Abstract
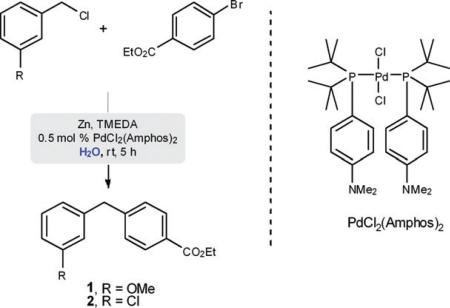
A remarkably simple entry to unsymmetrical diarylmethanes has been developed that relies on an in situ organozinc-mediated, palladium-catalyzed cross-coupling. Thus, by mixing a benzyl and aryl halide together in the presence of Zn metal and a Pd catalyst, diarylmethanes are formed at room temperature without assistance by a surfactant; hence, “on water”.
Diarylmethanes have recently come into focus given the presence of this subunit in a substantial number of biologically active molecules.1 The majority of approaches to this functional group array rely on cross-couplings; e.g., reactions using organoboron derivatives, catalyzed by palladium,2 or Grignards catalyzed by copper,3 nickel,4 or iron5 salts. Organozinc reagents are very attractive for this purpose given their low cost of preparation, high functional tolerance, and their low toxicity. Surprisingly, since the original work of Negishi et al.,6 few studies have been reported on the synthesis of diarylmethanes based on organozinc reagents with catalysis by palladium,7 rhodium,8 nickel,9 or cobalt.10 Nonetheless, organozinc reagents are routinely used stoichiometrically and hence, need to be prepared in a separate step.11 Recently, we have described a new process for effecting cross-coupling reactions that avoid entirely the prior preparation of organo-zinc reagents.12 Thus, zinc-mediated, palladium-catalyzed couplings are now possible between two organic halides, all of which takes place selectively (including insertion by zinc metal and Pd catalysis) using nanomicelles in water at room temperature.13 In this report, we describe an alternative approach: that is, an “on water” phenomenon (and hence, in the absence of a surfactant) that leads directly to unsymmetrical diarylmethanes.
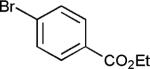
This investigation was initiated by treatment of 3-methoxybenzyl chloride (2 equiv.) and ethyl 4-bromobenzoate in pure water (Table 1) with zinc dust and a Pd catalyst (0.5 mol% PdCl2(Amphos)2).14 In the presence of TMEDA as the additive of choice,12 product 1 (R = OMe) is formed in high yields (entries 1–3) compared to that obtained in its absence (entry 4). Interestingly, the use of a surfactant for micellar catalysis in this reaction type does not enhance the reaction rate, or improve the yield of the coupling. Using the more reactive 3-chlorobenzyl chloride, even less TMEDA (e.g., 25 mol%) is required to realize a high yield of 2 (entry 3). In fact, if too much TMEDA is present, zinc insertion is uncontrollable and the product from protio-quenching, rather than 2, is formed (entries 1 and 2).15
Table 1.
Optimization of the reaction between substituted benzyl chlorides and ethyl 4-bromobenzoatea
 | ||||
|---|---|---|---|---|
| Entry | Type of zinc | TMEDA (n equiv.) | Isolated yield of 1 (%) | Isolated yield of 2 (%) |
| 1 | Dust | 1 | 92 | 52 |
| 2 | Dust | 0.5 | 96 | 66 |
| 3 | Dust | 0.25 | 95 | 92 |
| 4 | Dust | 0 | 53 | 65 |
| 5 | Powder | 1 | 84 | 62 |
| 6 | Powder | 0.5 | 82 | 86 |
| 7 | Powder | 0.25 | 94 | 96 |
| 8 | Powder | 0 | 76 | 74 |
Conditions: benzyl chloride (4 mmol), ethyl 4-bromobenzoate (2 mmol), zinc (6 mmol), PdCl2(Amphos)2 (0.01 mmol), degassed water (5 mL).
It is assumed that TMEDA is stabilizing the organozinc species by chelation, in water, rather than solely acting as a surface-cleaning and/or activating agent. Without TMEDA (entries 4 and 8), lower yields are obtained notwithstanding complete consumption of the starting benzyl chloride. Thus, the empirically-determined, optimized amount of TMEDA shown reflects a balance between stabilization and activation. In changing to zinc powder, the same trend was observed for both starting benzyl chlorides that ultimately led to products 1 and 2. These were obtained in excellent isolated yields (94% and 96%, respectively) employing only 25 mol% of TMEDA. However, in the case of electron-poor benzylic chlorides, zinc powder (rather than zinc dust) is the preferred precursor of this coupling partner.
