Abstract
Reduced executive cognitive ability is associated with alcohol dependence (AD) and other comorbid externalizing disorders. Working memory capacity, short-term memory, conditional associative learning, and intelligence were assessed in a sample (N = 477) with variation in lifetime histories of externalizing problems (conduct disorder, adult antisocial behavior, substance problems); this included a subsample (n = 285) with a DSM-IV diagnosis of AD. Individuals with both AD and a history of childhood conduct disorder (CCD) scored lower on cognitive measures compared to those with AD and no history of CCD. Structural equation models showed that reduced ability in all cognitive domains was predicted by a latent externalizing factor reflecting covariation among lifetime problems with alcohol, drugs, childhood conduct, and adult antisocial behavior, and was not uniquely related to any one problem. Further, for those with AD, the externalizing factor was associated with reductions in all the domains of cognitive ability. The results suggest that the reduced executive cognitive ability observed in AD individuals is partly accounted for by a general latent externalizing factor, rather than alcohol-related problems per se.
Keywords: Alcohol dependence, externalizing disorders, comorbidity, cognition, conduct disorder
Reduced Cognitive Ability in Alcohol Dependence: Examining the Role of Covarying Externalizing Psychopathology
Diminished executive cognitive abilities in working memory, conditional associative learning, and intelligence may contribute significantly to the development and maintenance of alcohol dependence (AD; Aytaclar, Tarter, Kirisci, & Lu 1999; Finn & Hall, 2004; Harden & Pihl, 1995; Pihl, Peterson, & Finn, 1990; Poon, Ellis, Fitzgerald, & Zucker, 2000). Limitations in these key domains of cognitive ability are also thought to contribute to a general predisposition to both disinhibited and poorly regulated behaviors, rather than a specific vulnerability to AD (Finn, 2002; Finn & Hall, 2004; Giancola, Zeichner, Yarnell, & Dickenson, 1996; Harden & Pihl, 1995). This disinhibitory predisposition can manifest as various externalizing behaviors, including childhood conduct disorder (CCD), adult antisocial behavior, AD, and other substance abuse (Barkley, 1997; 2001; Finn, 2002; Finn & Hall, 2004; Krueger et al., 2002). In fact, there is increasing evidence that AD and related externalizing problems are better conceptualized along a continuous latent dimension of externalizing problems, assessed as the quantity of AD symptoms and co-occurring externalizing problems (Krueger et al., 2004; Krueger, Markon, Patrick, & Iacono, 2005). Individuals at the high end of this dimension exhibit increased behavioral dysregulation, high levels of disinhibitory personality traits and a more chronic course of AD (Babor et al., 1992; Finn, Mazas, Justus, & Steinmetz, 2002).
Details relating to the nature of the association between the hypothesized latent dimension of externalizing problems and cognitive ability have not been fully characterized in previously published reports. The current study explicitly addresses this issue by examining both the overlap and statistical covariation among AD and other externalizing problems, and executive cognitive domains of working memory, short-term memory, conditional associative learning, and intelligence. This study also assesses the association between cognitive ability and this externalizing dimension within those with a diagnosis of AD. Because AD is heterogeneous with respect to externalizing problems, such as CCD, it is important to investigate whether cognitive abilities in those with AD are best predicted by covariation among externalizing problems or, more specifically, by the number of alcohol problems, or other specific types of externalizing problems (e.g., CCD, other substance abuse). In the following sections, we review the self-regulatory roles of working memory and conditional associative learning and the implications of comorbid externalizing psychopathology in alcohol dependence, and describe the scope and details of the design of the study.
Working Memory, Conditional Associative Learning, and Behavioral Regulation
Working memory represents one component of a broader system of inter-related executive cognitive processes (Zelazo & Frye, 1998) that plays a critical role in self-regulation and decision making (Barkley, 1997; 2001; Bechara & Martin, 2004; Finn, 2002; Kimberg & Farah, 1993). Behavioral regulation may be related to specific aspects of working memory including time and capacity limits of short-term activation along with other central processes including resistance to distraction, mental manipulation, attentional control in divided attention /dual task contexts, and maintenance of memory traces over time (Baddeley & Logie, 1999; Cowan, 1999; Engle, Tuholski, Laughlin, & Conway, 1999; Finn, 2002). It is possible that larger working memory storage and processing capacities facilitate fluid shifting of attention during the decision-making process from more salient, temporally proximal (immediate) to less salient, distal (future) outcomes, while simultaneously allowing for appropriate weighting and consideration of future consequences of these decisions (i.e., less impulsive decisions; Finn, 2002; Finn & Hall, 2004; Oberauer, 2002). By contrast, reduced working memory capacity and functioning is related to poor impulse control and general behavioral disinhibition (Barkley; 1997; 2001; Finn, 2002; Finn & Hall, 2004; Hinson, Jameson, & Whitney, 2003), which reflect the basic dispositional processes underlying externalizing problems (Finn, 2002; Krueger et al., 2002; Slutske et al., 2002). Previously published studies suggest that working memory is required for activated self-directed speech, self-reflection, and maintaining representations for the purpose of problem solving to guide socially adaptive behavior (Barkley, 2001; Finn, 2002; Oberauer, 2002).
At a theoretical level, both Engle et al. (1999) and Finn (2002) proposed multidimensional models of working memory that distinguish short-term memory, indicated by performance on simple span tasks, from executive working memory, indicated by performance on complex span tasks that require both attention-shifting and maintenance. Engle et al. (1999) also showed that these two dimensions of working memory were distinct from measures of fluid intelligence. In our study, we adopted this model of working memory capacity and tested its viability with confirmatory factor analyses of multiple measures of performance on both simple and complex span tasks.
Conditional associative learning also is thought to play a central role in self-regulation (Finn & Hall, 2004; Giancola, 1995; Harden & Pihl, 1995; Kimberg & Farah, 1993). While involving working memory, conditional associative learning reflects an additional process of learning to regulate behavior based on specific event-behavior associations. Greater conditional associative learning capacity is thought to reflect an increased ability to learn context appropriate behaviors from experience (Giancola, 1995; Lau, Pihl, & Peterson, 1995; Pihl & Bruce, 1995). Poor conditional associative learning has been associated with increased social deviance, alcohol problems, and aggression (Finn & Hall, 2004; Giancola et al., 1996; Lau et al., 1995; Seguin, Pihl, Harden, Tremblay, & Boulerice, 1995).
As components of a larger system of behavioral regulation, executive working memory, short-term memory, intelligence, and conditional associative learning may each play a significant role in various forms of externalizing psychopathology. What is less clear, and what is examined here, is the extent to which ability in these cognitive domains is related to specific externalizing problems or whether cognitive ability is best accounted for by the covariation among externalizing problems.
Comorbid Externalizing Psychopathology in Alcohol Dependence
AD is highly comorbid with CCD, adult antisocial behavior, and other substance abuse (Driessen, Veltrup, Wetterling, Ulrich, & Dilling, 1998; Krueger et al., 2002; 2005). Recent evidence suggests that the high levels of co-occurrence among AD and other externalizing disorders is not coincidental, but instead reflects a broader latent dimension of externalizing psychopathology (Krueger & Markon, 2006; Krueger et al., 2002), a highly heritable dimension (Krueger et al., 2002; Kendler, Prescott, Myers, & Neale, 2003) that is genetically distinct from internalizing disorders (Kendler et al., 2003). Based on these findings, a better way to conceptualize the covariation among AD and other externalizing problems is as a continuous latent dimension of externalizing psychopathology.
From a clinical perspective, it is evident that many cases of AD presenting for treatment are comorbid with various externalizing disorders; increased comorbidity often is associated with a more chronic course and poorer treatment outcome (Babor et al., 1992; McLellan, Luborsky, Woody, O'Brien, & Druley, 1983; Rounsaville, Dolinsky, Babor, & Meyer, 1987). Thus, accurate characterization of the association between impairments in cognitive ability and comorbid externalizing problems in those with AD may provide significant implications for treatment (Bates, Labouvie, & Voelbel, 2002; Tapert & Brown, 1999).
The Present Study
The overarching purposes of this study were to assess the association between AD-related externalizing problems and different domains of cognitive ability and to test the primary hypothesis that externalizing problems in general, and increased levels of externalizing problems within those who satisfy the DSM-IV diagnostic criteria for AD, is predictive of reduced cognitive ability. More specifically, we propose that covariation among externalizing problems is associated with reduced cognitive ability and that specific externalizing problems are not uniquely associated with reduced cognitive ability beyond their covariation with other domains of externalizing problems. To examine this association, our focus is primarily on dimensional models of externalizing problems and cognitive ability in those with AD and in a larger sample that included non-AD individuals. However, we also present a traditional categorical model analysis of the association between AD, CCD, and cognitive ability to provide a detailed description of the characteristics of our sample and to facilitate comparisons between our results and those reported in the literature, which typically employ categorical models. Because reductions in executive cognitive ability are related to both antisocial traits (Finn & Hall, 2004; Harden & Pihl, 1995; Speltz, DeKlyen, Calderon, Greenberg, & Fisher, 1999) and alcohol abuse (Bates, Voelbel, Buckman, Labouvie, & Barry, 2005; Finn & Hall, 2004), we hypothesized that individuals with both AD and a history of CCD would show diminished cognitive functioning compared to individuals with AD alone.
Following the general approach proposed by Krueger and colleagues (Krueger et al., 2002), we used structural equation models (SEMs) to explore the association between the hypothesized latent externalizing problems factor (indicated by measures of lifetime problems with alcohol, marijuana, other drugs, childhood conduct, and antisocial behavior) and different domains of cognitive ability (assessed as latent factors for executive working memory capacity, short term memory capacity, and intelligence, and a single observed measure of conditional associative learning). In addition, SEMs were used to assess whether externalizing disorders were associated with the cognitive ability variables when controlling for the influence of years of education. Externalizing disorders are associated with fewer years of education and with lower academic achievement (Finn et al., 2002; Kessler, Foster, Saunders & Stang, 1995; Moffitt, 1993) which partly reflects poor self-regulation that characterizes externalizing disorders (Finn, 2002; Moffitt, 1993; Patterson, Capaldi, & Bank, 1991). However, longer exposure to academic environments can provide opportunities or experiences that may enhance cognitive ability independent of its association with externalizing problems (Lynam, Moffitt, & Stouthammer-Loeber, 1993). Therefore, we sought to assess the associations between externalizing disorders and cognitive ability that are independent of years of education.
Previous studies show that, while significantly inter-correlated, different domains of executive cognitive function and intelligence are separable (Engle et al., 1999; Friedman et al., 2008) and associated with unique genetic influences (Friedman et al., 2008). In this study, model specification searches were performed to explore whether any indicators of the latent externalizing problems factor were uniquely associated with any measure of cognitive ability beyond their covariance with other indicators of the latent factor.
In summary, the current study adds to the literature by including (1) an examination of the utility of a dimensional model of covariation among externalizing problems in a predictive account of executive cognitive ability, (2) an assessment of different domains of cognitive ability (executive working memory, short-term memory, and higher order cognitive abilities including intelligence and conditional associative learning) in a large community (non-treatment) sample representing a substantial range of variation in externalizing problems, and (3) an investigation into the association between variations in externalizing problems and cognitive ability in those with AD.
