Abstract
An association between platelets, angiogenesis, and cancer has long been recognized, but the mechanisms linking them remains unclear. Platelets regulate new blood vessel growth through numerous stimulators and inhibitors of angiogenesis by several pathways, including differential exocytosis of angiogenesis regulators. Herein, we investigated the differential release of angiogenesis stimulators and inhibitors from platelets. Activation of human platelets with adenosine diphosphate (ADP) stimulated the release of VEGF, but not endostatin whereas, thromboxane A2 (TXA2) released endostatin but not VEGF. Platelet releasates generated by activation with ADP promoted migration and formation of capillary structures by human umbilical vein endothelial cells (HUV-EC-Cs) in in vitro angiogenesis models. Conversely, TXA2-stimulated platelet releasate inhibited migration and formation of capillary structures. Because tumor growth beyond 1-2 mm3 is angiogenesis-dependent, we hypothesized that cancer cells preferentially stimulate platelets to secrete their pro-angiogenic payload. In support of this, the breast cancer cell line MCF-7 stimulated secretion of VEGF and a pro-angiogenic releasate from platelets. Furthermore, the antiplatelet agent aspirin inhibited platelet–mediated angiogenesis after exposure to ADP or MCF-7 cells providing a potential mechanism for how aspirin may impact malignancy. Manipulation of differentially mediated release of angiogenic factors from platelets may provide a new modality for cancer treatment.
Introduction
Platelets are best known for their role in hemostasis and thrombosis. Platelets have additional roles in inflammation, angiogenesis and wound healing. The association between hemostasis and malignancy was first recognized by Professor Armand Trousseau in 1865 when he described cases of primary thrombophlebitis occurring in patients with occult malignancy. Because of these original findings, clinical data have confirmed the association of thrombosis and malignancy.1 Further establishing a link between abnormalities of the coagulation system and cancer, studies have shown that platelet count can be a prognostic factor; with patients presenting with thrombocytosis having a poor survival in a variety of cancers.2–4
Tumor cells and platelets exist in a symbiotic-like relationship with the survival of tumor cells directly linked to their interaction with platelets.5 Platelets aid cancer cells in completing their journey to metastatic sites in a variety of ways including coating tumor cells to help them evade the immune system, shielding tumor cells from high shear forces, aggregating tumor cells and platelets to embolize to new extravasation sites, and facilitating the adhesion of tumor cells to the vascular endothelium.6,7 However, these mechanistic aspects of the interaction between tumor cells and platelets are under appreciated.
Platelet activation by tumor cells may be one mechanism by which platelets are influenced by the presence of malignancy. It is recognized that platelets and tumor cells interact leading to platelet aggregation via known platelet agonists. Using several different cell lines it has been demonstrated that various human and animal tumor cells have the ability to aggregate platelets and that this capacity correlates with the tumor's metastatic potential.8–15 In addition, platelet activation has been established in several malignancies.6 Further support of the interaction between platelets and tumor cells is the recent advancements demonstrating antiplatelet agents can impact malignancy. Patients with malignancy who ingest aspirin, a known platelet inhibitor, have decreased rates of metastatic spread and improved survival.16
We hypothesized that tumor cells could manipulate the platelet's ability to regulate angiogenesis by “hijacking” the platelet which prompted us to ask; do platelets regulate physiologic and pathologic angiogenesis? This could occur through multiple mechanisms, including promoting the interaction of tumor cells and platelets, increasing the net angiogenic potential of the platelet by manipulating its angiogenic protein content, or alternatively inducing preferential release of pro-angiogenic factors from platelets. It is well-established that platelets carry a multitude of angiogenesis regulatory proteins in their α granules, and the normal ranges of angiogenesis regulators have been characterized in human platelets and in the platelets of patients with malignancy.17
Our laboratory has shown that platelets store angiogenic factors in distinct α granules and that these granules can be differentially released in the presence of the thrombin agonists; with PAR1 resulting in the release of VEGF and PAR4 resulting in release of endostatin.18 Others have shown that differential release is not limited to angiogenic factors and also affects known hemostatic factors.19 The platelet agonist, adenosine diphosphate (ADP) has also been shown to induce differential release.20 While these studies have focused on the secretion of individual angiogenesis regulatory proteins, the effect on the overall angiogenic potential of the released contents and physiology of the platelet remains unresolved.
In the present report, we expand our understanding of how platelets regulate new blood vessel growth. The results suggest that platelet agonists as well as tumor cells can manipulate new blood vessel growth by stimulating the differential release of angiogenesis regulatory proteins from platelets. In addition, our data suggests that the antiplatelet drug, aspirin may block the differential release of angiogenic regulators from platelets thereby inhibiting angiogenesis.
Methods
Preparation of resting platelets
Human blood collection was performed in accordance with ethics regulation with Brigham and Women's Hospital Institutional Review Board approval. Platelets were isolated from approximately 10 healthy volunteers as described.18 Volunteers did not ingest aspirin or nonsteroidal anti-inflammatory drugs for at least 10 days. For the experiments involving aspirin, volunteers consumed aspirin for at least 5 days. Platelet number was counted by FACS and adjusted to 2 × 108/mL. The resting state of the platelets was confirmed by P-selectin antibody labeling. In vitro aspirin exposure was performed by pre-treating platelet-rich plasma with 100μM of lysine-aspirin (gift of Dr Tanya Laidlaw, Brigham and Women's Hospital) for 1 hour before platelet isolation.
Activation of platelets
Release of α granules was examined in vitro in response to 25μM ADP (Biodata), 100μM Thromboxane A2 (Caymen), 10μM PAR4, or exposure to MCF-7 cells (3 × 106/mL) in fresh media. Platelets were exposed to the agonist for 10 minutes at 37°C before collecting the releasate or processed for immunofluorescence microscopy.
Immunofluorescence microscopy
Mouse anti-VEGF antibody was obtained from Lab Vision and rabbit anti-endostatin antibody was the generous gift of the Folkman laboratory. Platelet immunofluorescence labeling was performed as previously described and samples analyzed on a Nikon TE 2000 Eclipse microscope equipped with a Nikon 100×/1.4 NA objective and a 100-W mercury lamp.18 Images were acquired with a Hamamatsu Orca IIER CCD camera and analyzed using Molecular Devices Metamorph software.
Angiogenic protein quantification
VEGF and endostatin concentrations were determined in triplicate using the Quantikine human ELISA assay according to the manufacturer's instructions (R&D Systems) using 200 μl of platelet releasate. Concentrations of VEGF and endostatin were corrected for platelet count and statistical significance was determined using the Student t test.
