Abstract
The regulation of IL-17A and IL-22 production differs between human and murine γδ T cells. We find that human γδ T cells expressing Vγ2Vδ2 T cell receptors are peripherally polarized to produce IL-17A or IL-22, much like CD4 αβ Th17 T cells. This requires IL-6, IL-1β, and TGF-β, whereas expansion and maintenance requires IL-23, IL-1β, and TGF-β. In contrast, IL-17A and IL-22 production by murine γδ T cells is innately programmed during thymic ontogeny but requires IL-23 and IL-1β for maintenance. Murine γδ cells producing IL-17A and IL-22 play important roles in microbial, autoimmune, and inflammatory responses. However, the roles played by human IL-17A- and IL-22-producing γδ T cells are less clear but are also likely to be important. These observations highlight differences between humans and murine γδ T cells and underscore the importance of IL-17A- and IL-22-producing γδ T cells.
Keywords: Interleukin-17A, Interleukin-22, γδ T cell, Vγ2Vδ2 T cells, Isoprenoid metabolism
Introduction
γδ T cells have properties of both innate and adaptive immune cells. Although expressing adaptive T cell antigen receptors (TCRs) by rearranging V, D (for the δ chain), and J gene segments, human and mouse γδ T cells utilize a limited set of Vγ and Vδ genes. In cases where the γδ ligands and/or presenting molecules have been identified, they have been found to be ubiquitous nonpeptide compounds [such as lipids or (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP)] presented by major histocompatibility complex (MHC) class Ib molecules such as CD1 or by an unknown presenting molecule (for phosphoantigens). In other cases, the γδ T cells do not recognize an exogenous or endogenous antigen at all—instead they directly recognize an MHC class Ib molecule, such as H-2T or MICA/MICB. In these ways, recognition by γδ TCRs more resembles recognition of nonpeptide compounds by pattern recognition receptors than the recognition by conventional αβ TCRs of peptide antigens presented by MHC class I or II molecules.
Despite the identification of γδ T cells in 1986 [1], major questions still remain unanswered about their functional roles. Although normally constituting a small proportion of total T cells in humans and mice, certain infections or other stimuli can expand γδ T cells to high levels. Also, in many cases γδ T cells express invariant TCRs or TCRs with restricted V gene diversity, resulting in high frequencies of T cells with a defined specificity (although largely unknown for murine γδ T cells). Moreover, these invariant γδ T cells can be highly enriched at specific anatomic locations. From the study of other unconventional T cells, such as invariant natural killer αβ T (iNKT) cells that recognize lipids presented by CD1d [2, 3], it is clear that small populations of T cells that respond early during immune responses can greatly influence the eventual outcome.
Discoveries over the last decade have now defined a new lineage of T cells—the Th17 lineage—that produces IL-17A, IL-17F, and IL-22. Through studies of the role of IL-23 in autoimmune diseases, it became clear that deletion of the shared IL-12β chain ameliorated disease because of the loss of IL-23 function, not IL-12 function, and that, in most cases, traditional Th1 cells producing IFN-γ did not cause autoimmune pathology. This rapidly led to the delineation of the Th17 differentiation schema with identification of the cytokines and transcription factors involved (detailed below). Members of the IL-17 cytokine family (IL-17A through IL-17F) are proinflammatory cytokines that possess a diverse array of functions ranging from neutrophil recruitment to induction of wound repair and tissue remodeling that begin to function early in responses. Similarly, IL-22 can also mediate inflammation and stimulate the production of antimicrobial peptides, and plays a prominent role in skin inflammation and repair.
Although initial studies focused on Th17 and Th22 CD4 αβ T cells, Th17 αβ cells require time to develop and, therefore, would not be available during the crucial early phases of immune responses (within hours) where IL-17A plays critical roles in recruiting neutrophils to sites of infection and in initiating inflammatory responses. In these situations, γδ T cells, other innate lymphocytes (such as iNKT cells and lymphoid tissue inducer cells), Paneth cells, and neutrophils play sentinel functions by releasing IL-17A [4]. γδ T cells are important sources of IL-17A and IL-22 during infections and autoimmune diseases, and secrete IL-17A earlier in disease than conventional CD4 or CD8 αβ T cells. For example, in murine tuberculosis, γδ T cell production of IL-17A actually exceeds that of Th17 cells [5]. Furthermore, murine γδ T cells can produce IL-17A, IL-22, and IL-21 in response to IL-23 and IL-1β without exposure to exogenous antigens [6] or with exposure only to toll-like receptor (TLR) and dectin-1 ligands [7, 8].
From a therapeutic perspective, it is essential to determine the role played by these cells in disease as well as the mechanisms regulating their development. Moreover, although it is clear that IL-17A-producing γδ T cells are important in mice and that they exist in humans [9], little is known about the roles of human IL-17A-producing γδ T cells in infections and autoimmune diseases. In this review, we first cover the basic differentiation scheme of conventional T cells, including recently described Th17 T cells, then discuss the regulation and function of IL-17A and IL-22 production by unconventional γδ T cells in humans and in mice.
Biology of IL-17A and IL-22
IL-17A is a multifactorial cytokine, which promotes inflammation. It does this by increasing production of inflammatory cytokines, chemokines, matrix remodeling proteins, adhesion molecules, antimicrobials, and acute phase reactants. The major consequence of IL-17A signaling is the recruitment of neutrophils to areas of inflammation.
Because the IL-17A receptor (IL-17RA) is expressed ubiquitously throughout the body, virtually every cell in the body can participate in IL-17A-mediated inflammation. The principle effect of IL-17A is propagation of proinflammatory cytokines; for example, fibroblasts and epithelial cells respond to IL-17A by producing IL-6 and G-CSF [10] as well as the chemokines IL-8/CXCL8, CXCL1, CXCL2, CCL20, CCL2, and CCL7 [11–14]. IL-17A signaling also induces the expression of matrix metalloproteinases (MMP) such as MMP-1 [15], -3 [16, 17], -9 [18], and -13 [17, 19], and aggrecanase-1 [17]. MMPs are involved in tissue remodeling, and can contribute to tissue repair or tissue destruction. IL-17A also stimulates the production of receptor activator of nuclear factor kappa-B ligand (RANKL, also known as TNFSF11), a key stimulator of osteoclast differentiation and activation for bone resorption [20].
IL-17A also induces endothelial cells to upregulate the adhesion molecules E-selectin, VCAM-1 and ICAM-1, which enable neutrophils and other cells responding to the proinflammatory cytokines to exit the vasculature [21]. IL-17A also boosts mucosal immunity by induction of antimicrobials, such as the β-defensins and mucins, which are rapidly produced by airway epithelial cells in response to IL-17A [22, 23]. Lastly, IL-17A boosts systemic innate immunity through the induction of acute phase reactants, produced by the liver. One such reactant, C-reactive protein [24], is directly released by hepatocytes treated with IL-17A and functions to activate the complement system.
IL-17A signaling also acts at the molecular level to stabilize mRNA transcripts within target cells. This is achieved through activation of the MAPK pathway and targets mRNA transcripts containing Au-rich elements in myeloid cells. Examples of such transcripts include Cxcl-1 [25], Il-6 [26, 27], Il-8 [28], and G-csf [29]. IL-22 is often produced alongside IL-17A. IL-22 acts on non-immune cells, specifically epithelial cells and other cells of the skin, gut, lungs, and kidneys. Similar to IL-17A, IL-22 induces antimicrobial peptides, acute phase reactants, and MMPs, but uniquely mediates re-epithelialization and inhibition of keratinocyte differentiation [30–32].
Lineage commitment and differentiation of conventional αβ T cells: what lessons can we learn for γδ T cells?
To differentiate conventional naive αβ T cells from memory T cells, naive CD4 αβ T cells must be exposed to their peptide antigens bound to MHC class II molecules while naive CD8 αβ T cells must be exposed to their peptide antigens bound to MHC class I molecules. This process is generally initiated by professional antigen-presenting cells (APC) that express not only the MHC molecules with bound peptide antigens, but also costimulatory ligands such as CD80 (B7-1) and CD86 (B7-2). Engagement of other receptors expressed on T cells such as the CD4 and CD8 accessory molecules, costimulatory and inhibitory receptors (such as CD28, CTLA-4, and JCAM), CD2 and SLAM family receptors, TNF-family receptors (such as CD27), and integrins (such as LFA-1), to their ligands also enhances or are required for naive T cell activation. The binding of the αβ TCR to its cognate MHC-peptide antigen along with the other receptor interactions activates the T cell and converts it to a memory (educated) T cell. The affinity of the MHC–peptide complex for the TCR and the length of time that the TCR is bound also affect T cell differentiation and function.
While converting to memory T cells, naive T cells commit to different functional lineages. There is a strong contribution to function that is determined by the expression, or lack of expression (in the case of γδ and innate αβ T cells), of CD4 and CD8 accessory receptors on the naive T cells. Thus, expression of the CD4, CD8αβ heterodimer, CD8αα homodimer, or the lack of expression of CD4−CD8− in both αβ and γδ T cells delineates (or is a marker for) some of the functional capabilities of the cells, principally cytotoxicity versus helper activity.
During peripheral conversion of naive to memory, the types of cytokines produced by the memory T cell (Th1, Th2, Th9, Th17, Th22, TFH, etc.) depend on the type of APC, the cytokines present during activation, and the receptors/ligands expressed by the APC. In turn, the types of APC and surrounding cytokines are strongly dependent on the triggering of innate receptors [TLRs, nucleotide-binding oligomerization domain-containing proteins (NODs), nucleotide-binding domain leucine-rich repeat containing NOD-like receptors, mannose receptors, etc.] by pathogen-associated molecular patterns associated with the antigen source (e.g. viral, bacterial, or parasitic infections). These innate sensors direct T cell differentiation by engaging innate receptors on APC and surrounding cells which, in turn, stimulates the expression of specific cellular ligands and cytokines. Innate signals can also direct T cell differentiation by engaging innate receptors expressed by the T cells themselves. Therefore, the types of innate receptors engaged by the pathogen-associated molecular patterns play a major role in determining the downstream lineage commitment of T cells.
The cytokines and receptor–ligand interactions signal naive T cells to increase production or activity of various transcriptional activators/suppressors that then turn on and off genes to commit the T cells to different functional lineages. Then epigenetic changes serve to firmly commit the T cells to a particular functional lineage in many but not all cases. Depending on the number of times the T cells are activated through their TCRs, the number of cell divisions they undergo, and the conditions under which they are activated (i.e. surrounding cytokines and receptor ligand interactions), the memory T cells then undergo progressive differentiation to a more effector/terminally differentiated state with the associated loss or gain of various surface receptors and effector molecules.
The challenge of studying memory and lineage commitment of γδ T cells and unconventional αβ T cells is that, in many cases, the TCRs of these cells function more like pattern recognition receptors than foreign antigen receptors. That is, the antigens recognized by γδ T cells so far have been either nonpeptide conserved molecules, such as prenyl pyrophosphate isoprenoid metabolites and lipids, that are expressed by a variety of pathogens and have mammalian homologs, or endogenous self proteins (such as MICA/MICB, CD1, or H-2T MHC class Ib molecules). The presence of these self-ligands or ubiquitous nonpeptide antigens can rapidly convert naive γδ T cells into memory cells. Exposure to self-ligands may explain why many murine T cell V gene subsets acquire “memory” T cell phenotypes and commit to a functional lineage in the thymus. In contrast, although acquisition of a “memory” phenotype can take place in the thymus, many human neonatal Vγ2Vδ2 T cells exhibit naive phenotypes. In this case, commitment by human Vγ2Vδ2 T cells to Tγδ17 or other lineages can take place in the periphery, as for most αβ T cells.
Differentiation of Th17 CD4 αβ T cells
When considering the commitment of γδ T cells to the Th17 lineage, it is useful to consider the requirements for the differentiation of naive CD4 αβ T cells to Th17 cells that has been extensively studied in both humans and mice [33].
