Abstract
Background
The aim of this survey was to determine the prevalence of and factors associated with Helicobacter pylori (H. pylori) colonization in HIV-infected, highly active antiretroviral therapy-naïve Ugandan children aged 0-12 years.
Methods
In a hospital-based survey, 236 HIV-infected children were tested for H. pylori colonization using a faecal antigen test. A standardized interview with socio-demographic information and medical history was used to assess risk factors. A cluster of differentiation 4 (CD4) cell percentage was prevalent in most children.
Results
The overall prevalence of H. pylori in the HIV-infected children was 22.5%. Age-specific prevalence was as follows: up to one year, 14.7%; 1-3 years, 30.9%; and 3-12 years, 20.7%. HIV-infected children who were more seriously affected by their disease (low CD4 cell percentage or WHO clinical stage II-IV) were less likely to be colonized with H. pylori. There was a trend for a lower prevalence of H. pylori in children who had taken antibiotics for the preceding two weeks (21.6%) than in those who had not taken antibiotics (35.7%). There was no statistically significant difference in prevalence by gender, housing, congested living, education of the female caretaker, drinking water or toilet facilities.
Conclusions
HIV-infected, HAART-naïve Ugandan children had a lower prevalence of H. pylori colonization compared with apparently healthy Ugandan children (44.3%). Children with a low CD4 cell percentage and an advanced clinical stage of HIV had an even lower risk of H. pylori colonization. Treatment with antibiotics due to co-morbidity with infectious diseases is a possible explanation for the relatively low prevalence.
Background
Sub-Saharan Africa accounts for 67% of all people living HIV, and carries the highest burden of the global HIV epidemic [1]. In Uganda, it has been estimated that 1.1 million people, including 120,000 children, were living with HIV in 2008 [2]. The gastrointestinal tract is the largest immunological site of the body and HIV infection profoundly impacts on gut function [3,4]. HIV-infected children are affected by numerous gastrointestinal problems [5].
Helicobacter pylori, which can cause chronic gastritis, is associated with recurrent peptic ulcers and gastric cancer [6,7]. It is one of the most common causes of bacterial infection in man [8,9]. It was first isolated and cultured from the antrum of patients with gastritis by Warren and Marshall in 1983 [10]. H. pylori colonization is thought to be acquired early in life. Early colonization in children living in poor socio-economic conditions has been demonstrated, and several studies have shown a high prevalence of H. pylori among people in low-income countries [11-15]. The overall prevalence was 44.3% in our recently published study on apparently healthy, urban Ugandan children [15].
Published data on H. pylori infections in HIV-infected persons are mainly based on adults [16] and are from non-epidemic areas [17], and many studies have undertaken serological tests [18] or have been conducted on persons referred for gastrointestinal complaints [19]. These studies report diverging estimates of the prevalence of H. pylori [20]. To the best of our knowledge, there seem to be no studies on the prevalence of H. pylori in HIV-infected children living in sub-Saharan Africa. In a study designed to describe the findings in HIV-infected South African children who underwent gastroscopy, rates of H. pylori colonization were reported, and only one out of 26 children was colonized [21].
There are currently four distinct methodologies for H. pylori detection and/or identification: (1) 13Urea breath test [22,23]; (2) gastroscopy with biopsies and culture; (3) serology tests; and (4) antigen tests. The 13Urea breath test or invasive methods, such as gastroscopy with biopsies and/or urease tests, used to be the "gold standard" for detection of H. pylori. A 13Urea breath test is time consuming and personnel dependent. A gastroscopy should be performed when a child presents clinical symptoms for diagnosis and is not justified for mere identification of H. pylori. Serological tests are available, but have several drawbacks: (1) they do not discriminate between current and past infections; (2) they show low specificity in children and are thus of little use [24,25]; and (3) no data is available about the specificity and sensitivity of serological tests in HIV-infected individuals with immunodeficiency and altered antibody production.
The faecal monoclonal antigen test has a high sensitivity, specificity and accuracy in children: 91-100, 84-96 and 94-96%, respectively [26-28]. In a review on the incidence of H. pylori in HIV-infected patients [20], the use of the faecal antigen test is recommended for further studies due to its higher specificity in this population. It can be used on humans of all age groups, gives a rapid result without being invasive, and is not affected by acid-regulating medicines.
Our main objective was to determine the prevalence and factors associated with H. pylori colonization in HIV-infected, highly active antiretroviral therapy- (HAART-) naïve children aged 0-12 years in urban Kampala, Uganda.
Methods
Study site and data collection
The survey was conducted from February to October 2008 at the Department of Paediatrics and Child Health, Mulago National Referral Hospital, Kampala, a government-run hospital. It assumes the role of the local hospital for the people living in the area of Mulago Hill and, at the same time, the role of a national referral hospital for Uganda. Participants were enrolled from the general paediatric medical wards, the acute care unit, the ward for malnutrition and the paediatric infectious diseases clinic.
