 wks but became insignificant at 12 wks, 6 patients required CISC |
| 1/3 pts continued taking anticholinergics during study period | Placebo = 18 | Follow-up in Btx group only at 24 weeks | Secondary: changes in OAB symptoms of urgency, frequency and UUI episodes, as well as PVR, Pdetmax, RDV | Reduction in frequency/day, -7.9, p < 0.0001 and -6.2, p=0.003 at 4 and 12 wks (vs. placebo - 1.03 at 4 weeks and - 1.14 at 12 weeks. | ||||
| Reduction in urgency/day -9.2 (vs. placebo -1.29, p=0.0047) at 4wks | ||||||||
| Reduction in mean IE/day by -3.1 (vs. placebo - 0.74, p=0.03), -3.5, p=0.008 at 4 and 12 wks (-3.5 vs. -.71, p = .0076) | ||||||||
| Effects perceived bw 3d and 2wks postinjection | ||||||||
|
| ||||||||
| Flynn, 200910 | Women only with idiopathic OAB Mean age = 66 Mean IE/day = 8 Mean IIQ-7 = 54 Mean UDI-6 = 49 Mean pads/day = 5 Mean voids/day = 11 All anticholinergics stopped 10d prior to study |
Intervention: Randomized to either 200 or 300 U BTX-A injections into 10-12 detrusor sites, trigone-sparing | Intervention = 15 | 6 weeks | Primary: number of IE/day as recorded on 3-day bladder diaries, UDI-6, IIQ-7 | Randomized = 1 Double blind = 1 Described/appropri ate randomization = 1 Described/appropri ate blinding = 1 Withdrawal/dropo uts described = 1 |
Mean improvement for multiple measures of UI ranged from 40 to 70% and persisted through 6 weeks; including significant improvements in IIQ- 7 and UDI-6 scores | 4/15 BTX-group (26%) with PVR≥ 200 ccs |
| All subjects had failed at least one anticholinergic medication as well as behavioral treatment | Control: placebo injections | Placebo = 7 | With 3 and 6 week follow-up intervals | Secondary: 24-hr pad weights; number of pads/24-hrs, daily frequency and diurnal urinary frequency, MCC, volume of first uninhibited detrusor contraction, presence of stress leakage on CMG; absolute value of detrusor pressure at peak flow, peak urine flow and PVR. | Reduction in mean IE/day from baseline of 7.9 to 4.0 (p<0.005) at 3 weeks, and to 3.35 (p<0.002) at 6 weeks | 1 CISC in BTX group | ||
| No difference in # voids/day, diurnal voiding frequency or nocturia | 4 UTI, 2 (13%) in the BTX group and 2 (28%) in the placebo group (NS) | |||||||
| Of UDS measures, only MCC trended towards improvement from 336 cc to 378cc, not statistically significant (p=0.0818). | No de novo SUI | |||||||
| Increase PVR in BTX group from a baseline of 25 cc to 107 cc (p=0.0025) | ||||||||
| No significant change in the placebo group (30 cc to 27 cc [NS]) | ||||||||
IE = incontinence episodes, IIQ = Urinary Incontinence Impact Questionnaire (long form), PGI-I = Patient Global Impression of Improvement, IIQ-7 = Incontinence Impact Questionnaire-7 (short form), MCC = maximum cystometric capacity, RDV = reflex detrusor volume
We pooled data on incontinence episodes from the randomized studies. For each study, the mean difference between the botox and placebo group at followup was used. If a study did not report the mean difference at followup, then it was estimated by subtracting the mean at followup for the placebo group from the mean at followup for the BTX group10. Post-void residual urine volume data was also pooled similarly between the three randomized placebo-controlled trials. Because zero PVR events were often observed in the placebo group, we calculated odds ratios (ORs) by using the Peto method11. We also pooled data on health-related quality of life because two of the three available randomized trials utilized the short form of the urogenital distress inventory (UDI-6)1, 5, and one used the 28-item long form (UDI)6. The standardized effect size was calculated by taking the difference between the follow-up mean for the placebo group from that of the BTX group. This estimate was then divided by the weighted average standard deviation of the two means.
Pooled estimates were calculated using the DerSimonian & Laird random effects model12. A test of heterogeneity was performed using the I-squared statistic13. Publication bias was examined using Begg's funnel plot, Begg rank correlation14 and Egger regression asymmetry test15. All analyses were conducted in Stata 10.016.