Th17 differentiation begins with the ligation of the TCR in the presence of IL-6 and/or IL-21. This activates STAT3, which induces and increases expression of the master transcription factor, retinoid-related orphan receptor γt (RORγt, produced from the RORC gene) [34], and/or RORα (produced from the RORA gene) [35]. RORγt and RORα are key transcription factors required for IL-17A and IL-17F production. STAT3, in the presence of RORγt, binds to the IL17A and IL17F promoters [36], initiating IL-17A and IL-17AF mRNA production. IL-1β, through induction of IRF4, appears to stabilize the Th17 phenotype [37, 38]. Additionally, the RUNX1 transcription factor may further promote the differentiation of Th17 cells since it upregulates RORγt expression and IL17A transcription [39]. Within these developing Th17 T cells, IL-6 also induces expression of IL-23R [40]. This enables further STAT3 signaling through the binding of IL-23 to the IL-23R. Continued IL-23/IL-23R signaling through STAT3 is required by committed Th17 precursors for terminal differentiation of these cells into effector Th17 cells and maintenance of their phenotype in vivo [41]. In mice, TGF-β appears to be required to maximally differentiate naive cells into Th17 cells [42]. TGF-β has been suggested to indirectly suppress Th1 and Th2 differentiation through inhibition of STAT4 and GATA-3 transcription factors, respectively [43]. Human Th17 CD4 αβ T cells also require TGF-β for maximal differentiation of Th17 cells [44–46] probably through a similar mechanism [47]. Thus, triggering naive CD4 αβ T cells with MHC/peptide antigens in the presence of IL-6 and/or IL-21, IL-1β, and TGF-β directs their differentiation to the Th17 lineage. IL-23 serves to reinforce and maintain this lineage commitment.
Human IL-17A-producing γδ T cells
Human γδ V gene subsets: expansion of Vγ2Vδ2 T cells in infancy driven by microbial expression of HMBPP and other phosphoantigens
Humans express six functional Vγ gene segments and three major Vδ gene segments. Five of the Vγ gene segments (Vγ1.2, Vγ1.3, Vγ1.4, Vγ1.5, and Vγ1.8; also termed Vγ2, Vγ3, Vγ4, Vγ5, and Vγ8) belong to a single family showing 71–91% amino acid homology. The Vγ1 family also shows 42–48% amino acid homology with murine Vγ5 (using the Tonegawa nomenclature). The sixth functional Vγ gene segment, Vγ2 (also termed Vγ9), is distinct from all murine Vγ gene segments. In adults, the Vγ2 gene segment is commonly found paired with the Vδ2 gene segment (also distinct from all known murine Vγ gene segments). The Vγ2 gene segment can also pair with the Vδ1 gene segment (showing 58% amino acid homology with murine Vδ6), although Vδ1 is most commonly paired with a Vγ1 family member. The Vδ3 gene segment is the third most common segment in adults and shows 66% amino acid homology with murine Vδ5. Although Vα gene segments can recombine with the Cδ constant region and pair with Vγ gene segments, these TCRs constitute only a small fraction of total γδ T cells in most individuals.
Unlike mice, humans do not exhibit a predominance of γδ T cells at epithelial surfaces, nor are invariant TCRs commonly found at localized anatomic sites. There is evidence, however, for an early wave of γδ T cells, from fetal thymus and liver, expressing invariant Vγ2 gene segments [48] paired with oligoclonal Vδ2 gene segments [49–51]. However, by birth, the thymus has switched to Vδ1 and the Vγ1Vδ1 subset predominates [49, 52]. At birth, the repertoire of γδ TCR pairs is quite diverse with significant fractions of neonatal γδ T cells expressing Vγ1Vδ2 and Vγ2Vδ1 TCRs that are seldom seen in adults [52].
Between the ages of 1 and 10 years, however, the Vγ2Vδ2 T cell population expands to predominate among adult γδ T cells [53]. This expansion is independent of genetic background, since identical twins can have different V gene repertoires and there is no evidence for inheritance of V gene expression in families [53]. Instead, environmental exposure to microbes is likely responsible for this expansion. This expansion is limited to T cells expressing Vγ2 paired with Vδ2 and to a lesser extent, Vγ1 paired with Vδ1; other combinations that are present in neonates become almost non-existent in adults [52].
Because of this extensive activation and expansion, Vγ2Vδ2 T cells become the major subset in adult peripheral blood γδ T cells [53] with almost all exhibiting memory phenotypes (data not shown; [53, 54]). The Vγ2Vδ2 TCR (also termed Vγ9Vδ2) specifically recognizes HMBPP [55], an essential metabolite in isoprenoid biosynthesis pathways of many bacteria and all Apicomplexan parasites. Activation of Vγ2Vδ2 T cells in vivo is dependent on the production of this essential metabolite [56]. A related prenyl pyrophosphate, isopentenyl pyrophosphate (IPP), is similarly recognized by Vγ2Vδ2 T cells [57]. However, unlike HMBPP, IPP is present in both microbial and human isoprenoid biosynthesis. IPP is normally sequestered inside host cells, and does not activate Vγ2Vδ2 T cells unless the cells have been treated with bisphosphonates [58] or alkylamines [59] to block farnesyl diphosphate synthase, thereby increasing IPP levels.
Although never the predominant T cell population, Vγ1Vδ1 T cells (and rarely Vγ2Vδ2 T cells) are enriched in intraepithelial (but not lamina propria) tissues in the gut, constituting up to 37% of total T cells [60, 61]. They are also found in the skin, constituting about 5% of total T cells [61, 62]. A significant fraction of Vγ1Vδ1 and Vγ2Vδ1 T cells (up to 66% of duodenal γδ T cells) respond to self and foreign lipids presented by CD1 molecules [63–66]. Antigens identified include pollen-derived phosphatidyl ethanolamine [67] and lipid A [68]. Thus, a significant proportion of Vδ1 T cells recognize self and foreign lipids presented by CD1. Members of the Vγ1Vδ1 T cell subset also recognize the MHC class I-related molecules, MICA and MICB [69, 70]. Such recognition is TCR-dependent [71, 72] but is independent of antigen processing, presentation, and β2M [72]. In addition, Vδ1 and Vγ2Vδ2 T cells also express NKG2D. This C-type lectin also binds MICA, MICB, and UL16-binding proteins, and, together with TCR ligation, provides T cell costimulation [71, 73]. In response to activation, Vγ1Vδ1 and Vγ2Vδ2 T cells similarly produce IFN-γ and TNFα, and mediate cytolysis.
Finally, human γδ T cells expressing Vδ1, Vδ3, and Vδ5 have been found to expand in response to cytomegalovirus (CMV) infection after in utero infection [74] in normal adults [75], and in adults after kidney transplantation [76, 77]. In patients with in utero infection, an invariant Vγ1.8Vδ1 TCR is greatly enriched. γδ clones expressing this Vγ1.8Vδ1 TCR secrete IFN-γ in response to CMV-infected fibroblasts and kill CMV-infected target cells [74]. Similarly, Vδ1 and Vδ3 T cells expand greatly (up to 15–42% of total T cells in some cases) after renal transplantation in patients who develop a CMV infection. These γδ T cells may be further expanded in vitro when exposed to lysates of CMV-infected cells [76] and γδ clones derived from these cells specifically lyse CMV-infected target cells [78]. Thus, although the CMV-associated ligand(s) and the restricting molecules have not been defined, there is evidence for specific recognition of a CMV-associated compound.
Peripheral differentiation of neonatal Vγ2Vδ2 T cells into Tγδ17 and Tγδ1/17 cells requires antigen stimulation and IL-6, IL-1β, and TGF-β
In our studies on neonatal Vγ2Vδ2 T cells [9], we found that Vγ2Vδ2 T cells behave much like naive αβ T cells since they can be polarized into Th17-like lineage cells, which we term Tγδ17. Approximately 50% of neonatal Vγ2Vδ2 T cells are phenotypically naive (based on the expression of CD27 and CD28 without expression of CD45RO). Polarization of neonatal Vγ2Vδ2 T cells to produce IL-17A (termed Tγδ17 T cells) requires IL-6, IL-1β, and TGF-β coupled with TCR stimulation by the antigen, HMBPP. Neutralization of IL-6 or the absence of exogenous TGF-β or IL-1β, greatly reduces generation of Tγδ17 cells. Neutralization of IL-23, in contrast, increases the numbers of Tγδ17 T cells. Costaining for IFN-γ revealed that IL-23, in conjunction with IL-6, IL-1β, and TGF-β, induces Tγδ17 T cells to produce IFN-γ in addition to IL-17A (termed Tγδ1/17). A similar phenomenon was observed with Th17 CD4 T cell clones, where IL-23 and/or IL-12 induces IFN-γ production [79]. These results suggest that under these conditions, IL-23 may function to drive production of IFN-γ.
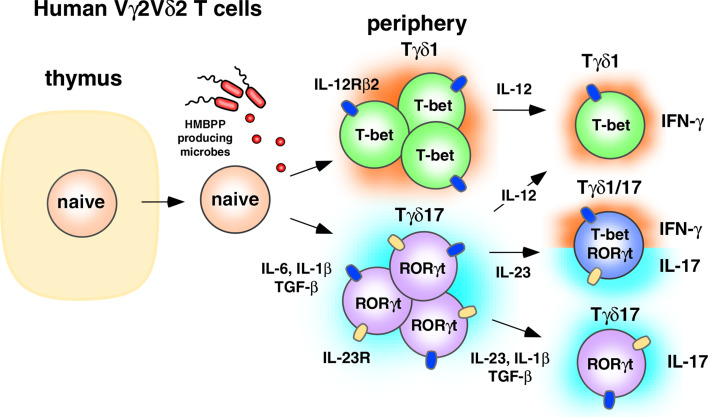
Our results suggest the following model (Fig. 1). In naive Vγ2Vδ2 T cells, IL-6 binding to the IL-6R activates STAT3 which in turn binds and activates the IL-23R, RORC/A, and IL-17A/F genes. IL-1β stabilizes the Tγδ17 phenotype by inducing IRF4 [37, 38]. TGF-β inhibits IL-12-mediated STAT4 signaling, thereby inhibiting differentiation into Th1-like cells (termed Tγδ1) that produce IFN-γ. Using intranuclear staining, we verified that neonatal Vγ2Vδ2 T cells producing IL-17A have increased levels of RORγt, consistent with the hypothesis that it is also a master regulator of Tγδ17. Studies examining RUNX1 levels in Tγδ17Vγ2Vδ2 T cells are planned to determine whether this transcription factor also plays a role.
Fig. 1.
Regulation of IL-17A-producing human γδ T cells. Human Vγ2Vδ2 T cells are peripherally programmed to become Tγδ17 through the effects of IL-6, IL-1β, and TGF-β in the presence of HMBPP. These cells can then be maintained as Tγδ17 through the action of IL-23, IL-1β, and TGF-β, or they can acquire IFN-γ potential through IL-23 signaling. Tγδ1/17 T cells commonly lose the ability to produce IL-17A altogether and revert functionally to Tγδ1-like cells through IL-12 signaling
In the presence of IL-23, the IL-17A-producing Tγδ17 T cells (which now express the IL-23R) acquire the ability to produce IFN-γ via an uncharacterized mechanism [9]. This acquisition of the ability to produce IFN-γ by Tγδ17Vγ2Vδ2 T cells is not unique to γδ T cells. Both human Tc17 [80, 81] and human Th17 [82, 83] αβ T cells show some level of instability/plasticity, and over time, many of these cells begin to produce IFN-γ and lose the ability to produce IL-17A. Similar to Tc17 αβ T cells, IL-17A production by Tγδ17 and Tγδ1/17Vγ2Vδ2 T cells is unstable in vitro and most of the cells eventually lose the ability to make IL-17A and, instead, produce IFN-γ (Ness-Schwickerath and Morita, unpublished observations). Future studies with sorted Tγδ17 populations are needed to confirm the role of IL-23 in the conversion to Tγδ1.