We decided in advance on an enrolment period of nine months. The data was collected by a doctor with experience in data collection and in paediatric HIV pre- and post-test counselling, as well as diagnosis and treatment of paediatric HIV. She was fluent in the local languages and English. GN and EH trained her in stool sampling, interview technique and ethical issues. GN was available for consultation if necessary. Children admitted during the enrolment period to the wards we have mentioned were invited to participate in the study if they were HIV infected, but HAART naïve, aged 0-12 years, and only after receiving informed consent from their caretaker. The ward matrons were asked to identify the eligible children and their caretakers. All those so identified children and caretakers were invited to participate.
The HIV status of the children was known before enrolment from routine testing as part of the medical service at the Department of Paediatrics and Child Health. HIV testing followed the Ugandan national guidelines [29] that closely follow the World Health Organization (WHO) guidelines. Children over 18 months of age were tested using a rapid blood test with a sensitivity rate > 98%. To confirm positive test results, a second test with a different antigenic specificity was used. If there was discordance between the two tests, an ELISA test (tie-breaker) was used to make a final diagnosis. For children under 18 months of age, a polymerase change reaction test was used to give a reliable HIV diagnosis.
Study population
The study population (Figure 1) consisted of 246 HIV-infected, HAART-naïve children aged up to 12 years. Only 4.1% of the eligible children (10/246) were not included in the final analysis as a result of: no H. pylori test performed (six); failure to produce a stool sample in three days (three); and providing an incomplete questionnaire (one). In 219 of the 236 participants, CD4 cell counts expressed as percentages were available. We classified CD4 cell percentage as high or low with limits defined by age: (1) for children < 12 months, high if CD4 cell percentage was > 25%: (2) for 12-36 months, high if CD4 cell percentage was > 20%; and (3) for ≥36 months, high if CD4 cell percentage was > 15%. The limits chosen were concurrent with those recommended for starting HAART according to the WHO guidelines available at the time of the study [30]. All children were clinically categorized using the WHO staging system for HIV-infected children [31] since it is recommended for evaluating the need to start up HAART in children when CD4 cell counts are unavailable [30].
Figure 1.
Study profile.
H. pylori stool antigen test
Stool samples requested from each participating child were collected in airtight containers at the time of the encounter, the end of the day, or the following morning. They were transported from the ward to the laboratory twice daily and stored in a +4°C fridge for a maximum of 24 hours before analysis by the H. pylori stool antigen test, HpSA®ImmunoCardSTAT, as per the manufacturer's instructions. A standard positive control test was performed after every 20 tests, all of them being verified as positive. The HpSA®ImmunoCardSTAT is a rapid lateral-flow immunoassay that utilizes a monoclonal anti-H. pylori antibody as the capture and detector antibody. Approximately 100 μl of stool was transferred into the sample diluent vial and vortexed for 15 seconds. Four drops of the specimen were applied to the test and the result was read after five minutes. The results were reported as positive or negative based on the manufacturer's cut-off values.
Statistical analysis
Data from the questionnaires, the results of the faecal antigen test and CD4 cell percentages were doubly entered using EpiData version 3.1 (http://www.epidata.dk). Data quality was ensured through careful selection and training of research assistants, supervision, and field editing by use of the "check" module at data entry combined with double data entry and validation. The "checks" at data entry were limits set by the study team to ensure that it was not possible to enter obviously wrong information. For example, a child can measure only between 48 cm and 180 cm (it is not possible to enter other data), and many answers can only be "yes" or "no".
After entering all data twice in separate files, the two separate data files were validated by comparison and any non-matching data were checked manually against the original paper form. The data were exported to SPSS version 17.0 for statistical analysis. To explore the prevalence of H. pylori and its association with other factors, bivariate logistic regression and multiple logistic regression were performed. Adjustments were made in the multiple logistic regression analysis for age, sex, CD4 cell percentage, clinical WHO staging, type of housing, number of people in the same household, education of the mother or female caretaker, sources of drinking water, toilet type (pit latrine), sharing of the toilet with other families, reported abdominal pain, wealth index, and drugs taken.
Due to the lack of known CD4 cell percentages in 17 participants, the multiple logistic regression analysis involved only 219 participants. The confidence interval (CI) reported was set at 95%, and the significance level was set at 0.05. To explore the socio-economic status of the participants, principal component analysis (PCA) was used. Twelve questions encompassed socio-economic status (composed of assets in the household, sources of power available for the family, standard of housing for the child, and if the family were farmers or owned their own land and/or house), and we carried out PCA for these questions. The model captured ~79% of our results; the Kaiser-Meyer-Oklin value was 0.79, exceeding the recommended value of 0.6, and the Barletts Test of Sphericity reached statistical significance. PCA revealed the presence of three components with Eigen values exceeding 1. The first principal component was used as our wealth index, explaining 30.5% of the variance. The wealth index was ranked and categorized into three tertiles (1 - poorest, 2 - poorer, 3 - least poor) that were equally distributed.