Results
Description of the Studies Identified by the Literature Search
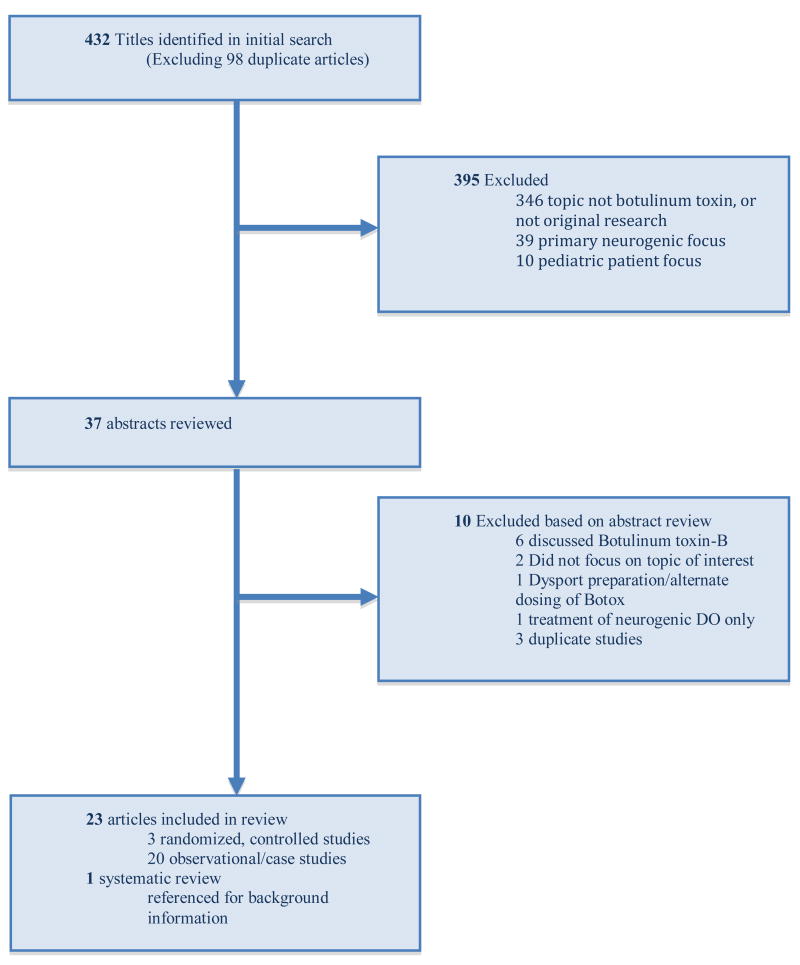
Our literature search identified 432 titles, from which we selected 37 for more detailed review. Of these, twenty-three full articles were included in the final review (Figure 1). Three randomized controlled trials addressing the use of Botulinum toxin-A were identified and included in our review. Reasons for article exclusion are shown in Figure 1. Overall, across all 23 studies included, 951 patients were enrolled.
Figure 1. Literature Flow (Flow of Eligible Studies of Botulinum toxin-A for OAB).
Across the studies, mean or median patient age was between 44 and 81 years. Sample size ranged from 7 to 110 patients and the proportion of women was 40%-100%, with seven studies including women only. BTX was injected transurethrally in all cases, but was given in varying doses, concentrations, and locations in the bladder. Some series included injections into both the bladder neck and external sphincter. Follow-up ranged from four weeks to 6 years, with average follow-up typically between six and nine months8. Outcome measures, such as incontinence (leakage) episodes, pad use, leakage severity, voiding frequency, and urodynamic parameters, were recorded, but not in a standardized or consistent fashion.
Efficacy of Botulinum-toxin A in Randomized control trials (RCTs)
We identified three randomized placebo-controlled trials that assessed the efficacy of BTX (Table 1). Brubaker et al. compared 200U of BTX vs. placebo in women with urge urinary incontinence (UUI) refractory to at least two first line therapies. Participants had to have documented detrusor overactivity on urodynamics, and at least six UUI episodes in a 3-day voiding diary. Flynn et al. compared 200U or 300U to placebo in men and women with more than two daily UUI episodes on voiding diary and 24-hour pad weights of greater than 100 grams. Patients were not required to have urodynamically proven detrusor overactivity. Anticholinergics were stopped at least ten days prior to the procedure. The study by Sahai et al. included men and women with urodynamically proven DO with or without urge incontinence. The studies by Brubaker and Sahai allowed for the continuation of established anticholinergic use during the study1, 6, 17. Botox injections were performed similarly between studies: 15-20 sites in 6 cc of solution were given in Brubaker's study, 20 cc were distributed in 20 sites in Sahai's study, and in Flynn's study 10-12 sites were each injected with 0.2 cc per site.