Method
Participants
Recruitment
Participants were recruited through advertisements placed in local newspapers and around the community. The ads / flyers were designed to attract respondents that varied from low to high levels of impulsive and disinhibited traits and alcohol use. This approach has been effective in attracting responses from individuals varying in disinhibited traits and from alcohol dependent, antisocial, and generally disinhibited participants (Bauer & Hesselbrock, 1993; Finn et al., 2002; Widom, 1977). The range of ads / flyers targeted “daring, rebellious, defiant individuals,” “carefree, adventurous individuals who have led exciting and impulsive lives,” “impulsive individuals,” “heavy drinkers wanted for psychological research,” persons with a “drinking problem,” persons who “got into a lot of trouble as a child,” persons “interested in psychological research,” “quiet, reflective and introspective persons,” and “social drinkers.”
Telephone screening interview
All respondents were given a telephone screening interview that began with a brief description of the study, followed by a series of questions assessing the study exclusion criteria, current and lifetime alcohol and other drug use, lifetime symptoms of alcohol and other drug abuse and dependence, childhood conduct disorder, and adult antisocial personality. Respondents meeting the study inclusion criteria (noted below) were provided with additional details of the study. Potential participants were informed that the entire study would take approximately 10 hours to complete and that these 10 hours would be divided over three 2–3 hour testing sessions; they were also told that they must abstain from using alcohol and other drugs for 12 hours before each session. Only those who met the group inclusion criteria after the diagnostic interview in the first session were allowed to continue to participate in the study.
Study exclusion criteria
Participants were excluded from the study if they [1] were not between 18 and 30 years of age, [2] could not read and speak English, [3] had never consumed alcohol, [4] had less than a sixth grade level of education, [5] reported having suffered from any serious head injuries, or [6] had a history of psychosis.
Group inclusion / exclusion criteria
The inclusion criteria for the group without a history of either AD or CCD (noAD/noCCD group) were no history of AD, CCD, antisocial personality (ASP), alcohol abuse or any other substance abuse or dependence. Participants in this group reported [1] consuming no more than 4 standard drinks on no more than one occasion in a typical month and no more than 6 drinks on any occasion in their life, [2] using marijuana no more than twice a week and less than 25 times in their life, and [3] not using any other mood altering drug in the last 6 months or recreationally more than 4 times in their life. If a subject failed to meet any one of the criteria, he or she was excluded from further participation. The inclusion criteria for the AD-alone group were meeting DSM-IV criteria for AD and not for a history of either CCD or ASP. The inclusion criteria for the ADCCD group were meeting DSM-IV criteria for both AD and a history of CCD. Subjects were not excluded from either AD group if they had current or past other drug abuse or dependence. The inclusion criteria for the CCD-alone group were meeting DSM-IV criteria for a history of CCD and not meeting DSM-IV criteria for AD. Histories of alcohol abuse or other substance abuse or dependence were allowed in the CCD-alone group to increase the representativeness of this sample in terms of the degree of heterogeneity of covarying externalizing problems.
Test session exclusion criteria
At the beginning of each session, participants were asked about their alcohol and drug use in the past 12 hours and were given a breath alcohol test using an AlcoSensor IV (Intoximeters, Inc., St. Louis, MO). Participants were rescheduled if they reported consuming any drug within the past 12 hours, had a breath alcohol level greater than 0.0%, reported feeling hung over, or appeared high, overly sleepy, or were unable or unwilling to answer test session questions.
Sample characteristics
The entire sample consisted of 477 young adults; 141 (79 men, 62 women) met DSM-IV diagnostic criteria for AD with no history of CCD (the AD-alone group), 144 (82 men, 62 women) with both AD and a history of CCD (the ADCCD group), 67 (35 men, 32 women) with a history of CCD and no AD (the CCD-alone group), and 125 (60 men, 65 women) with no history of CCD, AD, alcohol abuse, or other substance abuse (the noAD/noCCD group). The ethnicity of the sample was 78% Caucasian, 14% African American, 5% Asian, 2% Hispanic, and 1% Pacific Islander. Table 1 presents descriptive statistics and sample proportions for various demographic measures and current medications for each group. Table 2 presents group data on history of alcohol use, problems with AD (and alcohol abuse in the CCD-alone sample) and recent use of alcohol and other substances. Table 3 displays data on the prevalence and history of lifetime abuse or dependence on marijuana and other drugs. Approximately two thirds of the participants in the AD and the CCD-alone groups reported a history of marijuana and / or other drug abuse or dependence. Much of the reported marijuana abuse / dependence was current; a majority of the other reported drug abuse / dependence was in the past. Finally, Table 4 displays lifetime problems with alcohol, conduct, adult antisocial behavior, marijuana, and other drugs. Lifetime problems in each domain were quantified by summing positive responses to subsets of questions in respective sections of the diagnostic interview described below. As shown in Table 4, there was substantial variation within each group on lifetime problems with alcohol, childhood conduct, adult antisocial behavior, marijuana, and other drugs.
Table 1.
Demographic Characteristics and Current Medication by Group
| noAD/noCCD | CCD-alone | AD-alone | ADCCD | ||
|---|---|---|---|---|---|
| n (male/female) | 125 (60/65) | 67 (35/32) | 141 (79/62) | 144 (82/62) | |
| Variable | |||||
| Mean age in years (SD) | 21.2 (2.1) | 22.0 (2.9) | 22.0 (2.8) | 22.6 (3.3) | |
| Mean years of education (SD) | 14.6 (1.7) | 13.4 (2.1) | 14.1 (1.8) | 12.9 (2.2) | |
| % Currently student | 91.2 | 50.7 | 69.5 | 36.8 | |
| % Currently employed | 48.0 | 53.7 | 39.7 | 42.2 | |
| Hours / week (SD) | 15.9 (9.5) | 24.7 (8.0) | 20.9 (10.3) | 31.1 (12.6) | |
| Dollars / hour (SD) | 8.16 (3.1) | 8.68 (2.6) | 7.89 (3.0) | 8.56 (2.5) | |
| Current Medications | |||||
| % Antidepressant | 1.6 | 5.9 | 9.9 | 11.1 | |
| % Anti-anxiety | 0.0 | 1.5 | 2.8 | 1.4 | |
| % ADHD stimulant | 1.6 | 3.0 | 5.7 | 2.8 | |
Note. CCD = a history of childhood conduct disorder; AD = a history of alcohol dependence; CCD-alone = CCD without AD; AD-alone = AD without CCD. All current psychotropic medications were reported as prescribed by a physician. Antidepressant medications included all those prescribed for major depression, mostly SSRIs. Anti-anxiety medications included all those prescribed for anxiety, mostly benzodiazepines. ADHD stimulant medications included all those prescribed to treat ADHD symptoms, mostly Ritalin and Adderall.
Table 2.
History of Alcohol Use, Abuse and / or Dependence, and Recent Substance Use by Group
| Measure | noAD/noCCD | CCD-alone | AD-alone | ADCCD | |
|---|---|---|---|---|---|
| Mean (SD) age of onset of: | |||||
| Regular drinking | 18.56* (1.9) | 16.23* (2.6) | 16.33 (2.1) | 14.81 (2.7) | |
| Abuse and / or dependence | -- | 15.50** (2.3) | 17.52 (2.4) | 15.98 (2.6) | |
| Mean (SD) duration of: | |||||
| Abuse and/or dependence | -- | 5.32** (3.5) | 5.08 (2.9) | 6.82 (3.4) | |
| Mean (SD) recency of: | |||||
| Abuse and / or dependence | -- | 2.27** (2.8) | 0.45 (1.1) | 0.79 (1.7) | |
| Recent substance use: | |||||
| Alcohol frequency | 1.68 (1.2) | 2.48 (2.0) | 3.68 (2.1) | 3.88 (2.3) | |
| Alcohol quantity | 2.50 (1.8) | 3.33 (3.8) | 6.49 (4.4) | 7.70 (5.3) | |
| Marijuana frequency | 0.14 (0.5) | 2.39 (2.8) | 2.72 (3.0) | 3.52 (3.2) | |
| Stimulant (including cocaine) frequency |
-- | 0.25 (1.0) | 0.34 (1.1) | 0.43 (1.1) | |
| Opiate frequency | -- | 0.01 (1.2) | 0.06 (0.3) | 0.15 (0.6) | |
| Sedative frequency | -- | 0.21 (0.9) | 0.57 (1.7) | 0.82 (1.8) | |
| Other frequency | -- | 0.15 (.09) | 0.33 (1.3) | 0.61 (1.9) | |
Note. CCD = a history of childhood conduct disorder; AD = a history of alcohol dependence CCD-alone = CCD without AD; AD-alone = AD without CCD; Onset age of regular drinking = age when subject reported the initiation of drinking at least once / month for 6 months or more. Onset of abuse / dependence = age at which 3 or more symptoms of abuse / dependence were experienced during the same 6 month period. Duration of alcohol abuse / dependence = time from onset to the most recent experience of abuse / dependence symptoms. Recency of abuse / dependence is in years since most recent episode (a value of 0 = currently active abuse / dependence). Alcohol, marijuana and other drug use frequency is in days per week; alcohol use quantity = number of standard drinks per occasion.
22 noAD/noCCD and 2 CCD-alone subjects reported never drinking regularly, but had consumed alcohol at least once.
Table 3.
History of Other Substance Abuse and Dependence by Group
| Measure | noAD/noCCD | CCD-alone | AD-alone | ADCCD | |
|---|---|---|---|---|---|
| % Abuse / dependence other than alcohol | 0.0 | 56.7 | 58.2 | 79.2 | |
| % Abuse / Dependence (ever) | |||||
| Marijuana | 0.0 | 40.3 | 52.5 | 74.3 | |
| Cocaine | 0.0 | 16.4 | 11.3 | 23.6 | |
| Stimulant(s) | 0.0 | 22.4 | 15.6 | 29.2 | |
| Opiate(s) | 0.0 | 16.4 | 16.3 | 27.8 | |
| Sedative(s) | 0.0 | 6.0 | 15.6 | 25.7 | |
| Other | 0.0 | 7.5 | 7.8 | 15.3 | |
| % Current | |||||
| Marijuana | 0.0 | 15.0 | 23.4 | 39.6 | |
| Cocaine | 0.0 | 0.0 | 0.01 | 0.05 | |
| Stimulant(s) | 0.0 | 0.03 | 0.02 | 0.03 | |
| Opiate(s) | 0.0 | 0.0 | 0.01 | 0.02 | |
| Sedative(s) | 0.0 | 0.0 | 0.04 | 0.8 | |
| Other | 0.0 | 0.0 | 0.01 | 0.04 | |
| Mean duration in years (SD) | |||||
| Marijuana | -- | 3.19 (2.1) | 2.65 (2.7) | 4.23 (3.3) | |
| Cocaine | -- | 0.39 (0.4) | 0.77 (1.3) | 1.54 (1.0) | |
| Stimulant(s) | -- | 0.74 (0.7) | 0.97 (1.5) | 1.02 (1.2) | |
| Opiate(s) | -- | 1.17 (1.7) | 1.26 (1.6) | 1.29 (1.5) | |
| Sedative(s) | -- | 0.16 (0.1) | 1.79 (2.3) | 1.54 (2.0) | |
| Other | -- | 0.22 (0.2) | 1.23 (1.1) | 1.63 (3.0) | |
| Mean recency; years since (SD) | |||||
| Marijuana | -- | 2.39 (2.7) | 1.74 (2.3) | 1.77 (2.8) | |
| Cocaine | -- | 3.26 (2.0) | 3.35 (2.6) | 3.05 (3.0) | |
| Stimulant(s) | -- | 3.52 (3.4) | 2.84 (2.2) | 2.93 (2.3) | |
| Opiate(s) | -- | 4.29 (3.3) | 2.83 (2.7) | 2.71 (2.8) | |
| Sedative(s) | -- | 4.34 (2.3) | 3.30 (3.5) | 2.38 (2.5) | |
| Other | -- | 6.98 (2.5) | 2.95 (2.0) | 2.32 (2.0) | |
Note. CCD = a history of childhood conduct disorder; AD = a history of alcohol dependence; CCD-alone = CCD without AD; AD-alone = AD without CCD. Duration is the mean years of abuse / dependence calculated using the total number of reported months between onset of abuse / dependence and most recent experience with abuse / dependence. Recency is mean number of years since the last reported occurrence of abuse and / or dependence.