IL-23 with IL-1β and TGF-β helps maintain adult Tγδ17 cells but there is significant conversion into Tγδ1/17 and Tγδ1, IFN-γ-producing cells
In contrast to neonatal Vγ2Vδ2 T cells, adult Vγ2Vδ2 T cells are almost exclusively memory cells (about 98% of blood Vγ2Vδ2 T cells; Jin et al., unpublished observations). Consistent with this difference, the cytokine requirements for IL-17A production by adult Vγ2Vδ2 T cells is significantly different from those for neonatal Vγ2Vδ2 T cells [9]. In adults, optimal expansion of IL-17A-producing Vγ2Vδ2 T cells requires IL-1β, TGF-β, and IL-23, but does not require IL-6 (Fig. 1). Although the small population of naive Vγ2Vδ2 T cells in adults hypothetically could be polarized to Tγδ17 under these conditions, the lack of a requirement for IL-6 suggests that the majority of IL-17A-producing cells are memory Vγ2Vδ2 T cells already committed to the Tγδ17 and Tγδ1/17 lineages.
Given the plasticity of the Th17 lineage, it is likely that some fraction of IFN-γ-producing Vγ2Vδ2 T cells are actually Tγδ17 lineage cells that have failed to maintain IL-17A production. These former Tγδ17Vγ2Vδ2 T cells, however, may maintain permissive histone modifications at the IL-17A/F, RORC, or RORA loci, such that signaling by IL-1β, IL-23, and TGF-β could restore IL-17A production. They may also have other differences in chemokine receptor expression, cytotoxicity, or accessory molecule expression that confer specialized functional roles on these cells when compared with true Tγδ1 cells. Consistent with this possibility, CD4 Th17 αβ T cells producing IFN-γ but not IL-17A, that were stimulated under polarizing conditions for Th17 cells produce the Th17-lineage IL-22 cytokine and CCL20 (MIP-3A/LARC) chemokine [83].
In contrast to neonatal Vγ2Vδ2 T cells, adult Tγδ17 cells (IFN-γ−) are less frequent and were observed in only four out of ten donors [9]. Thus, most Vγ2Vδ2 T cells that produce IL-17A also produce IFN-γ. However, within the four donors with expandable Tγδ17 T cells, these cells could be detected ex vivo prior to expansion. The cytokines required for Tγδ17 expansion in adult blood were similar to those expanding Tγδ1/17 cells. This suggests that Tγδ17Vγ2Vδ2 T cells can persist in humans, as do αβ T cells. Because captive, specific pathogen-free rhesus macaques have much higher frequencies of Tγδ17 cells than human donors, it is likely that the type and number of infections may determine whether these cells persist in vivo. It is likely that frequent Th1 immune responses (involving ample IL-12) cause many human Tγδ17Vγ2Vδ2 T cells to transition to Tγδ1/17 cells, and then ultimately to Tγδ1-like cells.
Regulation of IL-22 production by human Vγ2Vδ2 T cells
Generation of neonatal IL-22-producing (Tγδ22) Vγ2Vδ2 T cells require conditions very similar to those required to generate IL-17A-producing Vγ2Vδ2 T cells, namely, IL-1β, IL-6, and TGF-β [9]. Adult Tγδ22Vγ2Vδ2 T cells, much like adult Tγδ1/17, require IL-23, IL-1β, and TGF-β, and are independent of IL-6 [9]. However, under these conditions, fewer Vγ2Vδ2 T cells converted to the Tγδ22 lineage than to the Tγδ17 (or Tγδ1/17) lineage. This may reflect the requirement for TNF-α (not included in our study) and inhibition by TGF-β (since the 1 ng/ml concentration we used inhibited Th22 priming in the study by Duhen et al.) [84]. Additionally, the lack of ligands for the aryl hydrocarbon receptor (AHR; discussed below for IL-22-producing murine γδ T cells) might have inhibited Th22 development since we did not include AHR ligands, nor did we use Iscove’s DMEM which has high levels of aromatic amino acids and favors Th17/Th22 differentiation [85]. Unlike murine CD4 αβ T cells, very few if any Vγ2Vδ2 T cells stimulated ex vivo produce both IL-17A and IL-22 cytokines [9]. Similarly, very few in vitro-polarized IL-17A-producing Vγ2Vδ2 T cells coproduce IL-22. This suggests that the Tγδ17 (and Tγδ1/17) lineage is distinct from the Tγδ22 lineage. As with Tγδ17 cells, IL-23 reduces the number of neonatal Tγδ22Vγ2Vδ2 T cells. Again we believe this is because under some conditions or at high concentrations, IL-23 can drive Tγδ22 cells to transition into Tγδ1 cells (perhaps by stimulating IL-12 production or by direct action). The Tγδ22 population, like the Tγδ1/17 population, consists of mixed populations of IFN-γ+ and IFN-γ− cells. Future studies examining polarization to Tγδ22 in the presence of AHR ligands or additional cytokines such as IL-6 and TNF-α [84] are in progress.
Role of IL-17A-producing Vγ2Vδ2 T cells in human microbial immunity
Relatively few studies have examined the role of human γδ T cells producing IL-17A and IL-22 in human immunity and autoimmunity. In our samples from ten healthy adult donors, an average of 1.1% of Vγ2Vδ2 T cells produced IL-17A. A similar, non-overlapping proportion of Vγ2Vδ2 T cells produced IL-22 (1.2%). Since Vγ2Vδ2 T cells constitute 5.25 ± 2.1% of total CD3+ T cells, IL-17A- and IL-22-producing Vγ2Vδ2 T cells occur at frequencies of 1 in 2,762 and 1 in 1,864 T cells, respectively. Moreover, several donors showed increased frequencies of IL-17+ Vγ2Vδ2 T cells (up to 1 in 906 T cells). Furthermore, in specific pathogen-free rhesus macaques, there was a fivefold increased baseline frequency of IL-17-producing Vδ2 T cells (5.6 ± 1.3%, ranging from 1.1 to 13.4%). This suggests that continued bacterial infections in humans may drive Tγδ17 cells to lose the ability to produce IL-17A.
Although the frequencies appear low, adult Vγ2Vδ2 T cells recognize most bacterial and Apicomplexan protozoan pathogens by virtue of their recognition of essential prenyl pyrophosphates, such as HMBPP, and a phosphoantigen produced by Gram-positive cocci (data not shown). Numerous bacterial and protozoan infections in humans (detailed in Table 1) are associated with major expansions of Vγ2Vδ2 T cells such that in some individuals, Vγ2Vδ2 T cells can constitute up to 50% of circulating T cells (one in two T cells) with increases to 10–25% commonly found (Table 1).
Table 1.
Expansion of human γδ/Vγ2Vδ2 T cells in response to infection
| Infection | γδ T cells, mean (max) % of T cells | Reference | |
|---|---|---|---|
| Normal subjects | Patients | ||
| Bacterial | |||
| Tuberculosis | 6 | 14 (35) | [175] |
| 2 | 6 (17) | [176] | |
| TB contacts | 5 | 10 (18) | [177] |
| TB meningitis | 3 | 4 (80% Vγ2Vδ2) | [178] |
| Leprosy reversal reaction | 5 | 29a | [179] |
| Tularemia | 3 | 33 | [180] |
| 7 | 31 (48) | [181] | |
| 5 | 25 (50) | [182] | |
| Salmonellosis | 5 | 18 (48) | [183] |
| Legionellosis | 5 | 15 (42) | [184] |
| Brucellosis | 4 | 29 (48) | [185] |
| Q-fever (Coxiella burnetii) | 4 | 16 (30) | [186] |
| Ehrlichiosis | 5 | 57 (97) | [187] |
| Meningitis (H. influenzae) | 7 | 27 (37) | [188] |
| Meningitis (N. meningitidis) | 7 | 25 (42) | [188] |
| Meningitis (S. pneumoniae) | 7 | 35 (46) | [188] |
| Listeriosis | 2 | 12 (33) | [189] |
| Protozoal parasites | |||
| Acute malaria (non-endemic) | 4 | 16 (26) | [190] |
| 5 | 16 (27) | [191] | |
| 3 | 18 (46) | [192] | |
| Malarial paroxysm | 4 | 11 (27) | [193] |
| Toxoplasmosis | 4 | 9 (15) | [194] |
| Leishmaniasis, visceral | 8 | 44 | [195] |
| Leishmaniasis | 3 | 13 (18) | [196] |
| Leishmaniasis, localized | 4 | 20a | [179] |
a% γδ T cells among CD3+ T cells in skin lesions.
Essentially all adult Vγ2Vδ2 T cells recognize prenyl pyrophosphate antigens due to the extensive use of germline encoded regions of the Vγ2Vδ2 TCR for prenyl pyrophosphate recognition [86] and the selection for Jγ1.2 and a hydrophobic Vδ2 CDR3 residue that occurs during infancy [52, 87–90]. For example, 91 out of 94 adult Vγ2Vδ2 T cell clones (97%) were antigen-responsive [88, 91, 92]. Thus, the frequency of Vγ2Vδ2 T cells is actually the antigen-specific frequency and, therefore, is very high at 1 in 19 T cells [9]. In contrast, the frequency of αβ T cells specific for a particular peptide/MHC complex among naive cells is usually very low: 1:158,000–1:1,875,000 for CD4 [93] and 1:33,000–1:164,000 (four of six were >1:142,000) for CD8 [94]. Since essentially all IL-17A- and IL-22-producing Vγ2Vδ2 T cells are antigen-specific, during primary infections, Vγ2Vδ2 T cells and other unconventional T cells are likely to be important sources of early IL-17A and IL-22 until naive CD4 and CD8 αβ T cells can be expanded and differentiated into memory Th17/Tc17 and Th22/Tc22 cells.
Thus far, IL-17A production by γδ T cells has only been described in two human diseases, active tuberculosis and HIV infection. In response to active tuberculosis infection, Peng et al. [95] demonstrated increases in the proportion of peripheral blood γδ cells producing IL-17A, and decreases in the proportion producing IFN-γ. Similarly, exposure of peripheral blood mononuclear cells from either tuberculosis patients or healthy controls to M. tuberculosis antigens for 7 days dramatically expanded IL-17A+ γδ T cells [95]. These results suggest that M. tuberculosis infection in vivo may expand blood IL-17A+ γδ T cells. Although the role of γδ production of IL-17A in granuloma formation cannot be easily addressed in humans, murine studies would suggest an important role. Additional studies in this patient population are needed to define the γδ subsets producing IL-17A and the mechanism by which the γδ T cells become activated. Based on our results, we would hypothesize that M. tuberculosis infection stimulates IL-23 and IL-1β cytokine production by lung APCs, which together with TLR ligands and HMBPP, activate and expand IL-17A+ Vγ2Vδ2 T cells.
To model human tuberculosis infections, Yao et al. [96] infected cynomolgus and rhesus macaques with M. tuberculosis and monitored cytokine production by T cells at various time points. After 4 weeks, statistically significant increases were observed in the percent of circulating T cells producing IL-22, but not IL-17A. However, in contrast to the work by Peng et al., they did not detect differences among the Vγ2Vδ2 T cells. There are several potential explanations for these differences. First and most significantly, Yao et al. stimulated Vγ2Vδ2 T cells only with HMBPP ex vivo, whereas Peng et al. restimulated Vγ2Vδ2 T cells with PMA and ionomycin (which allows maximal determination of IL-17A-producing cells). Second, Peng et al. study, examined cytokine production during reactivation of tuberculosis in chronically infected patients, whereas Yao et al. examined cytokine production at late time points in primary tuberculosis. And lastly, Yao et al. injected M. tuberculosis directly into the lung parenchyma via bronchoscopy, whereas Peng et al. examined patients who had acquired TB through a natural infection.