Brubaker et al. found that 60% of patients receiving BTX had a clinical response, as measured by the Patient Global Impression of Improvement scale. The median duration of response was 373 days (vs. 62 days for placebo, p <0.0001)6. Flynn et al. also found statistically significant decreases in pad usage among BTX recipients at six weeks, but found no difference in nocturia, urinary frequency, peak flow or detrusor pressure between groups5. Sahai found significant decreases in urinary frequency and UUI episodes in the BTX arms at 4 and 12 weeks1.
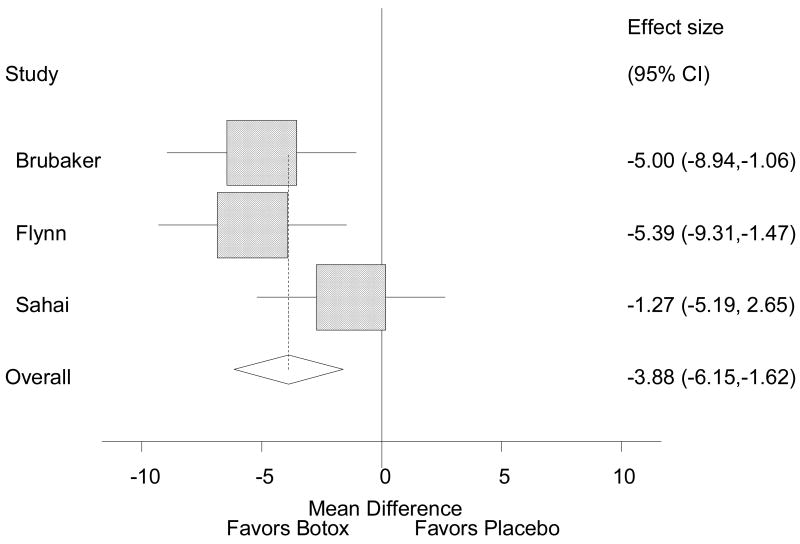
Data on the daily number of incontinence episodes were pooled from these RCTs, and are presented in Figure 2. The pooled random effects estimate of effect across all three studies was -3.88 (95% C.I. -6.15, -1.62), meaning that patients treated with BTX had 3.88 fewer incontinence episodes per day compared to patients treated with placebo. I-squared statistic for heterogeneity was zero, indicating no heterogeneity. Neither the Begg's test nor the Egger's test for publication bias was statistically significant (p = 1.00 and p = 0.725, respectively), indicating no publication bias.
Figure 2. Reduction in number of daily incontinence episodes after BTX.
Efficacy of Botulinum-toxin A in Case Series
Eleven large case series included 749 patients, or 70% of of the total number of patients in all the case series 8. Changes in voiding frequency were assessed in sixteen studies 8. In ten of these studies, the decrease in mean number of daily voids from baseline was statistically significant (p<0.05), with absolute reductions in voiding frequency of 1.2 to 7.0 (decrease by 12% to 50%). The overall leakage frequency ranged from 0.8 to 7.0 episodes daily at baseline and 0.1 to 3.0 at 4-6 weeks follow-up after Botox-A injection (p<0.05). Among the seven studies that assessed pad usage, the average number of pads decreased from a range of 3.9 to 5.0 at baseline evaluation, to 0 to 3.0 pads/day at follow-up 8.
QOL Summary
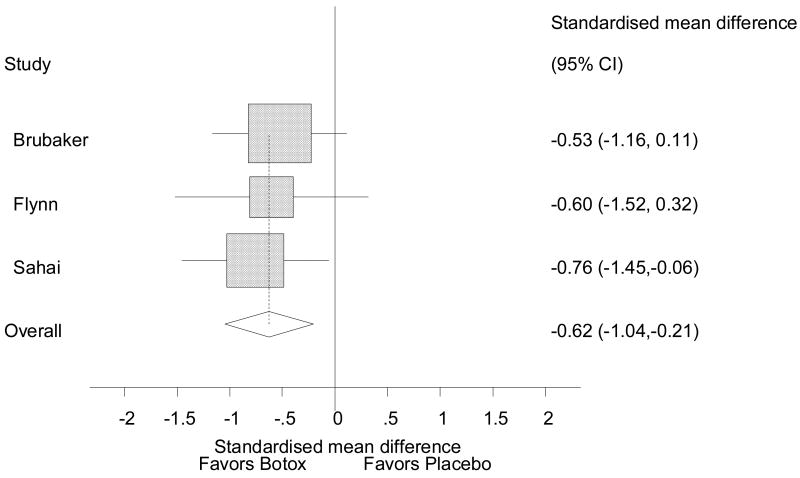
Quality-of-life (QOL) was assessed with standardized instruments in all three of the randomized controlled clinical trials identified5, 6. Flynn et al. and Sahai demonstrated significant improvements in IIQ-7 and UDI-6 scores5, and Brubaker et al. identified significant improvements in QOL using the long form of the UDI. The pooled estimate was an improvement in QOL with an effect size of 0.6 (Figure 3)18. An effect size of 0.6 translates into a 15 point improvement on the UDI-6. Egger's and Begg's tests revealed no publication bias (p= 0.587 and p = 1.00, respectively), and I-squared statistic for heterogeneity was zero, indicating no heterogeneity. Overall, the case studies analyzed demonstrated significant improvements in quality of life after Botox injection.