Table 4.
Lifetime Externalizing Problems and Percent Antisocial Personality by Group
| Measure | noAD/noCCD | CCD-alone | AD-alone | ADCCD | |
|---|---|---|---|---|---|
| Mean (SD) lifetime problems with: | |||||
| Alcohol | 4.22 (4.6) | 15.63 (12.1) | 37.39 (16.3) | 46.81 (14.5) | |
| Marijuana | 0.46 (2.4) | 10.60 (10.6) | 11.28 (11.6) | 17.39 (11.8) | |
| Other drug | 0.02 (0.3) | 13.52 (21.3) | 15.52 (27.3) | 28.46 (33.7) | |
| Conduct | 4.82 (3.6) | 17.12 (5.1) | 12.68 (5.5) | 20.63 (4.6) | |
| Adult antisocial | 3.79 (3.5) | 15.88 (6.8) | 12.03 (6.5) | 21.68 (6.9) | |
| % Antisocial Personality Disorder | 0.0 | 31.3 | 0.0 | 31.9 | |
Note. CCD = a history of childhood conduct disorder; AD = a history of alcohol dependence; CCD-alone = CCD without AD; AD-alone = AD without CCD. Lifetime problems with alcohol, marijuana, and other drugs are based on the number of positive responses to subsets of questions on the respective sections of the SSAGA. Other drug lifetime problems represent a total of problems with cocaine, other stimulants, opiates, sedatives, or other substances, such as hallucinogens, and inhalants. Conduct problems are positive responses to those questions assessing childhood conduct disorder. Adult antisocial problems are positive responses to the questions assessing antisocial personality. All values represent averages for entire sample within each group. Included in the group percentages of Antisocial Personality Disorder are participants that met DSM-IV diagnostic criteria for adult antisocial personality disorder.
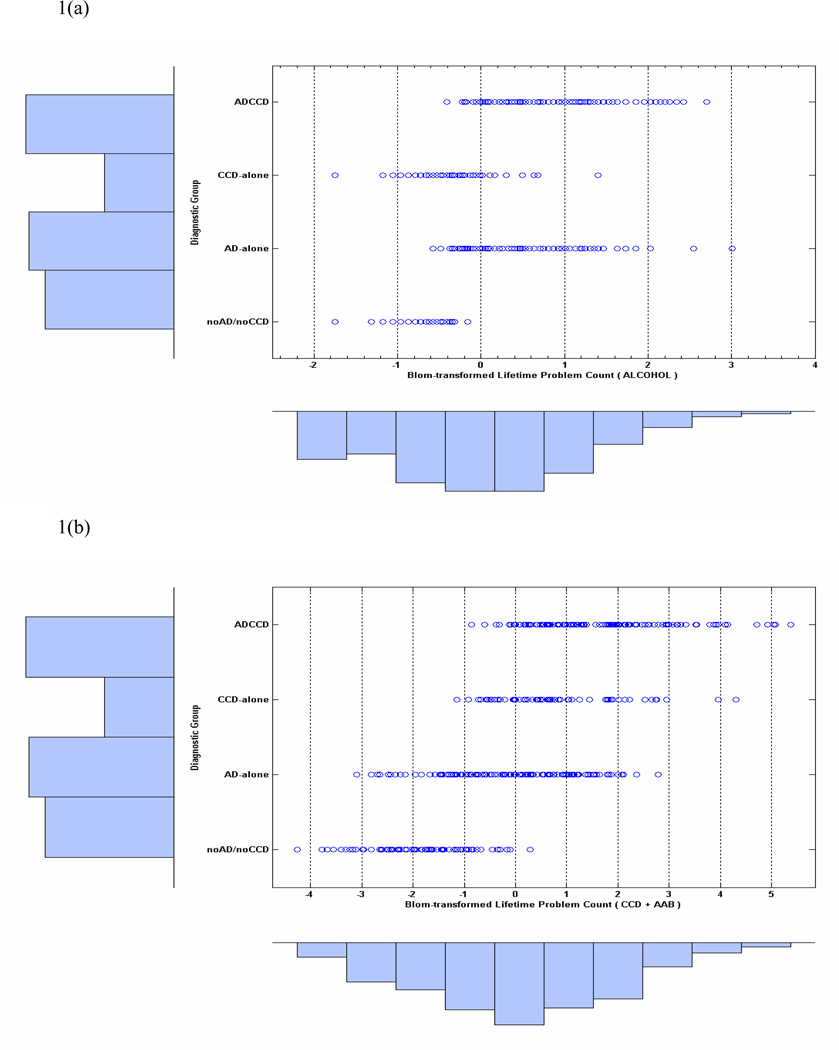
The frequency distribution below the x-axis in Figure 1(a) shows the Blom-transformed lifetime alcohol problems for the full sample (N = 477). The transformed counts are shown separately for each diagnostic group in the graph (open circles) with the relative number of cases in each group depicted by the marginal histogram to the left of the y-axis. Figure 1(b) depicts the combined (i.e., summed) Blom-transformed lifetime problems with childhood conduct and adult antisocial behavior. Although none of the participants in the noAD/noCCD group met diagnostic criteria for alcohol abuse / dependence or conduct disorder, a number reported some lifetime problems relating to alcohol use and conduct problems (see Table 4). The ADCCD, AD-alone, and CCD-alone groups also showed substantial variation on measures of lifetime problems with marijuana and other drugs. All groups displayed a range in severity of the different categories of problems, with ADCCDs having the most problems and noAD/noCCD group, the least. The extent of the variation within each group and across groups, and the overlap in variation between groups, indicate that the sample is well suited for an examination of a dimensional model of alcohol problems / dependence and general disinhibitory problems associated with AD.
Figure 1.
(a). Dot-plots of the distribution of Blom-transformed alcohol lifetime problem counts by group. The marginal distribution of problem counts combined across all groups is represented by the frequency histogram below the abscissa. Sample sizes are shown by the histogram displayed on the left ordinate. Figure 1(b). Distribution of Blom-transformed total lifetime antisocial problems (CCD + AAB) for each group.
Assessment Materials
Recent alcohol and other substance use
Participants were interviewed and asked whether they consumed alcohol or other drugs on each day of a typical week. If participants answered ‘yes’, they were asked to report the amount they usually consumed on that day. Alcohol use was quantified as the average frequency of drinking (days per week) and the average quantity consumed per day over the past 6 months. Drug use was quantified as the average frequency of use (days per week) for marijuana and other drugs, including stimulants, sedatives, hallucinogens, opiates or inhalants over the past 3 months. Table 2 presents the group means for these data.
Diagnostic interview
The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994) which uses criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV; American Psychiatric Association, 1994) was used to determine whether responses from potential participants satisfied diagnostic criteria for alcohol dependence (AD), childhood conduct disorder (CCD), adult antisocial personality, marijuana abuse / dependence, and drug abuse / dependence. The severity of lifetime problems associated with alcohol use, childhood conduct, adult antisocial behavior, MD, marijuana use, and other drug use were measured by summing positive responses to subsets of SSAGA questions in the respective diagnostic sections. Tables 3 and 4 present these data.
Short-term memory capacity
Short-term memory (STM) capacity was assessed with the digits forward (DF) and digits backward (DB) scales of the Digit Span subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS-R; Wechsler, 1981) and the letter-number sequencing (LN) task from the WAIS-III (Wechsler, 1997). Both DB and DF are commonly used measures of STM capacity and general attentional capacity in studies of working memory (Engle et al., 1999; Finn, 2002; Finn & Hall, 2004). There were missing data on this measure from one ADCCD subject. The LN task involves the presentation of increasingly larger sets of letters and numbers (e.g., G-6-2-B). The subject is asked to recall the numbers in numerical order (e.g., 2–6) and the letters in alphabetical order (e.g., B-G). There were no missing data on the letter-number sequencing task.
Executive working memory capacity
Executive working memory was assessed using two different complex-span tests, the Operation-Word Span test (OW; Conway & Engle, 1994) and a modified version of the Auditory Consonant Trigram test (Brown, 1958), that we refer to as the Auditory Consonant (AC) test. Numerous studies indicate that such complex span tests are valid measures of executive working memory capacity and reflect the capacity for resistance to distraction, mental manipulation, attentional control, and maintenance of memory traces over time (Engle et al., 1999; Kane, Poole, Tuholski, & Engle, 2006; Unsworth & Engle, 2007; 2008). The OW test involves competition for attentional resources and the maintenance of activation of mental representations in a dual task context. The test requires solving a simple mathematical operation while remembering a word (6/3 + 2 = 4 DOG). The subject reads the math operation aloud, responds “yes” or “no” to indicate if the answer is correct or not and then says the word; one half of the mathematical operations are correct. After a series of operation-word pairs (varying from 2 to 6), the subject is asked to recall the words in the exact order they were presented. Performance on this measure is quantified as the total number of correctly recalled words. There were missing data on the OW test from 2 noAD/noCCD, 1 CCD-alone, 1 AD-alone, and 4 ADCCD participants.
In its original form, the AC test involves recalling three-consonant nonsense strings after counting backward for varying periods of time. This test presumably taps divided attention and the strength of the maintenance / decay of the contents of working memory over time (Brown, 1958; Stuss, Seethem, & Poirier, 1987). To increase working memory load, we modified the test by including four- and five-consonant nonsense strings in addition to the original three-consonant strings. Greater loads are expected to amplify group differences. In this test, the experimenter reads aloud a string of consonants at a rate of one letter per second, followed by a three-digit number. The subject counts backward by threes from that number for either 18 or 36 seconds and is asked then to recall the original consonant string. Counting backward is assumed to interfere with rehearsal of the original consonant string. For all string lengths, two were followed by 18-second delay intervals and two were followed by 36-second delay intervals. Performance on this test was quantified as the total number of correct consonants recalled across all string lengths and delay intervals. There were missing data on the AC test from 2 noAD/noCCD, 1 CCD-alone, 1 AD-alone, and 5 ADCCD participants.