In a second human study, Fenoglio et al. [97] examined γδ T cells from healthy and HIV+ individuals and found increased frequencies of circulating γδ T cells (mostly Vδ1) and a higher propensity for IL-17 and IFN-γ production by both Vδ1 and Vγ2Vδ2 T cells. More than one-third of Vδ1 and approximately one-half of Vγ2Vδ2 T cells isolated ex vivo contained intracellular IL-17A [97]. However, the ability to produce IL-17A was rapidly lost in vitro unless Candida albicans extract or M. tuberculosis purified protein derivative was included in the cultures for Vδ1 or Vγ2Vδ2 T cells, respectively. In the absence of the bacterial products, the ability to produce IL-17 by either subset was lost. This suggests that the bacterial products stimulate the correct cytokines to retain the ability to produce IL-17A and that in their absence, IL-17-producing γδ T cells convert to Tγδ1 T cells. Consistent with this possibility, IL-17A-producing Vδ1 and Vγ2Vδ2 T cells also produced IFN-γ, were CD161+, and expressed RORγt and T-bet [97]. These results are similar to our findings. Unfortunately, their analysis did not extend to IL-22 production, nor did it address the specific factors present within the bacterial products that were required to retain IL-17A production. However, human memory CD4 αβ T cells specific for C. albicans include many Th17 cells [82] and IL-17A is essential for the control of C. albicans infections in mice [98]. Stimulation by C. albicans induces strong IL-17 responses due to the production of β-glucans and mannans that engage dectin-1 and mannose receptors, respectively, on APC [99]. This stimulates production of prostaglandin E2, which in turn, stimulates IL-6 and IL-23 production, thus favoring development of Th17 cells [99–101]. Similarly, M. tuberculosis infection stimulates both murine Th17 αβ T cells and Tγδ17 γδ T cells [5] through production of ligands for TLR4 and dectin-1 [102]. Thus, rather than being specific for antigens in C. albicans extracts or purified protein derivative, these microbial products likely stimulate cytokine production to favor the maintenance and/or differentiation of Tγδ17 cells.
There is evidence to suggest that IL-17 production by Vγ2Vδ2 T cells in neonates and infants will be highly relevant to their resistance to infections. Newborns have intrinsic defects in both APC and conventional T cells resulting in poor adaptive immune responses to infection [103, 104]. Defective IL-12 production by neonatal APC is in part responsible for poor Th1 αβ immunity. Instead of producing the Th1 cytokine, IL-12, neonatal APC produce IL-23, IL-1β, and IL-6 [105–108]. Because γδ T cells are the first T cells to develop, do not require professional antigen processing or presentation, and respond to shared microbial isoprenoid antigens, they are uniquely poised to mount protective immune responses in neonates [109]. In fact, nearly every Vγ2Vδ2 T cell will become activated prior to the age of 1 year by self or environmental antigens [53, 54]. From our studies on neonatal Vγ2Vδ2 T cells, we know that the IL-1β, IL-6, and IL-23 cytokines promote the differentiation of Tγδ1/17 T cells in vitro, and we propose that the same process occurs in vivo.
We speculate that neonatal Vγ2Vδ2 T cells activated in response to a broad range of HMBPP-producing microbes, are polarized by the high levels of IL-23, IL-1β, and IL-6 into Tγδ17 or Tγδ1/17 T cells which, in the absence of strong Th1 immunity, function to immediately upregulate protective innate immune mechanisms. Because of repeated infections, most of these Tγδ17 and Tγδ1/17 T cells lose their ability to produce IL-17A and only produce IFN-γ in adults. However, they are likely to retain some of the functional characteristics of Tγδ17 cells. A method to determine the proportion of adult Vγ2Vδ2 T cells derived from Tγδ17 awaits the identification of markers for Tγδ17 cells that have lost IL-17A production, but the proportion could be substantial.
Role of IL-17A-producing Vγ2Vδ2 T cells in human autoimmunity
The role of IL-17-producing Vγ2Vδ2 T cells in human autoimmunity is also poorly studied. Human γδ T cells coproducing IL-17A and IFN-γ have been found in the synovial fluid and synovium of patients with rheumatoid arthritis [110]. Pollinger et al. found that all of the patients with rheumatoid arthritis examined had IL-17- and IFN-γ-coproducing γδ T cells in the inflamed synovium. Moreover, in synovial fluid, the IL-17+, IFN-γ+ γδ T cells occur at a frequency equal to that of the IL-17+, IFN-γ+ CD4 T cells. The authors attempted to translate the results seen in their murine collagen-induced arthritis (CIA) model of human rheumatoid arthritis, and concluded that IL-17-producing γδ T cells do not drive bone destruction [110]. However, only three patients were examined in this study, and all were undergoing cortisone treatment. Thus, more patients will need to be studied before firm conclusions can be drawn. Moreover, human and murine γδ T cells have quite different antigen specificities, anatomic localization, and cytokine potentials, and, therefore, are unlikely to function similarly in most diseases. Finally, these findings do not preclude a contribution by γδ T cells to joint inflammation. Further studies in rheumatoid arthritis and other autoimmune diseases are needed to assess the pathogenic potential of IL-17-producing γδ T cells, especially during the initiation of autoimmunity, where a small population of T cells can direct the subsequent development of specific T lineages.
IL-17A-producing murine γδ T cells
Murine γδ T cells develop in thymic waves with poorly defined TCR specificity
Murine γδ T cells begin to develop prior to αβ T cells in thymic waves characterized by specific V gene combinations. The first waves produce invariant Vγ5Vδ1 and Vγ6Vδ1 TCRs that lack significant junctional diversity and exhibit tropism to the skin (dendritic epidermal T cells, DETC) and epithelial tissues, respectively [111] (in the nomenclature of Heilig and Tonegawa [112]). Postnatally, the thymus then switches to the production of highly diversified V gene segments (such as Vγ4, Vγ1, and Vδ1) on γδ T cells that localize to secondary lymphoid organs [113]. A subset of these γδ T cells, expressing a variety of Vγ and Vδ regions, recognize the nonclassical MHC class I molecules, T10 and T22, through their expression of a CDR3δ motif of nine amino acids primarily encoded by Dδ2 and containing six invariant residues [114]. T22 and T10 molecules are upregulated on activated immune cells, suggesting a role for these T10-/T22-specific γδ T cells during microbial responses [115]. Besides the H-2T molecules, a subpopulation of murine Vγ4 T cells are restricted to lipid-presenting CD1d molecule in coxsackievirus-induced myocarditis [116]. The relationship between these Vγ4 T cells and Vγ4Vδ4 T cells exhibiting limited diversity that are found in CIA [117] is unknown. Additionally, a ligand for the invariant skin Vγ5Vδ1 TCR is expressed by stressed keratinocytes, although the exact identity of the molecule is unknown. There have also been examples of murine γδ T cells specific for the herpes protein, gI [118], and H-2 MHC class II proteins (although this recognition does not involve peptides) [119]. Beyond these examples, most of the natural murine γδ antigens/ligands remain unknown. To date, IL-17A production has been documented for Vγ1 [120], Vγ4 [117, 120], Vγ6Vδ1 [8], and Vγ5Vδ1 DETC [121] γδ subsets.
Regulation of IL-17A-producing murine γδ T cells: thymic imprinting of distinct γδ T cell subsets for IL-17A production
Unlike αβ T cells, murine γδ T cells can be developmentally imprinted to produce IFN-γ or IL-17A. Utilizing a T22 tetramer to identify H-2T-specific cells, Jensen et al. [122] found that tetramer-positive antigen-inexperienced γδ T cells that lack CD122 (IL-2Rβ) produce IL-17A, whereas antigen-experienced γδ T cells expressing CD122 produce IFN-γ. Similarly, IL-17A-producing peritoneal Vγ6Vδ1 T cells lack CD122 but express CD25 (IL-2Rα) and originate from IL-17A-producing thymic precursors [123]. The appearance of IL-17A-producing thymic γδ T cells peaks at embryonic day 19, arguing against a role for exogenous antigen(s).
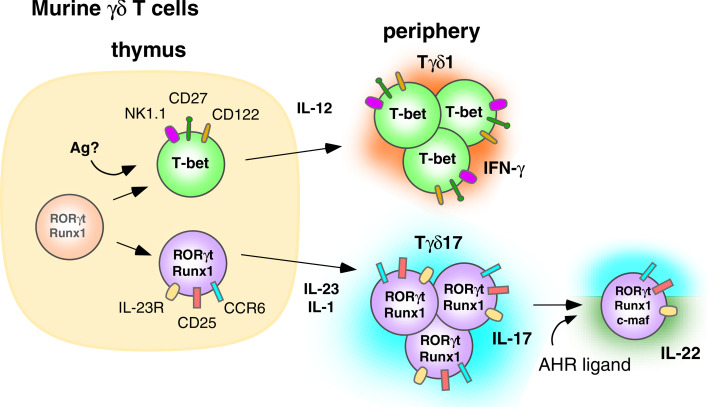
CD27 plays a critical role in determining whether γδ T cells produce IFN-γ or IL-17A (Fig. 2). CD27 expressed on thymic γδ T cells engages its ligand, CD70 (presumably expressed on thymic epithelial cells), while the γδ TCR likely binds to endogenous antigens resulting in trans-conditioning of thymic γδ T cells to produce IFN-γ [120]. CD27 ligation induces expression of the lymphotoxin-β receptor and other trans-conditioning genes, including Crem, N24a2, Rgs2, and Rgs1, in thymic Vγ4 and Vγ1 γδ T cells [120]. Thymic γδ T cells have high baseline expression of Rorc (which encodes ROR-γt) and Runx1, but require CD27 signaling to upregulate Tbx21 (which encodes T-bet) expression and to derepress the IFN-γ locus, which then enables them to produce IFN-γ [120] like CD4 αβ T cells [124]. In agreement with the other studies, the majority of CD27-negative, IL-17A-producing thymic γδ T cells did not express CD122 [122, 123].
Fig. 2.
Murine γδ T cells are thymically programmed to produce IL-17A in the absence of exogenous antigen. IL-23 and IL-1 are required by peripheral Tγδ17 T cells to maintain IL-17A production. Ligation of the AHR by these cells leads to the coproduction of IL-17A and IL-22. Murine γδ cells appear to show firm commitment to the Tγδ17 lineage and most do not acquire the ability to produce IFN-γ
Further characterization of adult thymic γδ T cells has revealed two distinct subsets, one being CD27−, CD122−, CCR6+, and NK1.1− producing IL-17A, and the other being CD27+, CD122+, CCR6−, and NK1.1+ producing IFN-γ. Unlike human CD4 αβ and Vγ2Vδ2 T cells producing IL-17A, the segregation between IL-17A and IFN-γ production in the thymus appears stable and irreversible. No IL-17A+, IFN-γ+ dual-positive γδ cells were observed [120]. Moreover, culturing IL-17A-producing CCR6+ γδ T cells with polarizing cytokines for the Th1 lineage (IL-12 and IL-18), or culturing IFN-γ-producing, NK1.1+ γδ cells with polarizing cytokines for the Th17 lineage (IL-23), fails to alter the cytokines produced [125]. Thus, unlike human αβ and γδ T cells, there appears to be little plasticity in thymic derived murine γδ T cells producing IL-17A.
In addition to CD27, SCART2 scavenger receptors (related to CD5 and CD6 in humans and WC1.1 in cattle and sheep) are predominantly expressed on murine γδ T cells and also identify γδ T cells producing IL-17A [126]. SCART2hi γδ T cells were found in peripheral lymph nodes and nearly all were Vγ4+, constituting about 25% of total Vγ4+ cells [126]. SCART2hi γδ T cells were also found in the dermis but did not express Vγ5Vδ1 DETC TCRs.
As discussed above for human αβ and Vγ2Vδ2 T cells, polarizing cytokines play a vital role in the differentiation of T cells into Th17/IL-17A-producing cells. In contrast to αβ and neonatal Vγ2Vδ2 T cells, neutralization of IL-6 has little effect on innate production of IL-17A by murine fetal thymic γδ T cells [7, 123, 127]. Furthermore, IFN-γ does not interfere with innate IL-17A production by thymic γδ T cells [120]. IL-6 is required at some point for optimal IL-17A production since about 75% fewer peripheral IL-17A+ γδ T cells were noted in IL-6−/− mice [128].
Conflicting reports exist concerning the requirement for TGF-β in the innate programming of IL-17A-producing thymic γδ T cells. In fetal thymic organ cultures, neutralization of TGF-β had no effect on the development of γδ T cells producing IL-17A [123]. In contrast, in γδ T cells from very young TGF-β-deficient mice prior to the development of lymphoproliferative disease, TGF-β is required for the innate programming of IL-17A production by thymic and peripheral γδ T cells [129]. One explanation for the different conclusions is that TGF-β exerts its effects indirectly by inhibiting STAT4 and GATA-3 in γδ T cells but has no effects on the expression of ROR-γt. This has been observed for αβ Th17 T cell differentiation where if the ability of αβ T cells to differentiate into Th1 and Th2 lineages is blocked, then TGF-β is not required for their differentiation into Th17 cells [43].