Angiogenesis antibody array
To assess the overall angiogenic potential of the releasate generated by platelets after exposure to various stimulants, the RayBio Human Angiogenesis Antibody C-1000 (RayBiotech, Inc) was screened according to the protocol.
Capillary tube formation assay
Capillary tube formation was used to assess the angiogenesis potential of releasates generated from resting platelets or platelets stimulated with agonists using the Millipore Capillary Tube Formation Assay kit in duplicate. After 6 hours of incubation, tube formation was quantified. 5 fields were imaged (at 4× and 20× magnification) with differential-interference-contrast microscopy using a Zeiss Axiovert microscope and the degree of tubulogenesis was quantified by counting branch points. Independent assays were averaged and statistical analysis was performed using the Student t test.
HUVEC Migration
The bottom chamber of a transwell plate (Corning) was precoated with 0.5% gelatin. HUVECs in serum free media were seeded in the media and 1 × 104/mL cells were inoculated into the upper chamber of each Transwell with the releasate from 2 × 108/mL platelets generated under experimental conditions placed in the bottom chamber. After 24 hours of incubation, the cells were fixed and stained with Diff-quik (Siemens), and the number of cells on the bottom of the filters was counted for 3 microscope fields. The results are shown as the percentage of cells that migrated to the bottom of the filter. Each experiment was performed in duplicate. Results from independent assays were averaged and statistical analysis was performed using the Student t test.
Results
ADP mediated release of VEGF from washed platelets
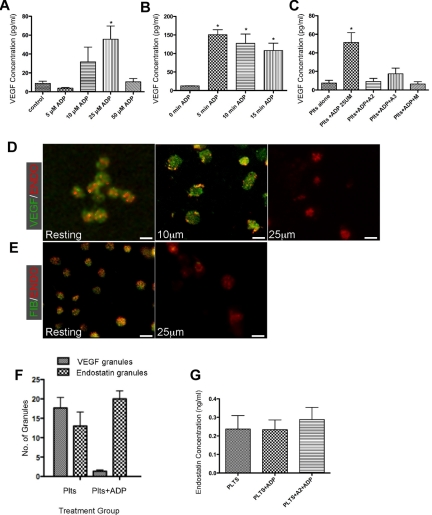
To determine whether physiologic agonists could stimulate the preferential release of angiogenesis regulatory proteins from human platelets, we treated resting platelets with a variety of platelet agonists and assayed for release of pro-angiogenic VEGF and anti-angiogenic endostatin. In Figure 1A, washed platelets are treated with various concentrations of ADP to determine the dose required for maximal VEGF release after 10 minutes of stimulation. At 25μM, a statistically significant increased amount of VEGF was present in the releasate from platelets. The amount released was 55.71 ± 13.92 pg VEGF/mL. In Figure 1B, addition of ADP at the maximal concentration was found to release VEGF after > 5 minutes of stimulation. The net mean for VEGF release from washed platelets exposed to 25μM ADP for 5 minutes was 150.3 ± 13.72 pg VEGF/mL compared with 12.50 ± 0.500 pg VEGF/mL for washed platelets that were unstimulated. Treatment of washed platelets with 25μM ADP for 10 minutes yielded 102.8 ± 23.47 pg VEGF/mL and 15 minutes yielded 107.9 ± 20.08 pg VEGF/mL. Based on these findings, stimulation of washed platelets with 25μM ADP led to a maximal VEGF release at 5 minutes, which was an 87.5% increase in VEGF in the releasate of washed platelets. To test the specificity for ADP receptors, 3 ADP antagonists were used that blocked either P2Y1 (A2P5P and A3P5P) or P2Y12 (MRS2395; Sigma-Aldrich). Figure 1C demonstrates that all of the antagonists blocked ADP-mediated VEGF release. This was verified by immunofluorescence as pretreatment of platelets with A2P5P before exposure to ADP was not associated with release of VEGF. Activation of platelets in response to ADP and other agonists was verified by determination of cell surface P-selectin expression by phycoerythrin-conjugated anti–P-selectin (CD62) antibody labeling and detection by flow cytometry.
Figure 1.
ADP mediates release of VEGF from platelets. (A) VEGF concentration in the releasate generated from platelets activated with various concentrations of ADP. (B) Time course of VEGF concentration in the releasate generated from platelets (plts) activated with 25μM ADP. (C) VEGF concentration in releasate generated from platelets resting or activated with ADP alone or pre-incubated with ADP antagonists (A2P5P [A2], A3P5P [A3], and MRS2395 [M]). (D) Immunofluorescence of platelets resting or activated with 10μM or 25μM ADP and labeled with VEGF (green) or endostatin (red). Scale bar is 2μm in size. (E) Immunofluorescence of platelets resting or activated with 25 μM ADP and labeled with endostatin (red) or Fibrinogen (green). (F) Number of granules labeled with VEGF or endostatin in resting platelets or platelets incubated with ADP. Numbers represent average granule counts from immunofluorescence images. (G) Endostatin concentration in releasate generated from resting platelets or platelets activated with 25μM ADP alone or with preincubation with A2. * indicates P < .05.
To visualize the differential release at the cellular level, we used immunofluorescence antibody labeling techniques. The washed platelets were stained with an antibody for VEGF and endostatin in double labeling experiments. In Figure 1D, in comparison to resting platelets treatment of platelets with 10 μm ADP resulted in retention of α granules which were labeled separately with both VEGF and endostatin with scant areas of overlap as shown in these representative images. The addition of 25μM ADP led to retention of α granules that predominantly stained positive for endostatin with release of VEGF (Figure 1D and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Because our previous work showed that VEGF and fibrinogen were stored in the same granule population, we repeated our immunofluorescence with staining for fibrinogen instead of VEGF. In Figure 1E, fibrinogen staining was no longer present in α granules after stimulation of the platelets with ADP (25μM). To quantify the difference in granule staining we counted the number of VEGF and endostatin staining granules to verify that after treatment with ADP, less VEGF containing granules were visible as shown in Figure 1F. This suggests that the VEGF (and fibrinogen) have been released from the platelets and present in the releasate, as confirmed in the VEGF ELISAs (Figures 1A-C). Because of the intra- and inter-variability in levels of VEGF in individuals these images and representative concentrations depict qualitative evidence that platelet activation with ADP can influence the release of VEGF from platelet α granules. Because the immunofluorescence data would suggest that the endostatin remained localized to the α granules, we measured the releasate to determine the quantity of endostatin present. In Figure 1G there was no significant increase in endostatin concentration in the releasate of platelets that were treated with ADP versus resting platelets.
