Abstract
Objectives
While screening the activity of potential inhibitors of matrix metalloproteinases (MMPs), due to the limited water solubility of some of the compounds, they had to be solubilized in ethanol. When ethanol solvent controls were run, they were found to partially inhibit MMPs. Thus, the purpose of this study was to compare the MMP-inhibitory activity of a series of alcohols.
Methods
The possible inhibitory activity of a series of alcohols was measured against soluble rhMMP-9 and insoluble matrix-bound endogenous MMPs of dentin in completely demineralized dentin. Increasing concentrations (0.17, 0.86, 1.71 and 4.28 moles/L) of a homologous series of alcohols (i.e. methanol, ethanol, propanols, butanols, pentanols, hexanols, the ethanol ester of methacrylic acid, heptanols and octanol) were compared to ethanediol, and propanediol by regression analysis to calculate the molar concentration required to inhibit MMPs by 50% (i.e. the IC50).
Results
Using two different MMP models, alcohols were shown to inhibit rhMMP-9 and the endogenous proteases of dentin matrix in a dose-dependent manner. The degree of MMP inhibition by alcohols increased with chain length up to 4 methylene groups. Based on the molar concentration required to inhibit rhMMP-9 fifty percent, 2-hydroxyethylmethacrylate (HEMA), 3-hexanol, 3-heptanol and 1-octanol gave the strongest inhibition.
Significance
The results indicate that alcohols with 4 methylene groups inhibit MMPs more effectively than methanol or ethanol. MMP inhibition was inversely related to the Hoy's solubility parameter for hydrogen bonding forces of the alcohols (i.e. to their hydrophilicity).
Keywords: dentin matrix, MMPs, alcohol, HEMA, collagen
1. Introduction
Dentin matrix metalloproteinases (MMPs) are host-derived proteolytic enzymes bound to the matrix. The MMPs have been detected in hard and soft tissues during tooth development [1-3]. However, after development, the dentin matrix mineralizes, causing dentin collagen to become very stiff. All noncollagenous proteins bound to collagen, including MMPs, also become covered with apatitic nanocrystals making them immobile and nonfunctional. Mineralized dentin is stable structurally and functionally as long as it remains mineralized [4,5].
During adhesive bonding, dentin is acid-etched to remove the smear layer and to demineralize the relatively impermeable dentin matrix to a depth of 1-8 μm to expose the collagen fibrils of the matrix. These 100 nm diameter fibrils remain anchored to and continuous with the underlying mineralized dentin. During bonding, solvated liquid monomers flow around the demineralized collagen fibrils and envelop them with comonomers that are then polymerized. This mixture of resin and insoluble collagen fibrils is called the hybrid layer [6]. It is a type of in situ tissue engineering in that it completely transforms the physicochemical nature of the surface from a wet hydrophilic crystalline surface to a more hydrophobic polymer surface that allows adhesion to resin composites. Unfortunately, acid-etching also uncovers and activates matrix-bound MMPs [7-10]. These MMPs seem to remain active even after resin-infiltration [8,11-15]. As hydrolases, these enzymes add water across specific collagen peptide bonds, splitting peptide chains into fragments. If there is no water in the hybrid layer, the MMPs are inactive. For instance, incubation of resin-dentin bonds in oil instead of water allows those bonds to remain stable over time [4,5]. Resin-dentin bonds incubated in water show progressively lower bond strengths over 3-12 months [16-19]. Transmission electron microscopy of hybrid layers in specimens exhibiting progressive decreases in bond strength, reveal loss of cross-banded collagen fibrils in the hybrid layer [19-23]. We speculated that endogenous MMPs in hybrid layers are responsible for the degradation of bond strengths and that this might be prevented using chlorhexidine, an antimicrobial agent also known to inhibit MMP-2, 8 and 9 [24]. The success of 2% chlorhexidine at preserving hybrid layers in vivo was shown by Hebling et al.[20], Carrilho et al. [21], Brackett et al. [22,23] and Loguercio et al. [25]. They used 2% chlorhexidine digluconate in water. To avoid incorporating water into the hybrid layer, we screened alcoholic solutions of chlorhexidine diacetate for their ability to inhibit the endogenous MMPs of dentin matrix. When alcohol controls were run, we were surprised to find that alcohols inhibited the endogenous collagenolytic activity of dentin matrices (see results in this study).
To evaluate this phenomenon more thoroughly, a series of alcohols were used (methanol, ethanol, propanol, butanol, pentanol, hexanol, heptanol and octanol) to inhibit soluble rhMMP-9 and insoluble matrix-bound MMPs. The purpose of this study was to test the null hypotheses that there are no differences among various alcohols in their ability to inhibit MMPs, and that there are no differences in alcohol inhibition of MMPs regardless of their hydrophilicity.
