Abstract
The trabecular meshwork (TM)/Schlemm’s canal (SC) outflow pathway is the tissue responsible for maintaining normal levels of intraocular pressure. In the present study, we investigate the effects of mechanical stress on the expression of IL-6 in the TM meshwork, as well as the effects of this cytokine on outflow pathway function. Application of cyclic mechanical stress to human TM primary cultures resulted in a statistically significant increase in both secretion and transcription of IL-6, compared to nonstressed controls. Addition of TGF-β1, which has been reported to be upregulated in TM cells under mechanical stress, also induced a significant activation of both the transcription and secretion of IL-6. Moreover, anti-TGF-b1 antibodies partially blocked the stretch-induced IL-6 production. Injection of IL-6 into perfused porcine anterior segments resulted in a 30% increase in outflow facility, as well as increased permeability through SC cell monolayers. These results suggest a role for IL-6 in the homeostatic modulation of aqueous humor outflow resistance.
Keywords: Trabecular meshwork, Interleukin-6, Mechanical stress, TGF-β1, glaucoma
The trabecular meshwork (TM)/Schlemm’s canal (SC) outflow pathway system constitutes the main route by which the aqueous humor exits the anterior chamber of the eye [1]. Maintenance of appropriate levels of aqueous humor outflow resistance through this pathway is critical in sustaining normal levels of intraocular pressure (IOP) [2,3]. Although it has been hypothesized that the TM/SC outflow pathway may respond to transient changes in IOP by altering its resistance to aqueous humor [4–6], thereby maintaining normal IOP levels, there is little experimental evidence explaining such potential homeostatic mechanisms.
As a result of cyclic fluctuations of IOP with each heartbeat, the conventional outflow pathway is subjected to continuous cycles of stretch and relaxation that might be involved in tissue homeostasis and, consequently, in IOP regulation [7]. It has been well described that changes in IOP exert dramatic effects on the morphology of the outflow pathway [8–12]. Increased IOP results in distention and stretching of the outflow pathway and its contained cells, while decreased IOP leads to relaxation of the tissue. It has also been demonstrated that mechanical stress can trigger certain responses in TM cells, including cytoskeletal changes, induction of gene expression, and activation of regulatory pathways [13–21]. These cellular mechanisms could potentially provide means for TM cells to “sense” changes in IOP and generate homeostatic responses aimed at restoring normal IOP values.
One of the reported responses to increased IOP in perfused human anterior segments observed by gene array analysis is an increase in interleukin-6 (IL-6) mRNA [16]. IL-6 is well known to increase the permeability of vascular endothelium through effects on the paracellular pathway, induction of matrix metalloproteinases (MMPs), and vesicular trafficking [22–24]. This array of effects mediated by IL-6 suggests that this cytokine could affect aqueous humor outflow resistance in the outflow pathway by increasing the permeability of the inner wall cells of SC [25] and/or reducing extracellular matrix (ECM) resistance at the level of the juxtacanalicular tissue (JCT) [5,26–29].
We hypothesize that production of IL-6 in response to increased mechanical stress associated with elevated IOP could cause a reduction in aqueous humor outflow resistance and thus contribute to the maintenance of normal IOP levels.
In this study, we have investigated the effects of mechanical stress on IL-6 expression in TM cells, as well as the effects of IL-6 on both the permeability of SC cell monolayers and on outflow facility in perfused porcine eye anterior segments.
Materials and methods
Construction of recombinant adenoviruses
For the generation of the replication-deficient recombinant adenoviruses, AdIL6-LacZ and AdIL6-SEAP, a 1165 bp DNA fragment containing the IL-6 gene promoter region was amplified by PCR from 100 ng of human genomic DNA (Clontech, Palo Alto, CA) using the specific primers: IL6P-F 5′-GATCCTCGAGCAAGAGACACCATCCTGA-3′, and IL6P-R 5′-CACTTCGAAGCAGAATGAGCCTCAGACAT-3′, which contain the restriction sites for XhoI and HindIII, respectively. The PCR was performed at 94 °C for 30 s followed by 35 cycles of 94 °C for 15 s, 68 °C for 15 s, and 72 °C for 2 min, using the Advantage-HF PCR kit (Clontech, Palo Alto, CA). The PCR product was purified and cloned into TOPO TA (Invitrogen, Carlsbad, CA) for sequencing. The product with the correct sequence was released by digestion with XhoI and HindIII (New England BioLabs, Beverly, MA) and then introduced into a modified pShuttle (Stratagene, La Jolla, CA) containing either the LacZ gene or the SEAP reporter gene obtained from the commercially available plasmid pSEAP2-Basic (BD Biosciences Clontech, Palo Alto, CA). These two pShuttles containing the IL-6 promoter were used to generate the replicant-deficient recombinant adenoviruses AdIL6-LacZ and AdIL6-SEAP, respectively, using the AdEasy system (Stratagene, La Jolla, CA). High-titer viral stocks were purified with the BD Adeno-X virus purification kit (BD Biosciences Clontech, Palo Alto, CA) and then titered using the BD Adeno-X Rapid Titer kit (BD Biosciences Clontech, Palo Alto, CA).
