Abstract
Individuals who have, or are at risk for, various genetic disorders face many challenges concerning disclosures of genetic information in dating situations. We conducted a qualitative interview study of 64 individuals confronting Huntington’s disease, breast cancer, or Alpha-1 antitrypsin deficiency, examining what issues these individuals encountered, and how they viewed and addressed these—including issues of understandings, privacy, and disclosures of genetic information to various groups (e.g., family members). Incidental to the primary research questions addressed, participants also often described a series of dilemmas in dating situations that they and/or family members, friends, and fellow patients faced of whether to date, and if so, whether, what, how, why, and when to disclose their genetic risk or illness. At times, these individuals feared and experienced rejection, and hence delayed, avoided, or opposed disclosure, or disclosed indirectly or inadvertently. These data are reported in this paper and highlight the importance of patients, their loved ones, genetic counselors, and other health care providers being aware of these issues, and appreciating the complex factors involved, which can affect patients’ coping and social support. This paper, the first to explore several key aspects of disclosures of genetic information in dating, thus suggests needs for public and professional education, and future research in this area.
Keywords: Risk communication, Decision-making, Health behaviors, Confidentiality, Ethics, Family relationships, Reproductive choices, Reproductive decisions, Qualitative research
Introduction
Dating situations are critical to the possibility of establishing an ongoing supportive relationship—usually the most important of one’s adult life—and of having children. In dating, two individuals each consider whether to make extraordinary and unique investments into each other’s lives, and hence they closely and carefully assess each other. Yet, little attention has been given to disclosures of genetic information in these contexts—what challenges patients face, and how they approach these.
We recently conducted an in-depth qualitative study, exploring the research questions of what issues individuals who had or were at risk of a genetic disease faced, and how they viewed and addressed these—including issues of understandings, privacy, and disclosures of genetic information. We explored disclosure to various groups of individuals (e.g., family members, including siblings and children, friends, co-workers, physicians, and insurance companies), and have reported on several issues, including disclosures of HD within families and issues of discrimination (Klitzman 2010a, b). Participants also often described struggles with disclosure decisions in a key additional context—dating situations. Specifically, interviewees explained how they and their adult family members, friends, and fellow patients (e.g., through disease support groups), if not already in committed relationships, had to decide whether, when, and what to tell prospective partners. Disclosure in dating was not the primary initial research focus of this project, but emerged in the course of the data collection and analysis. Since qualitative research methods allow the investigator to probe areas as they arise within and between interviews, we then explored these areas further.
Disclosures of genetic information in dating situations have received a small amount of attention in film (Rudnick 2008), and Hoskins et al. (2008) examined several issues concerning dating among 11 asymptomatic women with BRCA 1/2 mutations, but many questions remain. Hoskins found that such disclosures can represent a process, and that individuals may want to raise the topic “naturally” in the course of conversation vs. avoid it. But questions arise concerning what other factors, if any, may be involved, and how individuals who have not yet been tested, or who have proceeded to develop symptoms and/or undergo treatment (e.g., surgery), as well as those confronting other diseases, face these issues.
We have found no other published studies that focus on disclosures of genetic information in dating situations, though prior research has probed other areas that may be indirectly related: disclosures of genetic information in non-dating situations (e.g., in families); disclosures of other, non-genetic diagnoses (particularly HIV and sexually transmitted infections [STIs]) in dating (Christianson et al. 2008; Lee and Craft 2002; Klitzman and Bayer 2003); the general development and other aspects of dating relationships; and the effects of disease, once disclosed, within married couples. Yet, as we shall see, disclosures of genetic information pose several unique challenges.
Disclosures of health information during dating have been examined with regard to HIV and other STIs, indicating that men and women often have difficulty disclosing their infection in these situations, wrestling with dilemmas of whether, when, what, and how to tell, and how to assess trust and levels of moral responsibilities to disclose (Klitzman and Bayer 2003). These men and women frequently avoid or delay such disclosures, speaking in code or indirectly, or seeking to justify silence (e.g., not disclosing, but “practicing safer sex”). Given the health risks involved in non-disclosure, laws mandating disclosure of HIV infection to sexual partners exist in several states (Klitzman et al. 2004) and countries (Christianson et al. 2008).
Disclosures of other STIs in dating situations can produce anxiety (McCaffery et al. 2006). Men and women with genital herpes are less likely to tell casual than non-casual partners (Green et al. 2003), often delay disclosing the diagnosis to dates (Lee and Craft 2002), and “get to know prospective partners at a much slower pace,” creating a “screening period”, and usually telling only partners “whom they expect to remain in the relationship even after hearing” the diagnosis. Those unable to find such accepting partners decrease their dating activities.
Yet, HIV and other STIs involve not just stigma, but potential transmission of the virus to the person one is dating, whereas genetics does not involve infecting the person. Genetics can affect possible future children, but not all couples want to have children, and Preimplantation Genetic Diagnosis (PGD) can potentially prevent such transmission of the mutation, though PGD can pose problems, too, as discussed below.
The presence of ongoing health problems—but not initial disclosures of these—have also been examined in dating among older adults (i.e., over age 55), and has been found to be associated with comparative health, younger age, and mobility (Bulcroft and Bulcroft 1991; Carr 2004). But these studies have not examined how these individuals decide whether, when, and how to initially discuss their health concerns. Questions concerning disclosures about aspects of living with disabilities in relationships have also received attention, but have focused on the ways in which people with disabilities discuss assistance they may need (e.g., how much help they may require in a particular activity such as travel). However, these studies have not examined initial disclosures of disabilities in dating situations. Moreover, many disabilities are visible or readily apparent (e.g., having prostheses, or being wheelchair-bound). Hence, disclosures of genetic risk or disease, which are generally invisible, can pose dramatically different issues.
Several prior studies have explored other, psychosocial aspects of dating and spouse selection, examining socio-demographic differences in selection of prospective mates, focusing on age, race, social class (Hollingshead 1950; South 1991), and physical attributes such as weight, height (Belot and Francesconi 2006), and attractiveness (Li et al. 2002), suggesting that assortative mating often occurs whereby individuals choose mates who have similar traits as themselves.
Disclosures of genetic risks have been explored within established families (Wilcke et al. 1999; Hallowell et al. 2003), and Werner-Lin (2008) suggested how salient issues concerning BRCA mutations can change at each state of the life cycle, and how the meanings of these mutations may differ between single vs. partnered women, and women who did vs. did not wish to have children. BRCA 1/2 test results also can influence patients’ hypothetical reproductive decisions (Fortuny et al. 2009). For HD, too, some individuals undergo testing to help make reproductive decisions (Decruyenaere et al. 1996). Generally, members of couples married 20–30 years disclose cancer diagnoses to each other (Porter et al. 2005; Manne et al. 2004). But these studies have not examined how individuals confronting genetic disease approach disclosure decisions they face.
Moreover, in the case of dating, families have not yet been formed. Individuals may thus simultaneously face challenges in adjusting to the possibility of genetic risk, while also working towards establishing a relationship, and considering the possibility of having children.
Researchers have explored, too, the process of premarital relationship development in general. Surra (1990) outlined four models of such development: early determinism (which suggests that early properties of relationships affect later courses), incremental convergence and divergence (in which relationship properties emerge over time), progressive stages (in which relationships pass through stages during which each member evaluates the other’s qualities, weighing compatibilities and roles), and gradual differentiation (in which relationships alternate through periods of stability and instability). Questions arise as to whether genetics affects these processes and models or vice versa, and if so, how.
As suggested above, many questions persist concerning how exactly individuals broach the topic of genetic risk information in dating situations; whether they discuss genetic information, and if so, when, to what extent, how, and with whom. We thus probed these areas as they began to arise in the course of interviews conducted as part of the larger study mentioned above. This paper examines these issues: what concerns these individuals face in this context, how they respond to these, and what implications these decisions have.