Intelligence
General intelligence was measured using the Shipley Institute of Living Scale verbal and reasoning estimates of IQ (Zachary, 1986) and the Matrix Reasoning (MX) measure of non-verbal intelligence and reasoning (Wechsler, 1997). The Shipley is a convenient self-administered measure of intelligence that strongly correlates (median correlation = .79) with the WAIS Full Scale IQ (Zachary, 1986). As with other abbreviated measures of general intelligence, such as the Wechsler Abbreviated Scale of Intelligence (WASI, Psychological Corporation, 1999), the Shipley measure does not assess memory capacity. This effectively eliminates any potential overlap between our measures of working memory capacity and intelligence and, thus, allows for a more specific test of the roles for these two cognitive domains. The Matrix Reasoning (MX) measure assesses non-verbal intelligence. There were missing data on the Shipley measure from one noAD/noCCD and one ADCCD subject, and on the MX measure from 2 noAD/noCCD, 1 CCD-alone, 1 AD-alone, and 4 ADCCD participants.
Conditional associative learning
The Conditional Association Task (CAT; Petrides, 1985) was used to assess an additional, higher-order cognitive function involved specifically in conditional associative learning. This task has been used in previous studies of disinhibited populations to assess executive cognitive ability (Finn & Hall, 2004; Giancola et al., 1996; Gillen & Hesselbrock, 1992; Harden & Pihl, 1995). The task requires the subject to learn and recall the matched associations between six cards and six lights (LEDs); the six lights are randomly positioned on a metal box placed in front of the subject. Eighteen correct consecutive responses were required for successful completion of the task. As in earlier studies (Finn & Hall, 2004; Giancola et al., 1996; Harden & Pihl, 1995), CAT performance is quantified as the square root of the sum of total trials to criterion and total errors. Lower scores indicate better conditional associative learning. There were missing data on this measure from 1 noAD/noCCD, 2 CCD-alone, 3 AD-alone, and 2 ADCCD participants.
Procedure
In the first testing session, participants were required to read and sign an informed consent before testing began. The run time for the entire study was approximately 10 hours and involved three 2–3 hour testing sessions that assessed a range of factors associated with behavioral disinhibition in early-onset alcohol dependence, including assessments of psychopathology, substance use, cognitive ability, personality, decision making, behavioral self-control, life-transition problems, and social / drinking context. Participants were paid $10 per hour for their time in the laboratory.
The analyses reported in this paper reflect results for all participants completing the assessments of cognitive ability and psychopathology. As noted above, some participants did not complete all cognitive assessments because of a failure to attend all testing sessions or a failure to successfully follow task instructions. However, the missing data were fairly evenly distributed across groups. For the MANOVA, the data on cognitive measures for those with missing data were imputed using multiple regressions with available cognitive measures as predictors of missing data. Missing cognitive data for analyses using structural models with latent variables were computed using a full information maximum likelihood estimation algorithm.
Data Analysis
ADCCD versus AD-alone
The association between AD and CCD and cognitive ability was first analyzed using a 2 × 2 × 2 (AD by CCD by gender) multivariate analysis of variance (MANOVA) with all cognitive measures serving as the dependent variables. To test the hypothesis that the ADCCD group had lower overall cognitive ability compared to the AD-alone group a MANOVA was conducted within the AD group to assess the main effects of CD (analyzed as a planned comparison between the ADCCD and the AD-alone group).
Overview of multivariate dimensional analyses
Structural equation modeling (SEM) with maximum-likelihood estimation was used to test the association between the lifetime problem predictor variable (modeled as a single latent externalizing problems factor) and cognitive ability (modeled as three latent variables and one observed endogenous variable). The externalizing problems factor (EXT) reflects the statistical covariation among measures of lifetime problems with alcohol (alc), marijuana (mar), other drugs (drg), childhood conduct (ccd), and adult antisocial behavior (aab). The structural model was re-estimated to examine the externalizing problems latent factor and cognitive ability of in those with AD (i.e., both ADCCD and AD-alone groups). These models included years of education covarying with EXT, as well as predicting cognitive ability. Then, an SEM was used to assess the influence of recent alcohol use and drug use on the association between lifetime problems and cognitive ability in the full sample both with and without years of education as a covariate. The influence of recent use was not assessed in the AD subsample because the restricted range in the alcohol use indicator measures resulted in an inadmissible measurement model. The overall fit of each model was assessed using the chi-square goodness-of-fit index (χ2), the Bentler and Bonett (1980) normed fit index (NFI), and the root mean square error of approximation (RMSEA: Browne & Cudek, 1993) which is a residual based fit index. A good data-model fit is reflected by an NFI of 0.94 or greater and a RMSEA less than or equal to 0.05 (Bentler & Bonett, 1980; Browne & Cudek, 1993) or 0.06 (Hu & Bentler; 1999). An excellent data-model fit is reflected in a non-significant (p > .05) χ2 test statistic.
Measurement models for cognitive ability and externalizing problems
Confirmatory factor analysis (CFA) was used to test the feasibility of a two-factor measurement model for working memory. The CFA compared the fit of a one-factor working memory capacity model, where the single factor was indicated by all five measures of working memory capacity, with the fit of a two-factor model that included a short-term memory factor, indicated by DF, DB, and LN, and an executive working memory factor, indicated by the Operation Word Span (OW) and the Auditory Consonant Test (AC). The CFA revealed that the one-factor model did not fit the data very well χ2(5, N = 477) = 105.90, p < .00001, NFI = .88, RMSEA = 0.21. Consistent with the findings of Engle, et al. (1999), a two-factor model not only provided an acceptable fit the data well, χ2(4, N = 477) = 9.35, p = .053, NFI = .990, RMSEA = 0.05, but provided a significantly better fit than the one-factor model, with difference χ2 (1,477) = 96.55, p < .0001.
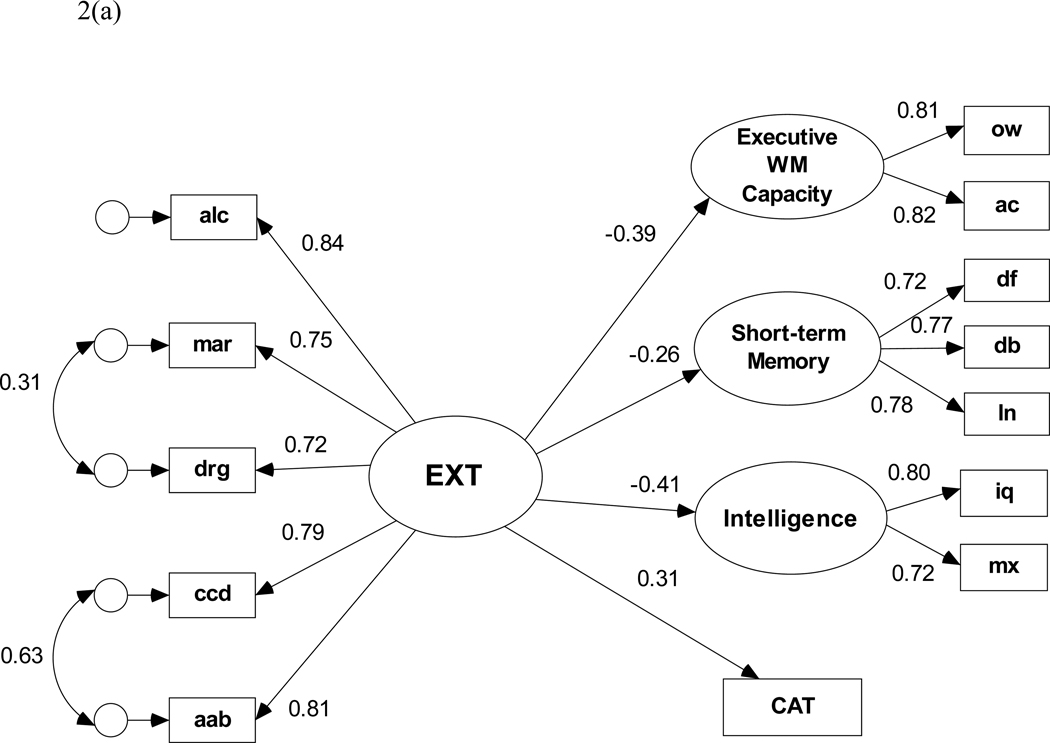
The measurement model for the EXT factor was fit before we proceeded with the structural analyses. Because of their skewed distribution, all measures of lifetime problems were Blom-transformed for the SEM analyses. Blom-transformation is considered an optimal approach to handling psychiatric symptom and problem counts in multivariate modeling analyses (Krueger et al., 2002; van den Oord et al., 2000). A specification search was performed by defining a set of candidate models with optional paths representing (1) all pairwise correlations of the residuals for the three substance-use manifest variables, and (2) correlation of the residuals for ccd and aab. Residuals for the three substance problem indicators were allowed to covary because these residuals are, in part, likely to reflect common variance associated with substance use problems that is not associated with the covariance between substance use problems and antisocial problems. Likewise, the residuals for two antisocial problem indicators also were allowed to covary, because they are assumed to reflect common variance in antisocial problems that are not associated with their covariance with substance use problems. Following Burnham and Anderson (1998), we selected the measurement model with the lowest rescaled Bayesian Information Criterion (i.e., BIC0 = 0) coefficient. BIC0 aids in selecting models by identifying which model among competing models reproduces the observed variances and covariances with the fewest estimated parameters (i.e., with the most parsimony). This model (illustrated on the left side in the SEM in Figure 2) has correlated residuals for lifetime problems with marijuana and other drugs as well as correlated residuals for ccd and aab and provides an excellent fit to the data with χ2(3, N = 477) = 4.25, p = .236, NFI = .997, RMSEA = 0.03.
Figure 2.
(a). Structural equation model of the predictive paths between the latent externalizing disorders factor and each cognitive ability variable for the full (N=477) sample. Path coefficients are standardized β weights and are all significant at p < .05.
