Abstract
Objectives
Complications after AAA repair resulting in re-intervention increase mortality risk, but have not been well-studied. Mortality after re-intervention is termed failure to rescue and may reflect differences related to quality management of the complication. This study describes the relationship between reoperation and mortality, and examines the effect of physician specialty on re-intervention rates and failure to rescue after AAA repair.
Methods
Data was extracted for 2616 patients who underwent intact AAA repair in 2005–2006 from a standard 5% random sample of all Medicare beneficiaries. Patient demographics, co-morbidities, hospital characteristics, repair type and specialty of operating surgeon were collected. Primary outcomes were 30-day reoperation and 30-day mortality. Logistic regression analysis identified characteristics predicting reoperation.
Results
A total of 156 reoperations were required in 142 (4.2%) patients. Early mortality was far more likely for patients requiring re-intervention than for those who did not (22.5% vs.1.5%; p<.0001). Of patients requiring reoperation, those requiring two or more interventions had an even higher mortality (54% vs. 20%; p=.0007). Despite equivalent reoperation rates between specialties (vascular surgeons 5.2%, others 5.6%, p=.67), the mortality after reoperation was nearly half for vascular surgeons compared with other specialties (16.2% vs. 32.3%; p=.04). The most common reason for reoperation was arterial complications (35.8%) accounting for the largest difference in mortality between vascular surgeons (30.7%) and other specialties (52.0%).
Conclusions
Postoperative complications requiring reoperation dramatically increase mortality after AAA repair. Despite similar complication rates, vascular surgeons showed lower mortality following reoperation.
INTRODUCTION
The relationship between major complications requiring re-operation and mortality after abdominal aortic aneurysm (AAA) repair has not been reported in the endovascular era. While many factors such as physician specialty, endovascular approach, and treatment in high-volume centers (1–6) have been associated with improved mortality after AAA repair, it is unclear if improved mortality rates reflect reduced complication rates or more effective management of these complications. The concept of “failure to rescue,” defined as early mortality after complications, is gaining importance in the literature as a marker of surgical quality. The impact of physician factors on failure to rescue for vascular procedures has not been investigated. This study describes the relationship between major complications requiring re-operation and mortality, and examines the effect of physician specialty on failure to rescue after open aneurysm repair (OAR) and endovascular aneurysm repair (EVAR).
METHODS
After obtaining institutional approval, a 5% random sample of Medicare beneficiaries was obtained from the Centers for Medicare & Medicaid Services (CMS) through the Chronic Condition Data Warehouse (CCW) for the years 2004 – 2006. Inpatient files, outpatient files, and denominator files were available. Each record contained demographics, physician and hospital identifiers, and diagnosis and procedure codes as classified by the International Classification of Diseases, 9th Clinical Modification (ICD-9, CM).
Data was extracted for all patients undergoing surgical treatment for intact AAA (ICD 9-CM codes 441.4, 441.9) between 1/1/05 and 12/31/06 who had been continuously enrolled in Medicare Part A and Part B for at least 365 days prior to the date of the index procedure to allow full characterization of baseline co-morbidities. Only patients with a procedural code for OAR or EVAR during the index hospitalization (codes 38.34, 38.44, 38.64, 39.52, 39.71) were included in the cohort. Ruptured aneurysms (441.3) were excluded, as were aneurysm diagnoses without an associated treatment code. Also excluded were those with incomplete enrollment in Medicare Part A and B for 12 months preceding surgery, enrollment in a Medicare HMO, or having railroad benefits at any time from entry into Medicare through 12/31/2006.
Patient demographic data collected included age, sex, race, and eligibility for Medicaid during the study period. Patient risk adjustment was estimated using the Centers for Medicare and Medicaid Services - Hierarchical Condition Categories (HCC) scale. This measure uses inpatient and ambulatory claims in the baseline year (2004) to calculate predicted expenditures, and is a validated reflection of the presence and complexity of medical comorbidities (7). A score of 1 represents the predicted cost of an average Medicare patient. Treatment variables included type of AAA repair, yearly hospital AAA repair volume, and operating physician specialty. Hospital volume was estimated by averaging the yearly number of procedures from 2004–2006 and multiplying by 20 to correct for the 5% sample, and was categorized into low volume (<50 cases per year) or high volume (≥50 cases per year). Physician specialty was determined from the Carrier files by unique physician identifier number (UPIN) for the index procedure.
Complications were defined as unplanned re-interventions and were categorized using a method modified by Morris et al (8). Procedure codes were counted as a complication if they occurred during the index hospitalization to ensure that subsequent planned procedures were not incorrectly counted as complications. For any given day, secondary procedure codes were classified into one of the eleven categories of complications. These categories were assigned in a hierarchical order: 1) retained foreign body, 2) post operative shock or hemorrhage, 3) repair of organ injury, 4) arterial complications, 5) abdominal infection, 6) colon resection, 7) re-operative laparotomy, 8) bowel obstruction, 9) respiratory complication, 10)amputation, and 11) wound complication. Each complication type was counted only once to avoid counting the same complication that may have required multiple procedures. For example, a patient may have required multiple drainage procedures for a post-operative abdominal infection, but infection itself would only be counted as one complication. In addition, multiple re-intervention types occurring on a particular date were counted as a single complication based upon the hierarchy above. For instance, claims with procedure codes for wound complication and abdominal infection on the same day would be recorded as an abdominal infection. By this means complications with multiple procedural codes were not over-counted.