2. Materials and methods
2.1 Experimental design
Two tiers of screening were used to evaluate the inhibitory activity of alcohols on MMPs. The first level of screening involved the use of a soluble recombinant human MMP-9 (rhMMP-9) in a colorimetric assay. The alcohols were diluted with distilled water to achieve concentrations of 0.17, 0.86, 1.71 and 4.28 mole/L if they were sufficiently soluble in water and did not cause precipitation of medium constituents. The second level of screening, used matrix-bound endogenous MMPs of completely demineralized dentin over a 30 day incubation. Both assays require the use of an incubation buffer containing calcium and zinc ions that are necessary to maintain enzyme structure and function. Preliminary experiments were done to determine the highest concentration of alcohols that could be achieved in the assay buffer. Some of the higher molecular weight alcohols were not completely soluble in the test buffers and could not be tested at all desired concentrations. Their highest soluble concentrations are listed in the tables that summarize the results. Because the second level of screening was labor-intensive and required 30 days, we only tested alcohols that reduced rhMMP-9 activity by over 50% and that were compatible with the calcium-zinc containing buffer. The alcohols that are listed are the highest concentrations that were completely soluble in the assay buffer along with the percent inhibition obtained at those concentrations. Lower concentrations of alcohols were then tested to permit calculation of the alcohol concentrations required to inhibit rhMMP-9 by 50%. This permitted comparison of the inhibitory potencies of each alcohol.
2.2 Use of soluble human recombinant MMP-9
This assay employed purified recombinant human MMP-9 (Cat#72009) and the Sensolyte Generic MMP colorimetric assay kit (Cat#72095) from AnaSpec, Inc. (San Jose, CA, USA) for screening anti-MMP activity of compounds of interest. The assay involves incubating a constant concentration of rhMMP-9 with a proprietary chromogenic substrate. The latter is a thiopeptolide that is cleaved by the MMPs and collagenases to release a sulfhydryl group. The sulfhydryl group reacts with 5,5’-dithiobis(2-nitrobenzoic acid) to produce the colored reaction product (2-nitro-5-thiobenzoic acid) which can be detected at 412 nm.
The thiopeptolide substrate solution was diluted to 0.2 mM with the supplied assay buffer in a 1:50 volume ratio. The 92-kDa rhMMP-9 was activated with 10 μg/mL of trypsin at 37°C for 2 h immediately before the experiment to generate its 68-kDa active form. The trypsin was then inactivated with trypsin inhibitor. The assay was performed in a 96-well plate using five replicate wells for each experimental and control variable. Each well in the experimental groups (i.e. the various alcohol concentrations) contained 2 μL of rhMMP-9 (19.6 ng/well), X μL of the potential MMP inhibitor and 50-(2 + x) μL of the thiopeptolide substrate solution. All wells also received 50 μL of substrate to give a total volume in each well of 100 μL..
The control groups consisted of: 1) a positive control containing rhMMP-9 only without the potential anti-MMP agent, 2) an inhibitor control containing rhMMP-9 and 10 μL of 20 μM GM6001, a known MMP inhibitor supplied in the assay kit, 3) a test compound control containing assay buffer and the potential anti-MMP agent, and 4) a substrate control containing assay buffer. Additional assay buffer was added to bring the total volume of all control wells to 50 μL prior to adding 50 μL of the thiopeptolide substrate solution to obtain the 100 μL volume. The alcohol inhibitors were preincubated with the enzyme for 30 min to prevent a burst of uninhibited enzyme activity that occurred if all of the reagents were mixed together.
The reagents were mixed completely by vibrating the plate gently for 30 sec and then read kinetically every 10 min for 60 min and absorbance was measured at 412 nm using a 96-well plate reader (Synergy HT, BioTek Instruments, Inc., Winooski, VT, USA).
Background absorbance was determined using the mean absorbance readings from the “substrate control” wells and subtracted from the readings of other wells containing the thiopeptolide substrate. The potencies of MMP-9 inhibition by the proprietary MMP inhibitor GM6001 (“inhibitor control”), and the alcohol inhibitors were expressed as percentages of the adjusted absorbance of the “positive control”, which was taken to be the maximum absorbance.
2.3 Preparation of demineralized dentin beams
Forty extracted human third molars were obtained with patient (18-22 yr old) informed consent under a protocol approved by the Human Assurance Committee of the Medical College of Georgia. Ninety percent of the teeth had completely formed roots. None of the teeth were carious. Half were impacted and half were erupted. The teeth were stored at 4°C in 0.9% NaCl supplemented with 0.02% sodium azide to prevent bacterial growth and used within three months after extraction. For each tooth, the enamel and superficial dentin were removed by horizontal sectioning 1 mm below the deepest central fissure using a diamond-coated copper disk (Isomet saw, Buehler Ltd., Lake Bluff, IL, USA) under water cooling. A 1 mm thick dentin disk consisting of mid-coronal dentin was prepared from each tooth. Two 5 × 2 × 1 mm dentin beams were prepared from the middle of each disk under water cooling to obtain eighty beams.
Prior to demineralization, a dimple was made on the occlusal side of each beam at the end to allow for repeated measurements to be performed on the same surface. The beams were tumbled at 3 rpm in 10% phosphoric acid for 6 h at 25 °C to completely demineralize the dentin. Absence of residual minerals was confirmed using digital radiography. The initial modulus of elasticity of each demineralized dentin beam was determined using three-point flexure (see below). The beams were distributed to seven experimental groups and one control group (N = 10) so that the mean initial elastic modulus of each group was similar in all groups.
2.4 Use of endogenous matrix-bound MMPs in beams of demineralized dentin
A recently developed assay [26] uses beams of human coronal dentin that are completely demineralized. Using a miniature 3-point flexure fixture, the stiffness of the beams is measured before and after demineralization. Mineralized dentin has a stiffness of about 17-18,000 MPa, while completely demineralized dentin has a stiffness of 2-3 MPa. The control beams were separately incubated in a simplified medium containing 2.5 mM calcium chloride, 0.05 mM ZnCl2, 5 mM HEPES buffer pH 7.4, 0.3 mM NaN3, and 150 mM NaCl, pH 7.4. Many high molecular weight alcohols caused precipitations of more complex media and could not be tested.