Figure 3. Improvement in QOL (standardized mean difference in scores for UDI-6 and UDI) after BTX.
Adverse events summary
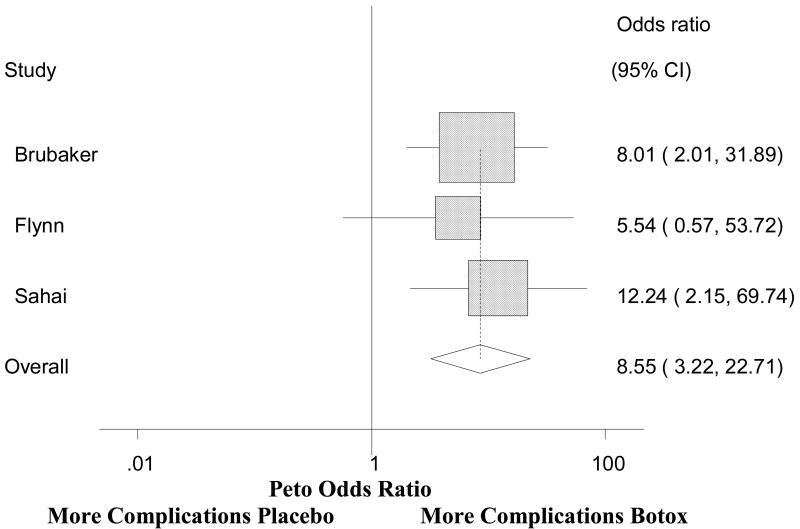
Urinary retention was the most commonly reported adverse event that occurred after BTX injection. In fact, the trial by Brubaker et al. was terminated early due to an elevated PVR of at least 200cc in twelve of 28 (43%) patients. All patients with a PVR of 200cc were placed on clean intermittent catheterization (CIC). Nine of the twelve women with an elevated PVR developed a UTI. The study by Flynn et al also reported a higher PVR in the patients treated with BTX toxin, but only one patient required CIC for symptomatic voiding difficulty. Six of sixteen patients (37.5%) treated with botulinum toxin in Sahai's study developed symptomatic retention of at least 150cc, resulting in the need for CIC. The pooled PVR using odds ratios is shown on Figure 411. The overall pooled analysis shows that the BTX group has almost 9 times the odds of having a PVR complication than the placebo group (8.55, 95% CI 3.22-22.71). Rates of post-procedure CIC varied among the case series from 0 to 41% and lasted up to 6 months. Other adverse events reported included UTIs, hematuria, dysuria. UTIs were associated with an elevated PVR and the need for CIC.
Figure 4. Risk of post-operative PVR >150cc after BTX.
Discussion
We identified three published randomized controlled clinical trials, and the pooled estimate of effect across all three was a statistically and clinically significant reduction in incontinence episodes and improvement in quality of life for patients treated with BTX compared with those treated with placebo. These findings suggest that BTX results in a significant improvement in OAB symptoms and quality of life among patients who fail or do not tolerate medical therapy. BTX expands treatment options for patients with medication-refractory OAB, and provides a feasible alternative to sacral modulation. In addition, BTX can be offered as an adjunct to sacral neuromodulation to maximize patient response.
The benefits of intravesical BTX must be balanced against a nearly 9-fold increase in the odds of urinary retention. In fact, the study by Brubaker et al. was terminated early because 43% of patients undergoing BTX had an elevation of PVR. Six of sixteen patients (37.5%) treated with Botox in Sahai's study developed symptomatic retention of at least 150cc, resulting in the need for CIC. In Flynn's study, four of 15 subjects (26%) receiving BTX had PVR≥ 200 ccs, but only one subject (7%) required intermittent catheterization. Since retention is not a rare consequence, patients should be willing to perform CIC or maintain an indwelling catheter before undergoing intravesical BTX. Given these estimates of benefits and harms, BTX deserves more study as a potential treatment option for those who do not desire, are not candidates for, or fail sacral neuromodulation, and who understand and are willing to accept the potential for an increased risk of urinary retention.