Perfusion of human and porcine eye anterior segments
Human cadaver eyes (ages 33–74) were obtained from donors with no history of eye disease less than 48 h postmortem and treated consistently with the tenets of the Declaration of Helsinki. Porcine eyes (age 6 months) were obtained from local abattoir and processed less than 3 h postmortem. Organ cultures of human or porcine anterior segments were performed using the method described by Johnson and Tschumper [30]. Briefly, eyes were bisected at the equator, and the lens, iris, and vitreous were removed. The anterior segments were then clamped to a modified petri dish and perfused at a constant flow of 3 μl/min with serum-free high-glucose DMEM supplemented with 110 mg/L sodium pyruvate, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 170 μg/ml gentamicin, and 250 μg/ml amphotericin using a microinfusion pump. Perfused anterior segments were incubated at 37 °C in 5% CO2. Intraocular pressures were continuously monitored with a pressure transducer connected to the dish’s second cannula and recorded with an automated computerized system. Transcorneal injection of IL-6 (500 ng in 50 μl, Sigma–Aldrich, St. Louis, MO) was performed once a stable baseline was reached, using a 27-gauge syringe. Tissue integrity and viability of the cells from the outflow pathway and the corneal endothelium were analyzed at the end of each perfusion experiment in semithin sections of four quadrants of the tissue.
Analysis of AdIL6-LacZ expression in perfused anterior segments
Anterior segments of human eyes were cultured as described above. After 48 h of perfusion, the segments were inoculated with 107 pfu AdIL6-LacZ in 100 μL of perfusion media at 3 μL/min. At day 2 post-infection, anterior segments were fixed by perfusion at 15 mm Hg in 1% paraformaldehyde, 0.2% glutaraldehyde, 0.02% NP40, and 0.01% sodium deoxycholate in PBS, removed from the perfusion system, and stained overnight at 37 °C in 1 mg/ml 5-bromo-4-chloro-3-β-D-galactoside, 5 mM K3Fe(CN), 5 mM K4Fe(CN)6 · 3H2O, and 2 mM MgCl2 in PBS for detection of β-galactosidase activity. After color development, the segments were post-fixed 2 h in 4% paraformaldehyde and then overnight in 30% sucrose. Tissue samples were embedded in OCT compound and frozen over dry ice for 1 h. Sections (8 μm) were cut in a cryostat, mounted onto gelatin-coated slides, and counterstained with neutral fast red (Vector Labs, Burlingame, CA). Six eyes from different donors were analyzed for the expression of the IL-6 promoter.
Cell cultures
Primary cultures of human TM and SC cells were prepared from cadaver eyes (ages 30–60) obtained less than 48 h post-mortem from donors with no history of eye disease, as previously described [31,32], and maintained at 37 °C in 5% CO2 in low glucose Dulbecco’s modified Eagle’s medium (DMEM) with L-glutamine and 110 mg/L sodium pyruvate, supplemented with 10% fetal bovine serum (FBS), 100 μM nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B. All the reagents were obtained from Invitrogen (Carlsbad, CA).
Cyclic mechanical stress application in cell culture
Human TM cells at passage 3 were plated on type I collagen-coated flexible silicone bottom plates (Flexcell, Hillsborough, NC). Once confluence was reached, culture medium was switched to serum-free DMEM, and cells were subjected to cyclic mechanical stress (5% stretching, 1 cycle/s) for the indicated times, using the computer-controlled, vacuum-operated FX-3000 Flexercell Strain Unit (Flexcell, Hillsborough, NC). Control cells were cultured under the same conditions, but no mechanical force was applied. When indicated 1 μg of either nonspecific IgG or anti-human TGF-β1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the culture medium before stress application.
Cell viability
Cell death was assayed by measuring the amount of lactate dehydrogenase (LDH) present in the culture medium as the result of damage to the plasma membrane, using the CytoTox 96 NonRadioactive Cytotoxicity Assay (Promega, Madison, WI).
Measurement of IL-6 concentration
The amount of IL-6 released to the culture medium was assessed with a commercially available sandwich enzyme-linked immunoassay kit (Biosource International, Camarilla, CA), according to the manufacturer’s instructions.
RNA extraction
Total RNA from HTM primary cultures was isolated using RNeasy kit (Qiagen, Valencia, CA), following the manufacturer’s protocol, and then treated with DNase. RNA yields were determined using the RiboGreen fluorescent dye (Molecular Probes, Eugene, OR).
Quantitative real-time PCR
First-strand cDNA was synthesized from total RNA (1 μg) by reverse transcription using oligo(dT) primer and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Real-time PCRs were performed in a 20 μl mixture containing 1 μl of the cDNA preparation, 1× iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), and 500 nm of each primer, in the BIO-RAD iCycler iQ system (Bio-Rad, Hercules, CA) using the following PCR parameters: 95 °C for 5 min, followed by 50 cycles of 95 °C for 15 s, 65 °C for 15 s, and 72 °C for 15 s. The fluorescence threshold value (Ct) was calculated using the iCycle iQ system software. The absence of nonspecific products was confirmed by both the analysis of the melt curves and by electrophoresis in 3% Super AcrylAgarose gels. β-Actin served as an internal standard of mRNA expression. The sequences of the primers used for the amplifications were: IL6-F 5′-CAAATTCGGTACATCCTCGACGGC-3′, IL6-R 5′ GGTTCAGGTTGTTTTCTGCCAGTGC-3′, β-Actin-F 5′-CCTCGCCTTTGCCGATCCG-3′, β-Actin R 5′-GCCGGAGCCGTTGTCGACG-3′.
SEAP reporter gene assay
HTM primary cultures were infected with either 25 or 50 pfu/ml AdIL6-SEAP. Three days after infection, cultures were shifted to serum-free media and treated with TGF-β1 treatment (2 ng/ml, Sigma–Aldrich, St. Louis, MO); activation of IL-6 promoter was quantified by determining the amount of the secreted alkaline phosphatase protein (SEAP) released to the culture medium using the Great EscAPe SEAP chemiluminescence detection kit (BD Biosciences Clontech, Palo Alto, CA), according to the manufacturer’s protocol.
