Abstract
As part of the randomized MRC Myeloma IX trial, we compared an attenuated regimen of cyclophosphamide, thalidomide, and dexamethasone (CTDa; n = 426) with melphalan and prednisolone (MP; n = 423) in patients with newly diagnosed multiple myeloma ineligible for autologous stem-cell transplantation. The primary endpoints were overall response rate, progression-free survival, and overall survival (OS). The overall response rate was significantly higher with CTDa than MP (63.8% vs 32.6%; P < .0001), primarily because of increases in the rate of complete responses (13.1% vs 2.4%) and very good partial responses (16.9% vs 1.7%). Progression-free survival and OS were similar between groups. In this population, OS correlated with the depth of response (P < .0001) and favorable interphase fluorescence in situ hybridization profile (P < .001). CTDa was associated with higher rates of thromboembolic events, constipation, infection, and neuropathy than MP. In elderly patients with newly diagnosed multiple myeloma (median age, 73 years), CTDa produced higher response rates than MP but was not associated with improved survival outcomes. We highlight the importance of cytogenetic profiling at diagnosis and effective management of adverse events. This trial was registered at International Standard Randomized Controlled Trials Number as #68454111.
Introduction
Induction therapy followed by high-dose chemotherapy with autologous stem-cell transplantation (ASCT) is the standard of care for myeloma patients who can tolerate this therapeutic approach.1,2 However, many patients are ineligible because of advanced age and/or comorbidities,3 and alternative, less toxic, approaches are needed for such patients. Since the 1960s, the standard therapy for patients ineligible for ASCT has been combination melphalan and prednisolone (MP). Although numerous combinations of conventional chemotherapeutic agents have been compared with MP over the years, none has improved overall survival (OS).4,5
Thalidomide kills malignant plasma cells directly and also has antiangiogenic properties and other effects on the bone marrow microenvironment that may synergize with chemotherapy to induce apoptosis.6,7 Thalidomide is active in elderly patients with newly diagnosed multiple myeloma (NDMM), particularly when combined with other active agents, such as dexamethasone8–10 or MP.11–14 Based on initial good responses, several studies have examined the impact of the addition of thalidomide to MP; and although some have shown improved OS,11,12 others have not.13–16
The combination of cyclophosphamide, thalidomide, and dexamethasone (CTD) is an effective and less myelosuppressive regimen than MP and can also be given as induction therapy before high-dose therapy and ASCT (G.J.M., F.E.D., W.M.G., S.E.B., A.J.S., N.N.C., G.C., S.F., J.L.B., H.R., C.R., M.T.D., R.G.O., F.M.R., N.H.R., G.H.J., and J.A.C., manuscript submitted, January 2011).17 Here we report the results of a large multicenter, randomized study (MRC Myeloma IX) in which we compared the efficacy and safety of an attenuated CTD regimen (CTDa) with that of the previous standard therapy, MP, in patients with NDMM who were ineligible for high-dose therapy and ASCT. The influence of age and cytogenetic profile on survival outcomes after these treatments was also assessed.
Methods
Criteria for enrollment
Patients 18 years of age or older with NDMM were eligible; exclusion criteria included pregnancy, acute renal failure, asymptomatic myeloma, solitary bone plasmacytoma, extramedullary plasmacytoma, and previous or concurrent active malignancies. A multicenter research ethics committee and local ethics committees approved the protocol; all patients gave written informed consent in accordance with the Declaration of Helsinki.
Study protocol and randomization
The MRC Myeloma IX study is a multicenter, phase 3, factorial-design trial (International Standard Randomized Controlled Trials Number 68454111). Younger, fitter patients entered the intensive treatment pathway (high-dose chemotherapy and ASCT, study results described elsewhere), whereas older, less-fit patients entered the nonintensive pathway (study results presented here). The pathway allocation was thus at the physician's discretion after informed discussion with patients. In the nonintensive pathway presented in this manuscript, patients were randomized to either MP (melphalan 7 mg/m2 per day and prednisolone 40 mg/day, both given on days 1-4 of each 28-day cycle) or CTDa. The CTDa regimen was composed of cyclophosphamide 500 mg/week; thalidomide 50 mg for 4 weeks and increased every 4 weeks in 50-mg increments to a maximum of 200 mg/day (thalidomide was reduced from a standard dose of 100 mg); and dexamethasone 20 mg/day on days 1 to 4 and 15 to 18 of each 28-day cycle (dexamethasone was reduced from a standard dose of 40 mg). The therapeutic aim for both treatment arms was to treat to maximum response, with a minimal of 6 cycles to a maximum of 9 cycles if the treatment was well tolerated. Initially, consideration of thromboprophylaxis was recommended for patients at risk of venous thromboembolism. From June 2006, thromboprophylaxis (eg, warfarin, low molecular weight heparin) was recommended for all patients receiving CTDa for the first 12 weeks of treatment. Randomization was on a 1:1 basis and open-labeled. Randomization was performed by the Clinical Trials Research Unit at the University of Leeds (Leeds, United Kingdom), using an automated 24-hour telephone system. The randomization used minimization based on treatment center, and hemoglobin (< 11.5 vs ≥ 11.5 g/dL for males and < 9.5 vs ≥ 9.5 g/dL for females), corrected serum calcium (< 2.6 vs ≥ 2.6mM), serum creatinine (< 140 vs ≥ 140μM), and platelet levels (< 150 vs ≥ 150 cells × 109/L). All patients (intensive and nonintensive pathways) were also randomized at study entry to a bisphosphonate, either sodium clodronate (1600 mg/day) or zoledronic acid (4 mg every 21-28 days), with continuation until progression. Eligible patients who completed induction therapy and had no evidence of disease progression or relapse were randomized to thalidomide maintenance therapy or no therapy. The results from the bisphosphonate and maintenance randomizations are presented elsewhere.18,19
Efficacy endpoints
The primary endpoints were response, progression-free survival (PFS), and OS. Secondary endpoints were quality of life (to be reported separately) and toxicity. Response was defined according to the modified European Group for Blood and Marrow Transplantation/International Bone Marrow Transplant Registry criteria.20 PFS was defined as the time from initial randomization to documented progression or death; progression was defined as relapse from complete response (CR) if the patient had achieved CR, or progressive disease if the patient has not achieved CR.20 Follow-up assessments by local investigators occurred every 4 weeks during induction therapy and every 3 months thereafter. Blood and urine samples were submitted for central review before study entry, after induction therapy (at the time of maximal response), and every 3 months thereafter until disease progression or relapse. Bone marrow aspirates, smears, and core biopsy in formalin were submitted for central review before study entry, after induction therapy (at the time of maximal response), and at relapse to determine plasma cell infiltration and phenotypic pattern.