Experimental beams were incubated in the same medium that contained twice the concentration of its constituents as the control group to permit dilution of the buffer by potential inhibitors to the media. Additional water was then added to dilute the final concentration of the medium to that of the control group. All beams were separately incubated at 37°C in a shaking water bath (Precision Model 2873, Thermo Scientific, Marietta, OH, USA) in 1 mL of media in polypropylene tubes with a rubber “O” ring in the screw cap to prevent evaporation of water or inhibitors. The following variables were measured: Decreases in dentin beam stiffness were measured by 3-point flexure at time 0 and 4 weeks using a Vitrodyne universal tester with a 1 N load cell on supports separated by 2.5 mm at 10% strain while the beams were under water. Flexural modulus (E) was calculated as:
As the ASTM D790-03 [27] standard for measuring the stiffness of polymeric beams is done at low strains and requires that the beam thickness be no more than one-sixteenth the length of the supported beam, our conditions violate those criteria and hence only provide an appropriate stiffness value. However, the method is capable of accurately detecting differences in stiffness between groups.
Loss of beam stiffness over time was used as an indirect measure of the hydrolysis of matrix by endogenous MMPs. The loss of beam stiffness of experimental beams incubated with potential inhibitors was compared to that of controls. After measuring the initial modulus of elasticity, the beams were rinsed with water for 30 sec to remove all media salts. They were then placed in sealed containers of anhydrous calcium sulfate (Drierite, W.A. Hammond Drierite Company, Ltd., Xenia, OH, USA) overnight. The next day, the dry mass of each beam was measured using a microanalytical balance to the nearest 0.01 mg. Pilot experiments indicated that these procedures yielded constant dry masses. These dry masses were measured on each beam at time 0 and 30 days. Each beam was rehydrated in water for 1 hr before being placed in its incubating medium. Previous work showed that this was sufficient time for complete re-expansion of the dried beams [28]. Loss of dry mass over time indicates solubilization of matrix by endogenous MMP activity because effective MMP inhibitors prevent the loss of dry mass over time.
The third index of matrix degradation over time was obtained by measuring the amount of collagen peptide fragments that were solubilized over 30 days of incubation. That is, each beam was incubated in 1 mL of medium. Any collagen peptide fragments that accumulated in the medium over 30 days could be quantitated by removing 400 μL of the 1 mL and mixing it with an equal volume of concentrated HCl to yield a final acid concentration of 6 N HCl in glass ampoules (Wheaton, Millville, NJ, USA). These vials were automatically sealed using an Ampulmatic Ampule Sealer (Bioscience, Inc., Allentown, PA, USA). The contents were hydrolyzed to amino acids in an oil bath at 118°C for 18 hr. After cooling, the glass vials were opened via prescored lines and placed in large glass dessicators containing NaOH pellets to trap HCl vapor and anhydrous calcium sulfate to trap water vapor. After 3 days in the vacuum dessicators, the dry contents of the vials were analyzed for hydroxyproline (HYP) using the colorimetric assay of Jamall et al. [29]. After color development, the absorbance of all specimens and standards was measured at 558 nm in a 96-well plate reader.
Effective inhibitors lowered the concentration of hydroxyproline (HYP) in the medium hydrolysate relative to that of controls which contained no inhibitors.
2.5 MMP inhibitors
All alcohols were purchased from Sigma Chemicals (St. Louis, MO, USA) and were used as received. They included methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol, tertbutanol, 2-hydroxyethylmethacrylate (HEMA), 1-pentanol, hexanols, heptanols, octanols, 1,2-ethanediol, and 1,3-propanediol.
2.6 Statistics
Plots were made of the degree of inhibition of rhMMP-9 by the highest soluble alcohol concentrations to permit regression analysis to permit calculation of percent inhibition versus the highest soluble alcohol concentration. Those alcohols that inhibited rhMMP-9 at their highest solubility were then reanalyzed at lower concentrations (e.g. 1.71, 0.86 and 0.17 moles/L) to determine the dose-response relationship of that alcohol. Using those regression equations, we calculated the highest soluble molar concentration of alcohols required to inhibit MMPs by 50% and designated this as Inhibitory Concentration 50 (IC50). This mathematical construct allows one to compare the same inhibitory activities of inhibitors with variable solubilities. The measured changes in beam stiffness, loss of dry mass and solubilization of collagen peptides were not normally distributed. As the normality and homoscedasticity assumptions of the data appeared to be violated, the data were expressed as least square means and the common standard error of the least square means. Least square means are the expected values of group or subgroup means for a balanced design involving the group variables with all covariates at their common mean values. Pair-wise multiple comparisons were performed using the Tukey test. Statistical significance was set at α = 0.05.