When other procedures occurred on the same day as the index procedure, they were counted as complications if the secondary procedure was not ordinarily anticipated for the index procedure. For example, a code for splenectomy on the same day as the index surgery would be presumed to be a direct complication of the surgery. However, a patient could have a code for femoral-femoral bypass which may be considered part of the index procedure if it occurred on the same day as the index procedure. If it occurred on a subsequent day it was considered a complication of surgery.
Analyzed outcomes included 30-day mortality (death within 30 days of the index procedure), and failure to rescue, defined as death after a post-operative complication requiring a secondary procedure. Variables were compared with Student’s t test, chi square, or Fisher’s exact test t-test when indicated. Data was considered statistically significant with a P-value ≤ .05. Multivariable logistic regression models were then used to determine independent correlates for treatment and outcome variables. Statistical analysis was performed using SAS version 8.0 (Cary, NC).
RESULTS
During the study period 2616 patients from the sample underwent intact aneurysm repair. The cohort was 75.7% male with a mean age was 75.8 +/− 6.5. Nearly all (93.9%) patients were Caucasian, and 8.5% of patients received Medicaid during at least a portion of the study period.
The index procedure was performed by vascular surgeons in 49.8% of cases. Index procedures were also performed by other specialists including general surgeons (20.3%), cardiothoracic surgeons (19.3%), and physicians with other or undefined specialties (10.6%). There were no significant differences in gender, co-morbidity or poverty between those treated by vascular surgeons and those treated by other surgeons (TABLE 1).
TABLE 1.
Demographics
| Factor | Total (2616) |
Vascular surgeons (1301) |
Other surgeons (1535) |
p value | |
|---|---|---|---|---|---|
| Age: | 45–64 | 3.4 | 3.2 | 3.6 | 0.33 |
| 65–74 | 39.0 | 38.1 | 39.9 | ||
| 75–84 | 48.7 | 48.3 | 49.1 | ||
| ≥85 | 8.9 | 10.5 | 7.3 | ||
| % male gender | 75.7 | 76.1 | 75.3 | 0.42 | |
| %Medicaid | 8.5 | 7.6 | 9.6 | 0.20 | |
| HCC score* | 0.97 | 0.99 | 0.95 | 0.15 | |
| Race: | Caucasian | 94.1 | 94.5 | 93.7 | 0.63 |
| African-American | 3.7 | 3.2 | 4.2 | ||
| Other | 2.2 | 2.3 | 2.1 | ||
Values represent % unless specified otherwise
Risk adjustment score of co-morbidity; 1.0 = average adjusted co-morbidity
On multivariate analysis (TABLE 2), only age greater than 85 was an independent predictor for receiving treatment by a vascular surgeon (OR 1.44, 95% CI 1.07 – 1.93; p < 0.02). Vascular surgeons were significantly more likely to perform AAA repair at high-volume centers (53% vs. 35%; p<0.0001). Vascular surgeons performed EVAR with similar frequency compared to other surgeons (61% vs. 58%, p= 0.08).
TABLE 2.