Safety assessment
Treatment-associated adverse events per regimen group were recorded. Thromboembolic events and acute renal failure were required to be reported for all patients if they occurred during the study period or until death or disease progression.
Cytogenetic characterization
Bone marrow aspirates were collected at study entry to determine the cytogenetic profiles of patients by fluorescence in situ hybridization (FISH) on CD138 purified plasma cells (Miltenyi Biotec), with patients classified as having “favorable” or “adverse” interphase FISH cytogenetic results. Adverse interphase FISH cytogenetic profiles were defined as gain(1q), t(4;14), t(14;20), t(14;16), and del(17p). Favorable interphase FISH cytogenetic profiles composed the remainder and included hyperdiploidy, del(1p32), t(11;14), and t(6;14).
Statistical analysis
The sample size was based on testing the hypothesis that CTDa is superior to MP in terms of PFS, OS, and response. It was anticipated that 850 patients (n = 425 per group) would be randomized in the nonintensive pathway. A total of 204 patients (n = 102 per group) would provide 80% power at 5% significance, to detect a 15% absolute difference in 5-year survival (2-tailed test). This was based on an estimated 15% 5-year survival rate of patients in the MP group. If 182 patients (n = 91 per group) were entered into the nonintensive pathway, the trial would be powered to detect an increase in CR from 20% with MP to 40% with CTDa (80% power at 5% significance). The anticipated number per group (n = 425) would provide > 80% power to detect this difference in response. The required number of events was 152.
Analyses were based on the intention-to-treat population (defined as all randomized patients, excluding those who withdrew consent) unless stated otherwise. For the primary endpoints of PFS and OS, the assumption of no interaction (chemotherapy effect depending on bisphosphonate and vice versa) between induction chemotherapy and bisphosphonates was prospectively tested before endpoint assessments. Cox models were used to obtain P values for the treatment effects and the interaction, without adjusting for the minimization factors. Cox proportional hazards models were used to compare treatment groups while adjusting for bisphosphonate treatment and the minimization factors (treatment center, and hemoglobin, calcium, creatinine, and platelet levels). Patients with missing follow-up data or those who were not known to have progressed or died at the time of analysis were censored at the last date they were known to be progression-free or alive, respectively. Proportional hazards were assessed by plotting the hazards over time for each treatment arm. For response, groups were compared with respect to the proportion achieving response (partial response, very good partial response [VGPR], or CR) using logistic regression to account for bisphosphonate treatment and the minimization factors.
Statistical analysis was performed using SAS Version 9 (SAS Institute) or Fortran software. All hypothesis tests are 2-sided and at the 5% significance level. Kaplan-Meier curves were constructed to compare survival outcomes between treatment groups and in various subgroups. The primary endpoints were ranked, and no claims on a rank lower than or equal to the first whose hypothesis could not be rejected were made. Improvement in response without benefit in survival would not change practice; therefore, PFS and OS have equal ranking, and response has a lower ranking.