TXA2-mediated release of endostatin from washed platelets
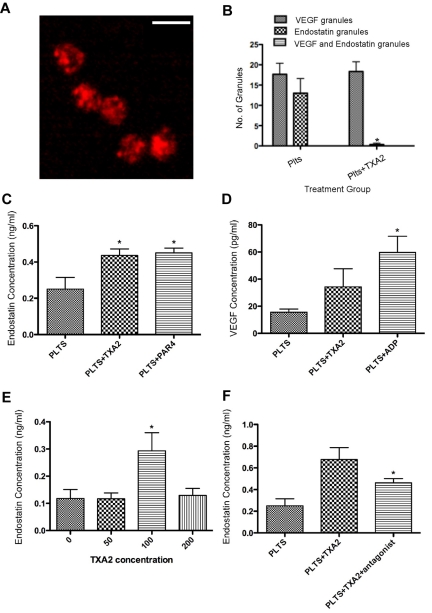
If ADP stimulates the preferential release of angiogenesis promoters, then other physiologic agonists may trigger the secretion of angiogenesis inhibitors. To test this hypothesis, we screened other platelet agonists including epinephrine, collagen, thrombin and thromboxane A2. We found that exposure to TXA2 led to the opposite effect of ADP. Using immunofluorescence microscopy, it is evident that the α granules retained VEGF but that endostatin-containing granules were no longer visible (Figure 2A and supplemental Figure 2, in comparison to the image of resting platelets in Figure 1D). To quantify the difference in granule staining we counted the number of VEGF and endostatin staining granules (Figure 2B). To verify that the endostatin that had been secreted was contained in the releasate, we used an endostatin ELISA. The concentration of endostatin in the releasate was significantly elevated in the platelet samples that had been activated by TXA2 in comparison to the releasate of resting platelets, and similar to the release observed after activation with PAR4 (Figure 2C). The amount of endostatin released from the resting platelet was 0.2508 ng/mL, whereas the amount released from platelets activated with TXA 2 was 0.4363 ng/mL (P = .01). In Figure 2D, we confirmed that TXA2 activation did not lead to a statistically significant release of VEGF in comparison to platelets. To further explore the release of endostatin by TXA2, a concentration curve was performed which revealed that a statistically significant amount of endostatin was released at a TXA2 concentration of 100μM (0.293 ng/mL P = .01; Figure 2E). Figure 2F shows that pretreatment with an antagonist of TXA2 led to a statistically significant decrease in endostatin release from platelets (P < .05). These studies show that platelets activated by TXA2 can differentially release endostatin from their α granules.
Figure 2.
TXA2 mediates release of Endostatin from platelets. (A) Immunofluorescence of platelets (plts) activated with TXA2 (100μM) and labeled with VEGF (red) and endostatin (green). Scale bar is 2μm in size. (B) Number of granules labeled with VEGF, endostatin, or VEGF and endostatin, in resting platelets or platelets incubated with TXA2. Numbers represent average granule counts from immunofluorescence images. (C) Endostatin concentration in the releasate of resting platelets or platelets activated with TXA2 or PAR4. (D) VEGF concentration in releasate generated from platelets resting or activated with TXA2 or ADP. (E) Endostatin concentration in the releasate generated from platelets activated with various concentrations of TXA2. (F) Endostatin concentration in the releasate generated from platelets pretreated with TXA2 antagonist before exposure to TXA2. * indicates P < .05.
Releasate from platelets activated with ADP has an overall pro-angiogenic potential
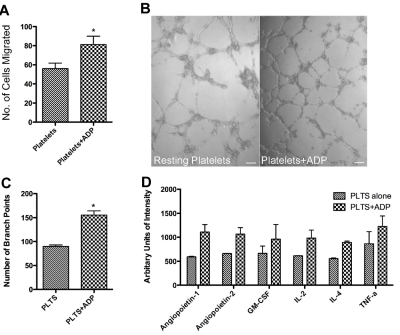
To examine the physiologic relevance of our findings, we analyzed the capacity of specific platelet releasates to promote angiogenesis. Based on the increased amount of VEGF in the releasate generated by ADP activation, we hypothesized that platelets stimulated with ADP would yield a more pro-angiogenic net potential. We used a variety of known angiogenesis assays including the EC migration assay and the capillary tube formation assay. Platelets from normal healthy controls were isolated and either allowed to rest or activated with the known platelet agonist ADP. The releasate from these activated platelets was collected and added to endothelial cells. Releasate from 2 × 108/mL ADP-activated platelets, in comparison to resting platelets, resulted in significantly more endothelial cell migration (Figure 3A; P < .05), suggesting that ADP-activated platelets generated a more pro-angiogenic releasate. We also used the capillary tube formation assay as another means of measuring the angiogenic potential of releasate derived from 2 × 108/mL ADP-activated platelets. Again, there is an increased angiogenic potential of the releasate derived from platelets activated with ADP (Figure 3B). As shown in Figure 3C, there is a statistically significant difference in the number of branch points between the 2 groups with mean number of branch points in the platelet group 89.75 ± 3.119 compared with 155.35 ± 8.9 in the platelet+ADP group (P < .05). Platelet buffer alone, or with the addition of ADP without platelets, did not demonstrate increased levels of endothelial cell migration or capillary tube formation (data not shown).
Figure 3.
Promotion of angiogenesis by ADP-stimulated platelet releasates. (A) Endothelial cell migration after exposure to the releasate from control, resting platelets (plts) or platelets activated with 25μM ADP. (B) Capillary tube formation with exposure to the releasate from resting platelets or platelets activated with 25μM ADP. The scale bar is 100μm in size. (C) Quantification of branch points generated by the releasate of platelets alone or platelets exposed to ADP. (D) Representative graph of angiogenic factors as measured by protein array that were found to have a 1.5-fold increase. * indicates P < .05.
Given that the overall net angiogenic potential of ADP-generated platelet releasate was pro-angiogenic, we identified the angiogenic factors present in the releasate. We therefore assessed the angiogenic protein content in the releasate of platelets exposed to ADP as shown in Figure 3D. Presence of angiogenic proteins in the releasate was detected using human angiogenesis antibody arrays. This array identified several pro-angiogenic factors that were elevated in the releasate of ADP activated platelets in comparison to the releasate from resting platelets. Some of the increased proteins include angiopoeitin 1 and 2, GM-CSF, IL-1α, IL-2, IL-4, p-9, Tie-2, and uPAR (supplemental Table 1). Factors that were associated with > 1.5-fold increase included Angiopoietin-1 and -2, IL-2, IL-4, and TNF-α.