Ethics
Ethical approval was obtained from Makerere University, Faculty of Medicine, Research and Ethics Committee in Uganda, and the Regional Committee for Medical and Health Research Ethics, West-Norway (REK-VEST) in Norway. The data collectors were trained in ethical issues prior to the survey. Oral and written information about the study was given to the caretakers either in English or the local language. Informed consent was obtained from all the caretakers of the participants in the study. If the doctor found the medical history of a participating child suspect of gastritis and the child tested positive for H. pylori, the child was given triple therapy of amoxicillin/claritromycin/omeprazole for one week. All children participating in the study were independently managed for their medical needs by the doctor in charge of the ward.
Results
The mean age (± SD) of the 236 participants who completed the study was 2.9 (2.8) years; for girls 2.8 (2.8) years and boys 3.1 (2.8) years. The youngest enrolled child was 1.5 months. There were 19 children younger than six months enrolled. The genders were equally represented in the survey: 121 (51.3%) girls and 115 (48.7%) boys.
The overall prevalence of H. pylori antigen in the 236 children was 22.5 % (Table 1).
Table 1.
Prevalence of Helicobacter pylori in Ugandan HIV-infected children by age groups
| Age categories | Total number N | H. pylori positive n | H. pylori prevalence % (95% CI) |
|---|---|---|---|
| 0 < 1 year | 68 | 10 | 14.7 (6-23) |
| 1 < 3 years | 81 | 25 | 30.9 (21-41) |
| 3 < 6 years | 53 | 12 | 22.6 (11-34) |
| 6 < 9 years | 22 | 4 | 18.2 (1-36) |
| 9 < 12 years | 12 | 2 | 16.7 (8-41) |
| Total | 236 | 53 | 22.5 (17-28) |
Age-specific prevalence was: (1) for up to one year, 14.7%: (2) for 1-3 years, 30.9%; and (3) for 3-12 years, 20.7%. The difference in prevalence between the youngest children and the group aged 1-3 years was significant, also after adjusting for the other factors in the multiple logistic regression analysis (Table 2). The lower prevalence after the age of three years was not statistically significant. There was no difference in colonization rates of H. pylori by gender.
Table 2.
Helicobacter pylori colonization in HIV infected, treatment naïve children and associated factors
| Number N | HP positive n (%) | Unadjusted odds ratio (95% CI) | p value | Adjusted odds ratio1 (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Age groups | ||||||
| 0 < 1 year | 68 | 10 (14.7) | 1 | 1 | ||
| 1 < 3 years | 81 | 25 (30.9) | 2.6 (1.1-5.9) | 0.02 | 2.8 (1.1-6.8) | 0.03 |
| 3 < 12 years | 87 | 18 (20.7) | 1.5 (0.6-3.5) | 0.34 | 1.2 (0.5-3.2) | 0.65 |
| Sex | ||||||
| Male | 115 | 22 (19.1) | 1 | - | ||
| Female | 121 | 31 (25.6) | 1.5 (0.8-2.7) | 0.23 | ||
| CD4 cell percentage2 | ||||||
| High | 115 | 35 (30.4) | 1 | 1 | ||
| Low | 104 | 13 (12.5) | 0.3 (0.2-0.7) | 0.002 | 0.3 (0.1-0.6) | 0.001 |
| Who classification | ||||||
| WHO stage I | 24 | 9 (37.5) | 1 | - | ||
| WHO stage II-VI | 212 | 44 (20.8) | 0.4 (0.2-1.1) | 0.07 | ||
| Type of housing | ||||||
| Permanent house | 105 | 23 (21.9) | 1 | - | ||
| Semi-permanent house | 131 | 30 (22.9) | 1.1 (0.6-2.0) | 0.86 | ||
|
Number of people in same household |
||||||
| 2-4 | 126 | 28 (22.2) | 1 | - | ||
| ≥5 | 110 | 25 (22.7) | 1.0 (0.6-1.9) | 0.93 | ||
|
Education of the mother/female caretaker |
||||||
| Completed primary | 83 | 21 (25.3) | 1 | - | ||
| school or higher | ||||||
| Incomplete primary school |
153 | 32 (20.9) | 0.8 (0.4-1.5) | 0.44 | ||
| Drinking water | ||||||
| Public tap | 138 | 28 (20.3) | 1 | - | ||
| Unprotected sources | 98 | 25 (25.5) | 1.4 (0.7-2.5) | 0.34 | ||
| Type of toilet | ||||||
| Open pit/pit latrine | 228 | 52 (22.8) | 1 | - | ||
| VIP latrine/flush toilet | 8 | 1 (12.5) | 0.5 (0.1-4.0) | 0.50 | ||
|
Sharing of toilet with other families |
||||||
| No | 72 | 16 (22.2) | 1 | - | ||
| Yes | 164 | 37 (22.6) | 1.0 (0.5-2.0) | 0.95 | ||
| Taken drugs last 3 months4 | ||||||
| No | 40 | 10 (25.0) | 1 | - | ||