For the main treatment comparison of OS, the hazards departed significantly from proportionality (P = .01 Kolmogorov-type supremum test based on 1000 simulations) with crossing Kaplan-Meier curves. Hazard ratios (HRs) were plotted over time to establish the changing HR pattern, and this graphical method was used to establish the time at which the HR changed. The data were then analyzed using piecewise hazards before and after those times as previously described.21
Results
Patients
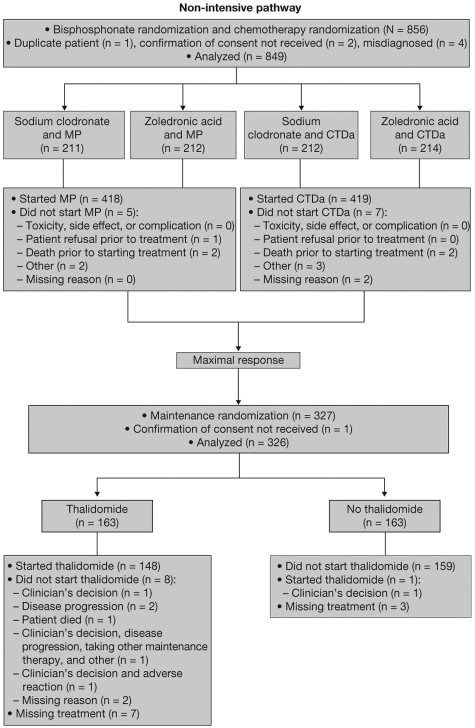
The trial commenced in 2003 and terminated in 2007, recruiting 1970 patients from 120 centers in the United Kingdom. Treatment disposition is shown in Figure 1. Treatment was initiated in 418 patients in the MP group and 419 patients in the CTDa group. A total of 161 patients in the MP group and 165 patients in the CTDa group (38.4% of all patients in the nonintensive pathway) completed induction and underwent second randomization to thalidomide maintenance therapy (n = 163) or no maintenance therapy (n = 163) in the second phase of the study.
Figure 1.
Consort diagram of the nonintensive pathway of the MRC Myeloma IX trial.
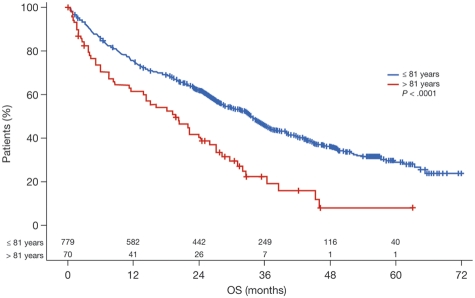
On January 20, 2009, the Trial Steering Committee agreed to disclose the chemotherapy results. Here, a cutoff date of October 5, 2009, was used. Patient characteristics were generally well balanced between treatment groups. Overall, a substantial proportion of patients (76% in each treatment group) had advanced-stage disease (International Staging System stage II or III; Table 1). Median β2-microglobulin level was 4.9 mg/L (range, 0.3-64.0 mg/L) and median age was 73 years (range, 57-89 years). In an exploratory analysis to determine the impact of age on outcomes, a cutoff point at 81 years was noted; patients older than this (n = 70) had significantly shorter survival than younger patients (P < .0001; Figure 2).
Table 1.
Patient characteristics at baseline
| Characteristic | MP (n = 423) | CTDa (n = 426) |
|---|---|---|
| Female sex, n (%) | 192 (45.4) | 184 (43.2) |
| Median age, y (range) | 73 (57-89) | 73 (58-87) |
| ISS disease stage, n (%) | ||
| I | 64 (15.1) | 46 (10.8) |
| II | 156 (36.9) | 156 (36.6) |
| III | 165 (39.0) | 168 (39.4) |
| Missing data | 38 (9.0) | 56 (13.1) |
| Median β2-microglobulin level, mg/L (range) | 4.9 (0.3-40.4) | 5.0 (0.4-64.0) |
| Paraprotein type, n (%) | ||
| IgG | 257 (60.8) | 248 (58.2) |
| IgA | 101 (23.9) | 100 (23.5) |
| IgM | 1 (0.2) | 2 (0.5) |
| No paraprotein | 5 (1.2) | 7 (1.6) |
| IgD | 3 (0.7) | 10 (2.3) |
| Light chain only | 49 (11.6) | 54 (12.7) |
| Missing data | 7 (1.7) | 5 (1.2) |
ISS indicates International Staging System; and Ig, immunoglobulin.
Figure 2.
Survival in patients assigned to the nonintensive pathway based on age, using the optimum age cutoff point (81 years).
Response
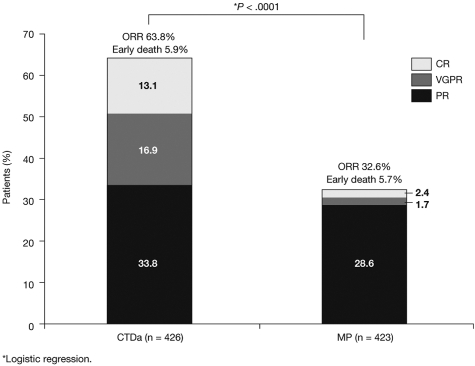
The overall response rate (ORR) was significantly higher in the CTDa group compared with the MP group (63.8% vs 32.6%, respectively; P < .0001). The increased ORR was primarily the result of increases in the CTDa group versus the MP group of CRs (13.1% vs 2.4%, respectively) and VGPRs (16.9% vs 1.7%; Figure 3).
Figure 3.
Response to MP and CTDa.