Releasate from platelets activated with TXA2 has an overall anti-angiogenic potential
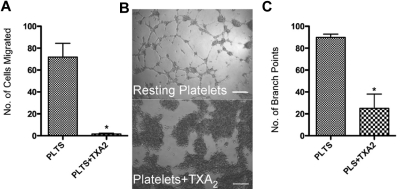
Based on the increased amount of endostatin in the releasate generated by TXA2 activation, we hypothesized that platelets stimulated with TXA2 would yield a more anti-angiogenic net potential. Platelets were activated with TXA2 and endothelial cells were exposed to the releasate. A significant decrease in endothelial cell migration was observed using the releasate generated from 2 × 108/mL platelets (Figure 4A). Strikingly, there was also no capillary tube formation visible (Figure 4B). This lack of capillary tube formation is reflected in a statistically significant decrease in the number of branch points with 89.75 ± 3.1 in the platelet group and 25.00 ± 13 in the platelet+TXA2 group (Figure 4C; P < .05). Platelet buffer alone or with the addition of TXA2 without platelets did not demonstrate increased levels of endothelial cell migration (data not shown).
Figure 4.
Inhibition of angiogenesis by thromboxane-stimulated platelet releasates. (A) Endothelial cell migration after exposure to the releasate from resting platelets (plts; control) or platelets activated with 100μM TXA2. (B) Capillary tube formation with exposure to the releasate from platelets activated with 100μM TXA2. The scale bar is 300μm in size. (C) Quantification of branch points generated by the releasate of platelets alone or platelets exposed to TXA2. * indicates P < .05.
MCF-7 cells stimulate differential release of VEGF and promote angiogenesis
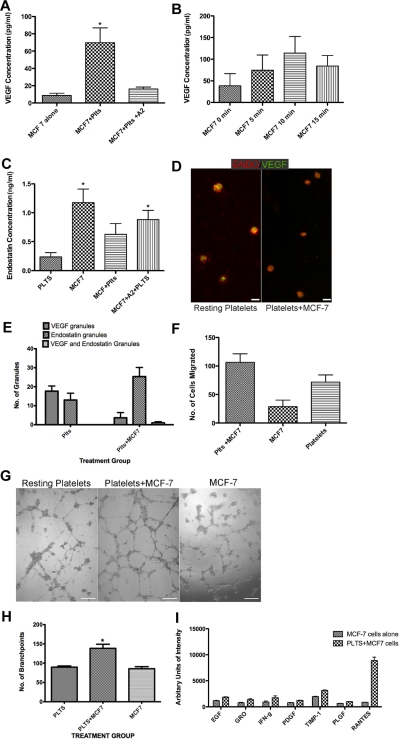
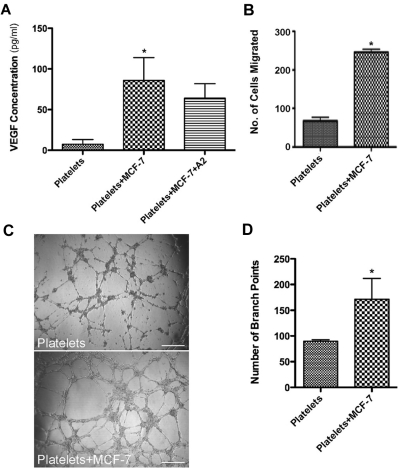
Because it is well known that tumor cells and platelets interact and that this interaction can lead to platelet activation we explored whether or not tumor cells could initiate differential release from platelet α granules. Interaction of platelets with MCF-7 cells led to a significant increase in VEGF concentration in the releasate (8.735 pg/mL ± 2.520 vs 69.82 pg/mL ± 17.03; P < .05; Figure 5A). The highest amount of VEGF released was produced after coincubation with the MCF-7 cells for 10 minutes (Figure 5B). The release of VEGF was partially blocked by exposure to the ADP antagonist, A2P5P suggesting that ADP is involved in the process of platelet activation. Platelets pre-exposed to A2P5P before interacting with the MCF-7 cells had a statistically significant decrease in VEGF release compared with the platelets that were directly exposed to the MCF-7 cells (69.82 pg/mL ± 17.03 vs, 16.24 pg/mL ± 2.21, P < .05; Figure 5A). To confirm that VEGF was only released during exposure to MCF-7 cells, as suggested by our immunofluorescence data, we performed an ELISA to measure endostatin and showed that the amount of endostatin measured in the releasate was not statistically significant (Figure 5C). In all of these experiments the MCF-7 cells activated the platelets leading to differential release of VEGF as measured both by ELISA and by immunofluorescence microscopy. There was not a significant increase in the amount of endostatin in the releasate in the platelets after coincubation with MCF-7 cells (0.2367 ng/mL ± 0.07 vs 0.6275 ng/mL ± 0.18; P = .08). The amount of endostatin released from the MCF-7 cells alone however was higher (1.174 ng/mL ± 0.236). We visualized these changes using immunofluorescence microscopy (Figure 5D). Platelets that have been exposed to MCF-7 cells retain endostatin but lack VEGF. To quantify the difference in granule staining we counted the number of VEGF and endostatin staining granules to verify that after exposure to MCF-7 cells, less VEGF containing granules were visible (Figure 5E). To make sure these findings were specific only to the tumor cell line, we demonstrated that nontumor associated human fibroblasts did not have an increase in VEGF release when incubated with platelets (data not shown).
Figure 5.
MCF-7 breast cancer cells induce the preferential release of VEGF from platelets. (A) VEGF concentration in the releasate generated from MCF-7 cells alone, platelets (plts) exposed to MCF-7 cells alone, or in the presence of A2. (B) VEGF concentration in the releasate generated from platelets exposed to MCF-7 cells for various lengths of time. (C) Endostatin concentration in the releasate from platelets alone, MCF-7 cells, platelets exposed to MCF-7 cells alone or in the presence of A2. (D) Immunofluorescence of platelets resting or after exposure to MCF-7 cells labeled with endostatin (red) and VEGF (green). Scale bar is 2um in size. * indicates P < .05. (E) Number of granules labeled with VEGF, endostatin, or VEGF and endostatin, in resting platelets or platelets incubated with MCF-7 cells. Numbers represent average granule counts from immunofluorescence images. (F) Endothelial cell migration after exposure to the releasate from platelets exposed to MCF-7 cells, the releasate from MCF-7 cells alone, or resting platelets (plts). (G) Capillary tube formation using the releasate from resting platelets, platelets exposed to MCF-7 cells, or MCF-7 cells alone. The scale bar is 300μm in size. (H) Quantification of branch points generated by the releasate of platelets alone, platelets exposed to MCF-7 cells, or MCF-7 cells alone. (I) Representative graph of angiogenic factors that were found to have a 1.5-fold increase. * indicates P < .05.