Methods
As shown in Table 1, and as described elsewhere (Klitzman 2010a, b), the Principal Investigator (PI) interviewed, for 2 h each, 64 individuals, who had or were at risk for one of three disorders—Huntington’s disease (HD), breast cancer (BRCA), and Alpha-1 antitrypsin deficiency (Alpha). We selected a heterogeneous group in order to more fully understand the range of issues and perspectives that could arise regarding genetic issues. The Columbia University Department of Psychiatry Institutional Review Board approved the study, and all participants gave informed consent.
Table 1.
Characteristics of the sample
| Disease
|
Total
|
||||
|---|---|---|---|---|---|
| BRCA (N=32) | HD (N=21) | Alpha (N=11) | (N=64) N (%) | ||
| Gender | Female | 32 | 9 | 7 | 48 (75%) |
| Male | 0 | 12 | 4 | 16 (25%) | |
| Age | 0–20 | 0 | 0 | 0 | 0 (0%) |
| 21–30 | 3 | 3 | 0 | 6 (9.4%) | |
| 31–40 | 8 | 12 | 0 | 20 (31.2%) | |
| 41–50 | 13 | 3 | 1 | 17 (26.6%) | |
| 51–60 | 3 | 3 | 5 | 11 (17.2%) | |
| 61–70 | 5 | 0 | 4 | 9 (14.0%) | |
| 71–80 | 0 | 0 | 1 | 1 (1.6%) | |
| Ethnicity | White | 21 | 18 | 11 | 50 (78.1%) |
| Black | 6 | 2 | 0 | 8 (12.5%) | |
| Asian | 2 | 0 | 0 | 2 (3.1%) | |
| Hispanic | 2 | 1 | 0 | 3 (4.7%) | |
| Other | 1 | 0 | 0 | 1 (1.6%) | |
| Marital status | Single | 19 | 9 | 2 | 30 (46.9%) |
| Married | 8 | 11 | 7 | 26 (40.6%) | |
| Divorced/Widowed | 5 | 1 | 2 | 8 (12.5%) | |
| Children | Yes | 16 | 8 | 9 | 33 (51.6%) |
| No | 16 | 13 | 2 | 31 (48.4%) | |
| Symptom status | Symptomatic | 20 | 6 | 11 | 37 (57.8%) |
| Asymptomatic | 12 | 15 | 0 | 27 (42.2%) | |
| Tested | Yes | 20 | 14 | 11 | 45 (70.3%) |
| No | 12 | 7 | 0 | 19 (29.7%) | |
| Test statusa | Positive | 8 | 10 | 11 | 29 (64.4%) |
| Negative | 11 | 4 | 0 | 15 (33.3%) | |
| Indeterminate | 1 | 0 | 0 | 1 (2.2%) | |
Percentages are of the total of those tested (45).
As described below, the participants varied in age, relationship status, and presence or absence of children. All were heterosexual.
To recruit participants, we distributed information about the study through clinics, studies at our institution, newsletters, flyers displayed on bulletin boards of our institution, and word of mouth. The recruitment materials stated, “Do you have, or are you at risk of, a genetic disease,” and did not mention, or refer to, disclosures in dating. Individuals contacted the principal investigator if they were interested. As inclusion criteria, participants had to be over 18, at risk of a genetic disorder (HD, Alpha, or BRCA), and able to give consent. Testing and symptom status were by self-report (except for those individuals confronting HD who were recruited through an HD clinic). With each participant, the PI conducted a confidential in-depth semi-structured interview concerning experiences of having, or being at risk for, disease. Interviews were conducted in the PI’s office or institution, or the subjects’ home or office, if they preferred. Several relevant sample sections of the semi-structured interview guide are attached (see Appendix), through which we sought to obtain detailed descriptions of the process of individuals’ views and decisions concerning genetic testing, disclosure of genetic risk or illness in dating situations, and related issues.
On theoretical grounds, Geertz (1973) has advocated studying aspects of individuals’ lives and social situations not by imposing external theoretical structures, but by trying to understand individuals’ own experiences, drawing on their own words and perspectives to obtain a “thick description”. Hence, to understand more fully the range of factors and issues that may be involved in genetic testing decisions, we used qualitative methods.
Data Analysis
We have adapted elements from grounded theory, as described by Strauss and Corbin (1990), to aid in understanding a complex social process. Specifically, grounded theory involves both deductive and inductive thinking, building inductively from the data to an understanding of themes and patterns within the data, and deductively drawing on frameworks from previous research and theories. For example, interviewees introduced topics such as interactions on dates that were then explored further in these and other interviews. This approach was informed by constant comparison in which data from different individuals were compared for similarities and differences to see whether these suggested hypotheses. Transcriptions and initial analyses of interviews were done during the period in which the interviews were being conducted and helped guide subsequent interviews. Interviews were conducted until “saturation” was reached (that is, “the point at which no new information or themes are observed in the data”) (Strauss and Corbin 1990; Guest et al. 2006).
Once the full set of interviews and initial analyses were completed, subsequent, more detailed analyses were conducted in two phases, primarily by the PI together with a research assistant (RA) who had social science training. In phase I of the subsequent coding, the PI and the RA independently examined a subset of interviews to assess factors that shaped participants’ experiences, identifying categories of recurrent themes and issues that were subsequently given codes. These two coders assessed similarities and differences among participants, examining themes and categories that emerged, ranges of variation within categories, and variables that may be involved. The coders systematically coded blocks of text to assign “core” codes or categories. While reading the interviews, a topic name (code) was inserted beside each excerpt of the interview to indicate the themes being discussed. The coders then worked together to reconcile their independently developed coding schemes into a single scheme, creating a coding manual, and examining areas of disagreement until reaching consensus. New themes that did not fit into the original coding framework were discussed, and modifications were made in the manual as needed.
In the next phase of the analysis, we subdivided thematic categories into secondary or sub-codes, and then refined and merged these, when suggested by associations or overlap in the data. Codes and sub-codes were then used in analysis of all of the interviews. Major codes (or categories) of text included, for example, occasions when participants disclosed their genetic risk in a dating situation. Sub-codes (or sub-themes) were conceptual and thematic subdivisions of these larger categories and included, for instance, reasons why the participant decided to disclose or not disclose to a prospective mate. To ensure coding reliability, two coders analyzed all interviews. To ensure trustworthiness, we triangulated the data with existing theoretical and empirical literature related to disclosures of, and responses to, genetic and other diseases (Hoskins et al. 2008; Werner-Lin 2008; Hallowell et al. 2003; DudokdeWit et al. 1997; Goffman 1963; Klitzman and Bayer 2003). These data also have a certain face validity that, we would suggest, further substantiates their trustworthiness. We have also presented below text from the interviews to allow readers to judge these data for themselves. Many of these participants discussed dating issues as these had arisen with themselves and/or family members (including siblings and children), friends, and fellow patients (e.g., in patient support groups and organizations). Some interviewees articulated certain themes more clearly and succinctly than did others, and thus, given space limitations, are quoted more below. We have indicated whether interviewees are symptomatic (Sx) or asymptomatic (Asx) and mutation-positive (+), negative (−), inconclusive, or untested (Unt).
Results
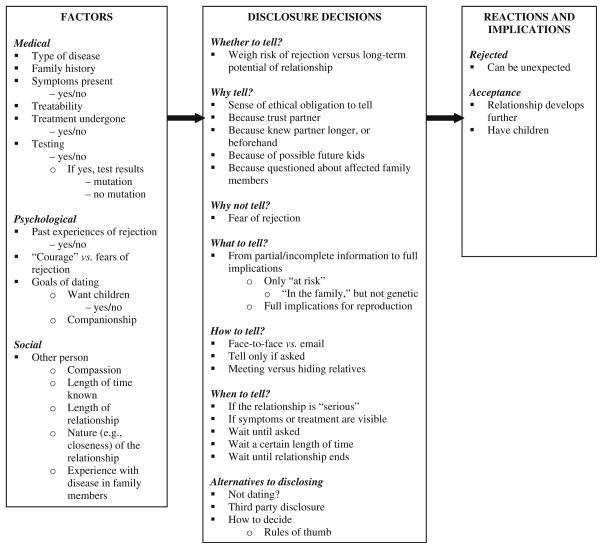
Overall, as illustrated in Fig. 1, these data suggest a model, whereby individuals confronting genetic disease who are considering, or involved in, dating face a series of dilemmas: whether, why, when, how, and what to disclose. These decisions were shaped by several medical, social, and psychological factors, and have several implications (e.g., whether the prospective mate accepts or rejects the individual). From a theoretical perspective, Goffman (1963) proposed a theory of stigma in which individuals try to manage “tainted” aspects of themselves and “to pass” as “untainted”. Here, individuals try not “to pass”, but to avoid rejection, and establish an ongoing relationship. This goal of avoiding stigma and rejection lead these individuals to wrestle with this series of questions.