3. Results
The results of the alcohol inhibitors on soluble rhMMP-9 are shown in Table 1. Methanol and ethanol showed relatively poor percent inhibition of rhMMP-9, compared to 1-propanol, 2-propanol, 1-butanol, 2-butanol or tert-butanol. The strong inhibitory activity of hydroxyethylmethacrylate (HEMA) was unexcepted. Higher molecular alcohols were less soluble and less inhibitory (Table 1). The diols were more inhibitory than the single alcohols; 1,2-ethandiol was more inhibitory than ethanol. To compare the inhibitory potential of alcohols, we compared their IC50 values. Lower IC50 values indicate higher inhibitory potency. Since the IC50 for 1-propanol was 2.7 moles/L compared to IC50 of 19 moles/L for methanol and ethanol, 1-propanol was far more inhibitory than either methanol or ethanol. The primary alcohol 1-propanol was more inhibitory than the secondary alcohol 2-propanol (Table 1). However, 2-butanol was more inhibitory than 1-butanol or tert-butanol. When compared to ethanol, HEMA inhibited MMP-9 nineteen times more than did the same concentration of ethanol (Table 1). As the molar concentration of 1-hexanol, and 3-octanol were increased, their inhibitory activity fell rapidly indicating a nonlinear dose-response. Thus, the IC50 values for these alcohols could not be calculated. As these alcohols also caused precipitation of buffer components when they were mixed with the demineralized dentin beam buffer medium, they could not be tested in that model system.
Table 1.
Inhibition of soluble rhMMP-9 by alcohols
| Chemical | Concentration | % Inhibition | IC50 (moles/L) |
|---|---|---|---|
| methanol | 4.28 M | 12.4 ± 1.57%A | 19.1 ± 2.86%a |
| ethanol | 4.28 M | 14.3 ± 2.06%A | 19.6 ± 0.36%a |
| 1-propanol | 4.28 M | 79.7 ± 0.18%F | 2.7 ± 0.13%d |
| 2-propanol | 4.28 M | 52.7 ± 0.18%D | 4.4 ± 0.13%e |
| 1-butanol | 1.71 M* | 56.1 ± 0.09%D | 1.6 ± 0.09%c |
| 2-butanol | 4.28 M | 78.7 ± 0.94%F | 0.5 ± 0.04%b |
| tert-butanol | 4.28 M | 91.1 ± 0.18%G | 2.7 ± 0.04%d |
| 1-pentanol | 0.86 M* | 28.5 ± 0.54%B | 1.7 ± 0.04%c |
| 2-HEMA | 4.28 M* | 99.4 ± 0.27%H | 0.1 ± 0.09%b |
| 1-hexanol | 0.17 M* | 28.3 ± 0.40%B | -- |
| 2-hexanol | 0.17 M* | 13.2 ± 0.67%A | 1.6 ± 0.09%c |
| 3-hexanol | 0.17 M* | 35.1 ± 1.16%E | 0.4 ± 0.04%b |
| 1-heptanol | -- | -- | -- |
| 2-heptanol | 0.17 M* | 13.7 ± 2.33%A | 3.0 ± 0.18%d |
| 3-heptanol | 0.17 M* | 17.1 ± 0.54%A | 0.5 ± 0.04%b |
| 1-octanol | 0.17 M* | 25.4 ± 1.21%B | 0.3 ± 0.22%b |
| 2-octanol | 0.17 M* | 21.5 ± 0.63%B,C | 2.7 ± 0.18%d |
| 3-octanol | 0.17 M* | 10.1 ± 1.79%A | -- |
| 4-octanol | 0.17 M* | 17.7 ± 0.27%A,C | 1.1 ± 0.04%c |
| 1,2-ethanediol | 4.28 M | 36.6 ± 0.18%E | 5.8 ± 0.36%e |
| 1,3-propanediol | 4.28 M | 84.4 ± 0.60%I | 1.1 ± 0.27%c |
Groups identified by different uppercase and lowercase superscript letters are significantly different (p±0.05). Values are mean ± SEM (n=5) as determined by least squares means method.
Higher molecular weight alcohols had lower solubilities than the lower molecular weight alcohols. The concentrations indicated are their highest soluble concentrations in the proprietary assay buffer. Alcohols highlighted in grey, inhibited rhMMP-9 by more than 50%. Their inhibitory activity was also assayed at lower concentrations of 1.71, 0.86 and 0.17 moles/L to obtain a dose-response relationship. That data is not shown but was used to obtain the IC50 values by plotting percent rhMMP-9 inhibition vs. alcohol concentrations.
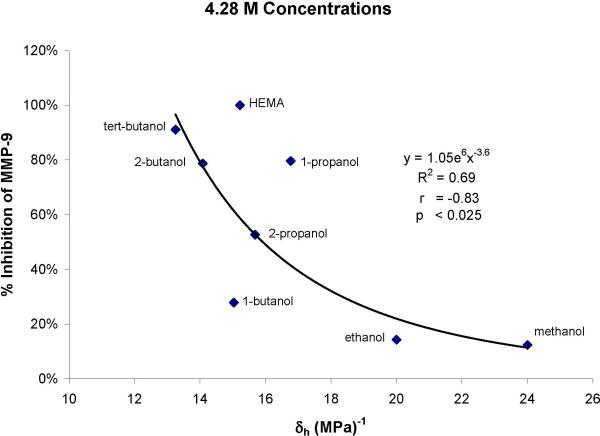
To determine the possible relationship between the hydrophilicity of the various alcohols and their ability to inhibit rhMMP-9, the Hoy's solubility parameters for the alcohols were calculated. These values are summarized in Table 2. Using the percent inhibition of rhMMP-9 by 4.28 moles/L of the alcohols, the correlation coefficients of these inhibitions were plotted against the Hoy's solubility parameters for hydrogen bonding forces (δh). Figure 2 shows that the relationship was inverse. That is, the more hydrophilic the alcohol, the lower was its ability to inhibit rhMMP-9 (Fig. 2). The correlation coefficient was -0.83 (p<0.025).