This study was limited by the relative lack of randomized controlled trials of intravesical Botox injection. There was extreme heterogeneity in the manner in which the case series document their outcome measures, and the case mix of each of these studies was variable, with significant differences in baseline OAB severity between studies. Of the three RCTs analyzed, only the study by Flynn et al. stopped anticholinergic therapy before undergoing BTX treatment. Hence, the studies by Brubaker and Sahai may be comparing BTX in addition to anticholinergic (rather than only placebo) in many patients. This may have changed the magnitude of any associations identified. However, the I-squared test for heterogeneity was not significant, indicating that the differences between groups seen in the three studies were similar, so any effect of anticholinergic drugs does not seem to be interactive with the effect of BTX. Some studies only provided mean changes from baseline values, others report a percentage change without any absolute value, and few included estimates of the variance (SD). This inconsistency in data reporting made it very difficult to synthesize the evidence. There was also significant variability in the dose of BTX used and in the location of the injections given. In some studies, BTX was injected into both the bladder and the bladder neck in order to decrease the risk of post-operative retention.
It is clear that many gaps remain in our knowledge of the optimal use of BTX for OAB. A European expert panel consensus conference recently convened to evaluate the evidence for the use of BTX in pelvic floor disorders19. They recommended placebo-controlled comparative trials to evaluate the safety and efficacy of both single and repeat injections, the duration of effect of BTX, and the timing for repeat injections19. Standardizing procedures will allow for formal comparisons to be made between studies. These include the dosage of drug, pattern of intravesical injections, and concomitant injection of the bladder neck and trigone. Anticholinergic use should be stopped well in advance of BTX injection. Management and monitoring of post-void residuals after BTX should also be standardized in order to detect clinically meaningful levels of retention between studies. Standaridizing outcomes would involve an expert panel meeting of clinicians to review and select the outcomes measures to be used. Such outcomes should involve domains of relevance to patients and that should have available measurement tools with adequate psychometric properties.
Conclusions
Intravesical injection of botulinum toxin resulted in significant improvement in OAB symptoms. The risk of post-operative urinary retention requiring CIC was significant, with an even higher number of patients experiencing an elevation in PVR. Several questions remain concerning the optimal administration of BTX for the OAB patient. Clearly more level I data from randomized controlled trials are needed to guide management.
Acknowledgments
Supported by the NIDDK (1 K23 DK080227-01, JTA)
Key of Definitions of Abbreviations
- BTX
botulinum toxin (type A)
- QOL
quality of life
- RCT
randomized controlled trial
- OAB
overactive bladder
- PVR
post-void residual
- MCC
mean cystometric capacity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sahai A, Khan MS, Dasgupta P. Efficacy of botulinum toxin-A for treating idiopathic detrusor overactivity: results from a single center, randomized, double-blind, placebo controlled trial. J Urol. 2007;177:2231. doi: 10.1016/j.juro.2007.01.130. [DOI] [PubMed] [Google Scholar]
- 2.Giannantoni A, Di Stasi SM, Stephen RL, et al. Intravesical resiniferatoxin versus botulinum-A toxin injections for neurogenic detrusor overactivity: a prospective randomized study. J Urol. 2004;172:240. doi: 10.1097/01.ju.0000132152.53532.5d. [DOI] [PubMed] [Google Scholar]
- 3.Schurch B, Stohrer M, Kramer G, et al. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- 4.Duthie J, Wilson DI, Herbison GP, et al. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005493.pub2. CD005493. [DOI] [PubMed] [Google Scholar]
- 5.Flynn MK, Amundsen CL, Perevich M, et al. Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder. J Urol. 2009;181:2608. doi: 10.1016/j.juro.2009.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brubaker L, Richter HE, Visco A, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol. 2008;180:217. doi: 10.1016/j.juro.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;15:7. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anger, J. T.: Available at www.uclahealth.org/jenniferanger.
- 9.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa T, Barbui C, Cipriani A, et al. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J, Thompson S, Deeks J, et al. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088. [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 17.Brubaker L, Kreder K, Richter HE, et al. Refractory urge urinary incontinence and botulinum a injection: the methods of the RUBI trial. J Appl Res. 2006;6:260. [Google Scholar]
- 18.Guyatt GH, Osoba D, Wu AW, et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 19.Apostolidis A, Dasgupta P, Denys P, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55:100. doi: 10.1016/j.eururo.2008.09.009. [DOI] [PubMed] [Google Scholar]