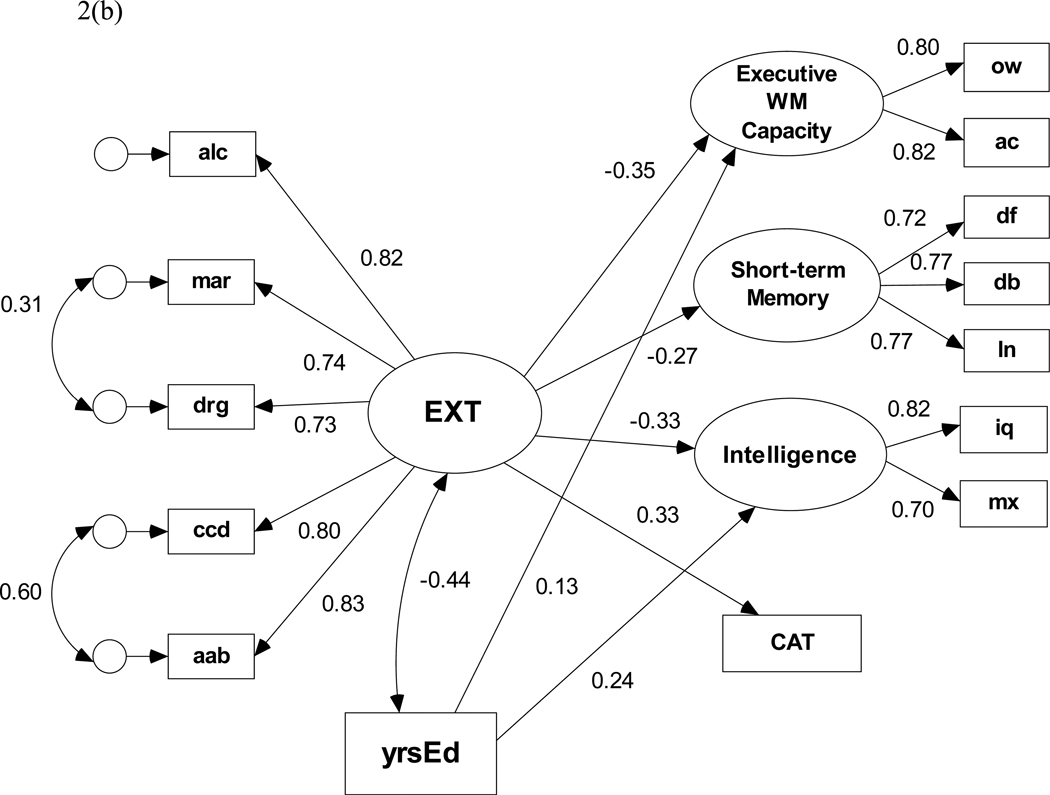
(b) Structural model based on the full (N=477) sample with years of education (yrsEd) covarying with EXT and predicting both increased executive WM capacity and higher intelligence.
SEM analyses
SEM analyses were conducted in three stages. The first model examined the association between the lifetime problem predictor variable, EXT, and the measures of cognitive ability analyzed as a latent factor for intelligence, a latent factor for short-term memory capacity, a latent factor for executive working memory capacity, and errors on the conditional associative learning task, a manifest variable. Model specification search was used to assess whether the individual indicators of the EXT factor were uniquely associated with the cognitive measures in the model beyond their covariance with other EXT indicators. Zero-based BIC0 was used to guide model respecification, i.e., whether including a direct path from any of the EXT manifest variables to the any of the domains of cognitive ability represented in the model would improve either fit and / or interpretability. We then re-estimated the best fitting model using years of education (yrsEd) as a covariate of EXT and as a (manifest) predictor variable of the cognitive factors. A specification search was performed to determine the most parsimonious model.
In the second stage of SEM, the same measurement model for EXT derived for the full sample, was re-estimated using data for those who met DSM-IV criteria for alcohol dependence; this model fit the data extremely well, χ2(3, N = 285) = 2.66, p = .446, NFI = .996, RMSEA = 0.000. This was done specifically to evaluate the influence of the covariation among lifetime problems on cognitive ability in those with alcohol dependence. The AD structural model was also evaluated with yrsEd as a covariate of EXT and predictor variable of the cognitive ability variables.
The third stage of SEM included latent factors representing recent alcohol and drug use in the model. Alcohol use indicators were average quantity consumed per occasion (aq) and average frequency of alcohol consumption (af). Drug use indicators were frequency of marijuana use (mj), opiate use (op), sedative use (sd) and stimulant use (stm). Alcohol and drug use indicators also were Blom-transformed to reduce distribution. Because using only one latent variable to model recent substance use resulted in a poor model fit, we adopted a measurement model with separate latent variables for alcohol use and drug use that fit the data adequately, χ2(8, N = 477) = 33.490, p < .001, NFI = .943, RMSEA = 0.082. The chi-square difference test between the one- and two-factor was statistically significant. The lowest zero-based BIC0 coefficient was obtained for the default model which had no direct paths from the recent use factors to any of the cognitive variables. This SEM tested the extent to which the observed association between the EXT latent factor and cognitive ability depended on recent substance use. As before, the most parsimonious model was evaluated using yrsEd as a covariate of EXT, RAU, and RDU and as a predictor variable of the cognitive ability variables.
Results
In the following sections, we first present results characterizing the differences among the diagnostic groups on counts of lifetime substance use problems and cognitive ability variables. We next describe the results regarding the primary research question related to the covariation of the externalizing problems, and whether a dimensional model of these problems provides a better predictive account of the cognitive ability variables than AD, or any one of the other problems.
Differences in Substance Use Problems by Group Membership and Gender
The ADCCD group had significantly more lifetime problems with alcohol, conduct, marijuana, and other drugs compared to all other groups (ps < .01). The group means on these measures are illustrated in Table 4. It is notable that the ADCCD group had more lifetime alcohol problems compared to the AD-alone group and more lifetime conduct problems compared to the CCD-alone group. There was a main effect of gender on all measures of alcohol consumption (ps < .05), but not on measures of alcohol or conduct problems or the use of marijuana or other drugs. There were no interactions involving gender on any measure.
Differences in Cognitive Ability by Alcohol Dependence and Conduct Disorder Diagnoses
Table 5 lists the group means for cognitive measures. The 2 × 2 × 2 (AD by CCD by gender) MANOVA revealed significant main effects of AD, F(8,462) = 2.71, p < .01, and CCD, F(8,462) = 4.42, p < .0001, but not gender, F(8,462) = 0.65, p = .74. There were no significant interactions. Overall, AD and CCD were associated with poorer performance on all measures of cognitive ability. A MANOVA examining the main effect of CD within the AD group revealed a significant main effect of CD, F(11,276) = 2.28, p < .05. Overall, the ADCCD group had lower levels of cognitive ability than the AD-alone group. Univariate ANOVAs (Table 5) indicate that the ADCCD group had significantly lower scores on the Shipley IQ, digits forward, digits backward, letter-number sequencing, and the AC test measures, Fs(1,283) = 9.49, 4.60, 5.78, 8.78 and 7.71, ps < .05.
Table 5.
Mean Scores for Cognitive Variables by Group
| Measure | noAD/noCCD | CCD-alone | AD-alone | ADCCD | Cohen’s d (AD-alone vs. ADCCD) |
|
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |||
| Shipley IQ | 108.4 (8.1) | 104.9 (10.4) | 105.2 (7.7) | 101.6 (12.3)⊕ | .35 | |
| Matrix Reasoning | 20.6 (3.3) | 19.82 (3.3) | 19.3 (3.7) | 18.4 (4.2) | .23 | |
| Conditional Associative Learning* | 9.06 (2.7) | 11.20 (3.8) | 10.40 (3.4) | 11.1 (3.7) | −.20 | |
| Digits Forward | 9.57 (2.1) | 9.49 (2.2) | 9.69 (1.9) | 9.17 (2.1)⊕ | .26 | |
| Digits Backward | 8.96 (2.8) | 8.33 (2.5) | 8.57 (2.2) | 7.89 (2.5)⊕ | .29 | |
| Letter/Number Sequencing | 12.7 (2.6) | 12.0 (3.0) | 12.16 (2.7) | 11.3 (2.5)⊕ | .33 | |
| Auditory Consonant | 32.73 (9.5) | 26.70 (12.5) | 28.16 (11.1) | 24.4 (12.0)⊕ | .33 | |
| Operation Word Span | 44.60 (10.8) | 41.45 (12.8) | 39.85 (11.9) | 38.2 (12.8) | .13 | |
Note. CCD = a history of childhood conduct disorder; AD = a history of alcohol dependence CCD-alone = CCD without AD; AD-alone = AD without CCD.
Lower scores on the Conditional Associative Learning Task reflect better performance (i.e., fewer errors).
denotes a significantly lower mean score than the AD-alone group (p < .05).
A Dimensional Model of Externalizing Problems and Cognitive Ability
Pearson correlations among the variables included in the SEMs (Table 6) illustrate strong relationships among the different indicators of the externalizing problems factor and between the measures of lifetime problems and cognitive ability (ps < .05). Years of education also correlates significantly (ps < .001) with all cognitive indicator variables. The initial SEM default model (i.e., with no direct paths from the lifetime problem indicators to the cognitive latent and observed variables) fit the data well, χ2(54, N = 477) = 95.53, p < .001, NFI = .971, RMSEA = 0.040. All four standardized regression weights between EXT and the cognitive ability variables (Figure 2(a)) are statistically significant (ps < .001). The model shows that EXT predicts lower executive working memory capacity, lower short-term memory capacity, lower intelligence, and more conditional associative learning errors. The lowest rescaled BIC0 coefficient was obtained for a model that included two additional structural paths; one direct path from alcohol problems to CAT and another from drug problems to executive working memory. These paths presumably reflect an association between executive working memory capacity and variance in lifetime alcohol and drug problems that is not shared with EXT. With these paths included in the SEM, the resulting data-model fit statistics are χ2(52, N = 477) = 81.83, p < .005, NFI = .975, RMSEA = 0.035. Although this model improved on the default model in terms of difference chi-square criterion, the specification search resulted in weak evidence to rule out the default model (BIC0 = 0.000 versus BIC0 = 1.372, respectively). Thus, the most conservative and parsimonious model would not include the direct paths from alcohol problems to CAT or from drug problems to executive WM. Figure 2(b) shows the minimum BIC0 structural model when years of education (yrsEd) is covaried with EXT and model specification search is performed with optional regression paths from yrsEd to each cognitive ability variable. Although there is a strong negative association between EXT and yrsEd, the derived fit statistics for this model are significantly poorer than for the previous model, χ2(64, N = 477) = 147.08, p < .001, NFI = .958, RMSEA = 0.052. Additionally, the BIC0 criterion favors a model in which only two regression paths (i.e., yrsEd → EWCM and yrsEd → Intelligence) are retained even though all four are statistically significant (ps < .001). Most important, all regression weights from EXT to cognitive ability remain statistically significant.
Table 6.