Table 2.
Hoy's solubility parameters for alcohols
| Alcohols | δ d | δ p | δ h | δ t |
|---|---|---|---|---|
| Methanol | 11.6 | 13.0 | 24.0 | 29.7 |
| Ethanol | 12.6 | 11.2 | 20.0 | 26.1 |
| 1-propanol | 13.3 | 10.3 | 16.8 | 23.8 |
| 2-propanol | 13.0 | 10.4 | 15.7 | 22.8 |
| 1,3-propanediol | 12.8 | 14.2 | 28.0 | 33.9 |
| 1-butanol | 13.6 | 9.5 | 15.0 | 22.4 |
| 2-butanol | 13.4 | 9.5 | 14.1 | 21.1 |
| Tert-butanol | 13.3 | 9.7 | 13.3 | 21.1 |
| 2-hydroxymethacrylate | 13.3 | 12.3 | 8.8 | 22.6 |
Values calculated with software from Computer Chemistry Consultancy (www.compchemconsul.com). They are listed in Figs. 2A and 2B.
Figure 2.
Plot of percent inhibition of rhMMP-9 by alcohols versus their Hoy's solubility of parameter for hydrogen bonding cohesives forces. The negative correlation coefficient shows that the more hydrophilic the alcohols (i.e. the higher their δh values), the less they inhibit the enzyme. All of these alcohols were soluble in the assay buffer at concentrations of 4.28 moles/L.
The alcohols at concentrations that inhibited rhMMP-9 by 50% or more were mixed with a buffer containing 2.5 mM Ca, 0.05 mM Zn and 5 mM HEPES buffer (pH 7.4) to determine if their continued presence in the incubation media for 30 days would inhibit the activity of matrix-bound endogenous rhMMPs of dentin.
When the inhibitory activity of the alcohols that inhibited rhMMP-9 50% or more (and did not cause precipitation of media) was tested against the endogenous MMPs in beams of demineralized dentin, we obtained a wide spectrum of inhibition that is summarized in Table 3.
Table 3.
Effects of alcohols on the loss of dry mass and dissolution of hydroxyproline from demineralized dentin beams
| Alcohols | Loss of dry mass (%) | % Inhibition | Solubilization of HYP* | % Inhibition |
|---|---|---|---|---|
| 4.28 M 1-propanol | -4.16 ± 0.21% (10)A | 80.2 | 2.39 ± 0.20 μg/mga | 78.2 |
| 2.30 M 2-butanol | -4.15 ± 0.11% (10)A | 80.2 | 1.88 ± 0.25 μg/mgb | 82.9 |
| 4.28 M tert-butanol | -3.52 ± 0.28% (10)A | 83.3 | 1.56 ± 0.18 μg/mgb | 85.8 |
| 4.28 M HEMA | -4.03 ± 0.09% (10)A | 80.8 | 1.58 ± 0.05 μg/mgb | 85.6 |
| 1.71 M HEMA* | -4.39 ± 0.12% (10)A | 79.1 | 1.80 ± 0.14 μg/mgb | 83.6 |
| 0.86 M HEMA* | -4.06 ± 0.10% (10)A | 80.7 | 1.30 ± 0.07 μg/mgb | 88.1 |
| 4.28 M 1,3-propanediol | -3.22 ± 0.31% (10)A | 84.7 | 0.14 ± 0.01 μg/mgc | 98.7 |
| buffer control | -21.02 ± 0.71% (10)B | 0 | 10.97 ± 0.58 μg/mgd | 0 |
Abbreviations: HYP = hydroxyproline; HEMA = 2-hydroxyethylmethacrylate. Values are means ± SEM (n = 10). Groups identified by different upper and lower case letters are significantly different (p<0.05). The power of the performed test with α=0.05 was 1.0. Although 2-butanol was soluble at 4.28 moles/L in the proprietary assay buffer used in the rhMMP-9 assay, when 2-butanol was mixed with the simplified buffer used in the beam assay, the highest soluble concentration was 2.3 moles/L. Although 4.28 and 1.71 moles/L 1-butanol inhibited rhMMP-9 more than 50%, these concentrations were not soluble when we mixed them with the simplified medium used in the dentin beam experiments. Therefore, we were unable to test 1-butanol in the beam studies.