Permeability assays in SC cell monolayers
Effects of IL-6 on SC cell monolayer permeability were measured using the method described by Langeler and van Hinsbergh [33], and modified by Lampugnani et al. [34]. Briefly, SC primary cultures at passage 3 were grown in Transwell cell culture chambers (0.4 μm pore size, polyester filters, from Corning Life Sciences, Acton, MA) for at least 2 weeks. Before the experiment, cells were washed, and culture media of both the upper and lower compartments were replaced by fresh serum-free medium. Type VI-A horseradish peroxidase (HRP, 0.126 μM, Sigma–Aldrich, St. Louis, MO) was added to the upper compartment. In order to measure baseline permeability levels for each monolayer, an aliquot of medium from the lower compartment was collected after 15 min of incubation at 37 °C. IL-6 (100 ng/ml, Sigma–Aldrich, St. Louis, MO) was then added to the upper compartment of the experimental wells. An identical volume of buffer without IL-6 was added to the control wells. Aliquots of medium from the lower compartment were collected at the indicated time intervals and kept on ice until enzymatic determination. HRP activity was assayed using the 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) liquid substrate system (Sigma–Aldrich, St. Louis, MO), according to the manufacturer’s instructions. Percentage of increase in permeability in the experimental wells treated with IL-6 compared to the nontreated controls was calculated at different times.
Results
Expression of IL-6 in the anterior segments of human eyes
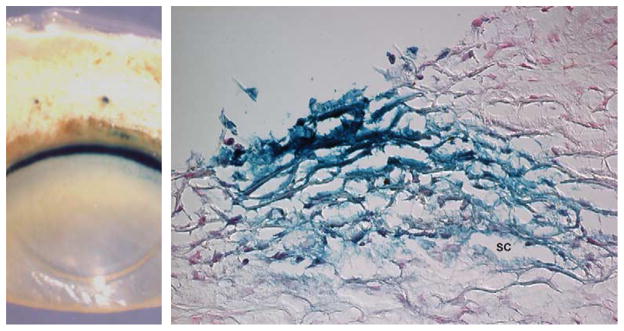
To first confirm the expression of IL-6 in the outflow pathway, perfused human anterior segments were transduced with the recombinant adenovirus AdIL6-LacZ, in which the expression of the reporter gene LacZ is driven by the human IL-6 promoter. Macroscopic analysis of stained anterior segments showed restricted expression of LacZ to the outflow pathway region as well as the blood vessels of the sclera (Fig. 1, left). Histological sections demonstrated strong β-galactosidase activity in the cells of the TM, including those from the uveal meshwork, corneoscleral meshwork, and juxtacanalicular tissue (JCT). Positive staining was also found in the endothelial cells from both the inner and outer walls of the SC (Fig. 1, right).
Fig. 1.
Expression analysis of the IL-6 promoter. Perfused anterior segments from human eyes were transduced with 107 pfu AdIL6-LacZ and analyzed for β-galactosidase staining 48 h.p.i. (Left) Macroscopic view of a stained anterior segment showing restricted expression in the outflow pathway and in blood vessels from the sclera. (Right) Cryosection from the same eye showing positive β-galactosidase staining in the cells of the TM and SC. Similar results were obtained in six independent experiments.
Increased expression of IL-6 in cyclically stressed HTM cells
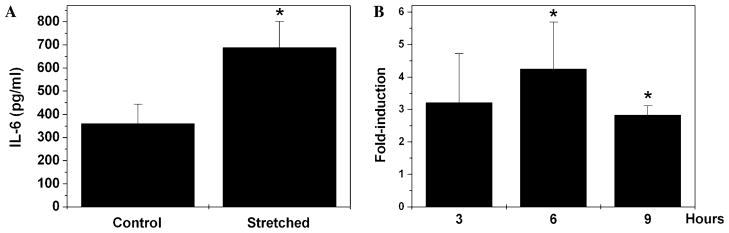
To analyze whether mechanical stress could modulate the expression of IL-6, we subjected three different sets of HTM cell primary cultures to cyclic mechanical stress (5% elongation, 1 cycle/s) for 12 h. Quantification of IL-6 concentration in the culture media demonstrated a significant increase (92.3 ± 10.4%) in secreted IL-6 in the cyclically stretched cultures, compared to the nonstretched controls (Fig. 2A). Analysis of cell viability by enzyme leakage showed viability values higher than 95%, similar to those found in the nonstretched cultures (data not shown). These data strongly indicate that the regime of cyclic mechanical stress applied in our experiments did not compromise cell viability, and therefore that the increased amount of IL-6 in the culture medium of stretched cultures did not result from cell lysis.
Fig. 2.
Effect of cyclic mechanical stretch on IL-6 production. Three different HTM cell cultures were subjected to cyclic mechanical strain (5% elongation, 1 cycle/s). (A) Concentration of IL-6 released to the culture medium was assayed by ELISA after 12 h of stretching. Data represent mean values ± SD. *Significantly different from control (t test, p < 0.0003, n = 3). (B) Induction of IL-6 transcription was analyzed at 3, 6, and 9 h by real-time PCR. β-Actin was used as an internal standard of mRNA expression. Data represent mean values ± SD of the fold-expression differences obtained from the Ct value. *Significantly different from control (t test, p < 0.05, n = 3).
To determine whether the observed increased secretion of IL-6 in the stretched cultures was dependent on the activation of IL-6 gene transcription or on the release of intracellular cytokine, we examined the expression levels of IL-6 mRNA after 3, 6, and 9 h of mechanical stress. As shown in Fig. 2B, cyclic mechanical stress significantly induced the activation of IL-6 transcription, reaching a maximum fold-induction 6 h after stress, followed by a gradual decrease. These results suggested that stretch-induced IL-6 secretion was primarily dependent on the activation of the IL-6 promoter.