Bivariate (Pearson product-moment) Correlations among Indicator Measures for EXT, Cognitive Variables, and Recent Substance Use Measures
|
EXT indicator measures |
Cognitive variables |
Recent substance-use indicator measures |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| yrsEd | −.26 | −.29 | −.38 | −.41 | −.41 | .25 | .33 | .16 | .16 | .23 | .39 | .25 | −.23 | −.12 | .04 | −.18 | −.15 | −.23 | −.11 |
| 1. alc | - | .65 | .59 | .66 | .69 | −.22 | −.26 | −.08 | −.17 | −.18 | −.27 | −.24 | .21 | .43 | .41 | .38 | .17 | .29 | .20 |
| 2. mar | - | .68 | .58 | .59 | −.20 | −.19 | −.04 | −.12 | −.18 | −.19 | −.17 | .23 | .30 | .23 | .52 | .24 | .32 | .30 | |
| 3. drg | - | .56 | .59 | −.29 | −.29 | −.11 | −.17 | −.22 | −.30 | −.23 | .30 | .19 | .12 | .42 | .33 | .43 | .31 | ||
| 4. ccd | - | .87 | −.24 | −.32 | −.09 | −.15 | −.23 | −.32 | −.23 | .28 | .28 | .23 | .39 | .10 | .26 | .21 | |||
| 5. aab | - | −.26 | −.30 | −.11 | −.18 | −.25 | −.30 | −.24 | .29 | .30 | .27 | .39 | .14 | .30 | .20 | ||||
| 6. ow | - | .66 | .43 | .42 | .50 | .56 | .46 | −.43 | −.13 | −.07 | −.20 | −.08 | −.18 | −.11 | |||||
| 7. ac | - | .38 | .45 | .47 | .54 | .49 | −.47 | −.10 | −.40 | −.18 | −.11 | −.19 | −.11 | ||||||
| 8. df | - | .58 | .55 | .36 | .22 | −.23 | −.04 | −.01 | −.04 | −.07 | −.10 | −.08 | |||||||
| 9. db | - | .58 | .33 | .35 | −.31 | −.11 | −.09 | −.06 | −.11 | −.07 | −.06 | ||||||||
| 10. ln | - | .41 | .37 | −.36 | −.08 | −.03 | −.12 | −.06 | −.12 | −.10 | |||||||||
| 11. iq | - | .58 | −.38 | −.15 | .01 | −.15 | −.04 | −.16 | −.03 | ||||||||||
| 12. mx | −.41 | −.08 | −.03 | −.09 | −.04 | −.15 | −.05 | ||||||||||||
| 13. cat | - | .07 | .01 | .23 | .12 | .15 | .11 | ||||||||||||
| 14. aq | - | .64 | .27 | .12 | .06 | .14 | |||||||||||||
| 15. af | - | .29 | .22 | .08 | .16 | ||||||||||||||
| 16. mj | - | .27 | .33 | .36 | |||||||||||||||
| 17. op | - | .33 | .35 | ||||||||||||||||
| 18. sd | - | .41 | |||||||||||||||||
| 19. stm | - | ||||||||||||||||||
Note. yrsEd = self-reported years of education completed. EXT indicator measures (1–5) were lifetime problem counts (Blom-transformed) for alc = alcohol, mar = marijuana, drg = drugs other than marijuana, ccd = childhood conduct disorder, and aab = adult antisocial behavior. Cognitive measures (6–13) were ow = operation word span, ac = auditory consonant, df = digits forward, db = digits backward, ln = letter number sequencing, iq = Shipley IQ estimate, mx = matrix reasoning, and cat = conditioned association task. Recent substance-use measures (14–19) were aq = alcohol quantity, af =alcohol frequency, mj = marijuana use frequency, op = opiate use frequency, sd = sedative use frequency, and stm = stimulant use frequency. Critical values of the correlation coefficient for a two-tailed test of the null hypothesis Ho: ρ = 0 with df=475 are: ± .09 (p<.05), ± .12 (p<.01), and ± .15 (p<.001).
Externalizing Problems and Cognitive Ability in Alcoholics
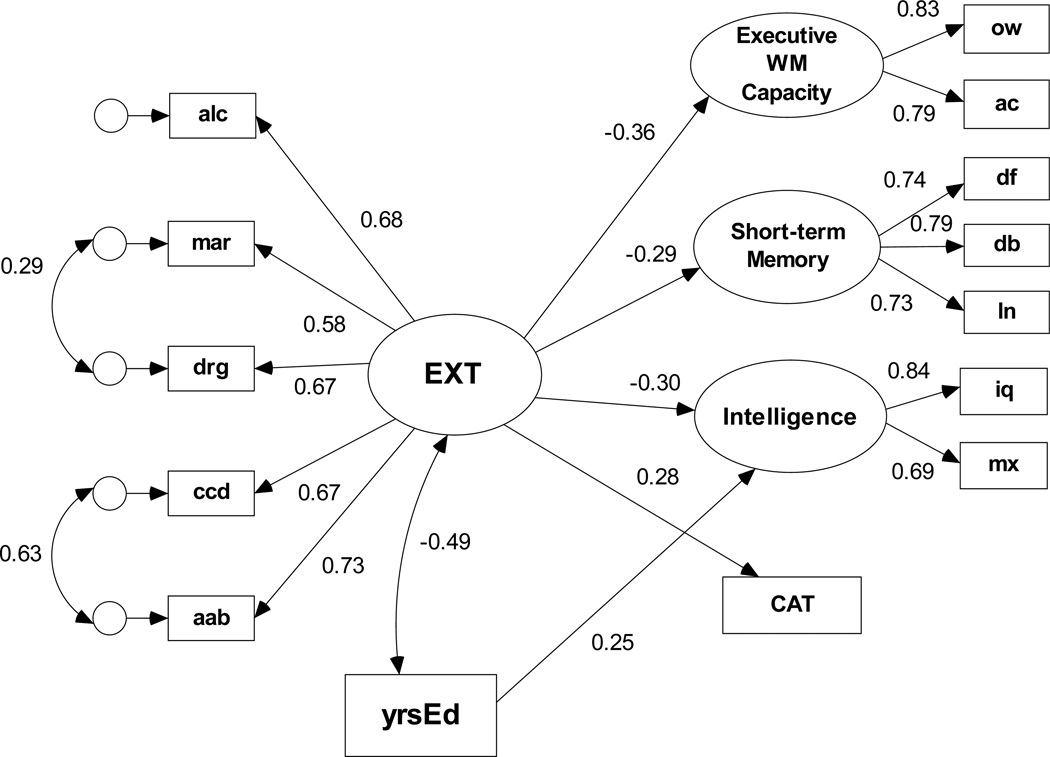
As stated above, the second SEM analyses used data for only those meeting DSM-IV criteria for alcohol dependence to evaluate the association between the covariation in lifetime externalizing problems and cognitive ability in those with alcohol dependence. Similar to the model estimated with the full sample, the default structural model for the AD subsample fit the data well, χ2(54, N = 285) = 67.37, p = 0.105, NFI = .959, RMSEA = 0.030 and had the lowest rescaled BIC0 coefficient. As in the full sample, including yrsEd as a covariate of EXT and as a predictor of the cognitive ability variables resulted in a poorer fit, χ2(65, N = 285) = 106.35, p < .001, NFI = .939, RMSEA = 0.047. Figure 3 displays the standardized regression weights for the BIC0=0.000 model. This model shows that higher scores on the EXT factor, which reflects greater number of lifetime problems with AD and co-occurring externalizing problems in those with alcohol dependence, are significantly (ps < .05) associated with lower executive working memory capacity, lower short-term memory capacity, lower intelligence, and more conditional associative learning errors. Additionally, there is a strong negative association between EXT and yrsEd; only a single regression path from yrsEd to Intelligence is retained in this model.
Figure 3.
Structural equation model of the predictive paths between the latent externalizing disorders factor and each cognitive ability variable for those meeting diagnostic criteria for alcohol dependence (n=285).
Recent Substance Use, Externalizing Problems, and Cognitive Ability
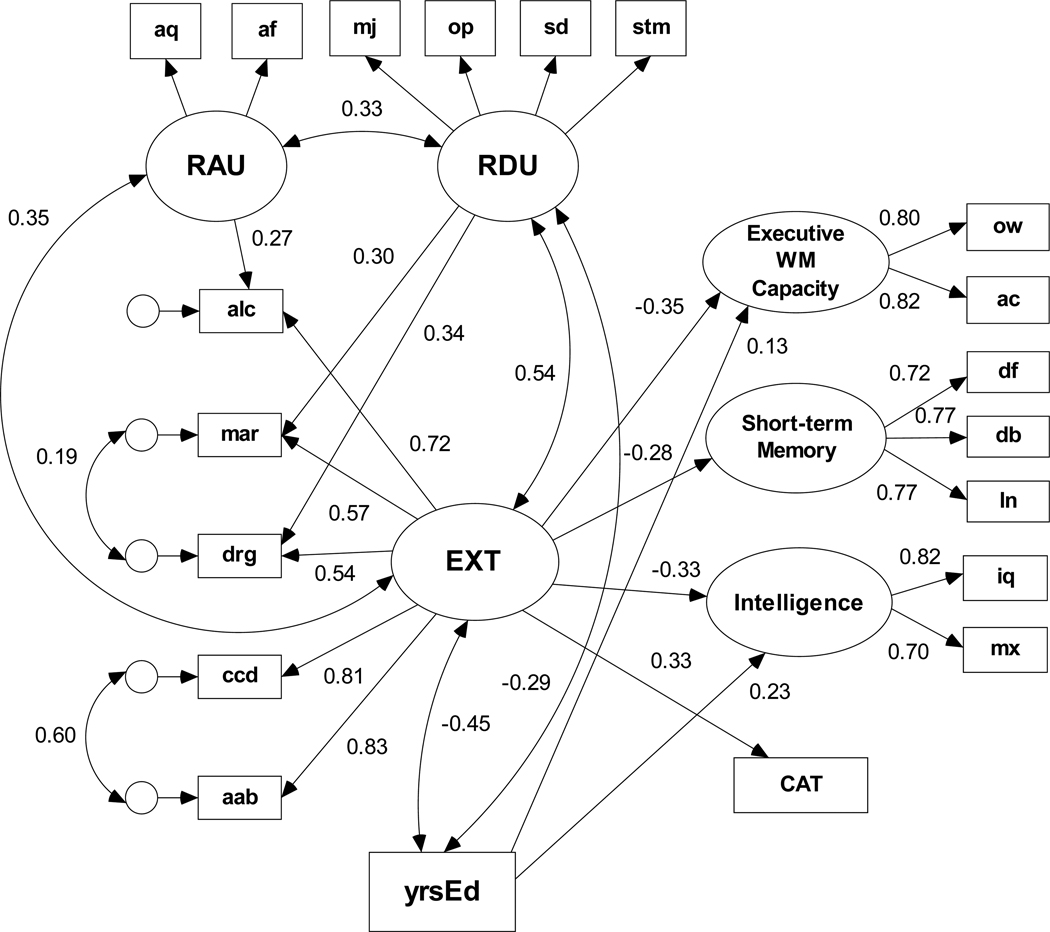
The structural model depicted in Figure 4 includes latent variables for alcohol use and drug use. The initial set of specification search candidate models included (1) covariance paths among EXT, recent alcohol use (RAU), and recent drug use (RDU) latent factors, (2) a direct path from RAU to the alcohol lifetime problem indicator, and (3) direct paths from RDU to both marijuana and other drug lifetime problem indicators. The direct paths to problem indicators of EXT represent the association between recent use and aspects of lifetime drug problems that are not shared with the other types externalizing problems. There were no direct paths from the lifetime indicators to the cognitive variables. The default model fit the data adequately, χ2(135, N = 477) = 294.61, p < .001, NFI = .932, RMSEA = 0.050 with only weak evidence to reject a model with a path from recent drug use to Intelligence (BIC0 = 0.927) or a model with a path from recent drug use to Executive WM capacity (BIC0 = 1.098). We then incorporated years of education as a covariate of EXT, RAU, and RDU and as a predictor variable with an optional path to each cognitive ability variable. Figure 4 displays the structural model that yielded a BIC0 = 0.000; all standardized regression weights (β) shown in Figure 4 were statistically significant (ps < .05). The model fit statistics are χ2(150, N = 477) = 349.82, p < .001, NFI = .923, RMSEA = 0.053. There is a significant negative association between yrsEd and both EXT and RDU but not RAU; yrsEd predicts both greater executive WM capacity and higher intelligence. As in previous models, the EXT factor predicts lower executive working memory capacity, lower intelligence, and more conditional associative learning errors even when years of education is included as a covariate.