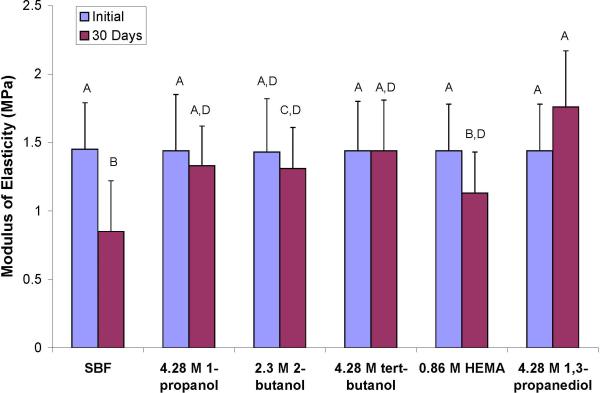
When the effects of most inhibitory alcohols to rhMMP-9 were evaluated on the stiffness of demineralized dentin beams (Fig. 1), the mean stiffness values of the five experimental groups revealed no significant change over 30 days, except for the controls incubated in buffer without any inhibitors. In that group, stiffness fell about 41% over 30 days (Fig. 1). As the stiffness of the beams in the experimental (i.e. inhibited) groups were significantly less than the control groups, the percent inhibition of the proteolytic activity of the beams was calculated by subtracting the inhibited values after 30 days from the initial values, divided by the initial stiffness value for that group times 100. This was also done for the loss of dry mass (Table 3) and solubilization of collagen peptides (Table 3).
Figure 1.
Changes in modulus of elasticity of demineralized dentin beams before and after 30 days of incubation in simulated body fluid (SBF) alone or containing polyol inhibitors.
When loss of dry mass among the groups was compared (Table 3), the demineralized dentin beams incubated in the control medium without any inhibitors lost about 21% of their dry mass over 30 days. The results showed that 4.28 M 1-propanol, 2.3 M 2-butanol, 4.28 M tertiary butanol, 4.28 M HEMA, 1.71 M HEMA, and 0.86 M HEMA all inhibited the proteolytic activity of bound proteases, because the percent loss of dry mass was reduced from 21% in controls to between 3-4%. This represented a 79-85% reduction in proteolytic activity.
When the incubation media were hydrolyzed to amino acids and analyzed for hydroxyproline, similar results were obtained (Table 3), which validated the use of dry mass loss as an index of matrix degradation. The percent inhibition of total endogenous proteases by alcohols was calculated by subtracting the alcohol-inhibited values from the uninhibited controls (i.e. buffer control), divided by the control values times 100. These values varied from 78.2% for 4.28 M 1-propanol to 98.7% for 4.28 M 1,3-propanediol.
4. Discussion
The results of this study show that many alcohols can inhibit both soluble rhMMPs and matrix-bound MMPs. This result requires rejection of the test null hypothesis that there are no differences among various alcohols in their ability to inhibit MMPs.
The molecular structures of the catalytic domains of MMP-2 and 9 are similar [30]. The domain consists of a 5-stranded β-pleated sheet, with three α-helices and connective loops. The protease domain contains one catalytic zinc, one structural zinc and three calcium ions. The substrate-binding cleft is formed by strand IV, helix B, and the extended loop region after helix B. Three histidines coordinate the active site zinc. The fourth ligand of the catalytic zinc is a water molecule [31] that actually hydrolyzes the specific peptide bond.
We speculate that alcohols in general that can inhibit MMPs, by forming a coordinate covalence bond between the catalytic zinc and the oxygen atom of the hydroxyl group of the alcohol. However, hydroxyl containing compounds might inhibit MMPs via an allosteric region [32]. We included HEMA in the series of alcohols because it is an ethanol-ester of methacrylic acid. Our results showing rhMMP-9 inhibition by HEMA confirm the report of Carlvaho et al. [33], but using entirely different methods and MMP-9 instead of MMP-2. HEMA contains both hydrophobic and hydrophilic ends. The actual binding of these alcohols to the MMPs seems to involve hydrophobic forces since the inhibitory activity of alcohols is inversely related to their Hoy's solubility parameters for hydrogen bonding forces. This is likely to be the mechanism of MMP inhibition at low to moderate alcohol concentrations. High alcohol concentrations may denature enzymes [34] by removing water from the enzymes and causing their denaturation.
The presence of bound water prevents interpeptide hydrogen bonding within the peptide chains of enzymes. Removal of that water allows rapid interpeptide hydrogen bonding that can disrupt the tertiary structure that is necessary for functional activity. Some proteins can “renature” when the alcohol is removed and the proteins are rehydrated. Other proteins are unable to reconstitute their original tertiary structure. The current study was not designed to test those issues. Future experiments need to be done to test the long-term effects of alcohols on soluble vs. insoluble matrix-bound MMPs.
Figure 2 shows the percent inhibition of rhMMP-9 by alcohols that inhibited more than 50% at 4.28 moles/L concentrations, plotted against the Hoy's solubility parameters for hydrogen bonding forces calculated by addition of the contributions of their molecular group constants using software from Computer Chemistry Consultancy (www.compchemconsul.com). In Fig. 2, note that the most hydrophilic alcohols, ethanol and methanol [δh of 20 and 24 (MPa)-1], gave the least inhibition, while the least hydrophilic alcohols, tertiary-butanol and HEMA, gave the highest inhibition of rhMMP-9. We speculate that alcohols with some degree of hydrophobicity may be required for optimum inhibition of MMPs. They may interact with the “methionine-turn” of the active site where methionine serves as a hydrophobic base beneath the three zinc ligands and preserves the active site of the enzyme [35].
Ethanol is a common solvent in dental adhesives, as is HEMA. However, HEMA can copolymerize with other adhesive monomers and may remain bound to MMPs for years. Dentsply/Caulk's XP Bond dental adhesive uses tertiary-butanol to solvate its comonomers. While tert-butanol inhibited rhMMP-9 almost as well as HEMA, tert-butanol is likely to be lost from the blend through evaporation during bonding, and via water sorption-displacement after bonding.