To examine the physiologic relevance of our findings, the capacity of specific releasates to promote angiogenesis was assessed. Based on the increased amount of VEGF in the releasate generated by MCF-7 cell mediated activation, we hypothesized that platelets exposed to tumor cells would yield a more pro-angiogenic net potential. The 2 × 108/mL platelets and MCF-7 cells interacted for 10 minutes and the releasate was tested in standard angiogenic assays. An increased endothelial cell migration was observed when platelets were exposed to MCF-7 cells in comparison to platelets alone or MCF-7 cells (Figure 5F). We also used the capillary tube formation assay to show that platelets exposed to MCF-7 cells generate a releasate that is pro-angiogenic, with a more complex meshwork of capillary tubes in comparison to the releasate of resting platelets or MCF-7 cells alone (Figure 5G). In Figure 5H, there is a statistically significant difference in the number of branch points between the 2 groups with a mean number of branch points in the platelet group of 89.75 ± 3.119 compared with 138.5 ± 10.6 in the platelet+MCF-7 group (P < .05). A statistically significant increase in capillary tube formation was not observed when MCF-7 cells alone were used.
Because the overall net angiogenic potential of the MCF-7 tumor cell platelet releasate was pro-angiogenic, we assessed the angiogenic protein content in the releasate of platelets exposed to MCF-7 cells (Figure 5I). Expression of angiogenic proteins in the releasate was detected using angiogenesis antibody arrays. In this comparison, we took into consideration factors released directly from the MCF-7 cells. The proteins that were elevated in the releasate of platelets exposed to MCF-7 cells included EGF, GRO, IFN-γ, PDGF-βb, PLGF, and TIMP-1, all of which were associated with > 1.5-fold increase (supplemental Table 1). Of note, the expression of Rantes was highly significant and was associated with a 10-fold increase in the releasate from platelets exposed to MCF-7 cells as opposed to the releasate from MCF-7 cells themselves.
Physical contact between platelets and MCF-7 cells is not necessary for differential release of VEGF
The preferential release of VEGF from platelets could be driven by direct interaction of MCF-7 cells with platelets, by the secretion of a factor from the tumor, or a combination of both. To discriminate between these possibilities, we used the Transwell apparatus. Platelets were placed in the top well in platelet buffer, and MCF-7 cells in media were placed in the lower chamber. Platelets were exposed to the MCF-7 cells in the transwells for various lengths of time and then the platelets in the top chamber were collected for analysis. In these experiments, the VEGF in the releasate from the platelets that had been exposed to the MCF-7 cells in the bottom chamber was significantly increased (7.406 pg/mL ± 5.8 vs. 85.92 pg/mL ± 28.10, P < .05; Figure 6A). Pretreatment of the platelets with the ADP antagonist A2P5P did not lead to a significant decrease in the amount of VEGF produced (85.92 pg/mL ± 28.10 vs. 64.21 pg/mL ± 17.17; P > .05). To examine the physiologic relevance of our findings, we again analyzed the capacity of specific releasates to promote angiogenesis. The platelets were exposed to MCF-7 cells for 10 minutes in the transwells and then the releasate was collected and used in standard angiogenic assays. There was increased endothelial cell migration when the platelets were exposed to MCF-7 cells in comparison to platelets alone (Figure 6B). In Figure 6C, the capillary tube formation assay showed that platelets exposed to MCF-7 cells in the transwell apparatus create a releasate that is pro-angiogenic, with more complex meshwork of capillary tubes than that generated without exposure to MCF-7 cells. In Figure 6D, there is a statistically significant difference in the number of branch points between the 2 groups with a mean number of branch points in the platelet group of 89.75 ± 3.119 compared with 171.5 ± 40 in the platelet coincubated with MCF-7 in transwell group (P < .05).
Figure 6.
Physical contact between platelets and MCF-7 cells is not necessary for VEGF release. (A) VEGF concentration in the releasate generated in the transwell apparatus (TW) in platelets alone, or coincubated with MCF-7 cells with or without A2. (B) Endothelial cell migration after exposure to the releasate generated from platelets alone or coincubated with MCF-7 cells in transwell apparatus. (C) Capillary tube formation using releasate generated in the transwell apparatus with platelets alone (top) or coincubation with MCF-7 cells (bottom). The scale bar is 300μm in size. (D) Quantification of branch points generated from the releasate using transwell apparatus with platelets alone or coincubated with MCF-7 cells.
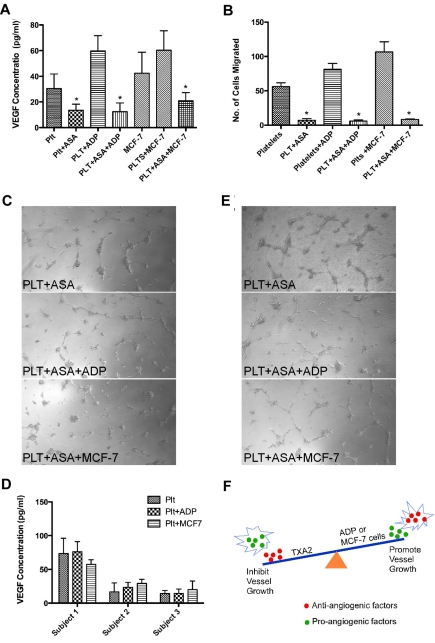
Aspirin inhibits the release of VEGF from platelets and suppresses angiogenesis mediated by MCF-7 cells or ADP
To determine whether aspirin could block the release of VEGF and also the angiogenic potential of platelets activated with MCF-7 cells or ADP, we performed these experiments in the presence of aspirin. Platelets were pretreated with aspirin (100μM) and the release of VEGF was assayed after exposure to MCF-7 cells or ADP. Platelets exposed to aspirin produced less VEGF in the resting state and also after exposure to ADP or MCF-7 cells (Figure 7A). In comparison to platelets without exposure to aspirin, there is a statistically significant decrease in VEGF release in each treatment group. Treatment with aspirin before exposure to ADP or MCF-7 cells led to a decrease in angiogenic potential of the releasate generated from platelets. In Figure 7B, we demonstrate that pretreatment with aspirin decreased endothelial cell migration either alone or after exposure to ADP or MCF-7 cells. The decreased angiogenic potential is also displayed in Figure 7C in which capillary tube formation was not observed after exposure to the releasate generated from platelets pretreated with aspirin before ADP or MCF-7 cell exposure. To look at the effect of aspirin on platelet-mediated angiogenesis in vivo, blood was drawn from 3 subjects who had been taking aspirin (81mg) for 5-7 days prior. In the case of all 3 subjects, the VEGF released was decreased in the resting state as well as after exposure to ADP or MCF-7 cells in comparison to platelets not exposed to aspirin (Figure 7D) and there was minimal capillary tube formation (Figure 7E). This demonstrates that pretreatment with aspirin decreases the amount of VEGF that is released from platelets in the resting state and also after activation with ADP or interaction with MCF-7 cells. Furthermore, the releasate generated from platelets after exposure to aspirin has a decreased angiogenic potential alone as well as after stimulation with ADP or interaction with MCF-7 cells.