Fig. 1.
Themes concerning disclosure of genetic information in dating
In brief, as described more fully below and outlined in Fig. 1, participants struggled with underlying questions of what obligations, if any, they had concerning whether, when, and what to disclose, and how to decide. Some chose to forego dating altogether, in part to avoid these dilemmas. Factors shaping these decisions were medical (e.g., type of disease, family history, symptoms, treatability, treatment undergone, testing and test results), psychological (e.g., fears and past experiences of rejection, and desires for children), and social (e.g., the prospective partner’s expectations and desires concerning the relationship and future children, compassion, and the length and nature of the relationship). These decisions occurred against the backdrop of dating, which can be difficult even in the absence of genetic risk, involving fragile and confusing choreographies, as mutual trust and social, and thus ethical, bonds develop from nothing to among the most important of one’s entire life. Disclosures of mutations often exacerbated these challenges.
These results are presented below within the framework of the series of questions with which these interviewees wrestled—i.e., based on these participants’ perceptions and experiences. Alternatively, one could potentially organize these data differently (e.g., focusing on the factors involved), but to do so would risk insufficiently presenting and hence understanding how these individuals themselves experienced and viewed these dilemmas.
At the same time, these questions (i.e., of whether, how, what, and when to disclose) are closely intertwined, along with the factors involved (e.g., desires for children), and cannot completely be disentangled. With each potential partner, a patient must answer each question, and these decisions usually affect each other. For example, what one decides to disclose can affect how one says it. When one decides to disclose can affect what one says. These interviewees’ narratives of disclosure often thus embody several issues and factors together. Rather than break up these narratives, we have hence presented each below in reference to a particular phenomenon, and then refer back to them in exploring other phenomena to which they relate as well. Overall, each of these dilemmas is conceptually somewhat distinct, and thus examined separately here for purposes of elucidation.
Of note, these concerns about rejection and dating generally arose with HD and BRCA more than with Alpha. Individuals with Alpha tended to become aware of the disease later in life (e.g., after their mid-40s), and they were usually the first ones in their family to be diagnosed. The diagnosis was first described in 1963 (Laurell and Erikson 1963), and before that, patients were usually misdiagnosed with emphysema. All of the interviewees confronting Alpha had symptoms and were receiving treatment (intravenous Prolastin). In contrast, individuals at risk of BRCA and HD were generally aware of their risk while younger (e.g., in their 20s), when dating often played critical roles in their lives.
Whether to Tell
While all participants felt that a clear obligation existed to tell the other person once one has definitively decided to get married, quandaries arose before this mutual commitment is firmly established. Once deciding to get married, one’s spouse’s right to the information was seen as outweighing one’s own right to privacy. Most interviewees valued privacy, but felt it was limited in this case, especially since another person’s health might be at stake. As a 70-year-old woman with Alpha said,
If you’re going to get married, your genetic make-up may affect not only your wife, but your children. There, you have an obligation to tell your wife—certainly if you’re going to have children. A person is entitled to genetic privacy, except as it affects somebody else. (A7/Sx, +)
Of note, questions arise of whether this obligation to tell is less if one does not wish to have children, and, if so, to what degree.
Yet, dating—before individuals have decided to marry, or enter long-term, committed relationships—presents other complexities. In these situations, individuals face multiple questions, and are frequently unsure how to proceed. Individuals had to weigh competing pros and cons—for example, balancing ethical obligations against potential risks of rejection. Participants often debated what to do, weighing the long-term potential of the relationship vs. the risk of rejection. A 39-year-old single woman with breast cancer, who has a mutation and underwent a mastectomy, said:
When you start a relationship, you think, “Now I’m gonna have to tell this person. Is this worth it?” If it’s a relationship where I need to tell, then I will. If not, I won’t. I dated men where it has come up very early on, and I realized this is not the person for me. That’s fine. (BC9/Sx, +).
Reactions to disclosure of genetic risk can thus serve as a test of the relationship itself.
Moreover, the fact that one has the mutation can be compounded by the fact that one has had symptoms, and potential visible and scarring treatment (i.e., a mastectomy) as well. In patients’ lived experiences, these issues can thus combine, creating additional obstacles, and possibly increasing the odds of rejection. Consequently, these factors can thus force individuals to consider the value (i.e., long-term potential) and hence seriousness of the relationship earlier than they otherwise would. As described below, questions emerge, too, of how one does or should determine whether one is ethically required to tell. On each side of these assessments, the factors are often themselves inherently unknown, and arguably unknowable a priori, causing stress.
Why Tell?
Individuals wrestled with why to tell or not tell.
Tell Because of Ethical Obligation
Those that disclosed did so primarily from a sense of ethical obligation to their prospective mate. Some readily told these partners because they had known and trusted them for a relatively long time—at times, before their first official romantic “date”. A 39-year-old woman at risk for HD, untested and without symptoms, told her future husband close to their first date because they had known each other for an extended period beforehand. “I probably told him within the first 24 h after our first date.” (HD12/Asx, −)
Tell Because of Disease in Family
Disclosure occurred, too, because a family member’s disease could not be hidden. The woman above continued,
“You’re gonna meet my dad. He’s a mess. He has this awful disease. So does my grandmother. I could probably have it too! And I could maybe give it to my children.” He had had a girlfriend who died in his arms of cancer a few years before. He had told me about that before we even got together. (HD12/Asx, −)
The fact that he had disclosed personal information to her about a former girlfriend’s death made it easier for her to disclose reciprocally. Disclosures can thus mutually facilitate each other, and can both result from, and enhance, trust. Eventually, she found she did not have the mutation. The fact that she did not have any early symptoms of HD, even though she had not yet undergone testing, may have also facilitated her disclosure.
Individuals may disclose, too, to avoid any dishonesty, concealment, or perceived lack of trust. “I try to be as honest as I can in relationships,” a 42-year-old single man with the HD mutation, but without symptoms, said. (HD5/Asx, +) The fact that he has this lethal mutation, rather than being merely at risk for it or for another less severe disease, heightened his sense of moral responsibility to disclose (i.e., the information was of momentous importance). The extent of the implications of the information can thus raise the perceived importance of disclosing it. Yet he also underscores the difficulties of disclosure, and sees honesty as an important goal.
Tell Because Children Would be Affected
The fact that the health of future children (i.e., third parties) may be affected added to the sense of moral responsibility to disclose. In dating, as opposed to other social relationships, disclosure occurred because of the possibility of having children with the disease. Hence, several felt that disclosure was easier if the prospective spouse was not interested in having kids. As the man above continued,
If she wants to have children, then our relationship won’t go anywhere. I’ve had a couple of relationships with women who already had children. That’s the simplest. (HD5/Asx, +)
Similarly, for women, the pressure to disclose can diminish after age 40. A divorced 43-year-old woman at risk for HD, who is untested and asymptomatic, said:
Now, because I’m 43, this weight is taken off my shoulders. It’s safer. So, when guys meet me, they don’t usually think of family. I was obsessed with wanting children. I adore kids. I’m really, really good with kids, but I hit 40. Maybe HD has kind of pushed the idea of having kids of out of my life. (HD16/Asx, Unt)
Removal of the possibility of having children can thus considerably ease the burdens of disclosure; but difficulties, though lesser, can remain. Disclosure is now “simpler” and “safer,” but still not completely simple or safe. Thus, the goals of dating can vary, altering the reasons for, and results from, disclosure.