Although many bonding systems include relatively high concentrations (ca. 35-50 wt%) of HEMA, the resin-dentin bonds produced by these adhesives slowly degrade over time [16,17,19,21], contrary to the expectation that HEMA-containing adhesives should inhibit MMPs. Such results suggest that once polymerized, HEMA is no longer an effective MMP inhibitor.
HEMA has been shown to have a relatively high affinity for demineralized dentin [35] presumably due to weak force interactions with collagen (i.e. hydrogen bonding of the primary alcohol group with collagen and hydrophobic interactions of the methacrylate vinyl group with hydrophobic collagen residues). The inhibitory interaction of HEMA with MMPs may require rotational freedom to obtain optimal binding. When HEMA polymerizes to polyHEMA, it loses its rotational freedom and it undergoes volumetric shrinkage that may displace the hydroxyl group from MMPs just enough to cause a loss of inhibition. The inhibitory activity of the primary alcohol group on either the active or allosteric sites of MMPs may require more rotational freedom than is possible in polyHEMA.
Carvalho et al. [32] used MMP-2 activity in PAGE gels to assay the percent inhibition of the enzyme by increasing concentrations of monomeric HEMA. In their model, MMP-2 was inhibited about 80% by 5 wt% HEMA [32]. When we made multiple dilutions of HEMA, we found that 2.2 vol% HEMA inhibited rhMMP-9 by 98% (data not shown). Thus, HEMA may be a general nonspecific inhibitor of MMPs.
The results of this study indicate that alcohols in general and HEMA, in particular, can inhibit MMPs in vitro. Appropriate controls must be performed to correct for the potential inhibition of MMP activity by alcohols when evaluating the potential of bonding ingredients to inhibit MMPs.
In the current study, the total endogenous MMP activity of demineralized beams was assayed indirectly over 30 days in beams of demineralized dentin by measuring their loss of dry mass and solubilization of collagen peptides [26,38]. The results of evaluation of the ability of alcohols to inhibit matrix-bound MMPs (Table 2) indicate that five of the alcohols that inhibited MMP-9 more than 50%, also inhibited matrix-bound MMPs more than 50%. However, plots of concentrations of alcohols necessary to inhibit MMP-9 versus total endogenous matrix-bound MMP activity in demineralized dentin beams showed poor correlations (R2 = <0.5, not shown). Collagen may compete with MMPs for alcohol hydrogen bonding. Thus, the dose-response relationship of soluble rhMMP-9 may differ from that of matrix-bound MMPs.
Acknowledgments
This study was supported in part by grants R01 DE015306-06 (PI. David H. Pashley), R21 DE019213-01 (PI. Franklin R. Tay) from the National Institute of Dental and Craniofacial Research and by Grant # 8126472 (PI. Arzu Tezvergil-Mutluay) from Academy of Finland. The authors are grateful to Mrs. Michelle Barnes for her secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heikinheimo K, Sato T. Expression of basement membrane type IV collagen and type IV collagnases (MMP-2 and MMP-9) in human fetal teeth. J Dent Res. 1995;74:1226–1234. doi: 10.1177/00220345950740051301. [DOI] [PubMed] [Google Scholar]
- 2.Tjäderhane L, Palosaari H, Sulkala M, Wahlgren J, Salo T. The expression of matrix metalloproteinases (MMPs) in human odontoblasts. In: Ishikawa T, Takahashi K, Maeda T, Suda H, Shimono M, Inoue T, editors. Proceedings of the International Conference of the Dental/Pulp Complex. Quintessence Publishing Co., Ltd.; Tokyo: 2002. pp. 45–51. [Google Scholar]
- 3.Bourd-Boittin K, Fridman R, Fanchon S, Septier D, Goldberg M, Menashi S. Matrix Metalloproteinase inhibition impairs the processing, formation and mineralization of dental tissues during mouse molar development. Exp Cell Res. 2005;304:493–505. doi: 10.1016/j.yexcr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Carrilho MRO, Tay FR, Pashley DH, Tjäderhane L, Carvalho RM. Mechanical stability of resin-dentin bond components. Dent Mater. 2005a;21:232–241. doi: 10.1016/j.dental.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Carrilho MRO, Carvalho RM, Tay FR, Yiu C, Pashley DH. Durability of resin-dentin bonds related to water and oil storage. Am J Dent. 2005b;18:315–319. [PubMed] [Google Scholar]
- 6.Nakabayashi N, Pashley DH. Hybridization of Dental Hard Tissues. Quintessence Publishing; Chicago: 1998. [Google Scholar]
- 7.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 8.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities of phosphoric acid-etched dentin by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–4476. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Nishitani Y, Yoshiyama M, Wadgaonkar B, Elrod D, Breschi L, Mannello F, Carvalho RM, Tjäderhane L, Tay FR, Pashley DH. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114:160–166. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 10.Tay FR, Pashley DH, Loushine RJ, Weller RN, Monticelli F, Osorio R. Self-etching adhesives increase collagenolytic activity in radicular dentin. J Endod. 2006;32:862–868. doi: 10.1016/j.joen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Reis AF, Giannini M, Pereira PNR. Long-term TEM analysis of the nanoleakage patterns in resin-dentin interfaces produced by different bonding strategies. Dent Mater. 2007;23:1164–1172. doi: 10.1016/j.dental.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Abdalla AI, Feilzer AJ. Four-year water degradation of a total-etch and two-self-etching adhesives bonded to dentin. J Dent. 2008;36:611–617. doi: 10.1016/j.jdent.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Reis A, Grande RHM, Oliveira GMS, Lopes GC, Loguercia AD. A 2-year evaluation of moisture on microtensile bond strength and nanoleakage. Dent Mater. 2007;23:862–870. doi: 10.1016/j.dental.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Jr, Tay FR, Dorigo EDS, Pashley DH. Chlorexidine stabilizes the adhesive interface over time: a 2-year in vitro study. Dent Mater. 2010;26:320–325. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res. 2009;88:1101–1106. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 16.Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, Pashley DH. Durability of resin-dentin bonds. J Adhes Dent. 1999;1:211–218. [PubMed] [Google Scholar]