| Yes | 196 | 43 (21.9) | 0.8 (0.4-1.9) | 0.67 | ||
|
Taken any antibiotics3 last 3 months |
||||||
| No | 61 | 17 (27.9) | 1 | - | ||
| Yes | 175 | 36 (20.6) | 0.7 (0.3-1.3) | 0.24 | ||
|
Taken any antibiotics last 2 weeks |
||||||
| No | 14 | 5 (35.7) | 1 | - | ||
| Yes | 222 | 48 (21.6) | 0.5 (0.2-1.6) | 0.23 | ||
|
Taken deworming medicine last 6 months |
||||||
| No | 125 | 23 (18.4) | 1 | - | ||
| Yes | 111 | 30 (27.0) | 1.6 (0.9-3.0) | 0.12 | ||
| Wealth index | ||||||
| Least poor | 78 | 12 (15.4) | 1 | - | ||
| Poorer | 80 | 19 (23.8) | 1.7 (0.8-3.8) | 0.19 | ||
| Poorest | 77 | 22 (28.6) | 2.2 (1.0-4.8) | 0.05 | ||
|
Reporting abdominal pain more than 3 times/week |
||||||
| No | 224 | 49 (21.9) | 1 | - | ||
| Yes | 12 | 4 (33.3) | 1.8 (0.5-6.2) | 0.36 |
1Adjusted for the 219 participants for whom CD4 percentage were available; adjustment was made for all categories included in the table.
2CD4 cell percentage was available for 219 of the 236 participants
3Last 2 weeks not included in this category
CD4 cell percentages were available for 219 participants. A low CD4 cell percentage was significantly associated with a lower H. pylori colonization rate, with an odds ratio (OR) and 95% confidence interval (OR ± 95% CI) of 0.33 (0.2-0.7), (Table 2). Participants with WHO stage II-VI had lower H. pylori colonization (20.8%) compared with those with WHO stage I (37.5%) (Table 3). The difference was not statistically significant (OR = 0.4; 95% CI 0.2-1.1) (Table 2).
Table 3.
Prevalence of Helicobacter pylori in Ugandan HIV-infected children by WHO stage
| WHO stage | Total number N | H. pylori positive n | H. pylori prevalence % (95% CI) |
|---|---|---|---|
| Stage I | 24 | 9 | 37.5 (17-58) |
| Stage II | 44 | 8 | 18.2 (6-30) |
| Stage III | 145 | 32 | 22.1 (15-29) |
| Stage VI | 23 | 4 | 17.4 (1-34) |
| Total | 236 | 53 | 22.5 (17-28) |
In the unadjusted analysis, the H. pylori colonization was higher among the poorest participants than among the other participants (OR ± 95% CI) of 2.2 (1.0-4.8), but the difference was not statistically significant after adjusting for the other factors in the analysis (Table 2).
There was no statistically significant difference in colonization rate in participants who had taken any kind of drugs or antibiotics within the last three months or two weeks before the survey assessment (Table 2). There was a lower colonization rate in children who had had antibiotics in the last three months (20.6 versus 27.9%) and in the last two weeks (21.6 versus 35.7%), but the difference was not statistically significant. Prophylaxis with cotrimoxazole was common (69.9%), but there was no different in the colonization rate between participants with or without prophylaxis.
There was no statistically significant difference in H. pylori prevalence by type of housing, congested living, education of female caretaker, drinking water sources, toilet facilities or reported abdominal pain (Table 2).
Discussion
In this large survey of HIV-infected children in an African urban setting, we identified a lower colonization rate of H. pylori in HIV-infected children compared with healthy children in the same area of Kampala, Uganda [15]. HIV-infected children more seriously affected by their disease (low CD4 cell percentage or WHO stage II-IV) were less likely to be colonized with H. pylori.
This is the first survey describing the prevalence of H. pylori colonization among HIV-infected, HAART-naïve Ugandan children. This is a novel survey in an epidemic area of HIV with focus on the prevalence of H. pylori in HAART-naïve children. Only two previous studies have provided data on prevalence of H. pylori in HIV-infected children [32,33], neither of them from endemic areas for HIV. A Belgian study [32] on 23 HIV-infected children of central African ethnic origin and born in Belgium used a serology test to detect H. pylori colonization. They found none of the tested children to be colonized compared with 19.2% of children in a control population.