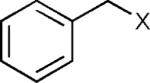
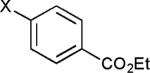
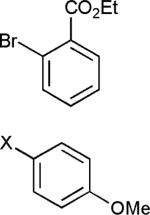
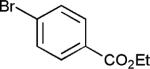
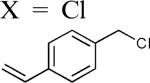
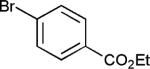
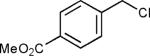
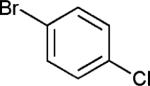
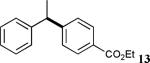
The reaction appears to be quite general, as reflected by the examples illustrated in Table 2. Simple benzyl bromide and benzyl chloride afford product 3 in high yields within a reaction time of three hours (entries 1 and 2). Both electron-rich or electron-poor benzyl bromides react very quickly with zinc metal; hence, competitive quenching and/or homo-coupling limit yields of desired cross-coupling products (entries 7 and 9). Otherwise, high yields are routinely obtained in all cases with relatively less reactive benzylic chlorides (entries 8 and 10–16). Moreover, only small amounts of homocoupling products are detected in these reaction mixtures. With respect to the aryl halide partner, it is worthy of note that aryl iodides also lead to high isolated yields of diarylmethanes; i.e., they do not compete for zinc insertion (entries 3 and 5). Steric effects do not appear to limit these reactions (entries 4, 13 and 15). A vast array of functionalities on both coupling partners are tolerated, including vinyl, trifluoromethyl, ester, aldehyde, chloride, and nitrile (entries 11–15). Finally 4-(1-chloroethyl)benzene leads to product 13 in excellent isolated yield (98%), whereas no product was detected using benzhydrylchloride.
Table 2.
Representative benzyl–aryl cross-couplingsa
| Entry | Benzyl halide | Aryl halide | Product | Yieldb (%) |
|---|---|---|---|---|
|
|
|
||
| 1 | X = Br | X = Br | 94c | |
| 2 | X = Cl | X = Br | 95d | |
| 3 | X = Cl | X = I | 96d | |
| 4 | X = Cl |
|
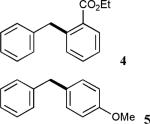
|
92 |
| 5 | X = Cl | X = I | 97d | |
| 6 |
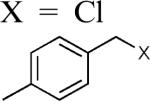
|
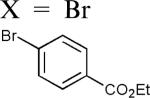
|
|
98d |
| 7 | X = Br | 70 | ||
| 8 |
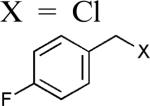
|
|
|
95 |
| 9 | X = Br | 57e | ||
| 10 | X = Cl | 96e | ||
| 11 |
|
|
|
74 |
| 12 |
|
|
|
79e |
| 13 |
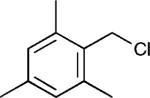
|
|
|
78 |
| 14 |
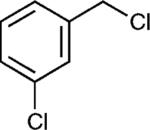
|
|
|
80e |
| 15 |
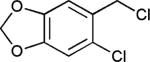
|
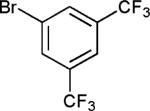
|
|
70 |
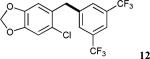
| 16 |
|
|
|
98f |
Conditions: benzyl halide (5 mmol), aryl bromide (2 mmol), zinc dust (6 mmol), PdCl2(Amphos)2 (0.01 mmol), TMEDA (0.5 mmol), degassed water (5 mL), rt, 8 h.
Isolated yield.
10 mol % TMEDA.
2 equiv. BnCl.
Zinc powder was used.
50 mol % TMEDA.
In summary, a remarkably straightforward method for preparing functionalized diarylmethanes, as described herein, has been uncovered. An aryl and benzylic halide, in the presence of inexpensive Zn and a Pd catalyst, combine in water alone at room temperature to afford the targeted products in high yields.