In contrast to the differing results on thymic innate programming, IL-23 clearly appears to have significant effects on IL-17A production by peripheral γδ T cells [7, 120, 130]. IL-1 has also been shown to play a critical role in IL-17A production by mature peripheral γδ T cells because IL-1R−/− mice show reduced production of IL-17A [6, 130]. Within mature splenic γδ T cells, IL-23 functions with IL-1β to further upregulate the constitutive expression of Rorc (RORγt) and Il23r [6]. This production of IL-17A in response to IL-23 is dependent on Tyk-2 [131], a member of the JAK family whose absence decreases phosphorylation of STAT3 [132]. Signals following IL-23R binding to IL-23 activate STAT3, which then binds and activates the Il-17a and Il-17f promoters [36]. IL-1R signaling appears to be required for maintenance of IL-23R expression in differentiated Th17 cells, and we assume that such signaling would be true of innately programmed γδ T cells [133]. Together, these results suggest a mechanism, distinct from that of αβ T cells, where γδ T cells acquire IL-17A-producing capacity and differentiate into “memory”-type T cells in the thymus, perhaps by responding to endogenous TCR ligands. Although these IL-17-producing γδ T cells respond to the normal cytokines that upregulate Th17 αβ T cell function (such as IL-23 and IL-1β), they are firmly committed to producing only IL-17A and do not exhibit the plasticity of human αβ and γδ T cells which can acquire the ability to produce IFN-γ and lose the ability to make IL-17A.
Regulation of IL-22-producing murine γδ T cells: requirement of the AHR
The development of murine γδ T cells producing IL-22 is less well understood. Thymic populations producing IL-22 have yet to be described. Therefore the bulk of knowledge comes from studies on peripheral γδ T cells and the development of IL-22-producing CD4 αβ T cells. The differentiation of αβ T cells into IL-22-producing cells (Th22 cells) requires engagement of the AHR (also known as the dioxin receptor). AHR is a ligand-dependent transcription factor that, upon engaging one of its ligands, binds the promoter region within dioxin-response genes and activates their transcription [134]. Ligands for AHR include numerous polycyclic aromatic hydrocarbons, both synthetic and naturally occurring. It is not known if there is a single, primary endogenous physiological ligand. Known ligands include 2,3,7,8-tetrachlorodibenzo-p-dioxin and the photoproduct of tryptophan, 6-formylindolo[3,2-b]carbazole (FICZ). AHR is upregulated in developing Th17 T cells [135] in response to the Th17 polarizing cytokines, TGF-β and IL-6, and in Tr1 regulatory cells in response to IL-27 [136]. AHR enhances naive T cell differentiation into Th17 cells by decreasing STAT1 activation, thereby enabling Th17 development [135]. The presence of AHR is required for production of IL-22 but not IL-17A or IL-17AF [85, 137].
Consistent with the reported effects of AHR activation on αβ T cells, injection the AHR ligand, FICZ, with heat-killed mycobacteria induces expansion of peritoneal γδ cells that produce both IL-17A and IL-22. IL-22 production is highly dependent upon the presence of the AHR because γδ T cells from AHR−/− mice do not produce IL-22 but do produce IL-17A [7]. Vγ6Vδ1 T cells in the lung are another peripheral IL-22-producing population that produce IL-22 in response to repeated stimulation by Bacillus subtilis in a lung fibrosis model [138]. This production of IL-22 by Vγ6Vδ1 T cells has been found to be critical to avoiding lung fibrosis in this model. Although some CD4 αβ T cells are reported to produce both IL-17A and IL-22, there were two distinct subsets of Vγ6Vδ1 T cells in the lung setting, those producing IL-22 and those producing IL-17A [138]. Very few Vγ6Vδ1 cells produced both cytokines, which is identical to what we observed with Vγ2Vδ2 T cells [9]. AHRd/d mice, that express an AHR with lower affinity, produce reduced amounts of IL-22 compared to wild-type mice. However, the levels of IL-17A and IL-17F are similar [138]. These results point to similarities between IL-22 production by Th17 αβ T cells and innately programmed γδ T cells since both require AHR.
Functions of IL-17A-/IL-22-producing murine γδ T cells in microbial immunity
IL-17A-producing murine γδ T cells are protective during many bacterial and fungal infections. An early indication of the importance of IL-17A production by γδ T cells is the ability of γδ and αβ NKT cells to control neutrophil levels in adhesion molecule-deficient mice [139]. The rapid production of IL-17A is an important property of γδ and unconventional αβ T cells and helps protect against microbial infections. This ability is critically dependent on their expression of the IL-23R [128].
γδ T cells can rapidly produce IL-17A, independent of foreign antigens, upon TLR and/or cytokine stimulation. This early production of IL-17A occurs in Escherichia coli infections where peritoneal IL-17A can be found as early as 1 h after infection and peaks at 6 h with neutrophil levels peaking at 24 h [8]. The IL-17A is produced by Vδ1 γδ T cell since production is significantly impaired in Vδ1-deficient mice [8]. Furthermore, IL-23 is sufficient to induce IL-17A production from γδ T cells purified from the infected peritoneum [8]. Similarly, IL-17A production by peritoneal γδ T cells rapidly reaches a peak 12 h after injection of heat-killed mycobacteria [7] or after sepsis due to cecal puncture [140]. IL-17A production requires TLR2 signaling and is amplified by IL-23 signaling, although it is not clear whether the γδ T cells were directly responding to the TLR stimulus [7].
In another study, lipopolysaccharide TLR4 and PAM3CysSerLys4 TLR2 ligands were directly injected into the peritoneal cavity where they stimulated the expansion of IL-17A+ γδ T cells [141]. Production of IL-23 and IL-1β by activated myeloid cells in the peritoneum stimulates CD27− γδ T cells to expand and produce IL-17A [141]. No direct response to TLR ligands is observed with purified γδ T cells in vitro. However, they do expand and produce IL-17A when cultured with exogenous IL-23 and IL-1β [141]. In contrast, infection with murine herpesvirus 4 or malaria parasites stimulates the expansion of CD27+ IL-17A− γδ T cells that produce IFN-γ [141].
Taken together, these results indicate that CD27 expression divides peritoneal γδ T cells into a CD27+ IFN-γ-producing subset that requires CD27 signaling and a CD27− IL-17A-producing subset that responds to IL-23 and IL-1β cytokines released by myeloid cells in response to innate immune signals [141]. All of these findings, however, do not preclude the requirement for recognition by the γδ TCR of self ligands since there is evidence that such self ligands are constitutively expressed on murine cells when detected by soluble multimeric γδ TCR [142, 143].
In addition to increasing neutrophil numbers and recruiting them to the sites of infection, IL-17A-producing γδ T cells can also promote the formation of abscesses and granulomas to help mediate containment of microbial infections. In cutaneous Staphylococcus aureus infections, IL-17A-producing skin Vγ5Vδ1 T cells play a critical role in immunity. Their production of IL-17A helps to recruit neutrophils into skin abscesses and limits the size of abscesses and the number of S. aureus bacteria that they contain [121].
In Listeria monocytogenes infections, IL-17A-producing Vγ6 paired with Vδ1 [144] and Vγ4 γδ T cells contribute to bacterial clearance by containing the bacteria within granulomas in the liver [145] and recruiting neutrophils and other myeloid cells. Without γδ T cells or IL-17A, bacterial numbers are more than 100-fold higher. Moreover, L. monocytogenes infections in γδ T cell-deficient mice are associated with large inflammatory lesions in the liver with necrotic hepatocytes [146, 147] that are indistinguishable from those seen in IL-17A-deficient mice [145]. IL-23 and IL-17R signaling are required to control L. monocytogenes systemic infections and γδ T cells are the principal source of IL-17A early in infection [148] although CD4−8− αβ T cells also produce IL-17A in the peritoneum [128].
The formation of lung granulomas during mycobacterial infections is critical to containing these infections. Here again, IL-17A production by murine lung γδ T cells, expressing Vγ4 and Vγ6 [149] paired with Vδ1 TCRs [144], are critical for the recruitment of granulocytes and monocytes into pulmonary granulomas in M. bovis BCG-infected mice [149, 150]. In fact, during M. tuberculosis infections in mice, IL-17A production is primarily from γδ and other unconventional T cells, rather than from CD4 and CD8 αβ T cells [5]. Both IL-23 and IL-17A are essential for protective vaccine responses to subsequent M. tuberculosis challenge [151], and mice unable to produce IL-17A have impaired maturation of BCG-induced granulomas with low numbers of γδ T cells [149]. An identical phenotype has been noted in mice lacking Vγ4 and Vγ6 T cells [149]. IL-17A is critical in M. tuberculosis infections since mice lacking IL-17A have 30-fold higher bacterial counts associated with poor granuloma formation [149].
Additional mechanisms for protection by IL-17A- or IL-22-producing γδ T cells include the induction of antimicrobial peptides and the remodeling of the extracellular matrix. Individually, IL-17A and IL-22 can upregulate expression of the antimicrobial peptides, β-defensin-2, S100A7, S100A8, and S100A9 by keratinocytes [32]. The combination of IL-17A and IL-22 is synergistic for induction of antimicrobials [152]. Such antimicrobials are especially important to mucosal immunity since they are broadly active and rapidly microbicidal. IL-17A also induces tissue remodeling proteins such as MMP-1, -3, -9 and -13, and aggrecanase-1 (see above). IL-22 also induces MMP-1 and MMP-3. MMP-9 and MMP-13 are important in bone homeostasis and repair, whereas MMP-1 is important in collagen remodeling and keratinocyte migration [32, 153]. MMP-3 is normally induced in response to skin injury and contributes to wound contraction [154].
Functions of IL-17A-/IL-22-producing murine γδ T cells in autoimmunity
IL-17A-producing γδ T cells can also prove pathogenic. Several autoimmune disease models have identified IL-17A-producing γδ T cells as being important in disease progression. As with any T cell population, inappropriate or sustained production of a proinflammatory cytokine can have devastating consequences. For example, in a model of CIA, oligoclonal IL-17A-producing Vγ4 γδ T cells accumulate in the lymph nodes and joints of collagen-injected mice [117]. Depletion of Vγ4 γδ T cells greatly reduces disease severity consistent with a significant reduction in pathogenic anticollagen IgG2a [117]. IL-17A production by the joint-infiltrating γδ T cells is regulated by IL-23 and IL-1β but not by collagen itself [155]. This study, however, found a heterogeneous population of γδ T cells that produce IL-17A expressing Vγ1, Vγ2, Vγ4, or Vγ6 paired with either Vδ1 or Vδ5. IL-17A production by γδ T cells is also observed in the methylated bovine serum albumin antigen-induced arthritis model—another model that uses complete Freund’s adjuvant (containing M. tuberculosis) with antigen to induce arthritis [156].
IL-17A induces the production of MMPs by synoviocyte and inhibits new matrix synthesis by chondrocytes, which together results in cartilage destruction of the joints [15, 157–159]. IL-17A also affects the delicate balance between bone formation by osteoblasts and bone resorption by osteoclasts. IL-17A favors osteoclast differentiation through the induction of RANKL. This results in unbalanced bone resorption and loss of bone mass [20]. Although equal numbers of αβ and γδ T cells produce IL-17A in joints with CIA, CD4 αβ T cells localize to bone and their deletion abrogates bone destruction. This suggests that CD4 αβ T cells help mediate bone destruction while γδ T cells increase overall joint inflammation [110].
In two different models of neuroinflammation, γδ T cells play an important role in early activation and recruitment of cells through the release of IL-17A. In a mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), γδ T cells producing IL-17A accumulate in the brain [6, 122, 160]. Like the CIA model, IL-17A production by these cells requires IL-1β and IL-23 [6]. Although γδ cells alone are not sufficient to reconstitute disease (αβ T cells are also required), γδ-deficient mice have reduced EAE severity [6]. In addition to producing IL-17A, γδ T cells also inhibit CD4 Treg responses in EAE and reverse Treg suppression of αβ T cells in vitro [161].