Survival
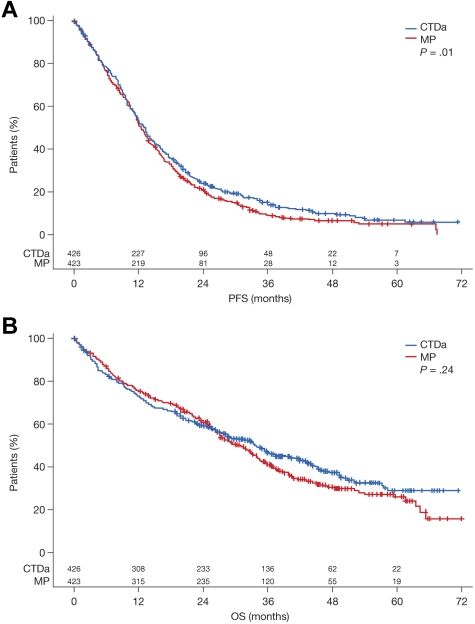
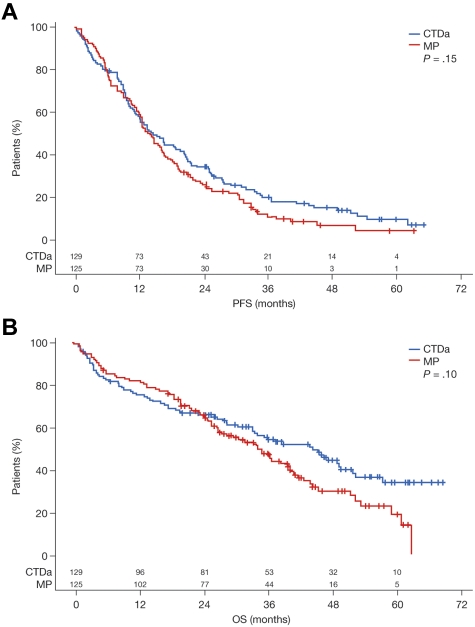
The median follow-up in patients entered into the nonintensive pathway was 44 months. The assumption of no treatment interaction for the PFS and OS endpoints was valid (PFS, P = .75; OS, P = .13), enabling the data to be analyzed in a factorial fashion as it was designed. Median PFS was 12.4 months in the MP group and 13.0 months in the CTDa group (HR = 0.82; 95% confidence interval [CI], 0.70-0.96; P = .01 in Cox model; Figure 4A). The median OS was 30.6 months in the MP group and 33.2 months in the CTDa group (HR = 0.89; 95% CI, 0.74-1.08; P = .24 in Cox model; Figure 4B). These findings suggest marginal benefit for CTDa. Moreover, although the Kaplan-Meier curves for OS suggest an early benefit for MP, the curves crossed after 18 to 24 months and remained separated thereafter in favor of CTDa, therefore suggesting an emergent survival benefit with CTDa.
Figure 4.
Survival according to treatment group with log-rank P value. (A) PFS. (B) OS.
Influence of cytogenetic profiles
Approximately half of patients in the nonintensive pathway underwent FISH cytogenetic profile testing at study entry. Of these, 42% had adverse interphase FISH, the remainder composing the favorable cytogenetic profile group. The 2 treatment groups were generally well balanced with regard to the prevalence of cytogenetic abnormalities at baseline (Table 2). For the favorable interphase FISH group, the median PFS and OS were 14 months (95% CI, 12-17 months) and 37 months (95% CI, 27-44 months), respectively. For the adverse interphase FISH group, the median PFS and OS were 12 months (95% CI, 10-13 months) and 24 months (95% CI, 20-28 months), respectively. The OS for patients with favorable interphase FISH was significantly longer compared with those with adverse interphase FISH (P < .001).
Table 2.
Interphase FISH cytogenetic profile results
| MP (n = 423) | CTDa (n = 426) | |
|---|---|---|
| Cytogenetic profile, n (%) | ||
| Favorable | 125 (58.1) | 129 (57.3) |
| Adverse | 90 (41.9) | 96 (42.7) |
| Cytogenetic abnormality, n/N (%)* | ||
| 13q− | 84/208 (40.4) | 102/221 (46.2) |
| 17p− | 19/208 (9.1) | 20/216 (9.3) |
| 1p− | 20/173 (11.6) | 23/176 (13.1) |
| 1q+ | 74/181 (40.9) | 78/190 (41.1) |
| t(4;14) | 21/211 (10.0) | 23/223 (10.3) |
| t(11;14) | 24/211 (11.4) | 30/223 (13.5) |
| t(14;16) | 9/212 (4.2) | 5/222 (2.3) |
| t(14;20) | 2/209 (1.0) | 3/220 (1.4) |
Multiple abnormalities can be present in the same patient.
In patients with favorable interphase FISH, although the beneficial effects of CTDa regimen on PFS and OS rates did not reach statistical significance (Figure 5), a survival benefit for patients randomized to CTDa subsequently emerged. In the favorable group, as with the overall population, there was a change in HRs after approximately 18 months with the Kaplan-Meier curves crossing and remaining separate thereafter, in favor of CTDa (Figure 5B; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This effect was not seen in the patients with adverse interphase FISH (supplemental Figure 1B).
Figure 5.
Survival according to treatment group in patients with favorable cytogenetics. (A) PFS. (B) OS.