Figure 7.
Aspirin inhibits VEGF release and angiogenesis mediated by ADP or MCF-7 cell exposure. (A) VEGF concentration in the releasate of platelets (plts) with or without pretreatment with aspirin (asa) alone or with exposure to ADP (25μM) or MCF-7 cells. (B) Endothelial cell migration after exposure to the releasate from platelets pretreated with aspirin alone or before exposure to ADP or MCF-7 cells. (C) Capillary tube formation using releasate from platelets pretreated with aspirin alone or before exposure to ADP or MCF-7 cells. (D) VEGF concentration in the releasate of platelets from subjects who ingested aspirin alone or after exposure to ADP or MCF-7 cells. (E) Representative images of capillary tube formation using platelet releasate from subjects who ingested aspirin with or without exposure to ADP or MCF-7 cells. (F) Schematic of the balance of pro- and anti-angiogenic factors and how this is influenced by platelet activation. Specific factors can influence which proteins are released from platelet α granules resulting in an overall pro- or anti-angiogenic effect. As diagrammed, TXA2 activation results in inhibition of blood vessel growth and ADP or MCF-7 cell regulated activation results in promotion of blood vessel growth.
Discussion
Because of their small size and the rheology of flowing blood, platelets are thrust against the endothelium by larger blood cells. We hypothesize that this proximity to, and the interaction with, the endothelium are key factors that allow platelets to modulate angiogenesis. Some of the first evidence suggesting that platelets influence new blood vessel development was reported by Gimbrone and others who showed that thrombocytopenia led to elevated vascular permeability.21,22,23
Platelets play a critical role in regulating angiogenesis in wound healing. During wound healing there is likely to be a spatial and temporal control of the release of angiogenic regulators to allow for a balance of anti- and pro-angiogenic factors to regulate hemostasis and angiogenesis simultaneously. In this paper we show that angiogenesis regulatory proteins can undergo differential release under in vitro physiologic conditions. In the presence of the known platelet agonists ADP and TXA2 there is differential release of VEGF and endostatin, respectively. Interestingly, agonist concentration and exposure times produced different concentrations of VEGF and endostatin which could be because of receptor saturation or the half life of these factors. The differential release of these angiogenic factors is partially blocked using specific antagonists. Our observations are consistent with publications of Ma et al and Bambace et al.20,24,25 While these previous studies have analyzed the release of individual angiogenesis regulatory proteins by specific platelet agonists, the agonist-driven releasate of platelets contains a complex inventory of multiple angiogenesis regulatory proteins. To fully appreciate the angiogenic potential of the platelet releasate in a more physiologic context, we performed various angiogenic assays. Our work reveals divergent angiogenic effects in the releasate generated by ADP and TXA2, demonstrating that platelets can stimulate or suppress angiogenesis (Figure 7F).
Because platelets have been implicated in several forms of pathologic angiogenesis, we focused on their interaction with tumor cells. The growth of a tumor beyond 1-2 mm3 requires the formation of new blood vessels. Like normal tissues, tumor cells require a blood vessel system to provide metabolites, oxygen, and a means of waste disposal. The role of platelets in tumor growth is supported by a large body of experimental and clinical data. Gasic and colleagues revealed the involvement of platelets in tumor progression, demonstrating that thrombocytopenia protected against metastasis.26 Additional studies demonstrated that some tumors can induce platelet activation. While cancer cells have been shown to aggregate platelets and this may correlate with increased metastatic disease, the underlying mechanisms are not fully understood.8,26–28 In our experiments we show that exposure to the tumor cell line, MCF-7, led to differential release of VEGF from platelet α granules, thus providing a mechanism to create a pro-angiogenic microenvironment for tumor growth. In support of this hypothesis, the releasate from MCF-7–stimulated platelets demonstrated an increased angiogenic potential. Interestingly, there is also an increase in the number of pro-angiogenic proteins present in the releasate, but many of the proteins are not the same as the pro-angiogenic proteins released during the process of activation with the platelet agonist ADP. This would imply that the process of platelet activation with MCF-7 cells involves stimulation with more than just ADP.
While our studies suggest that platelets may adhere to tumors and secrete their pro-angiogenic contents into the tumor microenvironment, additional mechanisms may stimulate this platelet-mediated pro-angiogenic shift. For example, because platelets take up several proteins, they may scavenge tumor-cell-released angiogenic stimulators from the vasculature of the tumor. In support of this concept, cancer patients have higher levels of platelet VEGF and could therefore contribute an additional boost to the ongoing angiogenesis. In addition, this hypothesis might help explain why high platelet counts coincide with poor prognosis in patients. Elevated levels of platelets may lead to more deposition of pro-angiogenesis regulatory proteins into the tumor microenvironment. The observation that thrombocytopenic mice fail to form tumors provides additional evidence that platelets may be contributing to tumor growth.29–31
The interaction of tumor cells and platelets is crucial to tumor survival and metastasis. Many studies have shown that tumor cells have the ability to induce platelet aggregation and that this is correlated positively with their metastatic potential in vivo.6,32–36 Our observation that MCF-7 tumor cells promote the differential release of pro-angiogenic proteins from platelets may explain why some of the inhibitors of platelet function, including aspirin and other antiplatelet agents can interfere with tumor metastasis and growth.12,37–40 By understanding the mechanisms involved in specific types of tumor cell-induced platelet activation (TCIPA) we can better target platelet agents that may be beneficial for decreasing angiogenesis and limiting tumor growth and metastasis.
There are many pharmacologic agents that are known to block platelet function and are thought to have an anti-mestastatic effect. Regular use of aspirin has been associated with a risk reduction for colon cancer.41 A recent study has shown that long-term use of aspirin can decrease the risk of death because of cancer. Many mechanisms have been suggested for how aspirin can impact malignancy including inhibition of cyclooxygenase, promotion of apoptosis, DNA mismatch repair, and inhibition of tumor cell proliferation by blockade of mitochondrial calcium uptake. Aspirin also functions as an inhibitor of TCIPA in vitro.12,37–40 Yet another mechanism by which aspirin could modulate cancer is as an irreversible inhibitor of platelet activation with subsequent decrease in VEGF release and decrease in angiogenic potential as shown in “Aspirin inhibits the release of VEGF from platelets and suppresses angiogenesis mediated by MCF-7 cells or ADP.” These anti-metastatic effects are not limited to aspirin as other antiplatelet agents such as P2Y12 and GPII/IIIa antagonists also inhibit TCIPA.34,42–47 Based on these findings, future work will focus on identifying the role platelet inhibition has on tumor cell-induced platelet activation and how this modulates the angiogenic capacity of the platelet releasate.