- 17.Okuda M, Pereira PNR, Nakajima M, Tagami J. Relationship between nanoleakage and long-term durability of dentin bonds. Oper Dent. 2001;26:482–490. [PubMed] [Google Scholar]
- 18.DeMunck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, Lambrechts P, Vanherle G. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003;82:136–140. doi: 10.1177/154405910308200212. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong SR, Vagas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, Qian F. Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Oper Dent. 2004;29(6):705–712. [PubMed] [Google Scholar]
- 20.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;86:529–533. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 21.Carrilho MR, Geraldeli S, Tay FR, de Goes MF, Carvalho RM, Tjäderhane L, Pashley DH. In vivo preservation of hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 22.Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Oper Dent. 2007;32(2):107–111. doi: 10.2341/06-55. [DOI] [PubMed] [Google Scholar]
- 23.Brackett MG, Tay FR, Brackett WW, Dib A, Dipp FA, Mai S, Pashley DH. In vivo chlorhexidine stabilization of hybrid layers of an acetone-based dentin adhesive. Oper Dent. 2009;34(4):381–385. doi: 10.2341/08-103. [DOI] [PubMed] [Google Scholar]
- 24.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8 and 9 by chlorhexidine. Clin Diag Lab Immunol. 1999;6:437–439. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loguercio AD, Stanislawczuk R, Polli LG, Costa JA, Michel MD, Reis A. Influence of chlorhexidine digluconate concentration and application time on resin-dentin bond strength durability. Eur J Oral Sci. 2009;117:587–596. doi: 10.1111/j.1600-0722.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 26.Carrilho MRO, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin stiffness associated with solubilization of collagen. J Biomater Mater Res Appl Biomater Part B. 2009;90B:373–380. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ASTM D790-03, Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials.
- 28.Agee KA, Becker TD, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Net expansion of dried, demineralized dentin matrix produced by monomer/alcohol saturation and solvent evaporation. J Biomater Res. 2006;79A:349–358. doi: 10.1002/jbm.a.30752. [DOI] [PubMed] [Google Scholar]
- 29.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 30.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Structure, function, biochemistry. Cir Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 31.Farkas E, Katz Y, Bhusare S, Reich R, Röschenthaler G-V, Königsmann M, Breuer E. Carbamoylphosphonate-based matrix metalloproteinase inhibitor metal complexes: solution studies and stability constants. Toward a zinc-selective binding group. J Biol Inorg Chem. 2004;9:307–315. doi: 10.1007/s00775-004-0524-5. [DOI] [PubMed] [Google Scholar]
- 32.Sela-Passwell N, Rosenblum G, Shoham T, Sagi I. Structural and functional bases for allosteric control of MMP activities: Can it pave the path for selective inhibition? Biochimica et Biophysica Acta. 2010;1803:29–38. doi: 10.1016/j.bbamcr.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho RV, Ogliari FA, de Sousa AP, Silva AF, Petzhold CL, Line SRP, Piva E, Etges A. 2-hydroxyethyl methacrylate as an inhibitor of matrix metalloproteinase-2. Eur J Oral Sci. 2009;117:64–67. doi: 10.1111/j.1600-0722.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert P, McBain AJ. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Crit Microbiol Rev. 2003;16:189–208. doi: 10.1128/CMR.16.2.189-208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharrock P, Grégoire G. HEMA reactivity with demineralized dentin. J Dent. 2010;38:331–335. doi: 10.1016/j.jdent.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Stöcker WL, Grams F, Bauman U, Reinmer P, Gomis-Ruth FX, McKay DB, Bode W. The metzincinstopological and sequential relations between the astacins, adamalysins, serralysins and matrixins (collagenases) define a superfamily of zinc peptidases. Proc Sci. 1995;4:823–840. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishitani Y, Yoshiyama M, Hosaka K, Tagami J, Donnelly A, Carrilho M, Tay FR, Pashley DH. Use of Hoy's solubility parameters to predict water sorption/solubility of experimental primers and adhesives. Eur J Oral Sci. 2007;115:81–86. doi: 10.1111/j.1600-0722.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 38.Tezvergil-Mutluay A, Agee K, Hoshika T, Carrilho M, Breschi L, Tjäderhane L, Nishitani Y, Carvalho RM, Looney S, Tay FR, Pashley DH. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin. Dent Mater. 2010 doi: 10.1016/j.dental.2010.07.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bode W, Gomis-Ruth FX, Stöckler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the “metzincins”. FEBS Lett. 1993;331:134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]