Figure 4.
Structural equation model based on the full (N=477) sample of the predictive paths between the latent externalizing disorders factor, recent alcohol and other drug use, and each cognitive ability variable. Path coefficients are standardized β weights. Only significant paths (ps < .05) are illustrated. Years of education (yrsEd) covaries negatively with EXT and RDU and predicts both increased executive WM capacity and higher intelligence.
Discussion
The fundamental question addressed in this study is whether reduced cognitive ability in the domains of working memory capacity, short-term memory capacity, intelligence, and conditional associative learning can be predicted by the covariance among AD and related externalizing problems (assessed as a single continuous latent factor) rather than by AD, or any one type of externalizing problem. Our results suggest that the reduced cognitive ability typically observed in AD individuals is associated with the covariation among lifetime problems with AD and related externalizing problems and not any specific disorder. Furthermore, the results indicate that externalizing problems were significantly associated with reduced ability in all cognitive domains independent of years of education. This pattern of association between externalizing problems and reduced cognitive ability also was observed in the AD subsample, i.e., covariation in externalizing problems in AD, not AD problems per se, is associated with lower cognitive ability. Consistent with this result, individuals with both AD and CCD had reduced cognitive ability compared to AD-alone individuals. Recent substance use did not appear to account for this association.
Externalizing Problems Factor and Reduced Cognitive Ability
Consistent with the work of Krueger and colleagues (2002; 2005), the dimensional analyses suggest that the co-occurrence of externalizing problems is best conceptualized as a continuous latent dimension of externalizing problems, possibly quantified in terms of the number of lifetime problems with AD and co-occurring externalizing problems. Adopting a dimensional approach to assessing the relationship between the covariation in externalizing problems and cognitive ability in AD may be superior to a categorical approach because it more fully accounts for both heterogeneity and variation in co-occurring externalizing problems. The results also suggest that, when examining cognitive ability in those meeting diagnostic criteria for AD, sub-diagnostic or diagnostic covarying externalizing problems are important and should be assessed. In general, this dimensional approach allows for, and captures, variations in severity of symptomatology, and more accurately depicts the underlying continuous nature of the relations among symptoms and other characteristics of interest, such as cognitive ability.
More specifically, our SEM results showed that a dimensional model of externalizing problems representing covariation among severity of lifetime problems with alcohol, marijuana, other drugs, childhood conduct disorder, and adult antisocial behavior fit the data well. The initial model indicated that the latent externalizing problems factor was associated with reduced cognitive ability. This model fit the data well for both the full sample and the AD subsample. Inclusion of latent factors for recent alcohol and drug use did not account for the association between the externalizing problems factor or reduced cognitive ability. Although there was weak evidence for unique paths from recent alcohol use to conditional association and from recent drug use to executive working memory, we question the statistical reliability of these paths. We believe the most parsimonious and conservative explanation of these associations is captured by a model with no direct (i.e., unique) paths based on contradiction between model selection BIC criterion and model fit statistics.
The structural model demonstrated that cognitive ability in those with AD varies as a function of the severity of lifetime externalizing problems. In a pattern similar to the analyses with the full sample, the externalizing problems latent factor was associated with reduced capacity on measures of executive working memory, short-term memory, intelligence, and conditional associative learning in those with a diagnosis of AD. These results are consistent with studies that suggest that childhood conduct problems and other drug use / abuse (Bates et al., 2002) are associated with greater severity of cognitive impairments in alcoholics (Bates et al., 2002; Sinah, Parsons, & Glenn, 1989; Tapert, Baratta, Abrantes, & Brown, 2002).
The cognitive abilities that co-varied with lifetime problems in our sample of AD participants may be relevant for successful recovery from AD and the development of adaptive self-regulatory strategies to maintain recovery. Research suggests that comorbid externalizing and internalizing problems are under-diagnosed in those with AD and are inadequately treated (Kanzler & Rosenthal, 2003). Reduced cognitive ability in substance-using populations may constrain the potential effectiveness of cognitive-based clinical interventions. In addition, studies suggest that treatment strategies that target comorbid conditions result in greater success when treating AD (Fals-Stewart & Schafer, 1992; Kanzler & Rosenthal, 2003; Smith, 2007). Our research suggests that, to the extent that successful treatment outcomes area tied to cognitive ability, intervention strategies would benefit from assessing and accounting for the full suite of externalizing problems, not only a specific diagnosis of interest.
Externalizing Problems, Years of Education, and Reduced Cognitive Ability
In each of the SEM analyses, the externalizing disorders factor was significantly associated with reduced ability when accounting for the influence of years of education which was significantly associated with all cognitive domains, but most strongly with executive working memory capacity and intelligence. Although the associations between externalizing problems and reduced cognitive ability were somewhat weaker when years of education was included in the model, the externalizing problems factor remained significantly associated with each domain of cognitive ability. Consistent with the work of Lynam et al. (1993), these results suggest that the association between externalizing problems and reduced cognitive ability is robust and not simply a byproduct of fewer years of education.
Childhood Conduct Disorder, Alcohol Dependence, and Reduced Cognitive Ability
Consistent with previous reports of greater self-regulatory problems in those with AD and a history of antisocial psychopathology (Finn et al., 2002; Mazas, Finn, & Steinmetz, 2000; Petry, 2002), the categorical analysis showed that the ADCCD group had lower average scores on a majority of measures of cognitive ability compared to the AD-alone group. As expected, both the AD-alone and the CCD-alone groups had lower cognitive ability relative to the noAD/noCCD group. An important limitation of the categorical approach is that other drug dependence and adult antisocial behavior problems co-varied with the presence of AD and CCD, and the number of AD or CCD symptoms differed across groups. For instance, the ADCCD group differed from the AD-alone group, both in terms of the presence of a diagnosis of CCD and in the number of lifetime problems with AD, marijuana abuse / dependence, other drug abuse dependence, and adult antisocial behavior compared with the AD-alone group. These results are consistent with the dimensional approach but provide a useful alternative to capturing variations in the number of AD and other comorbid externalizing problems within these AD groups.
Limitations, Implications, and Future Directions
The results reported here should be interpreted in light of the limitations of the cross-sectional design of the current study. First, all structural paths in our SEMs from the latent externalizing problems factor to the domains of cognitive ability were implemented as unidirectional rather than bidirectional paths. The models are intended only to illustrate associations among different measures of executive cognitive ability, lifetime disinhibitory disorders, and recent substance use. Note, however, that the same magnitude, sign, and pattern of the structural weights would be produced if covariance relationships were substituted for the regression paths in these models.
Second, it is important to note these results do not rule out the possibility that recent substance use affects cognitive ability. Research clearly indicates recent substance use does affect brain and neuro-cognitive function (Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007; Monti et al., 2005; Shafer et al., 1999). Because most of the alcohol dependent individuals assessed in this study are still actively abusing alcohol and some actively abusing illicit drugs, there is only weak evidence in our sample that reduced cognitive ability is due to the residual effects of recent or long-term substance use. Although the dimensional analyses suggest that lifetime problems are more strongly associated with reduced cognitive ability than current substance use, recent alcohol and drug use were consistently, although modestly, associated with reduced performance on all measures. The nature of the indicator measures of substance use and the cross-sectional design of this study make it difficult to adequately address specific details of the association between substance use and cognitive ability.
Third, although using a community-recruited sample has potential advantages over studying alcohol dependent individuals in treatment, the sample cannot be deemed representative of alcohol dependent individuals in general, whether recruited from treatment facilities or the community, due to self-selection biases and the recruitment criteria. Furthermore, the dimensional model of externalizing problems in the full sample assumes that this factor characterizes the covariation among the indicators in the entire population. Our sample was biased by our focus on recruiting large numbers of individuals with AD and some with CCD without AD. Thus, the sample is weighted toward externalizing problems and not representative of the population. Offsetting this concern to some degree are the similarities between our data and those of Krueger and colleagues (2002; 2005) in the magnitude of the regression coefficients for each indicator on the externalizing factor. Nonetheless, caution must be exercised in generalizing the results of the full sample analysis to the population at large. On the other hand, the model of externalizing problems within those with AD probably is a better reflection of the covariation among externalizing problems in those with a diagnosis of AD, because the lifetime comorbidity rates of other substance abuse, CCD, and antisocial personality mirrors population-based estimates (Grant et al., 2004; Regier et al., 1990).
A final limitation of the current study is that we did not assess other forms of psychopathology with known associations with AD, including ADHD and internalizing disorders (Driessen et al., 1998; Flory, Milich, Lynam, Leukefeld, & Clayton, 2003). For instance, reductions in cognitive ability have been associated with ADHD (Stevens, Quittner, Zuckerman, & Moore, 2002) and major depression (Paelecke-Habermann, Pohl, & Leplow, 2005). Future research should assess these domains when attempting to characterize the association between the covariation among externalizing disorders and cognitive ability.
Summary
The current study makes three important contributions to the literature on alcohol dependence, externalizing problems, and cognitive ability. First, to our knowledge, this is the first large-scale study of the covariation between cognitive ability and lifetime externalizing problems in a sample of young adults with early-onset AD. Second, the results show that the covariation in lifetime externalizing problems is associated with reduced capacity in the cognitive domains of working memory, short-term memory, conditional associative learning, and intelligence, and the association does not appear to be specific to any one type of externalizing problem assessed in the study. In addition, the results suggest that the association between externalizing problems and reduced cognitive ability is generally independent of years of education. These domains of cognitive ability are thought to be critically associated with self-regulation (Barkley, 2001; Finn, 2002; Kimberg & Farah, 1993), and the observed cognitive ability reductions in these domains likely reflect the self-regulatory, disinhibitory problems experienced by those with AD, CCD, and related disorders. Third, within those with AD diagnoses, higher scores on the latent externalizing problems factor were associated with reduced capacity in all domains of cognitive ability assessed in this study.