Supplementary Material
Footnotes
This article is part of a ChemComm ‘Catalysis in Organic Synthesis’ web-theme issue showcasing high quality research in organic chemistry. Please see our website (http://www.rsc.org/chemcomm/organicwebtheme2009) to access the other papers in this issue.
Electronic supplementary information (ESI) available: Experimental section and spectroscopic data. See DOI: 10.1039/b922280d
We are grateful to the NIH for support (GM 86485), and Johnson Matthey for generously supplying palladium catalyst PdCl2(Amphos)2 (Pd-132, catalog #C4138).
Notes and references
- 1.Gangjee A, Devraj R, Queener SF. J. Med. Chem. 1997;40:470. doi: 10.1021/jm9606913. [DOI] [PubMed] [Google Scholar]; Gangjee A, Vasudevan A, Queener SF. J. Med. Chem. 1997;40:3032. doi: 10.1021/jm970271t. [DOI] [PubMed] [Google Scholar]; McPhail KL, Rivett DEA, Lack DE, Davies-Coleman MT. Tetrahedron. 2000;56:9391. [Google Scholar]; Juteau H, Gareau Y, Labelle M, Sturino CF, Sawyer N, Tremblay N, Lamontagne S, Carriere M-C, Denis D, Metters KM. Bioorg. Med. Chem. 2001;9:1977. doi: 10.1016/s0968-0896(01)00110-9. [DOI] [PubMed] [Google Scholar]; Hsin LW, Dersch CM, Baumann MH, Stafford D, Glowa GR, Rothman RB, Jacobon AE, Rice KC. J. Med. Chem. 2002;45:1321. doi: 10.1021/jm010430f. [DOI] [PubMed] [Google Scholar]; Rosowsky A, Chen H, Fu H, Queener SF. Bioorg. Med. Chem. 2003;11:59. doi: 10.1016/s0968-0896(02)00325-5. [DOI] [PubMed] [Google Scholar]; Forsch RA, Queener SF, Rosowsky A. Bioorg. Med. Chem. Lett. 2004;14:1811. doi: 10.1016/j.bmcl.2003.12.103. [DOI] [PubMed] [Google Scholar]; Long Y-Q, Jiang X-H, Dayam R, Sachez T, Shoemaker R, Sei S, Neamati N. J. Med. Chem. 2004;47:2561. doi: 10.1021/jm030559k. [DOI] [PubMed] [Google Scholar]; Mertins K, Iovel I, Kischel J, Zapf A, Beller M. Adv. Synth. Catal. 2006;348:691. [Google Scholar]; Chappie TA, Humphrey JM, Allen MP, Estep KG, Fox CB, Lebel LA, Liras S, Marr ES, Menniti FS, Pandit J, Schmidt CJ, Tu M, William RD, Yang FV. J. Med. Chem. 2007;50:182. doi: 10.1021/jm060653b. [DOI] [PubMed] [Google Scholar]
- 2.Miyaura N, Yano T, Suzuki A. Tetrahedron Lett. 1980;21:2865. [Google Scholar]; Chowdhury S, Georghiou PE. Tetrahedron Lett. 1999;40:7599. [Google Scholar]; Botella L, Nájera C. J. Organomet. Chem. 2002;663:46. [Google Scholar]; Yamada YMA, Takeda K, Takahashi H, Ikegami S. J. Org. Chem. 2003;68:7733. doi: 10.1021/jo034354v. [DOI] [PubMed] [Google Scholar]; Chahen L, Doucet H, Santelli M. Synlett. 2003:1668. [Google Scholar]; Langle S, Abarbri M, Duchene A. Tetrahedron Lett. 2003;44:9255. [Google Scholar]; Nájera C, Gil-Moltó J, Karlström S. Adv. Synth. Catal. 2004;346:1798. [Google Scholar]; Bandgar BP, Bettigeri SV, Phopase J. Tetrahedron Lett. 2004;45:6959. [Google Scholar]; Nobre SM, Monteiro AL. Tetrahedron Lett. 2004;45:8225. [Google Scholar]; Kuwano R, Yokogi M. Org. Lett. 2005;7:945. doi: 10.1021/ol050078q. [DOI] [PubMed] [Google Scholar]; Singh R, Viciu MS, Kramareva N, Navarro O, Nolan SP. Org. Lett. 2005;7:1829. doi: 10.1021/ol050472o. [DOI] [PubMed] [Google Scholar]; McLaughlin M. Org. Lett. 2005;8:4875. doi: 10.1021/ol0517271. [DOI] [PubMed] [Google Scholar]; Flaherty A, Trunkfield A, Barto W. Org. Lett. 2005;8:4975. doi: 10.1021/ol051929x. [DOI] [PubMed] [Google Scholar]; Kuwano R, Yokogi M. Chem. Commun. 