Tell Because Hard to Hide Diagnosis
Disclosures can occur, too, because genetic risk or symptoms can simply no longer be concealed. A 37-year-old single woman with symptoms of HD, but no testing, told her boyfriend, because otherwise she’d have to lie about why she was losing jobs.
When I told him, he had problems with me trying to lie about jobs—why I lost them. (HD19/Sx, Unt)
She was displaying symptoms that led to these employment problems and that he may have perceived as well.
At a certain point, disclosures may also be inevitable, impossible to postpone because of treatment. A divorced 49-year-old woman with breast cancer said:
A young woman who had breast cancer started dating somebody, and asked me, “When do you tell?” The guy was going to find out if they got married and she’s going to have another chemo while dating, so, she has to say, “Oh by the way, this is going to happen.” (BC11/Sx, Unt)
Thus, disclosure may be forced by treatment side effects, rather than wholly dictated by aspects of the relationship itself.
Why Not Tell?
Individuals often balanced these reasons to disclose against competing rationales not to do so.
Fear of Rejection
Fears of possible rejection can discourage disclosure, even in a long-term relationship. Disclosures can lead to painful emotional break-ups with romantic partners. “One girl said to me, ‘I can’t go out with you anymore,’” an asymptomatic man with the HD mutation reported. “She was a bitch.” (HD5/Asx, +) He remained angry at her because of both what she decided, and how she told him.
Rebuff can occur because of not only symptoms, but effects of treatment as well. The presence of BRCA 1/2 mutations can prompt prophylactic removal of breasts or ovaries. Loss of ovaries can clearly prevent having children; and individuals often feared that the loss of one or both breasts could impair one’s attractiveness to potential partners. These surgeries can engender negative reactions. A 66-year-old divorced woman with the BRCA mutation and a unilateral mastectomy was rejected by boyfriends.
I’ve had bad reactions from lovers. It’s hard for me to deal with it, so naturally, it’s going to be hard for someone else to deal with it. I don’t feel comfortable with it, even after all these years. So I stay alone. (BC24/Sx, +)
Rather than disclose, she chooses silence, and with it, singlehood. Individuals’ views of their genetic risk or disease—e.g., negatively or uncomfortably—can thus shape prospective mates’ responses. Many women undergo reconstructive surgery after mastectomies, but not all do so, and such reconstruction may remain noticeable, affecting patients’ views of themselves and fears of others’ reactions. She underscores, too, how issues of dating can pose challenges and difficulties for older, as well as younger, people.
What to Tell?
Given these competing pros and cons of disclosure, individuals faced nuanced decisions of what exactly to tell—dilemmas of how much detail to provide. At times, they decided to offer only partial or incomplete information. For any given disease, it appeared easier to disclose simply “being at risk” than to divulge that one in fact had a “mutation”. An unmarried man reported that before recently testing mutation-positive for HD,
I used to be able to say, “I was at risk.” Now, I don’t have that option. I still seem to be without any signs, but I don’t want to be dishonest. (HD5/Asx, +)
Factual medical details shape an individual’s range of options. Here, too, honesty meant the most complete and up-to-date information.
Similarly, at times, individuals said that a disease was “in the family”, but not that it was “genetic” (i.e., that a mutation had been identified), and that a test was available. This additional information could prompt questions of whether the individual had in fact undergone such testing, or planned to do so.
An individual may also not divulge that he or she is at risk, but that a relative is. A 24-year-old single woman whose sister had been rejected said:
Now she’s seeing somebody who loves her and is crazy about her. But, she still hasn’t told him. She said to him, “My mom is a breast cancer survivor”. So she had relief that he would kind of understand. I don’t know if she’s going to tell him about her ovary and breast removal. (BC1/Asx, Unt)
This interviewee suggests a process, of gradually opening the door to the eventual full revelation, rather than disclosing complete information fully and abruptly all at once. Her comments raise a question of whether he may at any point have a right to know, and if so, when. As mentioned earlier, consensus emerged that if individuals are about to get married, disclosure would be essential, due to expectations of mutual trust. But it is not clear whether at a certain point before then, she needed to tell him, and if so, when—i.e., when exactly silence could undermine trust.
Individuals face dilemmas of exactly how much detail to provide concerning not only the eventual prognosis from a mutation, but also the course of the illness (e.g., the potentially long period of disturbing symptoms that can be involved and can burden a spouse). Some decided at a certain point to instill a sense of these difficulties, but these harsh details can be difficult to describe and hear, and can impede a budding romance. As an unmarried man with the HD mutation added,
I don’t think I’ve ever gone out with a girl where I’ve thought: she understands what 20 years of me dying is going to be like. I’ve tried to make them understand: if I start exhibiting symptoms, all bets are off. I don’t want to be in a relationship under those circumstances. Quite frankly, I think I’d kill myself if I got sick. (HD5/Asx, +)
He would prefer not to burden a spouse rather than be in a relationship—i.e., he values her well-being over his own, revealing a sense of altruism.
Individuals may also face decisions of whether to disclose or reveal affected family members. A 43-year-old divorced woman with a family history of HD, but no testing or symptoms, said:
The topic of my mom doesn’t come up. I just describe her as “aging a little, forgetful.” Not many people meet her. I just figure: if I ever get really close to someone, I’ll deal with it. I think I have to make sure this guy falls in love with me first before I tell him I’m defective [laugh]. (HD16/Asx, Unt)
She suggests that she does not present the full details. She says that her mother is “forgetful”, but not that her mother in fact has HD—disclosing the symptoms, but not the diagnosis. This interviewee does not feel that providing this partial knowledge is morally wrong because she does not yet have a strong bond with these men. Her humor disguises deep pain and moral uncertainty. She suggests, too, that she will “deal with” disclosure fully in the future if she needs to—that is, she postpones having to decide about these issues now. The act of broaching the topic slightly, as mentioned above, provides flexibility in eventually leading or not leading to further revelations.
Disclose Reproductive Implications
In dating, individuals must decide, too, whether to provide information concerning reproductive implications, and if so, when. Some, but not all, were aware of prevention methods—for example, the use of PGD to avoid transmitting mutations to offspring. Yet, these methods entail extra expense and potential complications. Nonetheless, a few individuals readily offered such information. A 29-year-old married woman with the HD mutation, but no symptoms, said that at-risk individuals would have to explain to dates,
You’re going to have to take into account, if you want kids: okay, you can have kids, but this is what you’ve got to do to ensure they don’t have this. (HD7/Asx, +)
Before she underwent testing, she had two children. She suggests the potential ordeal involved in undergoing IVF and PGD.
How to Tell?
Management of stigmatized information also forces questions of how to tell, involving both the content and the form of communication.
The Content of Information: Framing
Individuals had to decide how to frame the information. Many tried to not just give bare medical facts, but to cast the news as positively as possible, or at least avoid casting it entirely negatively. “I give information to the girls I date,” a man with the HD mutation said. “I usually email them good news as well as bad.” (HD5/Asx, +)
The Form of Communication: In Person or Not
Individuals also made decisions as to the medium of communication—e.g., in person or by email, which may not be optimal, given the sensitive nature of these topics. Interviewees also used email to disclose to, and update, immediate and extended family members; but were usually far more cautious in dating, feeling disclosures of details here were better done in person. Email can present both advantages and disadvantages, shielding a patient, allowing for some distance from possible immediate rejection, or difficult emotional fall out, and hence making disclosure appear easier in some ways. The recipient also has time to consider how best to respond, potentially offsetting the hurtful emotional sting of an immediate rebuff. Yet email also clearly has limitations, not readily permitting fuller understanding through verbal or nonverbal communication.
When to Tell?