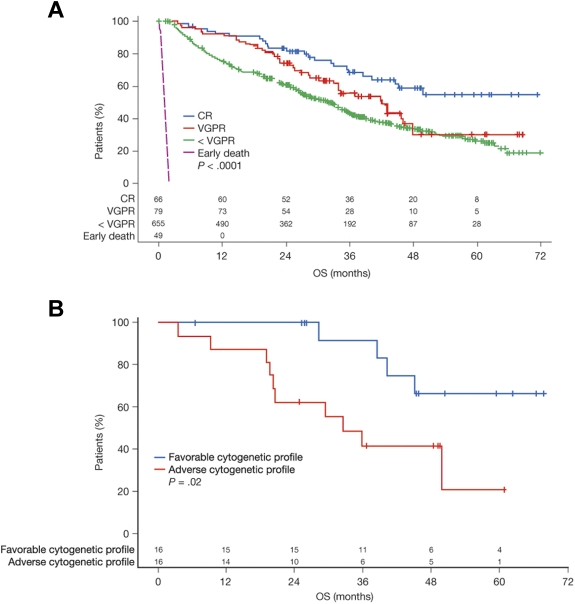
Influence of response
As expected, patients who achieved a CR had significantly better survival outcomes than patients who achieved a VGPR or less than VGPR, regardless of treatment regimen (P < .0001; Figure 6A). Median OS was not yet reached at the time of the analysis in the 66 patients who achieved CR (56 of whom were randomized to CTDa). However, these patients' cytogenetic profiles apparently continued to influence survival outcomes: patients with favorable interphase FISH profiles (n = 16) had significantly better OS than patients with adverse interphase FISH profiles (n = 16; P = .02; Figure 6B).
Figure 6.
OS among patients. Influence of (A) treatment response and (B) cytogenetic profile among patients achieving a CR.
Safety
The median number of induction therapy cycles delivered to each group was similar: MP, 6 (range, 0-18) and CTDa, 6 (range, 0-11). The dose of thalidomide was reduced in 31% of patients. Overall, 262 of 423 patients in the MP group died compared with 243 of 426 patients in the CTDa group (P = .16, Fisher exact test). The increased mortality in the MP group was attributed mainly to an increase in deaths related to disease progression and myeloma-related infections (Table 3). The rate of very early deaths (within 60 days of randomization) was comparable in each treatment group (5.9% vs 5.7%). The most common causes of deaths related to myeloma and/or treatment were: disease progression; infection resulting from disease, treatment, or both; and renal failure. Overall, 2 deaths in each group were attributed to thromboembolism.
Table 3.
Adverse events occurring in ≥ 10% of the patients
| Event | All grades, n (%) |
Grade 3 and 4, n (%) |
||||
|---|---|---|---|---|---|---|
| MP (n = 424) | CTDa (n = 427) | P* | MP (n = 424) | CTDa (n = 427) | P* | |
| Cytopenia† | 84 (19.8) | 66 (15.5) | .11 | 64 (15.1) | 47 (11.0) | .084 |
| Thromboembolic events‡ | 20 (4.7) | 68 (15.9) | < .0001 | |||
| Sensory neuropathy | 25 (5.9) | 101 (23.7) | < .0001 | 2 (0.5) | 11 (2.6) | .021 |
| Motor neuropathy | 11 (2.6) | 50 (11.7) | < .0001 | 5 (1.2) | 17 (4.0) | .016 |
| Constipation | 76 (17.9) | 175 (41.0) | < .0001 | 5 (1.2) | 15 (3.5) | .039 |
| Infection | 111 (26.2) | 137 (32.1) | .060 | 31 (7.3) | 55 (12.9) | .0086 |
| Rash | 30 (7.1) | 65 (15.2) | .00019 | 3 (0.7) | 7 (1.6) | .34 |
| Elevated AP level‡ | 40 (9.4) | 66 (15.5) | .0093 | |||
AP indicates alkaline phosphatase.
Fisher exact test.
Cytopenia resulting in dose modification or neutrophil count < 0.5 cells × 109/L.
Data were not collected according to adverse events grade.
The most frequently reported adverse reactions occurring in ≥ 10% of the safety population were cytopenia, sensory neuropathy, constipation, infection, rash, and elevated alkaline phosphatase levels. The majority of the adverse events were mild or moderate. Patients in the CTDa group had a higher incidence of sensory and motor neuropathy, thromboembolic events, constipation, infection, rash, and elevated alkaline phosphatase levels than did those in the MP group; however, the CTDa group had a lower incidence of cytopenia. The rates of grade 3 and 4 sensory (2.6%) and motor (4%) neuropathy with CTDa were low. The rate of renal insufficiency/failure was low (4.9%) and comparable between the treatment groups.
Twenty patients (4.7%) in the MP group experienced 22 thromboembolic events, and 68 patients (15.9%) in the CTDa group experienced 74 thromboembolic events (P < .0001, Fisher exact test). The increased incidence in the CTDa group was attributed primarily to an increase in deep vein thrombosis (34 events in the CTDa group and 11 events in the MP group) and pulmonary embolism (36 events and 7 events, respectively). A total of 35 patients (8.3%) in the MP group received thromboprophylaxis compared with 141 patients (33.1%) in the CTDa group, and only 18 events occurred while the patients were receiving anticoagulation treatment.
Discussion
In this component of the MRC Myeloma IX study, we show that the CTDa regimen, given as initial therapy in elderly NDMM patients considered ineligible for intensive therapy including ASCT, significantly increased ORR by 2-fold compared with MP (63.8% vs 32.6%). The depth of response seen with the CTDa regimen (CR and VGPR rates of 13% and 17%, respectively) was particularly encouraging, as the level of response correlated with survival outcomes. Overall, PFS and OS were comparable regardless of treatment, although there is clear evidence of an emerging OS benefit. These effects were most marked in the favorable cytogenetics group, defined by FISH. Importantly, our data indicate that patient cytogenetics continue to influence survival outcomes among patients who achieved a CR, underscoring the need for FISH cytogenetic profiling at the time of diagnosis. The influence of cytogenetics on survival outcomes has been noted previously in other studies of thalidomide-based therapy,22,23 where it was shown that patients with a favorable cytogenetic profile had improved PFS or event-free survival after treatment with thalidomide.