Involvement of TGF-β1 in the stretch-induced expression of IL-6 in HTM cells
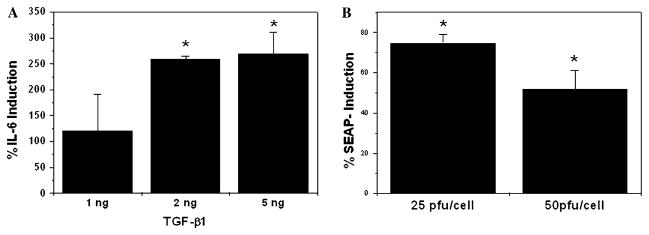
We have recently demonstrated both activation of latent TGF-β1 and induction of the TGF-β1 promoter in HTM cells under cyclic mechanical stress [16]. TGF-β1 has been reported to be a potent regulator of IL-6 expression in human fibroblasts, osteoblasts, and prostate epithelial cells [35–40]. To investigate the potential effect of TGF-β1 on IL-6 expression in HTM cells, three different HTM primary cultures were treated with increasing concentrations of TGF-β1 (1, 2, and 5 ng/ml) in the absence of serum. As shown in Fig. 3A, TGF-β1 treatment significantly induced (2.5 ± 0.05-fold, p < 0.05) the levels of secreted IL-6, assayed by ELISA at 20 h post-treatment (Fig. 3A). To analyze whether this increased secretion was mediated by the activation of the IL-6 promoter, HTM cells were infected with either 25 pfu/cell or 50 pfu/cell of recombinant-deficient adenovirus AdIL6-SEAP. Three days after infection, cells were shifted to serum-free medium and treated with 2 ng/ml TGF-β1. SEAP activity in the culture media assayed at 24 h post-treatment demonstrated significant activation of the IL-6 promoter in response to TGF-β1 treatment (Fig. 3B). The level of induction diminished with increasing concentration of the IL-6 promoter.
Fig. 3.
Induction of the IL-6 expression by TGF-β1 in HTM primary cultures. (A) Three different sets of HTM primary cultures were treated with increasing concentrations of TGF-β1 in serum-free media. Quantification of secreted IL-6 was assayed by ELISA at 20 h post-treatment. (B) Three independent HTM primary cultures were infected with AdIL6-SEAP (25 or 50 pfu/ml). At 3 days post-infection (d.p.i.), cultures were treated with TGF-β1 (2 ng/ml) for 24 h. The graph shows the relative percentage of SEAP activity induction in the culture media of TGF-β1-treated cultures when compared to nontreated controls. *Significantly different from control (t test, p < 0.05, n = 3).
In order to study the potential role of TGF-β1 in the stretch-induced production of IL-6, HTM cells were subjected to cyclic mechanical stress (5% elongation, 1 cycle/s, 12 h) in the presence of either nonspecific IgG or anti-human TGF-β1 antibody (1 μg). As shown in Fig. 4, the presence of an antibody against TGF-β1 significantly diminished (22.5% ± 6.2, p = 0.02) the stretch-induced production of IL-6.
Fig. 4.
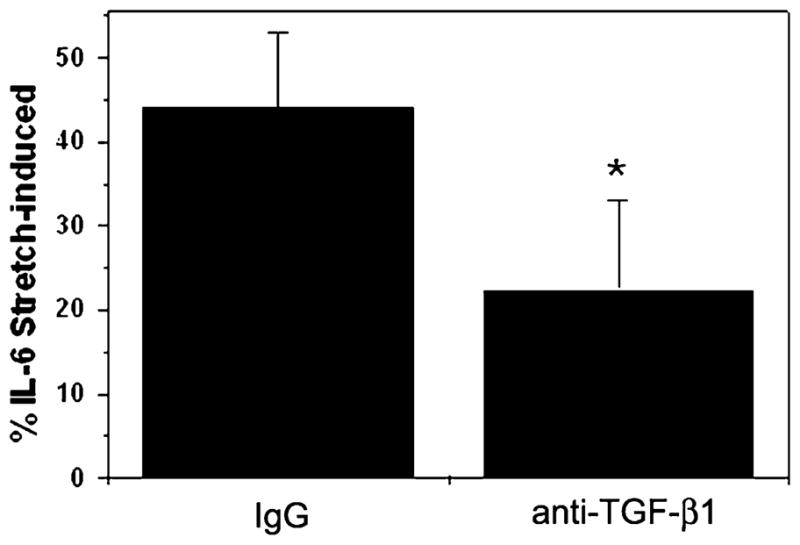
Effect of TGF-β1 in stretch-induced IL-6 production. Three different HTM cell cultures were subjected to cyclic mechanical strain (5% elongation, 1 cycle/s, 12 h) in the presence of anti-human TGF-β1 antibody (1 μg) or a nonspecific IgG. Concentration of IL-6 released to the culture media was assayed by ELISA. The graph shows the relative percentage of IL-6 induction compared to nonstretched control. *Significantly different from control (t test, p < 0.05, n = 3).
IL-6 increases outflow facility in perfused porcine anterior segments
To test the effects of IL-6 on outflow facility, porcine anterior segments were perfused for 48 h to obtain a stable IOP baseline. After this time, a volume of 50 μL containing either 500 ng of human IL-6 or PBS was injected into the anterior chamber through the cornea, and changes in outflow facility were monitored for 12 h. After an expected drop in pressure of both the control and IL-6-treated eyes resulting from the injection, eyes treated with IL-6 showed a significant increase in outflow facility when compared to their contralateral controls (Fig. 5A). Changes in outflow facility were not associated with loss of cells or visible alterations of the tissue structure as observed in semithin sections of the perfused tissue. (Fig. 5B).
Fig. 5.