An Italian study [33], using both serology and 13Urea breath tests in 45 perinatally HIV-infected children, found a prevalence of 17.7 and 20.0%, respectively. This was not different from a control population, but the HIV-infected and the control patients were both recruited from a socio-economic background predisposing them to H. pylori colonization; many of the children had a caretaker involved in intravenous drug abuse [33]. In 26 HIV-infected South African children who underwent gastroscopy, the rates of H. pylori colonization were reported, and only one child was colonized [21]. Our survey had a large sample size compared with other studies describing H. pylori prevalence in HIV-infected children [21,32,33].
In this survey, we used an active antigen method to investigate the colonization of H. pylori. A positive test is evidence of a current infection and not the possibility of a previous infection, which could have been the case had a serological test been used. If a test based on antibody detection was used, a participant with severe immunodeficiency could eventually show a false negative result due to an inadequate immune response. From other studies, we know that the antigen test used has high sensitivity and specificity in non-HIV-infected populations [26,27], and is recommended for screening in HIV-infected population [20].
A weakness is that we have no data on the specificity and sensitivity of this test in HIV-infected, immune-suppressed populations. Another weakness is that the number of children over six years of age was small compared with the rest of the study population, increasing the confidence interval of our estimates for H. pylori prevalence in the older age group. We failed to recruit more children over six years of age due to the natural history of AIDS and due to our inclusion criteria being HAART-naïve children.
In the adjusted multiple regression analysis, we had only 219 participants as 7.2% of the study population did not have their CD4 cell percentages measured. This made analysis more complex, but comparison of the models with 219 participants and all 236 participants showed no significant differences in OR with 95% CI or p values. In the analysis in Table 2, some of the factors had a much more skewed distribution and the survey did not have enough power to detect differences in the prevalence of H. pylori.
We have recently reported the prevalence of H. pylori in apparently healthy children in Kampala, Uganda [15]. Apparently healthy Ugandan children had an overall prevalence of H. pylori of 44.3%; HIV-infected Ugandan children had an overall prevalence of H. pylori of 22.5%. The prevalence in the HIV-infected children was lower in all age groups compared with apparently healthy children (Figure 2).
Figure 2.
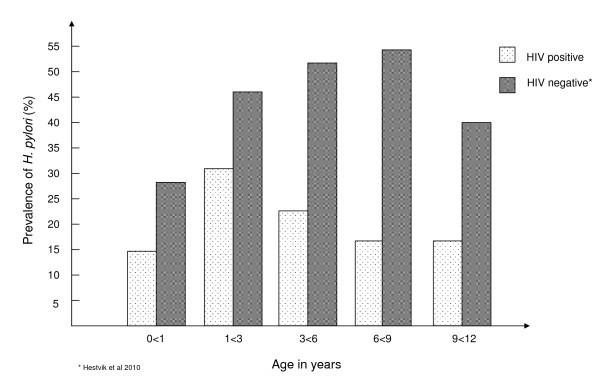
Comparison of prevalence of Helicobacter pylori in apparently healthy and HIV-infected Ugandan children by age.
Although there are limitations, the two studies have similarities. The two study populations have children aged 0-12 years, the gender distribution is similar, the same antigen test was used in both studies, and both studies were performed in urban areas. Sanitation conditions did not differ much between the two populations. The limitations of comparing the two studies are that one group is community based, receiving home visits, and the present study is hospital based, and that in the community-based study, 39% of the participants had taken antibiotics in the last three months versus 74% in the present study.
We identified a statistically significantly lower colonization rate of H. pylori in children who had low CD4 cell percentages. A CD4 cell percentage is more accurately used in young children due to the natural decline in the total lymphocyte count and the CD4 cell count [34]. To the best of our knowledge, there are no studies performed in child populations showing differences in prevalence of H. pylori according to the CD4 cell count. In adult populations, we find support for our findings: in a study from Argentina [35], the authors concluded that HIV-infected patients with H. pylori had a higher mean CD4 cell count than those without H. pylori; and a Zambian study [36] showed that HIV-infected adult patients with CD4 cell counts below 200 cells/mm3 were less likely to have positive H. pylori serologies (OR 0.29; 95% CI 0.09-0.93).
We found that H. pylori colonization was significantly higher in children aged 1-3 years than in children younger than one year of age. This is comparable to data for apparently healthy children from the same region [15], but it has not been described earlier among HIV-infected children in this age group. An Italian study [33] describing colonization of H. pylori by age only included three children younger than three years and none younger than one year of age.
We speculate that the colonization rates among the HIV infected are the same as in apparently healthy children, but among the HIV-infected children, accidental eradication is taking place due to the high use of antibiotics in these children; 74% of the children had taken antibiotics within the last three months and 95% of the survey participants were on antibiotics at time of enrolment or had taken antibiotics within the preceding two weeks.