Understanding platelet-mediated angiogenesis and its impact on tumor growth has broad-reaching implications. Physiologic angiogenesis is a fundamental biologic mechanism that occurs in embryonic development, as the formation of a vascular system is one of the initial events in organogenesis. It also occurs in adults during wound healing, in the placenta during pregnancy, in the cycling ovary, and during restoration of blood flow to damaged tissues. Angiogenesis is regulated by a very sensitive interplay of growth factors and inhibitors, and their imbalance can lead to disease. In cancer, diabetic eye disease, age-related macular degeneration, cutaneous and gastric ulcers, and rheumatoid arthritis, extreme angiogenesis feeds diseased tissue and damages normal tissue. Insufficient angiogenesis underlies conditions such as coronary heart disease, stroke and delayed wound healing, where limited vessel growth leads to poor circulation. It is currently estimated that 500 million people worldwide would benefit from either pro- or anti-angiogenic therapies.25 A better understanding of how angiogenesis regulatory proteins are transported and precisely delivered via platelets to areas of vessel development will yield strategies for targeted angiogenesis therapies.
Supplementary Material
Acknowledgments
The authors thank Dr Robert Flaumenhaft, Dr Marsha Moses, Dr Sandra Ryeom, and Dr Alex Zaslavsky for reading this manuscript; Dr Tanya Laidlaw for the aspirin; and Dr Roopali Roy for technical assistance with angiogenesis assays.
This work was supported by K08HL097070 awarded by the National Institutes of Health/National Heart, Lung, and Blood Institute to E.M.B. and PO1 CA045548-21 to J.E.I.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.M.B. designed and performed the research, analyzed the data, and wrote and revised the manuscript; B.A.M. performed experiments; and J.E.I. provided mentorship for the group and expertise in preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisabeth M. Battinelli MD/PhD, Division of Hematology, Department of Internal Medicine, Brigham and Women's Hospital, Karp, 5th floor, 1 Blackfan Circle, Boston, MA 02115; e-mail:ebattinelli@partners.org.
References
- 1.Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81(4):1012–1019. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monreal M, Fernandez-Llamazares J, Pinol M, et al. Platelet count and survival in patients with colorectal cancer-a preliminary study. Thromb Haemost. 1998;79(5):916–918. [PubMed] [Google Scholar]
- 3.Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9(9):1826–1830. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- 4.Sun NC, McAfee WM, Hum GJ, Weiner JM. Hemostatic abnormalities in malignancy, a prospective study of one hundred eight patients. Part I. Coagulation studies. Am J Clin Pathol. 1979;71(1):10–16. doi: 10.1093/ajcp/71.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 115(17):3427–3436. doi: 10.1182/blood-2009-10-247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honn KV, Tang D. Hemostasis and malignancy: an overview. Cancer Metastasis Rev. 1992;11(3–4):223–226. doi: 10.1007/BF01307178. [DOI] [PubMed] [Google Scholar]
- 7.Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102(6):V215–224. doi: 10.1016/s0049-3848(01)00285-7. [DOI] [PubMed] [Google Scholar]
- 8.Gasic GJ. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metastasis Rev. 1984;3(2):99–114. doi: 10.1007/BF00047657. [DOI] [PubMed] [Google Scholar]
- 9.Bastida E, Escolar G, Almirall L, Ordinas A. Platelet activation induced by a human neuroblastoma tumor cell line is reduced by prior administration of ticlopidine. Thromb Haemost. 1986;55(3):333–337. [PubMed] [Google Scholar]
- 10.Heinmoller E, Weinel RJ, Heidtmann HH, et al. Studies on tumor-cell-induced platelet aggregation in human lung cancer cell lines. J Cancer Res Clin Oncol. 1996;122(12):735–744. doi: 10.1007/BF01209121. [DOI] [PubMed] [Google Scholar]
- 11.Boukerche H, Berthier-Vergnes O, Penin F, et al. Human melanoma cell lines differ in their capacity to release ADP and aggregate platelets. Br J Haematol. 1994;87(4):763–772. doi: 10.1111/j.1365-2141.1994.tb06736.x. [DOI] [PubMed] [Google Scholar]
- 12.Alonso-Escolano D, Strongin AY, Chung AW, Deryugina EI, Radomski MW. Membrane type-1 matrix metalloproteinase stimulates tumour cell-induced platelet aggregation: role of receptor glycoproteins. Br J Pharmacol. 2004;141(2):241–252. doi: 10.1038/sj.bjp.0705606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jurasz P, Chung AW, Radomski A, Radomski MW. Nonremodeling properties of matrix metalloproteinases: the platelet connection. Circ Res. 2002;90(10):1041–1043. doi: 10.1161/01.res.0000021398.28936.1d. [DOI] [PubMed] [Google Scholar]
- 14.Pacchiarini L, Zucchella M, Milanesi G, et al. Thromboxane production by platelets during tumor cell-induced platelet activation. Invasion Metastasis. 1991;11(2):102–109. [PubMed] [Google Scholar]
- 15.Grignani G, Pacchiarini L, Ricetti MM, et al. Mechanisms of platelet activation by cultured human cancer cells and cells freshly isolated from tumor tissues. Invasion Metastasis. 1989;9(5):298–309. [PubMed] [Google Scholar]
- 16.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 376(9754):1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 17.Peterson JE, Zurakowski D, Italiano JE, Jr, et al. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol. 85(7):487–493. doi: 10.1002/ajh.21732. [DOI] [PubMed] [Google Scholar]
- 18.Italiano JE, Jr, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111(3):1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sehgal S, Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J Thromb Haemost. 2007;5(10):2009–2016. doi: 10.1111/j.1538-7836.2007.02698.x. [DOI] [PubMed] [Google Scholar]
- 20.Bambace NM, Levis JE, Holmes CE. The effect of P2Y-mediated platelet activation on the release of VEGF and endostatin from platelets. Platelets. 21(2):85–93. doi: 10.3109/09537100903470298. [DOI] [PubMed] [Google Scholar]