2005:5899. doi: 10.1039/b513372f. [DOI] [PubMed] [Google Scholar]; Burns JM, Fairlamb IJS, Kapdi AR, Sehnal P, Taylor JRK. Org. Lett. 2007;9:5397. doi: 10.1021/ol702291r. [DOI] [PubMed] [Google Scholar]; Molander GA, Elia MD. J. Org. Chem. 2006;71:9198. doi: 10.1021/jo061699f. [DOI] [PMC free article] [PubMed] [Google Scholar]; Henry N, Enguehard-Gueiffer C, Thery I, Guieffier A. Eur. J. Org. Chem. 2008:4824. [Google Scholar]; Alacid E, Náceja C. J. Org. Chem. 2009;74:2321. doi: 10.1021/jo802356n. [DOI] [PubMed] [Google Scholar]
- 3.Onuma K, Hashimoto H. Bull. Chem. Soc. Jpn. 1972;45:2582. [Google Scholar]; Ku Y-Y, Patel RR, Sawick DP. Tetrahedron Lett. 1996;37:1949. [Google Scholar]; Dohle W, Lindsay DM, Knochel P. Org. Lett. 2001;3:2871. doi: 10.1021/ol0163272. [DOI] [PubMed] [Google Scholar]; Kofink CC, Knochel P. Org. Lett. 2006;8:4121. doi: 10.1021/ol0616790. [DOI] [PubMed] [Google Scholar]
- 4.Park K, Kang M, Yie JE, Kim JM, Lee L-M. Tetrahedron Lett. 2005;46:2849. [Google Scholar]
- 5.Bedford RB, Huwe M, Wilkinson MC. Chem. Commun. 2009:600. doi: 10.1039/b818961g. [DOI] [PubMed] [Google Scholar]
- 6.Negishi E, King AO, Okukado N. J. Org. Chem. 1977;42:1821. [Google Scholar]
- 7.Betzemeier B, Knochel P. Angew. Chem., Int. Ed. 1997;36:2623. [Google Scholar]; Hargreaves SL, Pilkington BL, Russel SE, Worthington PA. Tetrahedron Lett. 2000;41:1653. [Google Scholar]; Angiolelli ME, Casalnuovo AL, Selby TP. Synlett. 2000:905. [Google Scholar]; Utas JE, Olofsson B, Åkermark B. Synlett. 2006:1965. [Google Scholar]
- 8.Hossain KM, Takagi K. Chem. Lett. 1999:1241. [Google Scholar]
- 9.Shade MA, Metzger A, Hug S, Knochel P. Chem. Commun. 2008:3046. doi: 10.1039/b803072c. [DOI] [PubMed] [Google Scholar]
- 10.Amatore M, Gosmini C. Chem. Commun. 2009:5019. doi: 10.1039/b809626k. [DOI] [PubMed] [Google Scholar]
- 11.Amatore and Gosmini have recently developed an elegant zinc-mediated, cobalt-catalyzed reaction between primary benzyl chlorides and aryl bromides in the presence of zinc (see ref. 10). However, the Co–Zn mix must be activated before performing the coupling (using 30 mol% allyl chloride, TFA in CH3CN).
- 12.Krasovskiy A, Duplais C, Lipshutz BH. J. Am. Chem. Soc. 2009;131:15592. doi: 10.1021/ja906803t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanda A, Fokin VV. Chem. Rev. 2009;109:725. doi: 10.1021/cr800448q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guram AS, Wang X, Bunel EE, Faul MM, Larsen RD, Martinelli MJ. J. Org. Chem. 2007;72:5104. doi: 10.1021/jo070341w. [DOI] [PubMed] [Google Scholar]
- 15.For extensive work on the pKa tolerance of organozinc reagents, see: Manolikakes G, Muñoz Hernandez C, Schade MA, Metzger A, Knochel P. J. Org. Chem. 2008;73:8422. doi: 10.1021/jo8015852.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