Effects of IL-6 on outflow facility. (A) Porcine anterior segments were perfused for 2 days to get a stable IOP baseline. Human IL-6 (500 ng) or PBS was administered by transcorneal injection. IOP was monitored, and changes in outflow facility compared to controls were calculated. Data represent mean values ± SD. *,**Significantly different from control (t test, *p < 0.05, **p < 0.005, n = 8). (B) Representative view of the outer and inner outflow pathway of semithin sections from perfused porcine anterior segments following IL-6 treatment.
IL-6 increases the permeability of SC cell monolayers
IL-6 has been reported to modulate vascular permeability through induced increased permeability of the vascular endothelium as well as ECM remodeling. To investigate the potential effects of IL-6 on the permeability of the SC endothelium, we measured HRP permeability through confluent SC cell monolayers grown in transwell chambers. As shown in Fig. 6, a significant increase (51%, p = 0.01) in SC cell monolayer permeability was observed 30 min after treatment with IL-6 (100 ng/ml), compared to the nontreated controls. At 60 min, the effect of IL-6 on permeability was partially diminished, suggesting some reversibility of action.
Fig. 6.
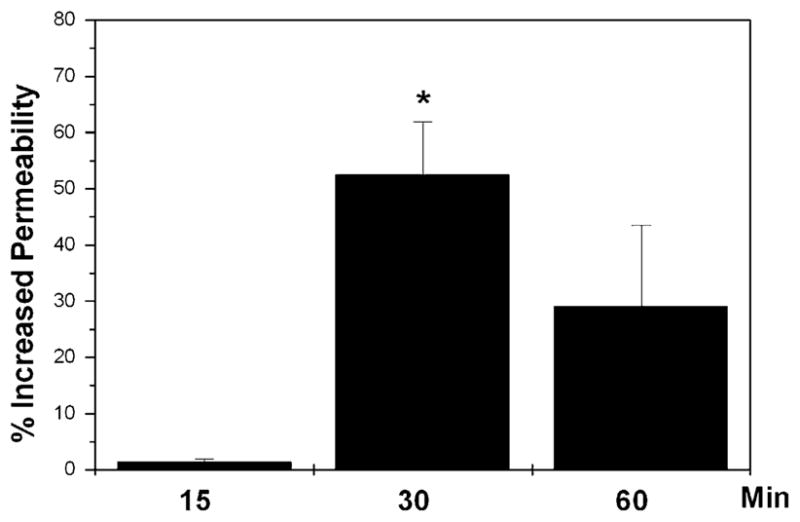
Effects of IL-6 on SC cell monolayer permeability. SC cell monolayers grown in transwell chambers were incubated with HRP and then treated with 100 ng/ml IL-6. HRP peroxidase activity in the lower chamber was measured at 0, 15, 30, and 60 min after the addition of the cytokine. Percentage of increase in permeability in the experimental wells treated with IL-6 compared to the nontreated controls was calculated at each time. Data represent mean values ± SD. *Significantly different from control (t test, p = 0.01, n = 3).
Discussion
In this study, we demonstrated the expression of IL-6 in the cells of the outflow pathway and its induction by cyclic mechanical stress. We also demonstrated the ability of IL-6 to increase outflow facility as well as SC cell monolayer permeability.
Since confirmation of IL-6 expression in the TM by immunohistochemistry is technically difficult, we used a recombinant adenovirus in which the reporter gene LacZ expression is driven by the IL-6 gene promoter. The observation that detectable expression of the IL-6 promoter within the perfused human anterior segments was primarily restricted to the cells of the TM and SC as well as blood vessels is consistent with a physiologic role for this cytokine in the outflow pathway.
Induction of IL-6 in response to different types of mechanical stresses has been reported in several cell types, such as vascular endothelial cells [41–43], vascular smooth muscle cells [44], cardiac myocytes [45], lung fibroblasts [46], and chondrocytes [47]. To investigate whether mechanical stress associated with mild fluctuations in IOP similar to those that may occur in normal physiologic conditions [7] could induce the production of IL-6 in TM cells, we selected a cyclic stretch regimen of 5% elongation per second as an in vitro model. Although in vitro stretch systems cannot fully represent the actual in vivo conditions, we believe that a moderate amount (5%) of stretching models the mechanical forces to which TM cells are normally exposed better than, e.g., larger levels of stress that might activate responses associated with cell damage. Under these conditions, we observed that mechanical stress induced a significant increase in both IL-6 mRNA expression and IL-6 secretion in HTM cell primary cultures.
A potential mechanism associated with the induction of IL-6 in the TM could involve the previously reported activation of TGF-β1 by mechanical stress in cultured HTM cells and perfused anterior segments [17]. TGF-β1 has been reported to be a powerful inductor of IL-6 expression in human fibroblasts, osteoblasts, and prostate cancer cells [35–40]. Our own results demonstrated that TGF-β1 also induces the expression of IL-6 in HTM cells. Moreover, addition of antibody against human TGF-β1 diminished the stretch-induced IL-6 production. Although TM cells are likely to have several redundant mechanisms involved in sensing and responding to mechanical stress, our results suggest that the initial activation of TGF-β-1 [17] may be one of the contributing factors leading to the induction of IL-6.
IL-6 is known to modulate vascular permeability through different mechanisms, including increased permeability of the vascular endothelium and the induction of MMP production, which results in ECM degradation. The observed increase in outflow facility induced by IL-6 in perfused anterior segments of porcine eyes could potentially be mediated by similar mechanisms.