Hospitalization therapy for bacterial infections, worms and protozoa are often given simultaneously if infections are present. These combined therapies can also be effective against H. pylori and eradicate it in a proportion of children. The use of antibiotics against opportunistic infections in HIV-infected populations is the most hypothesized explanation for lower colonization rates [19,20,37]. We could only show a trend of lower colonization among children who had used antibiotics. We assume that we could not show a significant difference between those who had used antibiotics and the others due to the large number of participants who had been treated with antibiotics in the past compared with those who had not been treated. The study was not designed to show those differences.
To use a clinical staging system for AIDS is useful and recommended when a CD4 cell count is not available, but it is not recommended for use for initiating HAART if a CD4 cell count is available [30]. We could not demonstrate a significant difference relating to the clinical HIV WHO stage and the prevalence of H. pylori, but there was a trend for lower colonization rates in more advanced stages (Table 3). We think this is because many of the criteria used for advanced staging are chronic or recurrent infection. These infections are treated with antibiotics, for example, amoxicillin against upper airway infections; this drug is also recommended as a part of the triple treatment of H. pylori [38].
There was no significant difference in prevalence by sex, type of housing, congested living, education of female caretaker, drinking water sources, toilet facilities, reported abdominal pain or wealth index. A possible explanation for the lack of such association, as described in non-HIV-infected children [11,14,15,39], is that the impact of the CD4 cell percentage is very strong and independent of the factors we have mentioned.
Conclusions
HIV-infected, HAART-naïve, urban Ugandan children had a lower prevalence of H. pylori colonization compared with apparently healthy Ugandan children. Children with more advanced HIV (a low CD4 cell percentage and advanced clinical stage of HIV) had lower rates of colonization of H. pylori; this might indicate that these children had more frequently been treated with drugs also effective against H. pylori. Treatment with antibiotics or other drugs effective against H. pylori, due to co-morbidity with infectious diseases, is a likely explanation for the relatively low prevalence.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EH participated in the conception, design and implementation of the study, statistical analysis, interpretation and writing of the manuscript. TT participated in the conception and design of the study, statistical analysis, interpretation and writing of the manuscript. DKM participated in implementation of the study and performed the HpSA tests. GN participated in design and implementation of the study. LG participated in design of the study, interpretation and writing of the manuscript. EO participated in the conception and design of the study, statistical analysis, interpretation and writing of the manuscript. JKT participated in conception, design and implementation of the study. All authors read and approved the final manuscript.
Contributor Information
Elin Hestvik, Email: elin.hestvik@cih.uib.no.
Thorkild Tylleskar, Email: thorkild.tylleskar@cih.uib.no.
Grace Ndeezi, Email: gracendeezi@yahoo.com.
Lena Grahnquist, Email: lena.grahnquist@ki.se.
Edda Olafsdottir, Email: edda.olafsdottir@helse-bergen.no.
James K Tumwine, Email: kabaleimc@gmail.com.
Deogratias H Kaddu-Mulindwa, Email: dhkmulindwa@med.mak.ac.ug.
Acknowledgements and funding
We would like to thank all the children, their caretakers, the data collectors and the laboratory technicians who participated in the survey. The survey was conducted as a part of the collaboration between the Department of Paediatrics and Child Health, Makerere University and the Centre for International Health, University of Bergen.
The study was funded by the University of Bergen and the GlobVac programme by the Research Council of Norway, grant no 172226 Focus on Nutrition and Child Health: Intervention Studies in Low-income Countries.