- 21.Gimbrone MA, Jr, Aster RH, Cotran RS, Corkery J, Jandl JH, Folkman J. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature. 1969;222(5188):33–36. doi: 10.1038/222033a0. [DOI] [PubMed] [Google Scholar]
- 22.Gore I, Takada M, Austin J. Ultrastructural basis of experimental thrombocytopenic purpura. Arch Pathol. 1970;90(3):197–205. [PubMed] [Google Scholar]
- 23.Kitchens CS, Weiss L. Ultrastructural changes of endothelium associated with thrombocytopenia. Blood. 1975;46(4):567–578. [PubMed] [Google Scholar]
- 24.Storrie MC, Scher M, McGuire J, Bokan J. Thrombocytopenia in the absence of leukopenia associated with the use of neuroleptics. J Clin Psychiatry. 1978;39(10):779–781. [PubMed] [Google Scholar]
- 25.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 26.Gasic GJ, Koch PA, Hsu B, Gasic TB, Niewiarowski S. Thrombogenic activity of mouse and human tumors: effects on platelets, coagulation, and fibrinolysis, and possible significance for metastases. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1976;86(3):263–277. doi: 10.1007/BF00286945. [DOI] [PubMed] [Google Scholar]
- 27.Radomski MW, Jenkins DC, Holmes L, Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991;51(22):6073–6078. [PubMed] [Google Scholar]
- 28.Pearlstein E, Ambrogio C, Gasic G, Karpatkin S. Inhibition of the platelet-aggregating activity of two human adenocarcinomas of the colon and an anaplastic murine tumor with a specific thrombin inhibitor, dansylarginine N-(3-ethyl-1, 5-pentanediyl)amide. Cancer Res. 1981;41(11 Pt 1):4535–4539. [PubMed] [Google Scholar]
- 29.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104(2):397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 30.Coughlin SR. Protease-activated receptors and platelet function. Thromb Haemost. 1999;82(2):353–356. [PubMed] [Google Scholar]
- 31.Pearlstein E, Ambrogio C, Karpatkin S. Effect of antiplatelet antibody on the development of pulmonary metastases following injection of CT26 colon adenocarcinoma, Lewis lung carcinoma, and B16 amelanotic melanoma tumor cells into mice. Cancer Res. 1984;44(9):3884–3887. [PubMed] [Google Scholar]
- 32.Tohgo A, Tanaka NG, Ogawa H. Platelet-aggregating activities of metastasizing tumor cells. IV. Effects of cell surface modification on thrombin generation, platelet aggregation and subsequent lung colonization. Invasion Metastasis. 1986;6(1):58–68. [PubMed] [Google Scholar]
- 33.Sugimoto Y, Oh-hara T, Watanabe M, Saito H, Yamori T, Tsuruo T. Acquisition of metastatic ability in hybridomas between two low metastatic clones of murine colon adenocarcinoma 26 defective in either platelet-aggregating activity or in vivo growth potential. Cancer Res. 1987;47(16):4396–4401. [PubMed] [Google Scholar]
- 34.Honn KV, Cicone B, Skoff A. Prostacyclin: a potent antimetastatic agent. Science. 1981;212(4500):1270–1272. doi: 10.1126/science.7015512. [DOI] [PubMed] [Google Scholar]
- 35.Tsuruo T, Kawabata H, Iida H, Yamori T. Tumor-induced platelet aggregation and growth promoting factors as determinants for successful tumor metastasis. Clin Exp Metastasis. 1986;4(1):25–33. doi: 10.1007/BF00053470. [DOI] [PubMed] [Google Scholar]
- 36.Mahalingam M, Ugen KE, Kao KJ, Klein PA. Functional role of platelets in experimental metastasis studied with cloned murine fibrosarcoma cell variants. Cancer Res. 1988;48(6):1460–1464. [PubMed] [Google Scholar]
- 37.Bradley CJ, Dauer RJ, Thurlow PJ, Connellan JM. Characterization of platelet aggregation induced by the human carcinosarcoma Colo 526: role of platelet activation, tumor cell cytoskeleton and tumor cell plasma membrane. Pathology. 1997;29(2):189–195. doi: 10.1080/00313029700169844. [DOI] [PubMed] [Google Scholar]
- 38.Jurasz P, Sawicki G, Duszyk M, et al. Matrix metalloproteinase 2 in tumor cell-induced platelet aggregation: regulation by nitric oxide. Cancer Res. 2001;61(1):376–382. [PubMed] [Google Scholar]
- 39.Bastida E, Almirall L, Ordinas A. Tumor-cell-induced platelet aggregation is a glycoprotein-dependent and lipoxygenase-associated process. Int J Cancer. 1987;39(6):760–763. doi: 10.1002/ijc.2910390617. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton J, Subbarao V, Granack K, Ts'ao C. Platelet interaction with a pancreatic ascites tumor. Am J Pathol. 1986;122(1):160–168. [PMC free article] [PubMed] [Google Scholar]
- 41.Serebruany VL. Aggressive chronic platelet inhibition with prasugrel and increased cancer risks: revising oral antiplatelet regimens? Fundam Clin Pharmacol. 2009;23(4):411–417. doi: 10.1111/j.1472-8206.2009.00710.x. [DOI] [PubMed] [Google Scholar]
- 42.Manegold PC, Hutter J, Pahernik SA, Messmer K, Dellian M. Platelet-endothelial interaction in tumor angiogenesis and microcirculation. Blood. 2003;101(5):1970–1976. doi: 10.1182/blood.V101.5.1970. [DOI] [PubMed] [Google Scholar]
- 43.Jurasz P, Alonso D, Castro-Blanco S, Murad F, Radomski MW. Generation and role of angiostatin in human platelets. Blood. 2003;102(9):3217–3223. doi: 10.1182/blood-2003-02-0378. [DOI] [PubMed] [Google Scholar]
- 44.Salgado R, Benoy I, Bogers J, et al. Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis. 2001;4(1):37–43. doi: 10.1023/a:1016611230747. [DOI] [PubMed] [Google Scholar]
- 45.Pinedo HM, de Gruijl TD, van Der Wall E, Buter J. Biological concepts of prolonged neoadjuvant treatment plus GM-CSF in locally advanced tumors. Oncologist. 2000;5(6):497–500. doi: 10.1634/theoncologist.5-6-497. [DOI] [PubMed] [Google Scholar]
- 46.Maragoudakis ME, Tsopanoglou NE, Andriopoulou P, Maragoudakis MM. Effects of thrombin/thrombosis in angiogenesis and tumour progression. Matrix Biol. 2000;19(4):345–351. doi: 10.1016/s0945-053x(00)00079-2. [DOI] [PubMed] [Google Scholar]
- 47.Pinedo HM, Verheul HM, D'Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352(9142):1775–1777. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.