The site of aqueous humor resistance in the human SC/TM outflow pathway has been localized to the JCT and inner wall of SC [5,12,28,48–51]. Although the outflow pathway of porcine eyes does not contain a true SC, it shows more features in common with that of the human eye than other species [52]. The functional analog of SC in porcine eyes is the angular aqueous plexus (AAP) formed by a number of endothelial-lined canals or vessels. Similar to SC, the endothelial cells lining these aqueous plexi have their long axis in the circumferential plane and are characterized by the presence of giant vacuoles. The JCT located beneath the AAP in the porcine eye is also analogous to the JCT of human eyes. The relative contribution of human SC (or porcine AAP endothelia) and the ECM in the JCT to the total resistance of the outflow pathway is still under debate. It is likely that changes in either of these parameters will have measurable effects on outflow facility. Recent experimental data based on the pharmacologic disruption of SC cells support this idea, and have led the authors to hypothesize that the resistance of the outflow pathway comprises a basic level of resistance generated by the ECM, with additional modulation and resistance created by the inner wall of SC [27]. Therefore, similar to what has been reported in the vascular system, the effects of IL-6 on the outflow pathway could be mediated by both increased monolayer cell permeability of the SC endothelium, as well as by the induction of MMPs and increased ECM degradation at the level of the JCT. Since reorganization of the cytoskeleton and destabilization of cell junctions occur faster than the reorganization of the ECM, effects on SC endothelial permeability appear as the more likely initial target for IL-6. The relatively quick effect of IL-6 on the permeability of human SC cell monolayers observed in cell culture experiments is consistent with the concept that the endothelium of the SC could potentially constitute the primary target for IL-6 within the outflow pathway. Furthermore, the seemingly observed longer lasting effect of IL-6 in organ culture compared to the SC cell monolayer permeability assay might suggest the existence of secondary mechanisms that possibly could involve ECM reorganization at the level of the JCT in the intact outflow pathway.
Altogether, the induction of IL-6 by mechanical stress in TM cells and the effects of IL-6 on outflow facility suggest a potential homeostatic mechanism aimed at restoring normal IOP values by increasing outflow facility in response to the mechanical stress produced by IOP elevations. Since our data also demonstrated expression of the IL-6 promoter in the endothelium of SC, an additional potential autocrine mechanism of IL-6 at the level of SC cells in response to elevated IOP cannot be ruled out.
We also hypothesize that the permanent activation of pro-inflammatory cytokines by chronic mechanical stress might lead to undesirable secondary effects. Both clinical observations and studies in transgenic mice have shown a link between IL-6 overexpression and several pathologic conditions, including atherosclerosis [53–55], Alzheimer’s disease [56–59], and rheumatoid arthritis [60–62]. It is worth mentioning that statins, which have become important in the treatment of patients with coronary artery disease [63,64] and also have been reported to have beneficial effects in POAG [65], are associated with a reduction in serum levels of IL-6.
In summary, our results suggest the presence of potential homeostatic mechanisms in the TM by which the mechanical stress associated with elevated IOP could promote the release of factors, like IL-6, capable of increasing outflow facility and restoring normal IOP levels. Furthermore, elevated levels of IL-6 resulting from chronic mechanical stress might contrarily lead to pathological effects. Further studies will be necessary to identify the specific mechanisms involved in the observed increase in outflow facility mediated by IL-6, and to evaluate the potential role of this cytokine in both the normal physiology and pathophysiology of this tissue.
Acknowledgments
The authors thank Taylor Hensley for his technical assistance. This work was supported in part by The Research to Prevent Blindness Foundation, The Glaucoma Foundation, and NIH Grants EY05722 and EY01894.
References
- 1.Bill A, Phillips CI. Uveoscleral drainage of aqueous humour in human eyes. Exp Eye Res. 1971;12:275–281. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- 2.Sommer A. Intraocular pressure and glaucoma. Am J Ophthalmol. 1989;107:186–188. doi: 10.1016/0002-9394(89)90221-3. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–1106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- 4.Vittitow J, Borras T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cell Physiol. 2004;201:126–137. doi: 10.1002/jcp.20030. [DOI] [PubMed] [Google Scholar]
- 5.Johnson M, Shapiro A, Ethier CR, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992;33:1670–1675. [PubMed] [Google Scholar]
- 6.Borras T, Rowlette LL, Tamm ER, Gottanka J, Epstein DL. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Invest Ophthalmol Vis Sci. 2002;43:33–40. [PubMed] [Google Scholar]
- 7.Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13:421–438. doi: 10.1097/01.ijg.0000131757.63542.24. [DOI] [PubMed] [Google Scholar]
- 8.Grierson I, Lee WR. The fine structure of the trabecular meshwork at graded levels of intraocular pressure. (1) Pressure effects within the near-physiological range (8–30 mmHg) Exp Eye Res. 1975;20:505–521. doi: 10.1016/0014-4835(75)90218-3. [DOI] [PubMed] [Google Scholar]