There was an emerging survival benefit after 18 months of treatment, particularly evident in patients with favorable interphase FISH. This effect was independent of whether maintenance was used or not. Although the patient numbers are small and these data should be interpreted with caution, this observation suggests that CTDa has important biologic effects against the myeloma clone, which can translate into clinically significant survival benefits. These beneficial effects seen after 18 months seem to be offset by the earlier toxicity, an observation that is important when selecting patients for treatment with combination regimens, including thalidomide, alkylating agents, and corticosteroids. Closer inspection of the effects of such regimens has revealed that performance status and the type of bisphosphonate used can have significant effects on early mortality rates.
Although some groups have reported an immediate and sustained survival benefit with the addition of thalidomide to MP,10,12 others have shown a late benefit with thalidomide therapy that emerges after 2-3 years,24,25 as seen here. Accounting for the relatively elderly, unselected, poorer-prognosis patients accrued to this study, the survival results achieved with the CTDa regimen compare well with many other studies evaluating thalidomide-containing regimens, such as melphalan, prednisone plus thalidomide, and thalidomide plus dexamethasone10,15,16 (Table 4). Survival outcomes in the MP group in the present study were relatively low compared with most other studies (Table 4).11–13 The median PFS and OS achieved with CTDa in the present study were 13 and 33 months, respectively, which is also lower than with melphalan, prednisone plus thalidomide in the meta-analysis by Waage et al (PFS, 20 months; OS, 39 months).26 This could be because this was an inclusive study with patients from many hospitals in the United Kingdom and, importantly, there was no age cutoff for the patients receiving more intensive treatment incorporating ASCT. This in turn led to patients in the nonintensive pathway being appreciably older and less fit than might have been the case in previous studies.
Table 4.
Summary of efficacy outcomes from selected trials evaluating thalidomide-based regimens in elderly patients with NDMM
| Study | Median follow-up, mo | Treatment | n | CR + PR, % | CR, % | PFS, mo | OS, mo |
|---|---|---|---|---|---|---|---|
| IFM 99-0612 | 51.5 | MPT | 124 | 76 | 13 | 27.5 | 51.6 |
| MP | 193 | 35 | 2 | 17.8 | 33.2 | ||
| GIMEMA13 | 38.1 | MPT | 159 | 76 | 16 | 21.8 | 45.0 |
| MP | 160 | 48 | 4 | 14.5 | 47.6 | ||
| Nordic15 | 42 | MPT | 182 | 57 | 13 | 15.0 | 29.0 |
| MP | 175 | 40 | 4 | 14.0 | 32.0 | ||
| HOVON16 | 39 | MPT | 165 | 66 | NA | 13* | 40 |
| MP | 168 | 45 | NA | 9* | 31 | ||
| IFM 01-0111 | 47.5 | MPT | 113 | 62 | 7 | 24.1 | 44.0 |
| MP | 116 | 31 | 1 | 18.5 | 29.1 | ||
| Ludwig et al10 | 28.1 | TD | 145 | 68 | 2 | 16.7 | 41.5 |
| MP | 143 | 50 | 2 | 20.7 | 49.4 | ||
| Morgan et al (current study) | 44 | CTDa | 426 | 64 | 13 | 13.0 | 33.2 |
| MP | 423 | 33 | 2 | 12.4 | 30.6 |
NA indicates not applicable.
Values are event-free survival, not PFS.
A survival benefit for thalidomide-based therapy has not been observed consistently across all trials,10–13,15,16 which could reflect a degree of selection. There are some important differences among these studies with regard to patient population and methodology. However, meta-analyses of the published results on melphalan, prednisone plus thalidomide versus MP show that the addition of thalidomide to alkylating agents and steroids results in improved PFS and a trend toward improved OS, giving significantly superior effects against the myeloma clone.26,27 There is, however, an increase in adverse events associated with these regimens, suggesting that the careful selection of patients within this older age group for treatment is extremely important.
In general, adverse events in the CTDa group were consistent with the known safety profile of thalidomide in multiple myeloma. Thromboembolic events were more frequent in the group receiving CTDa than MP (16% vs 5%, respectively), primarily the result of an increase in deep vein thrombosis and pulmonary embolism. The overall rate of thromboembolism in the current study is similar to, or lower than, that observed in previous studies of thalidomide-based therapies, which did not initially require thromboprophylaxis (grade 3 or 4 adverse events, 12%-13%).10–13 Furthermore, the number of thromboembolic events decreased dramatically in patients receiving anticoagulation therapy. Although CTDa was associated with a significantly higher rate of sensory and motor neuropathy, the rates of grade 3-4 neuropathy were low. Overall, more patients died in the MP group compared with patients in the CTDa group (62% vs 57%), mainly because of disease progression and myeloma-related infections.