Acknowledgments
This research was supported by National Institutes of Alcohol Abuse and Alcoholism grant R01 AA13650 to Peter. R. Finn. We thank Alissa Ellis for her contribution to this research.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Aytaclar A, Tarter RE, Kirisci L, Lu S. Association between hyperactivity and executive cognitive function functioning in childhood and substance use in early adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:172–178. doi: 10.1097/00004583-199902000-00016. [DOI] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of alcoholics I: Evidence for an empirically derived typology based on indicators of vulnerability and severity. Archives of General Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Logie R. Working memory: The multicomponent model. In: Mijake A, Shah P, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge, MA: Cambridge University Press; 1999. pp. 28–62. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory or ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. The executive functions and self-regulation: An evolutionary neuropsychological perspective. Neuropsychological Review. 2001;11:1–29. doi: 10.1023/a:1009085417776. [DOI] [PubMed] [Google Scholar]
- Bates ME, Labouvie EW, Voelbel GT. Individual differences in latent neuropsychological abilities at addictions treatment entry. Psychology of Addictive Behaviors. 2002;16:35–46. doi: 10.1037//0893-164x.16.1.35. [DOI] [PubMed] [Google Scholar]
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short-term neuropsychological recovery in clients with substance abuse disorders. Alcoholism: Clinical and Experimental Research. 2005;29:367–377. doi: 10.1097/01.alc.0000156131.88125.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. EEG, autonomic and subjective correlates of the risk for alcoholism. Journal of Studies on Alcohol. 1993;54:577–589. doi: 10.15288/jsa.1993.54.577. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:588–606. [Google Scholar]
- Brown J. Some tests of the decay of immediate memory. Quarterly Journal of Experimental Psychology. 1958;10:12–21. [Google Scholar]
- Browne MW, Cudek R. Alternative ways of assessing model fit. In: Bollen KA, K, Long JS, editors. Testing structural equation models. Newbury park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Bucholz K, Cadoret R, Cloninger CR, Dinwiddie S, Hasselbrock V, Nurnberger J, Reich T, Schmit I, Schuckit M. A new semistructured psychiatric interview for use in genetic linkage studies: A report of the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Engle RW. Working memory and retrieval: A resource-dependent inhibition model. Journal of Experimental Psychology: General. 1994;123:354–373. doi: 10.1037//0096-3445.123.4.354. [DOI] [PubMed] [Google Scholar]
- Cowan N. An embedded-process model of working memory. In: Mijake A, Shah P, editors. Models of Working Memory: Mechanisms of Active Maintenance and Executive Control. Cambridge, MA: Cambridge University Press; 1999. pp. 62–101. [Google Scholar]
- Driessen M, Veltrup C, Wetterling T, Ulrich J, Dilling H. Axis I and axis II comorbidity in alcohol dependence and the two types of alcoholism. Alcoholism: Clinical and Experimental Research. 1998;22:77–86. [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Finn PR. Motivation, working memory, and decision making: A cognitive-motivational theory of personality vulnerability to alcoholism. Behavioral and Cognitive Neuroscience Review. 2002;1:183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Finn PR, Bobova L, Wehner E, Fargo S, Rickert ME. Alcohol expectancies, conduct disorder, and early-onset alcoholism: Negative alcohol expectancies are associated with less drinking in non-impulsive versus impulsive subjects. Addiction. 2005;100:953–960. doi: 10.1111/j.1360-0443.2005.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Hall J. Cognitive ability and risk for alcoholism: short-term memory capacity and intelligence moderate personality risk for alcohol problems. Journal Abnormal Psychology. 2004;113:569–581. doi: 10.1037/0021-843X.113.4.569. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas C, Justus A, Steinmetz JE. Early-onset alcoholism with conduct disorder: Go/No-Go learning deficits, working memory capacity, and personality. Alcoholism: Clinical and Experimental Research. 2002;26:186–206. [PubMed] [Google Scholar]
- Flory K, Milich R, Lynam DR, Leukefeld C, Clayton R. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: Individuals with symptoms of attention-deficit / hyperactivity disorder and conduct disorder are uniquely at risk. Psychology of Addictive Behaviors. 2003;17:151–158. doi: 10.1037/0893-164x.17.2.151. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are mostly genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR. Evidence for dorsolateral and orbital prefrontal cortical involvement in the expression of aggressive behavior. Aggressive Behavior. 1995;21:431–450. [Google Scholar]
- Giancola PR, Zeichner A, Yarnell JE, Dickenson KE. Relation between executive functioning and the adverse consequence of alcohol use in social drinkers. Alcoholism: Clinical and Experimental Research. 1996;20:1094–1098. doi: 10.1111/j.1530-0277.1996.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Gillen R, Hesselbrock V. Cognitive functioning, ASP, and family history of alcoholism in young men at risk for alcoholism. Alcoholism: Clinical and Experimental Research. 1992;16:206–214. doi: 10.1111/j.1530-0277.1992.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:361–368. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- Harden PW, Pihl RO. Cognitive function, cardiovascular reactivity, and behavior in boys at high risk for alcoholism. Journal of Abnormal Psychology. 1995;104:94–103. doi: 10.1037//0021-843x.104.1.94. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. Journal of Experimental Psychology: Learning Memory and Cognition. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Poole BJ, Tuholski SW, Engle RW. Working memory capacity and top-down control of visual search. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:749–777. doi: 10.1037/0278-7393.32.4.749. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: The role of working memory in complex, organized behavior. Journal of Experimental Psychology: General. 1993;122:411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Foster CL, Saunders WB, Stang PE. Social consequences of psychiatric disorders, I: Educational attainment. American Journal of Psychiatry. 1995;152:1026–1032. doi: 10.1176/ajp.152.7.1026. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick PJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Nichol PE, Hicks BM, Markon KE, Patrick PJ, Iacono WG, McGue M. Using latent trait modeling to conceptualize an alcohol problems continuum. Psychological Assessment. 2004;16:107–119. doi: 10.1037/1040-3590.16.2.107. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick PJ, Iacono WG. Externalizing psychopathology in adulthood: A developmental-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psychology. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau MA, Pihl RO, Peterson JB. Provocation, acute alcohol intoxication, cognitive performance, and aggression. Journal of Abnormal Psychology. 1995;104:150–155. doi: 10.1037//0021-843x.104.1.150. [DOI] [PubMed] [Google Scholar]
- Lynam D, Moffitt T, Stouthamer-Loeber M. Explaining the relation between IQ and delinquency: Class, race, test motivation, school failure. Journal of Abnormal Psychology. 1993;102:187–196. doi: 10.1037//0021-843x.102.2.187. [DOI] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24(7):1036–1040. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP, Druley KA. Predicting response to alcohol and drug abuse treatments: Role of psychiatric severity. Archives of General Psychiatry. 1983;40:620–625. doi: 10.1001/archpsyc.1983.04390010030004. [DOI] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Monti PM, Miranda R, Nixon K, Sher KJ, Swartzwelder S, Tapert SF, White A, Crews FT. Adolescence: Booze, Brains, and Behavior. Alcoholism: Clinical and Experimental Research. 2005;29:207–220. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- Oberauer K. Access to information in working memory: Exploring the focus of attention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:411–421. [PubMed] [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. Journal of Affective Disorders. 2005;89:125–135. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Patterson GR, Capaldi D, Bank L. An early starter model for predicting delinquency. In: Pepler DJ, Rubin KH, editors. The Development and Treatment of Childhood Aggression. New York, NY: Plenum Press; 1991. pp. 139–168. [Google Scholar]
- Petrides M. Deficits on conditional associative-learning tasks after frontal- and temporal- lobe lesions in men. Neuropsychology. 1985;20:601–614. doi: 10.1016/0028-3932(85)90062-4. [DOI] [PubMed] [Google Scholar]
- Petry NM. Discounting of delayed rewards in substance abusers: Relationship to antisocial personality disorder. Psychopharmacology. 2002;162:425–432. doi: 10.1007/s00213-002-1115-1. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Peterson JB, Finn PR. Inherited predisposition to alcoholism: Characteristics of sons of male alcoholics. Journal of Abnormal Psychology. 1990;99:291–301. doi: 10.1037//0021-843x.99.3.291. [DOI] [PubMed] [Google Scholar]
- Poon E, Ellis DA, Fitzgerald HE, Zucker RA. Intellectual, cognitive, and academic performance among sons of alcoholics during the early school years: Differences related to subtypes of familial alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1020–1027. [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler abbreviated scale of intelligence. San Antonio, TX: Harcourt Brace; 1999. [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the epidemiological catchment area (ECA) study. Journal of the American Medical Association. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Robins LN, Regier DS, editors. Psychiatric Disorders in America. New York: Free Press; 1991. [Google Scholar]
- Rounsaville BJ, Dolinsky ZS, Babor TF, Meyer RE. Psychopathology as a predictor of treatment outcome in alcoholics. Archives of General Psychiatry. 1987;44:505–513. doi: 10.1001/archpsyc.1987.01800180015002. [DOI] [PubMed] [Google Scholar]
- Seguin J, Pihl R, Harden P, Tremblay R, Boulerice B. Cognitive and neuropsychological characteristics of physically aggressive boys. Journal of Abnormal Psychology. 1995;104:614–624. doi: 10.1037//0021-843x.104.4.614. [DOI] [PubMed] [Google Scholar]
- Sinah R, Parsons OA, Glenn SW. Drinking variables, affective measures, and neuropsychological performance: Familial alcoholism and gender correlates. Alcohol. 1989;6:77–85. doi: 10.1016/0741-8329(89)90077-3. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PAF, Bucholtz KK, Stathamm DJ, Martin NG. Personality and the genetic risk for alcohol dependence. Journal of Abnormal Psychology. 2002;111:124–133. [PubMed] [Google Scholar]
- Speltz ML, DeKlyen M, Calderon R, Greenberg MT, Fisher PA. Neuropsychological characteristics and test behaviors of boys with early onset conduct problems. Journal of Abnormal Psychology. 1999;108:315–325. doi: 10.1037/0021-843X.108.2.315. [DOI] [PubMed] [Google Scholar]
- Stevens J, Quittner AL, Zuckerman JB, Moore S. Behavioral inhibition, self-regulation of motivation, and working memory in children with attention deficit hyperactivity disorder. Developmental Neuropsychology. 2002;21:117–139. doi: 10.1207/S15326942DN2102_1. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Seethem LL, Poirier CA. Comparison of three tests of attention and rapid information processing across six age groups. Clinical Neuropsychology. 1987;1:139–152. [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youths. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four year outcomes. Journal of the International Neuropsychological Society. 1999;5:475–487. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: An examination of simple and complex span and their relation to higher order abilities. Psychological Bulletin. 2007;133:1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. Speed and accuracy of accessing information in working memory: An individual differences investigation of focus switching. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:616–630. doi: 10.1037/0278-7393.34.3.616. [DOI] [PubMed] [Google Scholar]
- van den Oord EJCG, Siminoff E, Eaves LJ, Pickles A, Silberg J, Maes H. An evaluation of different approaches to behavior genetic analyses with psychiatric symptoms scores. Behavior Genetics. 2000;30:1–18. doi: 10.1023/a:1002095608946. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale (Rev.) New York: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Test-III. Psychological Corporation, Harcourt Brace and Co.; 1997. [Google Scholar]
- Widom CS. A method for studying noninstitutionalized psychopaths. Journal of Consulting and Clinical Psychology. 1977;44:614–623. [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised manual. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]
- Zelazo PD, Frye D. Cognitive complexity and control: II. The development of executive function in childhood. Current Directions in Psychological Science. 1998;7:121–126. [Google Scholar]