- 9.Grierson I, Lee WR. Pressure-induced changes in the ultrastructure of the endothelium lining Schlemm’s canal. Am J Ophthalmol. 1975;80:863–884. doi: 10.1016/0002-9394(75)90284-6. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365–383. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone MA. Pressure-dependent changes in configuration of the endothelial tubules of Schlemm’s canal. Am J Ophthalmol. 1974;78:630–638. doi: 10.1016/s0002-9394(14)76301-9. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone MA. Pressure-dependent changes in nuclei and the process origins of the endothelial cells lining Schlemm’s canal. Invest Ophthalmol Vis Sci. 1979;18:44–51. [PubMed] [Google Scholar]
- 13.Booth A, Nguyen T, Polansky J. TIGR and stretch in the trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:1888–1889. [PubMed] [Google Scholar]
- 14.Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- 15.Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44:5174–5181. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez P, Epstein DL, Borras T. Genes upregulated in the human trabecular meshwork in response to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:352–361. [PubMed] [Google Scholar]
- 17.Liton PB, Liu X, Challa P, Epstein DL, Gonzalez P. Induction of TGF-beta1 in the trabecular meshwork under cyclic mechanical stress. J Cell Physiol. 2005;205:364–371. doi: 10.1002/jcp.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitton KP, Tumminia SJ, Arora J, Zelenka P, Epstein DL, Russell P. Transient loss of alphaB-crystallin: an early cellular response to mechanical stretch. Biochem Biophys Res Commun. 1997;235:69–73. doi: 10.1006/bbrc.1997.6737. [DOI] [PubMed] [Google Scholar]
- 19.Okada Y, Matsuo T, Ohtsuki H. Bovine trabecular cells produce TIMP-1 and MMP-2 in response to mechanical stretching. Jpn J Ophthalmol. 1998;42:90–94. doi: 10.1016/s0021-5155(97)00129-9. [DOI] [PubMed] [Google Scholar]
- 20.Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39:1361–1371. [PubMed] [Google Scholar]
- 21.WuDunn D. The effect of mechanical strain on matrix metalloproteinase production by bovine trabecular meshwork cells. Curr Eye Res. 2001;22:394–397. doi: 10.1076/ceyr.22.5.394.5500. [DOI] [PubMed] [Google Scholar]
- 22.Duchini A, Govindarajan S, Santucci M, Zampi G, Hofman FM. Effects of tumor necrosis factor-alpha and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J Investig Med. 1996;44:474–482. [PubMed] [Google Scholar]
- 23.Tamm I, Cardinale I, Krueger J, Murphy JS, May LT, Sehgal PB. Interleukin 6 decreases cell-cell association and increases motility of ductal breast carcinoma cells. J Exp Med. 1989;170:1649–1669. doi: 10.1084/jem.170.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamm I, Kikuchi T, Cardinale I, Krueger JG. Cell-adhesion-disrupting action of interleukin 6 in human ductal breast carcinoma cells. Proc Natl Acad Sci USA. 1994;91:3329–3333. doi: 10.1073/pnas.91.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein DL, Rohen JW. Morphology of the trabecular meshwork and inner-wall endothelium after cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci. 1991;32:160–171. [PubMed] [Google Scholar]
- 26.Bradley JM, Vranka J, Colvis CM, Conger DM, Alexander JP, Fisk AS, Samples JR, Acott TS. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- 27.Bahler CK, Hann CR, Fautsch MP, Johnson DH. Pharmacologic disruption of Schlemm’s canal cells and outflow facility in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2004;45:2246–2254. doi: 10.1167/iovs.03-0746. [DOI] [PubMed] [Google Scholar]
- 28.Ethier CR, Kamm RD, Palaszewski BA, Johnson MC, Richardson TM. Calculations of flow resistance in the juxtacanalicular meshwork. Invest Ophthalmol Vis Sci. 1986;27:1741–1750. [PubMed] [Google Scholar]
- 29.Samples JR, Alexander JP, Acott TS. Regulation of the levels of human trabecular matrix metalloproteinases and inhibitor by interleukin- 1 and dexamethasone. Invest Ophthalmol Vis Sci. 1993;34:3386–3395. [PubMed] [Google Scholar]
- 30.Johnson DH, Tschumper RC. Human trabecular meshwork organ culture. A new method. Invest Ophthalmol Vis Sci. 1987;28:945–953. [PubMed] [Google Scholar]
- 31.Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998;39:1804–1812. [PubMed] [Google Scholar]
- 32.Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- 33.Langeler EG, van Hinsbergh VW. Characterization of an in vitro model to study the permeability of human arterial endothelial cell monolayers. Thromb Haemost. 1988;60:240–246. [PubMed] [Google Scholar]
- 34.Lampugnani MG, Resnati M, Dejana E, Marchisio PC. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991;112:479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moller A, Schwarz A, Neuner P, Schwarz T, Luger TA. Regulation of monocyte and keratinocyte interleukin 6 production by transforming growth factor beta. Exp Dermatol. 1994;3:314–320. doi: 10.1111/j.1600-0625.1994.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 36.Franchimont N, Rydziel S, Canalis E. Transforming growth factor-beta increases interleukin-6 transcripts in osteoblasts. Bone. 2000;26:249–253. doi: 10.1016/s8756-3282(99)00275-6. [DOI] [PubMed] [Google Scholar]
- 37.Turner M, Chantry D, Feldmann M. Transforming growth factor beta induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine. 1990;2:211–216. doi: 10.1016/1043-4666(90)90018-o. [DOI] [PubMed] [Google Scholar]
- 38.Park JI, Lee MG, Cho K, Park BJ, Chae KS, Byun DS, Ryu BK, Park YK, Chi SG. Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene. 2003;22:4314–4332. doi: 10.1038/sj.onc.1206478. [DOI] [PubMed] [Google Scholar]
- 39.Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS, Lee TH, Bae YS, Ha KS, Lee ZW, Rhee SG, Choi I. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF-beta 1-induced IL-6 expression. J Immunol. 2000;165:2190–2197. doi: 10.4049/jimmunol.165.4.2190. [DOI] [PubMed] [Google Scholar]