In conclusion, we have shown that the CTDa regimen provides higher response rates than MP, and a median PFS, but not OS, which is superior to that achieved with MP in patients with NDMM who are ineligible for intensive therapy and ASCT. On further analysis, patients with a favorable interphase FISH profile were most likely to benefit from CTDa treatment, highlighting the importance of FISH assessment at the time of diagnosis. These results indicate that the CTDa regimen shows significant benefits in elderly patients; however, the relevance of this study's findings may be limited because of the increased use of novel agents, such as bortezomib and lenalidomide, for the initial treatment of myeloma. Furthermore, to benefit from these effects, patients have to survive early hazards, underscoring the need for adequate physician management of adverse events to allow patients to continue on therapy. Consistent with other studies,10,26,27 our data support the careful selection of patients with reasonable performance status and suggest that judicious dose adjustment of steroids to ensure patients stay on treatment is an important aspect of adopting such regimens.
Supplementary Material
Acknowledgments
The authors thank all the patients, investigators, and staff at the participating centers who made this study possible; Professor David Bowen for independent safety oversight; the staff at the Clinical Trials Research Unit, University of Leeds, for trial coordination, data management, and analysis; the Department of Immunology, University of Birmingham; Wessex Regional Genetics Laboratory, University of Southampton; Haematological Malignancy Diagnostic Service, Leeds Teaching Hospitals NHS Trust; and the Institute of Cancer Research, London, for central laboratory investigations; the MRC Leukemia Data Monitoring and Ethics Committee, the MRC Leukemia Trial Steering Committee, the National Cancer Research Institute Haematological Oncology Clinical Studies Group, Myeloma, United Kingdom; the National Institute for Health Research for support through the National Cancer Research Network and the support of the Biomedical Research Center at the Royal Marsden Hospital; and Excerpta Medica for editorial support in the preparation of this manuscript (funded by Celgene Corporation); and Dr Anna Georgieva for providing editorial support regarding references, consistency of language, formatting, and artwork.
This work was supported by the MRC Myeloma IX trial (obtained from the United Kingdom MRC) and Novartis, Schering Health Care, Chugai, Pharmion, Celgene, and Ortho Biotech (unrestricted educational grants), mainly to support trial coordination and the laboratory studies.
Lead investigators in the 20 centers with the highest trial enrollment were as follows: N. H. Russell (Nottingham University Hospitals, Nottingham, United Kingdom), G. Cook (Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom), H. Roddie (Western General Hospital, Edinburgh, United Kingdom), C. Rudin (Royal Devon and Exeter Hospital, Exeter, United Kingdom), D. W. Milligan and M. A. Lumley (Heart of England Foundation Trust, Birmingham, United Kingdom), J. Snowden (Royal Hallamshire Hospital, Sheffield, United Kingdom), H. Sayala (Hull and East Yorkshire Hospitals NHS Trust, Hull, United Kingdom), P. Chu (Royal Liverpool University Hospital, Liverpool, United Kingdom), D. Wright (Mid Yorkshire NHS Trust, Wakefield, United Kingdom), K. Gelly (Ninewells Hospital, Dundee, United Kingdom), D. Turner (Torbay Hospital, Torquay, United Kingdom), H. Jackson (University Hospital of Wales, Cardiff, United Kingdom), J. Craig (Addenbrooke's Hospital, Cambridge, United Kingdom), J. Tighe (Aberdeen Royal Infirmary, Aberdeen, United Kingdom), S. Shafeek (Worcestershire Royal Hospital, Worcester, United Kingdom), J. Neilson (Russells Hall Hospital, Dudley, United Kingdom), J. Cavet (Christie Hospital, Manchester, United Kingdom), A. McKernan (Royal Derby Hospital, Derby, United Kingdom), A. Kruger (Royal Cornwall Hospital, Truro, United Kingdom), M. P. Macheta (Blackpool Victoria Hospital, Blackpool, United Kingdom), A. Wood (James Cook University Hospital, Middlesbrough, United Kingdom), and A. Smith (Southampton General Hospital, Southampton, United Kingdom).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.J.M., J.A.C., and G.H.J. were the chief investigators; G.J.M., F.E.D., G.H.J., J.A.C., S.E.B., and M.T.D. designed the trial and developed the protocol; A.J.S. and W.M.G. developed the statistical analysis plan; G.J.M., F.E.D., J.A.C., R.G.O., G.C., N.H.R., C.R., H.R., and G.H.J. recruited patients; G.H.J. and F.E.D. reviewed safety data; M.T.D., R.G.O., F.M.R., and F.E.D. conducted central laboratory investigations; N.N.C. and S.E.B. coordinated the data collection and regulatory and governance requirements; W.M.G. developed the mathematical and statistical modeling; G.J.M., J.A.C., G.H.J., S.E.B., N.N.C., W.M.G., M.T.D., A.J.S., S.F., J.L.B., and R.G.O. contributed to the manuscript writing, generation of tables and figures, and/or interpretation of the data; G.J.M. developed an early draft; and all authors reviewed and approved the submitted manuscript.