References
- WHO. UNAIDS 2008 Report on Global AIDS epidemic. Geneva, Switzerland; 2008. [Google Scholar]
- Ministry of Health Uganda. UNGASS country progress report, Uganda, January 2008-December 2009. Kampala, Uganda; 2010. http://www.aidsuganda.org [Google Scholar]
- Sharpstone D, Gazzard B. Gastrointestinal manifestations of HIV infection. Lancet. 1996;14:379–383. doi: 10.1016/S0140-6736(96)01034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MR, Brann OS. Gastrointestinal manifestations of HIV infection. Curr Gastroenterol Rep. 2000;14:283–293. doi: 10.1007/s11894-000-0020-1. [DOI] [PubMed] [Google Scholar]
- Narwal S, Galeano NF, Pottenger E, Kazlow PG, Husain S, DeFelice AR. Idiopathic Esophageal ulcers in a child with AIDS. J Pediatr Gastroenterol Nutr. 1997;14:211–214. doi: 10.1097/00005176-199702000-00016. [DOI] [PubMed] [Google Scholar]
- Kindermann A, Lopes AI. Helicobacter pylori infection in pediatrics. Helicobacter. 2009;14(Suppl 1):52–57. doi: 10.1111/j.1523-5378.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect. 2009;14:971–976. doi: 10.1111/j.1469-0691.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;14(Suppl 1):3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. [DOI] [PubMed] [Google Scholar]
- Owen RJ. Bacteriology of Helicobacter pylori. Baillieres Clin Gastroenterol. 1995;14:415–446. doi: 10.1016/0950-3528(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Marshall. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;14:1273–1275. [PubMed] [Google Scholar]
- Ndip RN, Malange AE, Akoachere JF, MacKay WG, Titanji VP, Weaver LT. Helicobacter pylori antigens in the faeces of asymptomatic children in the Buea and Limbe health districts of Cameroon: a pilot study. Trop Med Int Health. 2004;14:1036–1040. doi: 10.1111/j.1365-3156.2004.01299.x. [DOI] [PubMed] [Google Scholar]
- Pelser HH, Househam KC, Joubert G, van der Linde G, Kraaij P, Meinardi M, McLeod A, Anthony M. Prevalence of Helicobacter pylori antibodies in children in Bloemfontein, South Africa. J Pediatr Gastroenterol Nutr. 1997;14:135–139. doi: 10.1097/00005176-199702000-00005. [DOI] [PubMed] [Google Scholar]
- Thomas JE, Dale A, Harding M, Coward WA, Cole TJ, Weaver LT. Helicobacter pylori colonization in early life. Pediatr Res. 1999;14:218–223. doi: 10.1203/00006450-199902000-00010. [DOI] [PubMed] [Google Scholar]
- Langat AC, Ogutu E, Kamenwa R, Simiyu DE. Prevalence of Helicobacter pylori in children less than three years of age in health facilities in Nairobi Province. East Afr Med J. 2006;14:471–477. doi: 10.4314/eamj.v83i09.46769. [DOI] [PubMed] [Google Scholar]
- Hestvik E, Tylleskar T, Kaddu-Mulindwa DH, Ndeezi G, Grahnquist L, Olafsdottir E, Tumwine JK. Helicobacter pylori in apparently healthy children aged 0-12 years in urban Kampala, Uganda: a community-based cross sectional survey. BMC Gastroenterol. 2010;14:62. doi: 10.1186/1471-230X-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichterfeld M, Lorenz C, Nischalke HD, Scheurlen C, Sauerbruch T, Rockstroh JK. Decreased prevalence of Helicobacter pylori infection in HIV patients with AIDS defining diseases. Z Gastroenterol. 2002;14:11–14. doi: 10.1055/s-2002-19637. [DOI] [PubMed] [Google Scholar]
- Vaira D, Miglioli M, Menegatti M, Holton J, Boschini A, Vergura M, Ricci C, Azzarone P, Mule P, Barbara L. et al. Helicobacter pylori status, endoscopic findings, and serology in HIV-1-positive patients. Dig Dis Sci. 1995;14:1622–1626. doi: 10.1007/BF02212680. [DOI] [PubMed] [Google Scholar]
- Fabris P, Bozzola L, Benedetti P, Scagnelli M, Nicolin R, Manfrin V, Scarparo C, De Lalla F. H. pylori infection in HIV-positive patients. A serohistological study. Dig Dis Sci. 1997;14:289–292. doi: 10.1023/A:1018801532136. [DOI] [PubMed] [Google Scholar]
- Panos GZ, Xirouchakis E, Tzias V, Charatsis G, Bliziotis IA, Doulgeroglou V, Margetis N, Falagas ME. Helicobacter pylori infection in symptomatic HIV-seropositive and -seronegative patients: a case-control study. AIDS Res Hum Retroviruses. 2007;14:709–712. doi: 10.1089/aid.2006.0174. [DOI] [PubMed] [Google Scholar]
- Romanelli F, Smith KM, Murphy BS. Does HIV infection alter the incidence or pathology of Helicobacter pylori infection? AIDS Patient Care STDS. 2007;14:908–919. doi: 10.1089/apc.2006.0215. [DOI] [PubMed] [Google Scholar]
- Cooke ML, Goddard EA, Brown RA. Endoscopy findings in HIV-infected children from sub-Saharan Africa. J Trop Pediatr. 2009;14:238–243. doi: 10.1093/tropej/fmn114. [DOI] [PubMed] [Google Scholar]
- Frenck RW Jr, Fathy HM, Sherif M, Mohran Z, El Mohammedy H, Francis W, Rockabrand D, Mounir BI, Rozmajzl P, Frierson HF. Sensitivity and specificity of various tests for the diagnosis of Helicobacter pylori in Egyptian children. Pediatrics. 2006;14:e1195–1202. doi: 10.1542/peds.2005-2925. [DOI] [PubMed] [Google Scholar]