In managing stigma, these interviewees also confronted dilemmas of when to tell. Potential rejection had to be weighed against reasons for disclosing, and individuals thus feared telling either too early or too late. But between these two extremes, to gauge the most appropriate time can be extremely difficult, given costs and benefits. A 36-year-old unmarried man with the HD mutation, but no symptoms, said,
I wonder when is the right time to tell them. On the one hand, you don’t want to tell them right away, because it’s just odd. On the other hand, you don’t want to get married to the person and they don’t know yet—it would be a betrayal of trust not to tell them for so long. I worry about that. I’ve been on dates since I found out, but nothing has really developed, such that I’ve felt obliged to tell them. It’s an important moral issue to tell someone not too late in the relationship. Too early would be odd. Too late would be wrong. You worry about doing the wrong thing. (HD4/Asx, +)
He struggled to avoid both the awkwardness of raising the topic too early and the moral reprehensibility of unduly delaying. But judging when exactly is the right time remained hard. As he suggests, this question entails ethical concerns—doing the “right,” not the “wrong” thing. Yet quandaries can arise in negotiating these conflicts—in balancing one’s own desires for, and right to, privacy vs. one’s sense of implicit responsibility to others. Resolution of these tensions can be highly subjective.
Telling When Close Enough
Individuals often struggle with deciding at what point exactly the relationship, and hence the obligation to tell are strong enough to counter fears of rejection. A divorced 43-year-old woman at risk of HD, but without symptoms or testing, said, “I can’t tell guys I date. Unless I get very close, I won’t let them know.” (HD16/Asx, Unt)
To make these decisions, some participants developed “rules of thumb”, establishing predetermined points at which they would tell. These rules may be firm or flexible, automatic or dependent on other issues. One unmarried man concluded that for HD, for which he had the mutation, disclosure within 2 weeks was appropriate.
I generally tell them within 2 weeks. I don’t want to get into a situation where somebody becomes attached, and doesn’t understand the situation. The last thing I would want is: get a couple of years into a relationship, and then turn around and tell somebody. I don’t know whether my approach works in my favor in the long run or not. I just started dating somebody last week. She’s 32. I don’t yet know enough about her to know what she really wants in life. The last woman I went out with was 37, and had children. It was a little bit easier. (HD5/Asx, +)
To understand another person’s desires and preferences constitutes a process over time in and of itself. A prospective spouse may also not yet have determined his or her long-term wishes.
While this man usually waited 2 weeks, others established benchmarks than required more subjective assessments. Some told only if and when the relationship appeared “serious,” or seemed to have long-term potential. A 61-year-old man with Alpha said,
I wait until I see whether it’s somebody that I might be interested in on a longer term basis. I didn’t bother telling anybody, until I met someone I wanted to date steadily and pursue the relationship. At that point, I would explain and say, “If this is a problem, then you should say so and move on.” I never had anybody back out because of it. Most women—maybe the kind that I picked—would be understanding. (A4/Sx, +)
He suggests, too, though, that he chose women who would be less likely to reject him. He also prefers that women give him their reaction when he tells them, rather than waiting and prolonging a relationship that will eventually end as a result of the disease. Here again, future symptoms—not reproductive implications—can impede dating. Though treatment (Prolastin) exists for Alpha, the disease can still progress, and cause further debilitation.
Telling Only if Asked
Others manage the stigma by disclosing their genetic risk—or saying they will do so—only when they are asked. A divorced 32-year-old woman with a strong family history of breast cancer, but no symptoms or genetic testing done herself, said she would tell a date if it came up, but otherwise would not volunteer the information.
If we were talking about that, and it came up, I would offer it. I wouldn’t withhold it. But I wouldn’t make it come up. (BC20/Asx, Unt)
She sees different degrees of moral obligation to disclose, related to omission vs. commission (i.e., feeling that it is permissible to omit the information unless the topic comes up). She may also feel less obliged to disclose since she herself has had no evidence of disease. The imperative to tell may thus be less here than for those who have a mutation and/or serious disease.
As suggested earlier, the topic can arise of other family members who may be affected. Family history can readily get discussed, and include aspects of family members’ lives that can lead to, or even necessitate, disclosure of disease. A single man, mutation-positive for HD, said,
That’s the problem with the girl I’ve just met. The “tell me about your family” discussion is going to be next. Unfortunately, with my family, there’s nowhere to go. It’s a messy background. It’s hard, especially if I then have to say I had a twin who suicided. It gets into muddy areas. (HD5/Asx, +)
In dating, a significant other may also meet one’s family members. In the case of HD, a family member’s symptoms can be readily visible, forcing the family history, and potentially one’s own risk, to the fore.
Telling Only After Breaking Up
Others resolve these conflicts by choosing to avoid telling as long and as much as possible, opting to end the relationship itself, if the truth should come out. A few waited until after they broke up, and disclosed only then, as friends, to former partners. The 43-year-old divorced woman at risk for HD, but without symptoms or testing, said,
I tell them after I date them, so that they stay my friends [laughing]. A guy I dated 6 years ago is one of my best friends now. (HD16/Asx, Unt)
Thus, she is able to obtain a degree of closeness and support, even if it limits the ultimate nature and extent of these relationships.
Reported Reactions to Telling
Rejection vs. Acceptance
As mentioned above, interviewees reported experiences and fears of rejection, but such rebuff did not always occur. In fact, rejections can be surprising and impossible to predict—at times coming from partners whom patients assumed would be understanding. A 24-year-old single woman with a family history of breast cancer said her sister had been rejected by a boyfriend—who was a physician—because of the disease, and a prophylactic oophorectomy and mastectomy. Such rebuffs can be very painful because they are so unexpected. This interviewee is speaking about someone else, so the full details are not wholly clear, but her description is of note, in and of itself.
My sister told one of her boyfriends that she had had cancer. He flipped out. A week after, they broke up. He was a doctor, but doesn’t know how to react to cancer! Here’s a potential wife, but she’s got cancer. He doesn’t want to marry someone who’s going to die. I said, “You’re better off without him.” But, it was traumatizing to her. (BC1/Asx, Unt)
Factors Affecting Reactions
Several factors may be involved in whether rejection occurs. Genetic risks vary widely in perceived severity and implications. HD, given its lethality and disturbing psychiatric symptoms, can stymie relationships. Rejection also occurred with breast cancer, given its potential lethality, and difficulties of treatment, including removal of breasts and ovaries. Women often feared that these surgeries would impair their physical attractiveness and reproductive abilities (Klitzman and Chung 2009). Overall, at least for breast cancer, symptoms and treatment appeared more likely to elicit rejection than having the mutation but being asymptomatic.
Disease in the potential partner’s family could also help reduce the likelihood of stigmatization and rejection. Some individuals may normalize mutations, even for HD, understanding and accepting the risk. A 35-year-old single man at risk for HD, who is asymptomatic and untested, said,
Everyone’s got something. My girlfriend said cancer runs in her family. Does that mean she shouldn’t have children? No. (HD3/Asx, Unt)
Yet, as he lacks any evidence of the disease, such routinization of possible risk may be easier for him than for those who knew they had symptoms or mutations.
Alternatives to Telling
To manage the stigma, a few individuals adopted alternatives to disclosing one’s risk to a prospective mate.
Not Dating as a Result
As suggested earlier, potential rejection leads some to hesitate or avoid dating altogether. The 66-year-old divorced woman who had a unilateral mastectomy and “bad reactions from lovers” said:
My response is a coward’s: I shy away from the whole situation. I’m totally terrified of dating…Do I tell guys? Should I not date? I really don’t know. I haven’t really made peace with it. It’s difficult enough to have a meaningful relationship anyway. So imagine if you’re physically handicapped! Some women are very brave, others are not. Some men are very compassionate, others not. (BC24/Sx, −)
Prior rejections can thus shape ongoing approaches to these dilemmas. She suggests, too, several psychological factors involved: the potential discloser’s courage, and the listener’s compassion. Again, effects of symptoms and treatment (i.e., being “handicapped”), rather than genetic risks per se, may pose the main obstacle.
Not surprisingly, such avoidance of dating altogether occurs among individuals at risk for HD as well. This avoidance may be partially unconscious, or the manifestation of deeper psychological anxieties. A 36-year-old single man had not been in a relationship since he found that he had the HD mutation. The prospect of having to disclose his risk of HD appeared to impede his dating, yet he had difficulty acknowledging that the mutation had this effect.