IL-17A-mediated neuroinflammation also plays a major role in the pathogenesis of ischemia–reperfusion brain injury. In a mouse model of middle cerebral artery occlusion followed by reperfusion, Shichita et al. [162] identified IL-17A-producing γδ T cells within the infarct areas. In IL-17A-, IL-23- or γδ T cell-deficient mice, the infarct volume is reduced. IL-17A is especially neurotoxic in that it induces blood–brain barrier disruption and mediates recruitment of cells into the CNS [162–164]. From these studies a common mechanism emerges in which neuroinflammation resulting in local IL-23 and IL-1β induces infiltrating γδ T cell production of IL-17A, independently of a defined γδ TCR antigen.
Besides classic autoimmune diseases, γδ T cells producing IL-17A and IL-22 also play important roles in the development of inflammatory responses during infections/environmental exposures that cause tissue damage. Thus, in hypersensitivity pneumonitis induced by repeated inhalation of the nonpathogenic B. subtilis bacteria, Vγ6Vδ1 T cells accumulate in the lung and produce IL-17A [165]. This accumulation requires live bacteria, whereas CD4 αβ T cell recruitment does not [166]. In the absence of IL-17A signaling, bacteria persist longer in the lung and the mice develop increased lung inflammation and fibrosis [165]. The absence of Vγ6Vδ1 T cells also leads to increased lung inflammation and fibrosis [166]. Besides IL-17A, Vγ6Vδ1 T cells also produce IL-22 [138]. Vγ6Vδ1 production of IL-22 requires the AHR and protects against lung fibrosis, whereas treatment with anti-IL-22 monoclonal antibodies increases fibrosis [138]. Similarly, the presence of IL-17A-producing γδ T cells shorten the recovery period in bleomycin-induced lung injury [167]. In contrast, in experimental silicosis both γδ and CD4 αβ T cells mediate inflammation and the mice go on to develop fibrosis even in the absence of IL-17A (or IL-22) and even when treated with IL-22 [168]. Finally, in another murine model of “Farmer’s lung” caused by Saccharopolyspora rectivirgula, Vγ6Vδ1 T cells again accumulate (albeit in lesser numbers) but there is no attenuation of lung fibrosis by γδ T cells although fibrosis does require IL-17A [169]. Thus, γδ T cells can mediate inflammation and can aid healing of lungs without fibrosis by producing IL-22, but their function varies according to the initiating stimuli.
IL-17A-producing γδ T cells also likely promote myocarditis in murine coxsackievirus B3-induced myocarditis [170, 171]. Murine Vγ4+ γδ T cells in infected mice are the earliest T cells infiltrating the myocardium where they are activated by CD1d and promote myocarditis [172]. A second population of γδ T cells expressing Vγ1 receptors suppresses the development of myocarditis [170]. Vγ4+ T cells can transfer myocarditis and kill infected cardiocytes [173], and their deletion decreases myocarditis [174]. Anti-IL-17A antibody treatment ameliorates disease and improves survival [171], suggesting that these Vγ4+ γδ T cells produce IL-17A as in other diseases.
Concluding remarks
Unlike murine γδ T cells, human Vγ2Vδ2 T cells acquire IL-17A production post-thymically through a mechanism similar to Th17 polarization of αβ T cells (Fig. 1). Naive Vγ2Vδ2 in the presence of HMBPP-producing microbes and the cytokines IL-6, IL-1β, and TGF-β upregulate expression of RORγt and become IL-17A-secreting Tγδ17 cells. In the absence of these cytokines, Vγ2Vδ2 T cells upregulate T-bet expression leading to the generation of Tγδ1 which have high levels of IL-12Rβ2 and respond to IL-12. Tγδ17 cells likely express both IL-12Rβ2 and IL-23R, enabling them to become Tγδ1/17 (through IL-23 signaling) or functionally Tγδ1 (through IL-12 signaling), or to maintain the Tγδ17 phenotype (through IL-23, IL-1β, and TGF-β signaling).
Murine thymic γδ T cells have basal expression of RORγt and Runx1 (Fig. 2), which in the absence of antigen and through thymic trans-conditioning, yield IL-17A-producing γδ T cells. Alternatively, γδ T cells, which possibly encounter antigen in the thymus, upregulate T-bet and become IFN-γ producing Tγδ1 cells. Phenotypically, the IL-17A-producing γδ cells express IL-23R, CD25, and CCR6 whereas IFN-γ-producing γδ cells express NK1.1, CD27, and CD122. The IL-17A-producing γδ T cells require IL-23 and IL-1 in the periphery to maintain and reinforce expression of RORγt and production of IL-17A, whereas Tγδ1 cells require IL-12. Ligation of the AHR and its association with c-MAF, mediates acquisition of IL-22 production by the Tγδ17 cells.
The involvement of IL-17A- and IL-22-producing γδ T cells in many diverse models of microbial, autoimmune, and inflammatory diseases underscores their importance in the initiation and resolution of acute inflammatory responses in a variety of settings. Despite their small numbers, γδ T cells can have large effects on the type of immune response and the disease outcome.
Acknowledgments
We thank Drs. Hong Wang and Grefachew Workalemahu for critical reading of the manuscript. This work was supported by grants to C.T.M. from the National Institute of Arthritis and Musculoskeletal and Skin Disease (AR045504) and the Bill & Melinda Gates Foundation (Exploration OPP1006946). Kristin Ness-Schwickerath was supported by the National Institute of Allergy and Infectious Diseases (T32 AI007511).
Abbreviations
- APC
Antigen-presenting cell
- AHR
Aryl hydrocarbon receptor
- CIA
Collagen-induced arthritis
- DETC
Dendritic epidermal T cells
- EAE
Experimental autoimmune encephalomyelitis
- FICZ
6-Formylindolo[3,2-b]carbazole
- HMBPP
(E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate
- IL
Interleukin
- IPP
Isopentenyl pyrophosphate
- MHC
Major histocompatibility complex
- MMP
Matrix metalloproteinase
- NKT
Natural killer T
- ROR
Retinoid-related orphan receptor
- STAT
Signal transducer and activator of transcription
- TCR
T cell antigen receptor
- TLR
Toll-like receptor
References
- 1.Brenner MB, McLean J, Dialynas DP, Strominger JL, Smith JA, Owen FL, Seidman JG, Ip S, Rosen F, Krangel MS. Identification of a putative second T-cell receptor. Nature. 1986;322:145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- 2.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr Opin Immunol. 2007;19:186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 5.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 6.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 9.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vγ2Vδ2 T cells. J Immunol. 2010;184:7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 12.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine. 2008;41:92–104. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao C-Y, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-κB-dependent signaling pathway. J Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 15.Chabaud M, Garnero P, Dayer J-M, Guerne P-A, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 16.Bamba S, Andoh A, Yasui H, Araki Y, Bamba T, Fujiyama Y. Matrix metalloproteinase-3 secretion from human colonic subepithelial myofibroblasts: role of interleukin-17. J Gastroenterol. 2003;38:548–554. doi: 10.1007/s00535-002-1101-8. [DOI] [PubMed] [Google Scholar]
- 17.Sylvester J, Liacini A, Li WQ, Zafarullah M. Interleukin-17 signal transduction pathways implicated in inducing matrix metalloproteinase-3, -13 and aggrecanase-1 genes in articular chondrocytes. Cell Signal. 2004;16:469–476. doi: 10.1016/j.cellsig.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Jovanovic DV, Martel-Pelletier J, Di Battista JA, Mineau F, Jolicoeur FC, Benderdour M, Pelletier JP. Stimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin-17 in human monocyte/macrophages: a possible role in rheumatoid arthritis. Arthritis Rheum. 2000;43:1134–1144. doi: 10.1002/1529-0131(200005)43:5<1134::AID-ANR24>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Rifas L, Arackal S. T cells regulate the expression of matrix metalloproteinase in human osteoblasts via a dual mitogen-activated protein kinase mechanism. Arthritis Rheum. 2003;48:993–1001. doi: 10.1002/art.10872. [DOI] [PubMed] [Google Scholar]
- 20.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roussel L, Houle F, Chan C, Yao Y, Bérubé J, Olivenstein R, Martin JG, Huot J, Hamid Q, Ferri L, Rousseau S. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 2010;184:4531–4537. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 22.Kao C-Y, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 23.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1β and IL-17A; the NF-κB paradigm. J Immunol. 2009;183:6236–6243. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel DN, King CA, Bailey SR, Holt JW, Venkatachalam K, Agrawal A, Valente AJ, Chandrasekar B. Interleukin-17 stimulates C-reactive protein expression in hepatocytes and smooth muscle cells via p38 MAPK and ERK1/2-dependent NF-κB and C/EBPβ activation. J Biol Chem. 2007;282:27229–27238. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 26.Andoh A, Shimada M, Bamba S, Okuno T, Araki Y, Fujiyama Y, Bamba T. Extracellular signal-regulated kinases 1 and 2 participate in interleukin-17 plus tumor necrosis factor-α-induced stabilization of interleukin-6 mRNA in human pancreatic myofibroblasts. Biochim Biophys Acta. 2002;1591:69–74. doi: 10.1016/S0167-4889(02)00250-1. [DOI] [PubMed] [Google Scholar]
- 27.Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, Okuno T, Fujiyama Y, Bamba T. IL-17 stimulates inflammatory responses via NF-κB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035–G1044. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 28.van den Berg A, Kuiper M, Snoek M, Timens W, Postma DS, Jansen HM, Lutter R. Interleukin-17 induces hyperresponsive interleukin-8 and interleukin-6 production to tumor necrosis factor-α in structural lung cells. Am J Respir Cell Mol Biol. 2005;33:97–104. doi: 10.1165/rcmb.2005-0022OC. [DOI] [PubMed] [Google Scholar]
- 29.Cai X-Y, Gommoll CP, Jr, Justice L, Narula SK, Fine JS. Regulation of granulocyte colony-stimulating factor gene expression by interleukin-17. Immunol Lett. 1998;62:51–58. doi: 10.1016/S0165-2478(98)00027-3. [DOI] [PubMed] [Google Scholar]
- 30.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, Khatcherian A, Gonzalez J, Pierson KC, White TR, Pensabene C, Coats I, Novitskaya I, Lowes MA, Krueger JG. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Danilenko DM, Bunting S, Ganesan R, Sa S, Ferrando R, Wu TD, Kolumam GA, Ouyang W, Kirchhofer D. The serine protease marapsin is expressed in stratified squamous epithelia and is up-regulated in the hyperproliferative epidermis of psoriasis and regenerating wounds. J Biol Chem. 2009;284:218–228. doi: 10.1074/jbc.M806267200. [DOI] [PubMed] [Google Scholar]
- 32.Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, Volk H-D, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 33.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 38.Huber M, Brustle A, Reinhard K, Guralnik A, Walter G, Mahiny A, von Low E, Lohoff M. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci U S A. 2008;105:20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 41.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor β is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V. A critical function for transforming growth factor-β, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 45.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-β are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santarlasci V, Maggi L, Capone M, Frosali F, Querci V, De Palma R, Liotta F, Cosmi L, Maggi E, Romagnani S, Annunziato F. TGF-β indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol. 2009;39:207–215. doi: 10.1002/eji.200838748. [DOI] [PubMed] [Google Scholar]
- 48.Delfau M-H, Hance AJ, Lecossier D, Vilmer E, Grandchamp B. Restricted diversity of Vγ9-JP rearrangements in unstimulated human γ/δ T lymphocytes. Eur J Immunol. 1992;22:2437–2443. doi: 10.1002/eji.1830220937. [DOI] [PubMed] [Google Scholar]
- 49.McVay LD, Jaswal SS, Kennedy C, Hayday A, Carding SR. The generation of human γδ T cell repertoires during fetal development. J Immunol. 1998;160:5851–5860. [PubMed] [Google Scholar]
- 50.Krangel MS, Yssel H, Brocklehurst C, Spits H. A distinct wave of human T cell receptor γ/δ lymphocytes in the early fetal thymus: evidence for controlled gene rearrangement and cytokine production. J Exp Med. 1990;172:847–859. doi: 10.1084/jem.172.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morita CT, Verma S, Aparicio P, Martinez AC, Spits H, Brenner MB. Functionally distinct subsets of human γ/δ T cells. Eur J Immunol. 1991;21:2999–3007. doi: 10.1002/eji.1830211215. [DOI] [PubMed] [Google Scholar]
- 52.Morita CT, Parker CM, Brenner MB, Band H. T cell receptor usage and functional capabilities of human γδ T cells at birth. J Immunol. 1994;153:3979–3988. [PubMed] [Google Scholar]