Conflict-of-interest disclosure: G.J.M. received payment for lectures including service on speakers' bureaus from Novartis, Celgene Corporation, and Ortho Biotech, as well as payment for the development of educational presentations and reimbursement of costs to attend scientific meetings from Celgene Corporation. F.E.D. received payment for lectures, including service on speakers' bureaus from Novartis, Celgene Corporation, and Ortho Biotech, as well as payment for the development of educational presentations and reimbursement of costs to attend scientific meetings from Celgene Corporation. W.M.G. and S.E.B. received reimbursement of costs to attend scientific meetings in the framework of this study from Celgene Corporation and Ortho Biotech. G.C. received consultancy fees and payment for lectures, including service on speakers' bureaus from Celgene Corporation and Ortho Biotech. R.G.O. was a speakers' bureau member of, and received support to attend scientific meetings from, Celgene Corporation and Ortho Biotech. G.H.J. received payment for lectures, including service on speakers' bureaus from Celgene Corporation. The remaining authors declare no competing financial interests.
A complete list of the members of the NCRI Haematological Oncology Study Group appears in the online supplemental Appendix.
Correspondence: Gareth J. Morgan, Section of Haemato-Oncology, Institute of Cancer Research, Brookes Lawley Building, 15 Cotswold Road, Belmont, Sutton, Surrey SM2 5NG, United Kingdom; e-mail: gareth.morgan@icr.ac.uk.
References
- 1.Kumar S, Giralt S, Stadtmauer EA, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114(9):1729–1735. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]
- 2.Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2003;4(6):379–398. [PubMed] [Google Scholar]
- 3.Mileshkin L, Prince HM. The adverse prognostic impact of advanced age in multiple myeloma. Leuk Lymphoma. 2005;46(7):951–966. doi: 10.1080/10428190500085024. [DOI] [PubMed] [Google Scholar]
- 4.Myeloma Trialists' Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. J Clin Oncol. 1998;16(12):3832–3842. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Rajkumar SV. Treatment of multiple myeloma: a comprehensive review. Clin Lymphoma Myeloma. 2009;9(4):278–288. doi: 10.3816/CLM.2009.n.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96(9):2943–2950. [PubMed] [Google Scholar]
- 7.Holstein SA, Tong H, Hohl RJ. Differential activities of thalidomide and isoprenoid biosynthetic pathway inhibitors in multiple myeloma cells. Leuk Res. 2010;34(3):344–351. doi: 10.1016/j.leukres.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3):431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar SV, Rosiñol L, Hussein M, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26(13):2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig H, Hajek R, Tóthová E, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113(15):3435–3442. doi: 10.1182/blood-2008-07-169565. [DOI] [PubMed] [Google Scholar]
- 11.Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664–3670. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 12.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370(9594):1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 13.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112(8):3107–3114. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 14.Beksac M, Haznedar R, Firatli-Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86(1):16–22. doi: 10.1111/j.1600-0609.2010.01524.x. [DOI] [PubMed] [Google Scholar]
- 15.Waage A, Gimsing P, Fayers P, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116(9):1405–1412. doi: 10.1182/blood-2009-08-237974. [DOI] [PubMed] [Google Scholar]
- 16.Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28(19):3160–3166. doi: 10.1200/JCO.2009.26.1610. [DOI] [PubMed] [Google Scholar]
- 17.Wu P, Davies FE, Horton C, et al. The combination of cyclophosphamide, thalidomide and dexamethasone is an effective alternative to cyclophosphamide-vincristine-doxorubicin-methylprednisolone as induction chemotherapy prior to autologous transplantation for multiple myeloma: a case-matched analysis. Leuk Lymphoma. 2006;47(11):2335–2338. doi: 10.1080/10428190600821955. [DOI] [PubMed] [Google Scholar]
- 18.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376(9757):1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan GJ, Davies FE, Gregory WM, et al. Thalidomide maintenance significantly improves progression-free survival (PFS) and overall survival (OS) of myeloma patients when effective relapse treatments are used: MRC Myeloma IX results [abstract]. Blood. 2010;116(9):623. [Google Scholar]
- 20.Bladé J, Samson D, Reece D, Apperley J, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation: Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 21.Gregory WM, Bolland K, Whitehead J, Souhami RL. Cautionary tales of survival analysis: conflicting analyses from a clinical trial in breast cancer. Br J Cancer. 1997;76(4):551–558. doi: 10.1038/bjc.1997.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offidani M, Corvatta L, Polloni C, et al. Thalidomide-dexamethasone versus interferon-alpha-dexamethasone as maintenance treatment after ThaDD induction for multiple myeloma: a prospective, multicentre, randomised study. Br J Haematol. 2009;144(5):653–659. doi: 10.1111/j.1365-2141.2008.07495.x. [DOI] [PubMed] [Google Scholar]
- 23.van Rhee F, Dhodapkar M, Shaughnessy JD, Jr, et al. First thalidomide clinical trial in multiple myeloma: a decade. Blood. 2008;112(4):1035–1038. doi: 10.1182/blood-2008-02-140954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 25.Barlogie B, Pineda-Roman M, van Rhee F, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112(8):3115–3121. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waage A, Palumbo A, Hulin C, et al. MP versus MPT for previously untreated elderly patients with multiple myeloma: a meta-analysis of survival of 1682 individual patient data from 6 randomized clinical trials [abstract]. Haematologica. 2010;95(suppl 2):235. Abstract 0567. [Google Scholar]
- 27.Kapoor P, Rajkumar SV, Dispenzieri A, et al. Melphalan and prednisone versus melphalan, prednisone and thalidomide for elderly and/or transplant ineligible patients with multiple myeloma: a meta-analysis. Leukemia. 2011;25(4):689–696. doi: 10.1038/leu.2010.313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.