- de Carvalho Costa Cardinali L, Rocha GA, Rocha AM, de Moura SB, de Figueiredo Soares T, Esteves AM, Nogueira AM, Cabral MM, de Carvalho AS, Bitencourt P. et al. Evaluation of [13C]urea breath test and Helicobacter pylori stool antigen test for diagnosis of H. pylori infection in children from a developing country. J Clin Microbiol. 2003;14:3334–3335. doi: 10.1128/JCM.41.7.3334-3335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri F, Pastore M, Clemente R, Festa V, Quitadamo M, Niro G, Conoscitore P, Rutgeerts P, Andriulli A. Helicobacter pylori infection may undergo spontaneous eradication in children: a 2-year follow-up study. J Pediatr Gastroenterol Nutr. 1998;14:181–183. doi: 10.1097/00005176-199808000-00010. [DOI] [PubMed] [Google Scholar]
- Lepper PM, Moricke A, Vogt K, Bode G, Trautmann M. Comparison of different criteria for interpretation of immunoglobulin G immunoblotting results for diagnosis of Helicobacter pylori infection. Clin Diagn Lab Immunol. 2004;14:569–576. doi: 10.1128/CDLI.11.3.569-576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Ozawa K, Okuda M, Nakayama Y, Yoshimura N, Konno M, Minoura T, Iinuma K. Multicenter comparison of rapid lateral flow stool antigen immunoassay and stool antigen enzyme immunoassay for the diagnosis of Helicobacter pylori infection in children. Helicobacter. 2004;14:669–673. doi: 10.1111/j.1083-4389.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Nares-Cisneros J, Jaramillo-Rodriguez Y, Martinez-Ordaz VA, Velasco-Rodriguez VM, Madero A, Mena-Arias G, Manriquez-Covarrubias L. Immunochromatographic monoclonal test for detection of Helicobacter pylori antigen in stool is useful in children from high-prevalence developing country. Helicobacter. 2007;14:354–358. doi: 10.1111/j.1523-5378.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- Falsafi T, Valizadeh N, Sepehr S, Najafi M. Application of a stool antigen test to evaluate the incidence of Helicobacter pylori infection in children and adolescents from Tehran, Iran. Clin Diagn Lab Immunol. 2005;14:1094–1097. doi: 10.1128/CDLI.12.9.1094-1097.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda MoH. Uganda National Policy Guidelines for HIV Counselling and Testing. Kampala, Uganda; 2003. [Google Scholar]
- WHO. Antiretroviral therapy of HIV infection in infants and children. Recommendations for a public health approach. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- WHO. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance. Geneva, Switzerland: WHO; 2005. [Google Scholar]
- Blecker U, Keymolen K, Lanciers S, Bahwere P, Souayah H, Levy J, Vandenplas Y. The prevalence of Helicobacter pylori positivity in human immunodeficiency virus-infected children. J Pediatr Gastroenterol Nutr. 1994;14:417–420. doi: 10.1097/00005176-199411000-00009. [DOI] [PubMed] [Google Scholar]
- Lionetti P, Amarri S, Silenzi F, Galli L, Cellini M, de Martino M, Vierucci A. Prevalence of Helicobacter pylori infection detected by serology and 13C-urea breath test in HIV-1 perinatally infected children. J Pediatr Gastroenterol Nutr. 1999;14:301–306. doi: 10.1097/00005176-199903000-00016. [DOI] [PubMed] [Google Scholar]
- Lugada ES, Mermin J, Kaharuza F, Ulvestad E, Were W, Langeland N, Asjo B, Malamba S, Downing R. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol. 2004;14:29–34. doi: 10.1128/CDLI.11.1.29-34.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos M, Araya V, Pskorz E, Quesada EC, Concetti H, Perez H, Cahn P. Coinfection: Helicobacter pylori/human immunodeficiency virus. Dig Dis Sci. 2004;14:1836–1839. doi: 10.1007/s10620-004-9580-5. [DOI] [PubMed] [Google Scholar]
- Fernando N, Holton J, Zulu I, Vaira D, Mwaba P, Kelly P. Helicobacter pylori infection in an urban African population. J Clin Microbiol. 2001;14:1323–1327. doi: 10.1128/JCM.39.4.1323-1327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciarelli AG, Marano BJ Jr, Gualtieri NM, Zuretti AR, Torres RA, Starpoli AA, Robilotti JG Jr. Lower Helicobacter pylori infection and peptic ulcer disease prevalence in patients with AIDS and suppressed CD4 counts. Am J Gastroenterol. 1996;14:1783–1784. [PubMed] [Google Scholar]
- Oderda G, Shcherbakov P, Bontems P, Urruzuno P, Romano C, Gottrand F, Gomez MJ, Ravelli A, Gandullia P, Roma E. et al. Results from the pediatric European register for treatment of Helicobacter pylori (PERTH) Helicobacter. 2007;14:150–156. doi: 10.1111/j.1523-5378.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- Malaty HM, Graham DY. Importance of childhood socioeconomic status on the current prevalence of Helicobacter pylori infection. Gut. 1994;14:742–745. doi: 10.1136/gut.35.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]