- 53.Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J Exp Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Roederer M. Ontogeny of γδ T cells in humans. J Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 55.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas A-K, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- 56.Puan K-J, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK, Samuelson MI, Märker-Hermann E, Pasa-Tolic L, Nieves E, Giner J-L, Kuzuyama T, Morita CT. Preferential recognition of a microbial metabolite by human Vγ2Vδ2 T cells. Int Immunol. 2007;19:657–673. doi: 10.1093/intimm/dxm031. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 58.Kunzmann V, Bauer E, Wilhelm M. γ/δ T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 59.Bukowski JF, Morita CT, Brenner MB. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/S1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 60.Deusch K, Luling F, Reich K, Classen M, Wagner H, Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the γ/δ T cell receptor, the CD8 accessory molecule and preferentially uses the Vδ1 gene segment. Eur J Immunol. 1991;21:1053–1059. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- 61.Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, Warnke RA, Bhan AK, Strominger JL, Brenner MB. Human lymphocytes bearing T cell receptor γ/δ are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ebert LM, Meuter S, Moser B. Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–4336. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 63.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4−CD8− cytolytic T lymphocytes. Nature. 1989;341:447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 64.Faure F, Jitsukawa S, Miossec C, Hercend T. CD1c as a target recognition structure for human T lymphocytes: analysis with peripheral blood γ/δ cells. Eur J Immunol. 1990;20:703–706. doi: 10.1002/eji.1830200336. [DOI] [PubMed] [Google Scholar]
- 65.Spada FM, Grant EP, Peters PJ, Sugita M, Melián A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self recognition of CD1 by γ/δ T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russano AM, Bassotti G, Agea E, Bistoni O, Mazzocchi A, Morelli A, Porcelli SA, Spinozzi F. CD1-restricted recognition of exogenous and self-lipid antigens by duodenal γδ+ T lymphocytes. J Immunol. 2007;178:3620–3626. doi: 10.4049/jimmunol.178.6.3620. [DOI] [PubMed] [Google Scholar]
- 67.Russano AM, Agea E, Corazzi L, Postle AD, De Libero G, Porcelli S, de Benedictis FM, Spinozzi F. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted γδ T cells. J Allergy Clin Immunol. 2006;117:1178–1184. doi: 10.1016/j.jaci.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Cui Y, Kang L, Cui L, He W. Human γδ T cell recognition of lipid A is predominately presented by CD1b or CD1c on dendritic cells. Biol Direct. 2009;4:47. doi: 10.1186/1745-6150-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 70.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial γδ T cells. J Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 72.Zhao J, Huang J, Chen H, Cui L, He W. Vδ1 T cell receptor binds specifically to MHC I chain related A: molecular and biochemical evidences. Biochem Biophys Res Commun. 2006;339:232–240. doi: 10.1016/j.bbrc.2005.10.198. [DOI] [PubMed] [Google Scholar]
- 73.Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, Bukowski JF. MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/S1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 74.Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, Van Rysselberge M, Twite N, Goldman M, Marchant A, Willems F. Human cytomegalovirus elicits fetal γδ T cell responses in utero. J Exp Med. 2010;207:807–821. doi: 10.1084/jem.20090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon M-E, Merville P, Moreau J-F, Déchanet-Merville J. Long-term expansion of effector/memory Vδ2− γδ T cells is a specific blood signature of CMV infection. Blood. 2008;112:1317–1324. doi: 10.1182/blood-2008-01-136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X, Michelson S, Méric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau J-F. Implication of γδ T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–1449. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lafarge X, Merville P, Cazin M-C, Bergé F, Potaux L, Moreau J-F, Déchanet-Merville J. Cytomegalovirus infection in transplant recipients resolves when circulating γδ T lymphocytes expand, suggesting a protective antiviral role. J Infect Dis. 2001;184:533–541. doi: 10.1086/322843. [DOI] [PubMed] [Google Scholar]
- 78.Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, Dromer C, Emilie D, Moreau J-F, Déchanet-Merville J. Shared reactivity of Vδ2neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med. 2005;201:1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yen H-R, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, Liang K-L, Bruno TC, Pyle KJ, Chan S-L, Anders RA, Trimble CL, Adler AJ, Lin T-Y, Pardoll DM, Huang C-T, Drake CG. Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol. 2009;183:7161–7168. doi: 10.4049/jimmunol.0900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kondo T, Takata H, Matsuki F, Takiguchi M. Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol. 2009;182:1794–1798. doi: 10.4049/jimmunol.0801347. [DOI] [PubMed] [Google Scholar]
- 82.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 83.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, Pierce R, McClanahan TK, Sadekova S, de Waal Malefyt R. Human Th17 cells comprise heterogeneous subsets including IFN-γ-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679–687. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 84.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 85.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H, Fang Z, Morita CT. Vγ2Vδ2 T cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J Immunol. 2010;184:6209–6222. doi: 10.4049/jimmunol.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.1365-2567.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davodeau F, Peyrat M-A, Hallet M-M, Gaschet J, Houde I, Vivien R, Vie H, Bonneville M. Close correlation between Daudi and mycobacterial antigen recognition by human γδ T cells and expression of V9JPC1γ/V2DJCδ-encoded T cell receptors. J Immunol. 1993;151:1214–1223. [PubMed] [Google Scholar]
- 89.Yamashita S, Tanaka Y, Harazaki M, Mikami B, Minato N. Recognition mechanism of non-peptide antigens by human γδ T cells. Int Immunol. 2003;15:1301–1307. doi: 10.1093/intimm/dxg129. [DOI] [PubMed] [Google Scholar]
- 90.Davodeau F, Peyrat MA, Hallet MM, Houde I, Vie H, Bonneville M. Peripheral selection of antigen receptor junctional features in a major human γδ subset. Eur J Immunol. 1993;23:804–808. doi: 10.1002/eji.1830230405. [DOI] [PubMed] [Google Scholar]
- 91.Tanaka Y, Sano S, Nieves E, De Libero G, Roca D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci U S A. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Libero G, Casorati G, Giachino C, Carbonara C, Migone N, Matzinger P, Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral γ/δ T cells. J Exp Med. 1991;173:1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J, Li BQ. Interleukin 17-producing γδ T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5:203–208. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC, Chen ZW. Differentiation, distribution and γδ T cell-driven regulation of IL-22-producing T cells in tuberculosis. PLoS Pathog. 2010;6:e1000789. doi: 10.1371/journal.ppat.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fenoglio D, Poggi A, Catellani S, Battaglia F, Ferrera A, Setti M, Murdaca G, Zocchi MR. Vδ1 T lymphocytes producing IFN-γ and IL-17 are expanded in HIV-1 infected patients and respond to Candida albicans. Blood. 2009;113:6611–6618. doi: 10.1182/blood-2009-01-198028. [DOI] [PubMed] [Google Scholar]
- 98.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 99.Smeekens SP, van de Veerdonk FL, van der Meer JW, Kullberg BJ, Joosten LA, Netea MG. The Candida Th17 response is dependent on mannan- and β-glucan-induced prostaglandin E2. Int Immunol. 2010;22:889–895. doi: 10.1093/intimm/dxq442. [DOI] [PubMed] [Google Scholar]
- 100.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-γ production by memory CD4+ T cells. Eur J Immunol. 2009;39:1301–1312. doi: 10.1002/eji.200838969. [DOI] [PubMed] [Google Scholar]
- 102.van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J, Kullberg BJ, van Crevel R, van der Meer JWM, Joosten LAB, Netea MG. Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukoc Biol. 2010;88:227–232. doi: 10.1189/jlb.0809550. [DOI] [PubMed] [Google Scholar]
- 103.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin Immunol. 2006;121:251–259. doi: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramsburg E, Tigelaar R, Craft J, Hayday A. Age-dependent requirement for γδ T cells in the primary but not secondary protective immune response against an intestinal parasite. J Exp Med. 2003;198:1403–1414. doi: 10.1084/jem.20030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol Res. 2000;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- 106.Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 107.Arulanandam BP, Van Cleave VH, Metzger DW. IL-12 is a potent neonatal vaccine adjuvant. Eur J Immunol. 1999;29:256–264. doi: 10.1002/(SICI)1521-4141(199901)29:01<256::AID-IMMU256>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 108.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gibbons DL, Haque SFY, Silberzahn T, Hamilton K, Langford C, Ellis P, Carr R, Hayday AC. Neonates harbour highly active γδ T cells with selective impairments in preterm infants. Eur J Immunol. 2009;39:1794–1806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- 110.Pollinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, Bay S, Keller R, Raulf F, Di Padova F, O’Reilly T, Horwood NJ, Patel DD, Littlewood-Evans A. Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011;186:2602–2612. doi: 10.4049/jimmunol.1003370. [DOI] [PubMed] [Google Scholar]
- 111.Allison JP, Havran WL. The immunobiology of T cells with invariant γδ antigen receptors. Annu Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- 112.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 113.Xiong N, Raulet DH. Development and selection of γδ T cells. Immunol Rev. 2007;215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 114.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien Y-H. Antigen recognition determinants of γδ T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 115.Crowley MP, Faher AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, Chien Y-h. A population of murine γδ T cells recognized an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- 116.Huber S, Sartini D, Exley M. Role of CD1d in coxsackievirus B3-induced myocarditis. J Immunol. 2003;170:3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- 117.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sciammas R, Johnson RM, Sperling AI, Brady W, Linsley PS, Spear PG, Fitch FW, Bluestone JA. Unique antigen recognition by a herpesvirus-specific TCR-γδ cell. J Immunol. 1994;152:5392–5397. [PubMed] [Google Scholar]
- 119.Schild H, Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, Bluestone JA, Matis L, Draper RK, Chien Y-h. The nature of major histocompatibility complex recognition by γδ T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 120.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ γδ T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 124.van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MH, Eldering E, van Lier RAW. CD27–CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int Immunol. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 125.Haas JD, Gonzalez FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, Forster R, Prinz I. CCR6 and NK1.1 distinguish between IL-17A and IFN-γ-producing effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 126.Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult γδ T cells. J Immunol. 2008;181:1710–1716. doi: 10.4049/jimmunol.181.3.1710. [DOI] [PubMed] [Google Scholar]
- 127.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Riol-Blanco L, Lazarevic V, Awasthi A, Mitsdoerffer M, Wilson BS, Croxford A, Waisman A, Kuchroo VK, Glimcher LH, Oukka M. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J Immunol. 2010;184:1710–1720. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, Letterio JJ, Min B. Spontaneous development of IL-17-producing γδ T cells in the thymus occurs via a TGF-β1-dependent mechanism. J Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing γ/δ T cells. Cell Host Microbe. 2010;7:140–150. doi: 10.1016/j.chom.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakamura R, Shibata K, Yamada H, Shimoda K, Nakayama K, Yoshikai Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by γδ T cells. J Immunol. 2008;181:2071–2075. doi: 10.4049/jimmunol.181.3.2071. [DOI] [PubMed] [Google Scholar]