- 40.Eickelberg O, Pansky A, Mussmann R, Bihl M, Tamm M, Hildebrand P, Perruchoud AP, Roth M. Transforming growth factor-beta1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblasts. J Biol Chem. 1999;274:12933–12938. doi: 10.1074/jbc.274.18.12933. [DOI] [PubMed] [Google Scholar]
- 41.Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int Suppl. 1998;67:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- 42.Cucina A, Sterpetti AV, Borrelli V, Pagliei S, Cavallaro A, D’Angelo LS. Shear stress induces transforming growth factor-beta 1 release by arterial endothelial cells. Surgery. 1998;123:212–217. [PubMed] [Google Scholar]
- 43.Sterpetti AV, Cucina A, Morena AR, Di Donna S, D’Angelo LS, Cavalarro A, Stipa S. Shear stress increases the release of interleukin-1 and interleukin-6 by aortic endothelial cells. Surgery. 1993;114:911–914. [PubMed] [Google Scholar]
- 44.O’Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta(1) Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 45.Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, Takahashi T, Kato T, Ogawa S. Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res. 1999;84:1127–1136. doi: 10.1161/01.res.84.10.1127. [DOI] [PubMed] [Google Scholar]
- 46.Kimoto S, Matsuzawa M, Matsubara S, Komatsu T, Uchimura N, Kawase T, Saito S. Cytokine secretion of periodontal ligament fibroblasts derived from human deciduous teeth: effect of mechanical stress on the secretion of transforming growth factor-beta 1 and macrophage colony stimulating factor. J Periodontal Res. 1999;34:235–243. doi: 10.1111/j.1600-0765.1999.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 47.Mohtai M, Gupta MK, Donlon B, Ellison B, Cooke J, Gibbons G, Schurman DJ, Smith RL. Expression of interleukin-6 in osteoarthritic chondrocytes and effects of fluid-induced shear on this expression in normal human chondrocytes in vitro. J Orthop Res. 1996;14:67–73. doi: 10.1002/jor.1100140112. [DOI] [PubMed] [Google Scholar]
- 48.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- 49.Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-h. [DOI] [PubMed] [Google Scholar]
- 50.Brubaker RF. The effect of intraocular pressure on conventional outflow resistance in the enucleated human eye. Invest Ophthalmol. 1975;14:286–292. [PubMed] [Google Scholar]
- 51.Bill A, Svedbergh B. Scanning electron microscopic studies of the trabecular meshwork and the canal of Schlemm—an attempt to localize the main resistance to outflow of aqueous humor in man. Acta Ophthalmol (Copenh) 1972;50:295–320. doi: 10.1111/j.1755-3768.1972.tb05954.x. [DOI] [PubMed] [Google Scholar]
- 52.McMenamin PG, Steptoe RJ. Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J Anat. 1991;178:65–77. [PMC free article] [PubMed] [Google Scholar]
- 53.Seino Y, Ikeda U, Ikeda M, Yamamoto K, Misawa Y, Hasegawa T, Kano S, Shimada K. Interleukin 6 gene transcripts are expressed in human atherosclerotic lesions. Cytokine. 1994;6:87–91. doi: 10.1016/1043-4666(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 54.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin- 6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 55.Song L, Schindler C. IL-6 and the acute phase response in murine atherosclerosis. Atherosclerosis. 2004;177:43–51. doi: 10.1016/j.atherosclerosis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Papassotiropoulos A, Hock C, Nitsch RM. Genetics of interleukin 6: implications for Alzheimer’s disease. Neurobiol Aging. 2001;22:863–871. doi: 10.1016/s0197-4580(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 57.Angelis P, Scharf S, Mander A, Vajda F, Christophidis N. Serum interleukin-6 and interleukin-6 soluble receptor in Alzheimer’s disease. Neurosci Lett. 1998;244:106–108. doi: 10.1016/s0304-3940(98)00136-0. [DOI] [PubMed] [Google Scholar]
- 58.Hull M, Fiebich BL, Lieb K, Strauss S, Berger SS, Volk B, Bauer J. Interleukin-6-associated inflammatory processes in Alzheimer’s disease: new therapeutic options. Neurobiol Aging. 1996;17:795–800. doi: 10.1016/0197-4580(96)00107-8. [DOI] [PubMed] [Google Scholar]
- 59.Hull M, Strauss S, Berger M, Volk B, Bauer J. The participation of interleukin-6, a stress-inducible cytokine, in the pathogenesis of Alzheimer’s disease. Behav Brain Res. 1996;78:37–41. doi: 10.1016/0166-4328(95)00213-8. [DOI] [PubMed] [Google Scholar]
- 60.Choy E. Interleukin 6 receptor as a target for the treatment of rheumatoid arthritis. Ann Rheum Dis. 2003;62(Suppl 2):i–i68. doi: 10.1136/ard.62.suppl_2.ii68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nawata Y, Eugui EM, Lee SW, Allison AC. IL-6 is the principal factor produced by synovia of patients with rheumatoid arthritis that induces B-lymphocytes to secrete immunoglobulins. Ann N Y Acad Sci. 1989;557:230–238. doi: 10.1111/j.1749-6632.1989.tb24016.x. discussion 239. [DOI] [PubMed] [Google Scholar]
- 62.Houssiau FA, Devogelaer JP, Van Damme J, De Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 63.Rupp S, Badorff C, Koyanagi M, Urbich C, Fichtlscherer S, Aicher A, Zeiher AM, Dimmeler S. Statin therapy in patients with coronary artery disease improves the impaired endothelial progenitor cell differentiation into cardiomyogenic cells. Basic Res Cardiol. 2004;99:61–68. doi: 10.1007/s00395-003-0441-3. [DOI] [PubMed] [Google Scholar]
- 64.Paulo S, Fernandes S, Vizinho R, Carneiro AV. Statin therapy in the primary and secondary prevention of coronary artery disease in patients with type 2 diabetes. A scientific review. Rev Port Cardiol. 2004;23:1461–1482. [PubMed] [Google Scholar]
- 65.McGwin G, Jr, McNeal S, Owsley C, Girkin C, Epstein D, Lee PP. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol. 2004;122:822–826. doi: 10.1001/archopht.122.6.822. [DOI] [PubMed] [Google Scholar]