Since I found out, I haven’t been intimately involved with anyone. I ask myself: at what point do I have to tell someone? I can’t just lay this on someone on the first date. I’ve only been on a few dates, not anything that has developed into a full-blown relationship. (HD4/Asx, +)
He ponders here an ethical dilemma: at what point is he morally obliged to disclose—that is, whether the ethical claims of a relationship dictate that he do so. Rather than not dating altogether, he tries to resolve the conflict by avoiding more than a handful of dates with any one woman. The lack of a longer term relationship appears to result in part from his concerns about whether to disclose his mutation. The short relationships didn’t impede disclosure. Rather, the fear of disclosure seemed to curtail the relationships.
Third Party Disclosures
When an individual has not divulged genetic risk information to a prospective mate, external others may face questions of whether they should intervene in some way—either pressuring the individual to disclose, or speaking to the patient’s potential mate themselves. At times, for instance, siblings felt that a brother or sister who has not yet disclosed to a significant other should do so. A 31-year-old single woman who tested mutation-negative for HD said that her brother’s current girlfriend may know about his mother who has HD—but not that he himself is at risk.
She’s seen my mom, but I’m sure he hasn’t told her that he is at risk. She’s only 21. I don’t want to discuss it with her and scare her off. (HD21/Asx, −)
According to this interviewee, her brother is embarrassed by their mother, whom he thus doesn’t take his partner to see much.
I’m sure he doesn’t take his girlfriend over to our mom a lot because of how mum is. He lives around the corner, but sees our mom only once a month. It’s embarrassment. (HD21/Asx, −)
These interviewees refrained from in fact proceeding to divulge the information to others’ partners, but occasionally pondered the possibility, and encouraged these patients to disclose—though usually without success. These external others thus had to decide how much to push for such disclosure vs. desist.
Factors Affecting Disclosure
Clearly, as indicated above, several factors can affect individuals’ decisions of how to manage this information. Medical factors, such as degrees of risk, treatment, and types of disease, can play roles here. Alpha, which is treatable, appeared easier to disclose than HD. The degree and certainty of impact on future offspring also appeared to affect these decisions. Psychological factors, such as whether the individual and his or her prospective mate desired children influenced these choices as well. Hence, older women, past child-bearing age, were able to avoid an important impediment to disclosure—potential partners’ expectations of having children.
Conclusions
These data suggest how individuals confronting genetic risk or illness face potential stigma and rejection in dating, and hence struggle to manage this information through a series of decisions of whether to date, and if so whether, what, how, and when to disclose their genetic risk. Despite fears of rejection, they often felt ethically obliged to disclose at a certain point, at which they felt that expectations of, and desires for, mutual trust in the developing relationship dictated that they be as forthcoming as possible, and that their partner thus had a right to the information. They felt that these implicit expectations of trust then outweighed fears of rejection. Yet, individuals had to balance these reasons to divulge this information against these anxieties, and thus decide exactly when, what, and how to disclose. Interviewees varied in decisions about when to disclose (i.e., sooner vs. later), based partly on other events, with some individuals delaying or avoiding disclosure as long as possible. Interviewees described a spectrum, too, in what to tell—from the disease being “in the family”, but not necessarily “genetic”, to its implications for possible offspring. Differences emerged in how disclosures occurred, from direct and planned to indirect and inadvertent (e.g., due to partners meeting relatives affected by the disease). Some individuals thus tried to disguise or hide ill family members’ symptoms from prospective mates. Several medical, psychological, and social factors shaped these decisions, including symptoms and severity of disease, testing status, and the newness of a relationship. These challenges led some individuals, particularly if affected by HD, not to date at all.
One could simply say that all individuals should disclose when a relationship becomes serious. But more nuanced problems emerge here, because determinations of when exactly a relationship seems serious enough to overcome fears of rejection can be unclear, and genetic information that pertains to oneself may arise in the course of discussions about parents or siblings, forcing decisions about disclosure. Divulging genetic information and STIs raise similar anxieties and delays, yet genetic information differs from STIs and many other kinds of medical information in that family members’ diseases can implicate an individual, potentially stigmatizing him or her, and thus at times prompting efforts to disguise one’s family medical history. Moreover, treatments exist for STIs, but not for all genetic disorders (e.g., HD).
These issues have reproductive implications, many aspects of which have received little attention. Disclosures of genetic risks differ from disclosures of other aspects of oneself, including other disabilities and diagnoses, since genetics can also affect future generations. This possibility makes these disclosures and decisions about them more serious, and hence difficult, as they potentially affect third parties—future descendants.
Increasingly, PGD has been used for HD, BRCA, and other disorders, potentially reducing the implications of disclosures, since PGD can eliminate transmission of the disease to offspring (Klitzman et al. 2008). But PGD is expensive, as it necessitates IVF, which can cost $20,000 a cycle, which insurance companies generally do not cover. PGD and IVF may also not be successful, with any one cycle of IVF generally having a “take home baby” rate of only approximately 26% (Canadian Fertility and Andrology Society 2007). PGD can involve complications as well, frequently causing side effects due to medications involved. Thus, not all individuals may want, or be able to afford, PGD. Patients may not even know about it, in part because it is still relatively new. Hence, it can be important that genetic counselors be prepared to discuss these issues with patients.
Hoskins et al. (2008) explored several aspects of dating experiences among 11 young asymptomatic women with BRCA 1/2 mutations, focusing on the fact that disclosure is a process over time, but additional issues arose here. Specifically, these interviewees show how this process can in fact consist of a series of distinct but related ethical, social, and psychological decisions that these individuals then have to confront (i.e., what, how, and when to disclose). Moreover, the present data highlight how dilemmas arise concerning not just the timing and method of disclosure, but the content as well—i.e., merely “being at risk” vs. “having a mutation” vs. “having symptoms”. While Hoskins suggested that women often wanted to raise the topic “naturally” in conversation vs. avoid it, the present data underscore a series of difficulties in determining exactly when to disclose. These individuals further illustrate how disclosures in dating (while relationships themselves are evolving) represent several complex processes over time, rather than single, all-or-none decisions. With time, individuals may divulge increasing numbers of details.
While Werner-Lin (2008) found that differences existed in the urgency of finding a life partner between single vs. partnered women, and those who wanted vs. did not want children, the current data reveal several additional issues, related to how individuals confront disclosure decisions as a result of these desires and how these desires can compete with other concerns. Additional factors include lengths and types of relationships, differences in specific diseases and their symptoms, treatability, presence and severity of symptoms, knowledge of having a mutation vs. being at risk but not yet tested, and extents of family history. Disclosure dilemmas confront not only individuals who have undergone genetic testing, but those who are untested. While Hoskins reported that disclosures of BRCA may bond couples more closely, and that subjects with more perceived risk often had better partner communication, these phenomena may occur much less with HD, a fatal, untreatable disease. Similarly, negative reactions from dates received little attention in Hoskins’ study, but arose here, in part because HD may present more frightening implications for many people. In addition, while young women at risk for breast cancer often test in order to inform family planning (Decruyenaere et al. 1996), these issues may differ with other genetic diseases such as HD, where the lack of treatment causes stress and fatalism that can offset the potential benefits of testing. While Evers-Kiebooms et al. (2000) and others have suggested that psychosocial issues may vary in some ways between different genetic disorders, the present data highlight additional factors that may be involved. For instance, presence and severity of symptoms may cut across diagnoses, and prove to be more important than specific diagnoses per se. Future research can explore these types of differences more fully.
The issues here resemble in some regards those concerning disclosures to other sets of people (e.g., family members), but differences emerge, too. With prospective mates, added stresses exist, related to possible transmission of a mutation to future children, and the fact that severe rejection can ensue—termination of the entire relationship—which ordinarily does not occur with immediate family members. In existing families, years of trust can profoundly shape disclosures, while dating relationships are generally far newer.