- 132.Shaw MH, Boyartchuk V, Wong S, Karaghiosoff M, Ragimbeau J, Pellegrini S, Muller M, Dietrich WF, Yap GS. A natural mutation in the Tyk2 pseudokinase domain underlies altered susceptibility of B10.Q/J mice to infection and autoimmunity. Proc Natl Acad Sci U S A. 2003;100:11594–11599. doi: 10.1073/pnas.1930781100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Staschke KA, Dong S, Saha J, Zhao J, Brooks NA, Hepburn DL, Xia J, Gulen MF, Kang Z, Altuntas CZ, Tuohy VK, Gilmour R, Li X, Na S. IRAK4 kinase activity is required for Th17 differentiation and Th17-mediated disease. J Immunol. 2009;183:568–577. doi: 10.4049/jimmunol.0802361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld J-C, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 138.Simonian PL, Wehrmann F, Roark CL, Born WK, O’Brien RL, Fontenot AP. γδ T cells protect against lung fibrosis via IL-22. J Exp Med. 2010;207:2239–2253. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 140.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JLM, Ward PA. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22:2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 141.Ribot JC, Chaves-Ferreira M, d’Orey F, Wencker M, Gonçalves-Sousa N, Decalf J, Simas JP, Hayday AC, Silva-Santos B. Cutting edge: adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-γ- or IL-17-producing γδ T cells upon infection. J Immunol. 2010;185:6421–6425. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aydintug MK, Roark CL, Chain JL, Born WK, O’Brien RL. Macrophages express multiple ligands for γδ TCRs. Mol Immunol. 2008;45:3253–3263. doi: 10.1016/j.molimm.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aydintug MK, Roark CL, Yin X, Wands JM, Born WK, O’Brien RL. Detection of cell surface ligands for the γδ TCR using soluble TCRs. J Immunol. 2004;172:4167–4175. doi: 10.4049/jimmunol.172.7.4167. [DOI] [PubMed] [Google Scholar]
- 144.Hamada S, Umemura M, Shiono T, Hara H, Kishihara K, Tanaka K, Mayuzumi H, Ohta T, Matsuzaki G. Importance of murine Vδ1+ γδ T cells expressing interferon-γ and interleukin-17A in innate protection against Listeria monocytogenes infection. Immunology. 2008;125:170–177. doi: 10.1111/j.1365-2567.2008.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O’Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SHE. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 147.Fu Y-X, Roark CE, Kelly K, Drevets D, Campbell P, O’Brien R, Born W. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J Immunol. 1994;153:3101–3115. [PubMed] [Google Scholar]
- 148.Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- 149.Okamoto Yoshida Y, Umemura M, Yahagi A, O’Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 150.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 151.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 152.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Löffek S, Schilling O, Franzke C-W (2010) Biological role of matrix metalloproteinases: a critical balance. Eur Respir J (in press). doi:10.1183/09031936.00029109 [DOI] [PubMed]
- 154.Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, Banda MJ. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–265. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H, Nakamura T, Shimizu M, Kawabata D, Yukawa N, Hashimoto M, Sakaguchi N, Sakaguchi S, Yoshifuji H, Nojima T, Ohmura K, Fujii T, Mimori T. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60:2294–2303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- 156.Cornelissen F, Mus AM, Asmawidjaja PS, van Hamburg JP, Tocker J, Lubberts E. Interleukin-23 is critical for full-blown expression of a non-autoimmune destructive arthritis and regulates interleukin-17A and RORγt in γδ T cells. Arthritis Res Ther. 2009;11:R194. doi: 10.1186/ar2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chabaud M, Lubberts E, Joosten L, van Den Berg W, Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001;3:168–177. doi: 10.1186/ar294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van Bezooijen RL, van der Wee-Pals L, Papapoulos SE, Löwik CW. Interleukin 17 synergises with tumour necrosis factor α to induce cartilage destruction in vitro. Ann Rheum Dis. 2002;61:870–876. doi: 10.1136/ard.61.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moran EM, Mullan R, McCormick J, Connolly M, Sullivan O, Fitzgerald O, Bresnihan B, Veale DJ, Fearon U. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-α, Oncostatin M and response to biologic therapies. Arthritis Res Ther. 2009;11:R113. doi: 10.1186/ar2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wohler JE, Smith SS, Zinn KR, Bullard DC, Barnum SR. γδ T cells in EAE: early trafficking events and cytokine requirements. Eur J Immunol. 2009;39:1516–1526. doi: 10.1002/eji.200839176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, Korn T. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing γδ T cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 163.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CR. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24:1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 165.Simonian PL, Roark CL, Wehrmann F, Lanham AM, Born WK, O’Brien RL, Fontenot AP. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J Immunol. 2009;182:6540–6549. doi: 10.4049/jimmunol.0900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Simonian PL, Roark CL, Diaz del Valle F, Palmer BE, Douglas IS, Ikuta K, Born WK, O’Brien RL, Fontenot AP. Regulatory role of γδ T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. 2006;177:4436–4443. doi: 10.4049/jimmunol.177.7.4436. [DOI] [PubMed] [Google Scholar]
- 167.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, Putney L, Ferrick DA, Hyde DM, Love RB. IL-17 producing γδ T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 2008;31:167–179. doi: 10.1007/s10753-008-9062-6. [DOI] [PubMed] [Google Scholar]
- 168.Lo Re S, Dumoutier L, Couillin I, Van Vyve C, Yakoub Y, Uwambayinema F, Marien B, van den Brûle S, Van Snick J, Uyttenhove C, Ryffel B, Renauld J-C, Lison D, Huaux F. IL-17A-producing γδ T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. 2010;184:6367–6377. doi: 10.4049/jimmunol.0900459. [DOI] [PubMed] [Google Scholar]
- 169.Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, Born WK, O’Brien RL, Fontenot AP. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182:657–665. doi: 10.4049/jimmunol.0900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huber SA, Graveline D, Newell MK, Born WK, O’Brien RL. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 171.Fan Y, Weifeng W, Yuluan Y, Qing K, Yu P, Yanlan H. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of coxsackievirus B3-induced viral myocarditis reduces myocardium inflammation. Virol J. 2011;8:17. doi: 10.1186/1743-422X-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role for CD1d. Med Microbiol Immunol. 2005;194:121–127. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- 173.Huber SA, Born W, O’Brien R. Dual functions of murine γδ cells in inflammation and autoimmunity in coxsackievirus B3-induced myocarditis: role of Vγ1+ and Vγ4+ cells. Microbes Infect. 2005;7:537–543. doi: 10.1016/j.micinf.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 174.Huber SA, Graveline D, Born WK, O’Brien RL. Cytokine production by Vγ+-T-cell subsets is an important factor determining CD4+-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. J Virol. 2001;75:5860–5869. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Balbi B, Valle MT, Oddera S, Giunti D, Manca F, Rossi GA, Allegra L. T-lymphocytes with γδ+ Vδ2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis. 1993;148:1685–1690. doi: 10.1164/ajrccm/148.6_Pt_1.1685. [DOI] [PubMed] [Google Scholar]
- 176.Ito M, Kojiro N, Ikeda T, Ito T, Funada J, Kokubu T. Increased proportions of peripheral blood γδ T cells in patients with pulmonary tuberculosis. Chest. 1992;102:195–197. doi: 10.1378/chest.102.1.195. [DOI] [PubMed] [Google Scholar]
- 177.Ueta C, Tsuyuguchi I, Kawasumi H, Takashima T, Toba H, Kishimoto S. Increase of γ/δ T cells in hospital workers who are in close contact with tuberculosis patients. Infect Immun. 1994;62:5434–5441. doi: 10.1128/iai.62.12.5434-5441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dieli F, Sireci G, Di Sano C, Champagne E, Fournié J-J, Salerno JI. Predominance of Vγ9/Vδ2 T lymphocytes in the cerebrospinal fluid of children with tuberculous meningitis: reversal after chemotherapy. Mol Med. 1999;5:301–312. [PMC free article] [PubMed] [Google Scholar]
- 179.Modlin RL, Pirmez C, Hofman FM, Torigian V, Uyemura K, Rea TH, Bloom BR, Brenner MB. Lymphocytes bearing antigen-specific γδ T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 180.Sumida T, Maeda T, Takahashi H, Yoshida S, Yonaha F, Sakamoto A, Tomioka H, Koike T, Yoshida S. Predominant expansion of Vγ9/Vδ2 T cells in a tularemia patient. Infect Immun. 1992;60:2554–2558. doi: 10.1128/iai.60.6.2554-2558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Poquet Y, Kroca M, Halary F, Stenmark S, Peyrat M-A, Bonneville M, Fournié JJ, Sjöstedt A. Expansion of Vγ9Vδ2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66:2107–2114. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kroca M, Tärnvik A, Sjöstedt A. The proportion of circulating γδ T cells increases after the first week of onset of tularemia and remains elevated for more than a year. Clin Exp Immunol. 2000;120:280–284. doi: 10.1046/j.1365-2249.2000.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hara T, Mizuno Y, Takaki K, Takada H, Akeda H, Aoki T, Nagata M, Ueda K, Matsuzaki G, Yoshikai Y, Nomoto K. Predominant activation and expansion of Vγ9-bearing γδ T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kroca M, Johansson A, Sjöstedt A, Tärnvik A. Vγ9Vδ2 T cells in human legionellosis. Clin Diagn Lab Immunol. 2001;8:949–954. doi: 10.1128/CDLI.8.5.949-954.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bertotto A, Gerli R, Spinozzi F, Muscat C, Scalise F, Castellucci G, Sposito M, Candio F, Vaccaro R. Lymphocytes bearing the γδ T cell receptor in acute Brucella melitensis infection. Eur J Immunol. 1993;23:1177–1180. doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- 186.Schneider T, Jahn HU, Liesenfeld O, Steinhoff D, Riecken EO, Zeitz M, Ullrich R. The number and proportion of Vγ9/Vδ2 T cells rise significantly in the peripheral blood of patients after the onset of acute Coxiella burnetii infection. Clin Infect Dis. 1997;24:261–264. doi: 10.1093/clinids/24.2.261. [DOI] [PubMed] [Google Scholar]
- 187.Caldwell CW, Everett ED, McDonald G, Yesus YW, Roland WE. Lymphocytosis of γ/δ T cells in human ehrlichiosis. Am J Clin Pathol. 1995;103:761–766. doi: 10.1093/ajcp/103.6.761. [DOI] [PubMed] [Google Scholar]
- 188.Raziuddin S, Mir NA, el-Awad M el-H, Telmesani AW, al-Janadi M. γδ T lymphocytes and proinflammatory cytokines in bacterial meningitis. J Allergy Clin Immunol. 1994;93:793–798. doi: 10.1016/0091-6749(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 189.Jouen-Beades F, Paris E, Dieulois C, Lemeland J-F, Barre-Dezelus V, Marret S, Humbert G, Leroy J, Tron F. In vivo and in vitro activation and expansion of γδ T cells during Listeria monocytogenes infection in humans. Infect Immun. 1997;65:4267–4272. doi: 10.1128/iai.65.10.4267-4272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ho M, Webster HK, Tongtawe P, Pattanapanyasat K, Weidanz WP. Increased γδ T cells in acute Plasmodium falciparum malaria. Immunol Lett. 1990;25:139–142. doi: 10.1016/0165-2478(90)90105-Y. [DOI] [PubMed] [Google Scholar]
- 191.Roussilhon C, Agrapart M, Ballet J-J, Bensussan A. T lymphocytes bearing the γδ T cell receptor in patients with acute Plasmodium falciparum malaria. J Infect Dis. 1990;162:283–285. doi: 10.1093/infdis/162.1.283-a. [DOI] [PubMed] [Google Scholar]
- 192.Schwartz E, Shapiro R, Shina S, Bank I. Delayed expansion of Vδ2+ and Vδ1+ γδ T cells after acute Plasmodium falciparum and Plasmodium vivax malaria. J Allergy Clin Immunol. 1996;97:1387–1392. doi: 10.1016/S0091-6749(96)70208-7. [DOI] [PubMed] [Google Scholar]
- 193.Perera MK, Carter R, Goonewardene R, Mendis KN. Transient increase in circulating γ/δ T cells during Plasmodium vivax malarial paroxysms. J Exp Med. 1994;179:311–315. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Scalise F, Gerli R, Castellucci G, Spinozzi F, Fabietti GM, Crupi S, Sensi L, Britta R, Vaccaro R, Bertotto A. Lymphocytes bearing the γδ T-cell receptor in acute toxoplasmosis. Immunology. 1992;76:668–670. [PMC free article] [PubMed] [Google Scholar]
- 195.Raziuddin S, Telmasani AW, El-Awad ME, Al-Amari O, Al-Janadi M. γδ T cells and the immune response in visceral leishmaniasis. Eur J Immunol. 1992;22:1143–1148. doi: 10.1002/eji.1830220506. [DOI] [PubMed] [Google Scholar]
- 196.Russo DM, Armitage RJ, Barral NM, Barral A, Grabstein KH, Reed SG. Antigen-reactive γδ T cells in human leishmaniasis. J Immunol. 1993;151:3712–3718. [PubMed] [Google Scholar]