These data suggest that how, when, and what genetic information individuals present can affect each of the theoretical models of relationship development mentioned earlier (Surra 1990). For instance, disclosure of genetic risk may create instability in relationships. The information may emerge only over time, and can impair the relationship’s development. The extent of a relationship can affect genetic disclosure, which can in turn further affect the extent of the relationship (e.g., weakening or strengthening it). Decisions about genetic risk disclosures can thus potentially determine which model a relationship takes (e.g., rapid vs. more gradual development). Prior desires for a particular model (e.g., rapid vs. gradual) can potentially also shape disclosure decisions in ways that future research can explore further. Consistent with past literature suggesting the existence of assortative mating (i.e., that people often choose partners who have similar characteristics—e.g., values, height, weight—to themselves), the present data suggest that partners who are more familiar with serious disease in their own family may be more accepting.
Importantly, none of these individuals appear here to have been unethical in their disclosures. Rather, they highlight the challenges that people at risk of genetic diseases confront, with which enhanced professional, patient, and public awareness and education can help. Importantly, disclosure can represent a process in which patients slowly begin to broach the topic, and provide increasing details over time.
Practice Implications
These data have several critical sets of implications for professional practice, and provider, patient, family, and broader public education—to increase awareness and sensitivity to the series of dilemmas that arise here. Not all of these interviewees had met with genetic counselors, and those who had done so described how these counselors tended to focus on testing decisions, and did not always talk about disclosures. None of these interviewees mentioned genetic counselors or other providers discussing disclosures in dating situations.
Yet genetic counselors and other providers can help patients in facing this set of decisions and in thinking through these issues in advance. Importantly, providers can assist patients in viewing disclosures of genetic risk in dating as a process consisting of a series of decisions. Careful consideration of these choices can potentially help patients maximize their support and well-being, and avoid painful rejection. Still, to determine when exactly disclosure is optimal can be difficult. Patients may struggle to disclose neither too early (on a first or second date), nor too late (when marriage plans are underway). Predictions of a potential mate’s reactions may be inaccurate. Rejection, though feared, can potentially ensue, but does not always occur. Recognition that the patient has a series of choices can thus potentially be beneficial.
It is vital that at-risk individuals, their loved ones, genetic counselors, and other health care providers understand and appreciate these complex issues and factors, and address them as sensitively and effectively as possible. Providers’ full exploration of these issues can potentially help patients make these decisions (i.e., how much information to provide when, and how to respond to a date’s questions and concerns). Balancing these competing issues (e.g., how exactly to weigh perceived expectations of trust vs. fears of rejection) can be hard. But providers can assist patients by helping them decide what exactly to say when to whom, and how to do so, to try to avoid suboptimal disclosures (e.g., saying too much too early or too little too late). Patients’ fears of rejection may arise in part from patients’ own feelings about their genetic risk (e.g., potentially feelings of stigma or shame), and hence providers may be able to help patients overcome unrealistic fears that may impede abilities to lead satisfying lives.
Public education efforts can also reduce stigma and increase understanding of genetic disease. Education about PGD can be important, too, in reducing fears of future children being affected by genetic disease, since this technology can now lower these risks, though this procedure poses challenges, too. Potentially, patients and providers could also consider having prospective partners join genetic counseling sessions to help these partners understand the genetic risks involved.
These data underscore, too, how genetic counselors need to be flexible in their approaches, since these concerns can vary widely with the specific medical, psychosocial, and social details of each individual’s life. Differences may emerge not only between diseases, but among individuals at risk for any one disease. These data thus highlight the needs for genetic counselors and other providers to be aware of the range of variations that may arise due to these factors.
Several of these interviewees are older (e.g., in mid-40s through 60s). Yet the prior literature on genetic risks in dating has focused on younger women between the ages of 22 and 35 who may end up having children (Werner-Lin 2008; Hoskins et al. 2008). Dating among older adults, in general, has recently been receiving attention (Bulcroft and Bulcroft 1991). Such relationships can lead to enhanced social support for the members of the couple, avoiding isolation due to widowhood (Dickson et al. 2005; Carr 2004). The present data suggest that genetic counselors should consider discussing issues regarding disclosures in dating with not only younger patients, but older ones as well (e.g., in their 60s). These patients face issues related not to reproduction, but to concerns about potentially needing the prospective mate to be a caregiver.
Recent research on dating in general has also begun to examine the phenomenon of on-line dating (Gibbs et al. 2006). Over upcoming years, providers may thus need to be prepared to discuss these issues concerning disclosures of genetic risk in the context of Internet dating as well.
Implications for Research
These data suggest several areas for future research to explore these issues more fully—e.g., to investigate in further detail how these decisions concerning what, when, and how to disclose may affect whether rejection occurs or not, and how providers can best help patients confront these choices. Such studies can examine more thoroughly how often genetic counselors or other providers discuss these issues with patients, and how often and in what ways these discussions affect patients’ decisions, and thereby reduce possible rejection. This research can further assess how specific medical, social, and psychological factors (e.g., types and extent of present and future symptoms) shape relationship development vs. termination, and what differences emerge within particular subgroups (e.g., single women who want to have children) related to each of these factors.
Future investigations can explore, too, what specific educational or practice interventions can help, and how and to what degree. Prospective research can also follow participants longitudinally over time to probe in more detail how these simultaneous processes (i.e., working toward establishing a relationship, deciding about whether to have children, and adjusting to a genetic risk or mutation and its medical implications) occur in parallel, yet interact and influence each other.
Potential Study Limitations
These data may have several potential limitations. This qualitative study was designed to explore the ranges of issues that these individuals confront, not to quantitatively measure frequencies of specific attitudes. Hence, such rates and statistics are not presented, but can be measured in future research among larger samples. Future studies can also then analyze statistical associations between aspects of disclosure and medical, social, and psychological variables (e.g., gender, race and ethnicity, and disease and symptom status). Participants’ partners were not interviewed as part of the present study, but future research can do so. Still, these participants’ views and experiences are valuable in and of themselves as the first published data to explore several of these issues. These participants were interviewed at one point in time only, but described their past as well as present experiences. Future prospective investigations can probe these issues, too, over time.
Concerning potential gender differences, gender emerged as one possible factor here among many—including medical, psychological, and social variables, as described above. Given the size of the present study, clear-cut differences do not readily emerge that resulted unequivocally from gender, rather than from other factors. Indeed, Lee and Craft (2002), in their study of disclosures of genital herpes during dating, also found that their sample was too small to meaningfully compare men and women. But overall they found no differences between genders. Thus, though one might think that women may be more concerned with issues about having future children, men here, too, expressed interest in having children, and these wishes affected their decisions. Moreover, with breast cancer, this study included only women, making such a gender comparison impossible. However, future research on larger samples of individuals confronting genetic disease can more fully investigate whether significant gender differences emerge, in relation to these other factors, and if so, when.
In sum, as the first study we know of to examine many of these issues among individuals who confront several different genetic disorders, and are of varying mutation and symptom status, ages, and genders, these data offer valuable insights into the ranges of decisions concerning disclosures of genetic risk that arise in dating situations. These data suggest several sets of factors that genetic counselors and others should be aware of to help patients, and that future studies can further explore.
Acknowledgments
I would like to thank Wendy Chung, Karen Marder, Deborah Thorne, Carol Moskowitz, Jennifer Williamson, Edward Eden, Lori Tartell, Rubie Senie, Victor Grann, Carolyn Kumah, Melissa Conley, and Lisa Chin. This research was funded by a grant from the Ethical, Legal and Social Implications Program of the National Human Genome Research Institute (R01-HG002431-01).
Appendix
Selected Questions From Semi-Structured Interview Guide
Whom have you told or not told about your having, or being at risk for, a genetic disease?
Have you told friends, family members, or significant others?
In each case, what, when, and why did you decide to disclose?
How did you make these decisions?
What reactions did you encounter?
Are there people you chose not to tell? Who? Why?
How do you view these decisions?
Who was the most difficult person to tell? Why? What did you do?
Do you have any other thoughts about these issues?
References
- Belot M, Francesconi M. Can anyone be “the” one? Evidence on mate selection from speed dating. Bonn: Institute for the Study of Labor; 2006. [Google Scholar]
- Bulcroft RA, Bulcroft KA. The nature and functions of dating in later life. Research on Aging. 1991;13(2):244–260. [Google Scholar]
- Canadian Fertility and Andrology Society. Human assisted reproduction live birth rates for Canada. [Accessed on April 22, 2010];2007 Available from: http://cfas.cfwebtools.com/index.cfm?objectid=FB99277D-FF33-EC88-991F86750424B6A7.
- Carr D. The desire to date and remarry among older widows and widowers. Journal of Marriage and Family. 2004;66(4):1051–1068. [Google Scholar]
- Christianson M, Lalos A, Johansson EE. The Law of Communicable Diseases Act and disclosure to sexual partners among HIV-positive youth. Vulnerable Children and Youth Studies. 2008;3(3):234–242. doi: 10.1080/17450120802069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decruyenaere M, Evers-Kiebooms G, Boogaerts A, Cassiman J, Cloostermans T. Prediction of psychological functioning one year after the predictive test for Huntington’s disease and impact of the test result on reproductive decision making. Journal of Medical Genetics. 1996;33:737–743. doi: 10.1136/jmg.33.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson FC, Hughes PC, Walker KL. An exploratory investigation into dating among later-life women. Western Journal of Communication. 2005;69(1):67–82. [Google Scholar]
- DudokdeWit AC, Tibben A, Frets PG, Meijers-Heijboer EJ, Devilee P, Klijn JG, et al. BRCA1 in the family: a case description of the psychological implications. American Journal of Medical Genetics. 1997;71(1):63–71. doi: 10.1002/(sici)1096-8628(19970711)71:1<63::aid-ajmg12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Evers-Kiebooms G, Welkenhuysen M, Claes E, Decruyenaere M, Denayer L. The psychological complexity of predictive testing for late onset neurogenetic diseases and hereditary cancers: implications for multidisciplinary counselling and for genetic education. Social Science & Medicine. 2000;51:831–841. doi: 10.1016/s0277-9536(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Fortuny D, Balmana J, Grana B, Torres A, Ramon y Cajal T, Darder E, et al. Opinion about reproductive decision making among individuals undergoing BRCA1/2 genetic testing in a multicentre Spanish cohort. Human Reproduction. 2009;1(1):1–7. doi: 10.1093/humrep/den471. [DOI] [PubMed] [Google Scholar]
- Geertz C. Thick description: toward an interpretive theory of culture. New York: Basic Books; 1973. [Google Scholar]
- Gibbs JL, Ellison NB, Heino RD. Self-presentation in online personals: the role of anticipated future interaction, self-disclosure, and perceived success in internet dating. Communication Research. 2006;33(2):152–177. [Google Scholar]
- Goffman E. Stigma: Notes on the management of spoiled identity. Englewood Cliffs: Prentice-Hall; 1963. [Google Scholar]
- Green J, Ferrier S, Kocsis A, Shadrick J, Ukoumunne OC, Murphy S, et al. Determinants of disclosure of genital herpes to partners. Sexually Transmitted Infections. 2003;79:42–44. doi: 10.1136/sti.79.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82. [Google Scholar]
- Hallowell N, Foster C, Eeles R, Ardern-Jones A, Murday V, Watson M. Balancing autonomy and responsibility: the ethics of generating and disclosing genetic information. Journal of Medical Ethics. 2003;29:74–83. doi: 10.1136/jme.29.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Cultural factors in the selection of marriage mates. American Sociological Review. 1950;15(15):619–627. [Google Scholar]
- Hoskins LM, Roy K, Peters JA, Loud JT, Greene MH. Disclosure of positive BRCA 1/2-mutation status in young couples: the journey from uncertainty to bonding through partner support. Families, Systems & Health. 2008;26(3):296–316. doi: 10.1037/a0012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R. Genetic Discrimination Post-GINA: subtle and indirect discrimination. Journal of Genetic Counseling. 2010a;19(1):68–83. doi: 10.1007/s10897-009-9262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R. Misunderstandings concerning genetics among patients confronting genetic disease. Journal of Genetic Counseling. 2010b doi: 10.1007/s10897-010-9307-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitzman R, Appelbaum P, Chung W. Anticipating issues related to increasing Pre-implantation genetic diagnosis use: A research agenda. Reproductive Biomedicine. 2008;17(1) doi: 10.1016/s1472-6483(10)60188-5. http://www.rbmonline.com/4DCGI/Article/Detail?38%091%09=%203517%09. [DOI] [PubMed]
- Klitzman R, Bayer R. Mortal secrets: Truth and lies in the age of AIDS. Baltimore: Johns Hopkins University Press; 2003. [Google Scholar]
- Klitzman R, Chung W. Decision-making about prophy-lactic surgery among individuals with BrCA 1/2 genetic mutations: clinical and ethical conundrums. American Journal of Medical Genetics. 2009;152A(1):52–66. [Google Scholar]
- Klitzman R, Kirshenbaum SB, Kittel L, Morin SF, Daya S, Mastrogiacomo M, et al. Naming names: perceptions of name-based HIV reporting, partner notification, and criminalization of non-disclosure among persons living with HIV. Sexuality Research and Social Policy. 2004;1(3):38–57. [Google Scholar]
- Laurell CB, Erikson S. The electrophoretic alpha1 globulin pattern of serum in alpha antitrypsin deficiency. Scandinavian Journal of Clinical and Laboratory Investigation. 1963;15:132. [Google Scholar]
- Lee JD, Craft EA. Protecting one’s self from a stigmatized disease… once one has it. Deviant Behavior. 2002;23(3):267–299. [Google Scholar]
- Li NP, Bailey JM, Douglas TK, Linsenmeier JAW. The necessities and luxuries of mate preferences: testing the tradeoffs. Journal of Personality and Social Psychology. 2002;82(6):947–955. [PubMed] [Google Scholar]
- Manne S, Ostroff J, Rini C, Fox K, Goldstein L, Grana G. The interpersonal process model of intimacy: the role of self-disclosure, partner disclosure, and partner responsiveness in interactions between breast cancer patients and their partners. Journal of Family Psychology. 2004;18(4):589–599. doi: 10.1037/0893-3200.18.4.589. [DOI] [PubMed] [Google Scholar]
- McCaffery K, Waller J, Nazroo J, Wardle J. Social and psychological impact of HPV testing in cervical screening: a qualitative study. Sexually Transmitted Infections. 2006;82:169–174. doi: 10.1136/sti.2005.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter LS, Keefe FJ, Hurtwitz H, Faber M. Disclosure between patients with gastrointestinal cancer and their spouses. Psycho-Oncology. 2005;14:1030–1042. doi: 10.1002/pon.915. [DOI] [PubMed] [Google Scholar]
- Rudnick, Joanna . In The Family. USA: Kartemquin Films; Oct 1, 2008. [Google Scholar]
- South SJ. Sociodemographic differentials in mate selection preferences. Journal of Marriage and the Family. 1991;53:928–940. [Google Scholar]
- Strauss A, Corbin J. Basics of qualitative research—techniques and procedures for developing grounded theory. Newbury Park: Sage; 1990. [Google Scholar]
- Surra CA. Research and theory on mate selection and premarital relationships in the 1980s. Journal of Marriage and the Family. 1990;52:844–865. [Google Scholar]
- Werner-Lin A. Beating the biological clock: the compressed family life cycle of young women with BRCA gene alterations. Social Work in Health Care. 2008;47(4):416–437. doi: 10.1080/00981380802173509. [DOI] [PubMed] [Google Scholar]
- Wilcke JTR, Seersholm N, Kok-Jensen A, Dirksen A. Transmitting genetic risk information in families: attitudes about disclosing the identity of relatives. The American Journal of Human Genetics. 1999;65:902–909. doi: 10.1086/302531. [DOI] [PMC free article] [PubMed] [Google Scholar]