Multivariate logistic regression for factors predicting treatment by vascular surgeons
| Factor | O.R. | 95% CI | p value | |
|---|---|---|---|---|
| Age | 45–64 | .97 | .63 – 1.52 | .90 |
| 65–74 | Referent | |||
| 75–84 | 1.02 | .86 – 1.20 | .86 | |
| ≥85 | 1.44 | 1.07 – 1.93 | .015 | |
| % male gender | 1.01 | .84 – 1.21 | .91 | |
| Race: | Caucasian | Referent | ||
| African-American | .75 | .49 – 1.14 | .18 | |
| Other | 1.65 | .59 – 4.61 | .34 | |
| %Medicaid | .83 | .62 – 1.12 | .22 | |
| HCC score | 1.06 | .95 – 1.19 | .29 | |
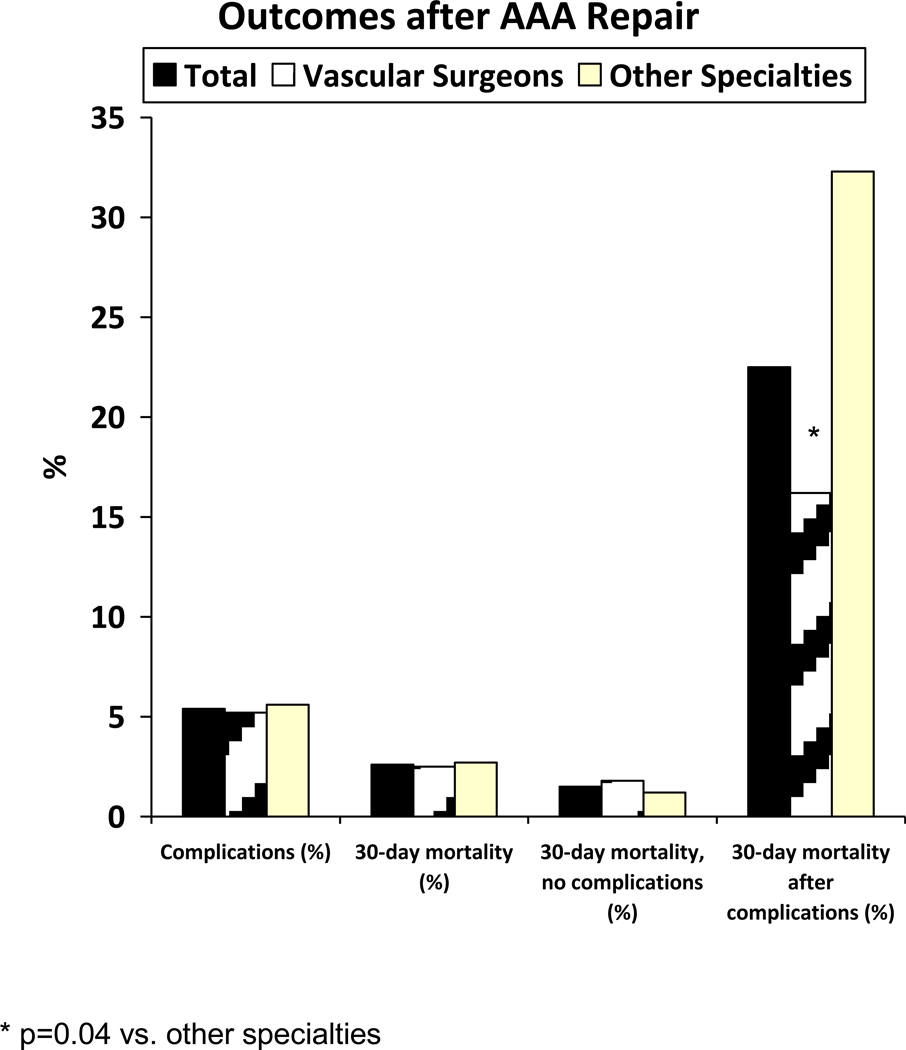
Overall mortality for the entire cohort was 2.6%. Mortality did not differ for patients cared for by vascular surgeons compared to others (2.5% vs. 2.7%, p=0.65; FIGURE 1). Mortality was equivalent by specialty for both OAR and EVAR (TABLE 3).
Figure 1.
Complications and Mortality after AAA repairs
TABLE 3.
Outcomes by specialty and by operation type
| Total (n=2616) |
Vascular surgeons (n=1301) |
Other surgeons (n=1315) |
p-value* | |
|---|---|---|---|---|
| All re-interventions (%) | ||||
| OAR | 9.5 | 9.0 | 10.0 | 0.58 |
| EVAR | 2.7 | 2.9 | 2.5 | 0.64 |
| Arterial re-interventions (%) | ||||
| OAR | 2.5 | 2.0 | 2.9 | 0.43 |
| EVAR | 1.6 | 1.9 | 1.3 | 0.42 |
| 30-day mortality (%) | ||||
| OAR | 5.6 | 3.8 | 5.4 | 0.20 |
| EVAR | 1.2 | 1.6 | 0.8 | 0.13 |
| 30-day mortality after re-interventions (%) | ||||
| OAR | 23.0 | 15.6 | 33.3 | 0.06 |
| EVAR | 21.4 | 17.4 | 26.3 | 0.48 |
OAR: Open aneurysm repair
EVAR: Endovascular aneurysm repair
p-value compares vascular surgeons to other surgeons
Mortality for patients requiring re-intervention was significantly higher than for those not requiring re-intervention (22.5% vs. 1.5%; p < 0.0001, figure 1). Mortality was also directly related to the number of reoperations required. Undergoing two or more re-interventions was associated with a significantly higher mortality compared to one required re-intervention (54% vs. 20%; p=0.0007, data not shown). Mortality was 50% after 2 or 3 re-interventions, and 100% after 4 re-interventions.
Post-operative complications requiring 152 re-interventions occurred in 142 (5.4%) patients. Re-interventions were more likely after OAR (9.5% vs. 2.7%, p<0.0001). The majority of complications were arterial (38%), respiratory (34%), or re-operative laparotomies (8.3%). There was no difference in reoperation rates between vascular surgeons and other surgeons (5.2% vs. 5.6%, p=0.65). Re-intervention rates after OAR and EVAR were similar for both surgeon types (TABLE 3).
Despite equivalent need for reoperation among specialties, the mortality after reoperation was nearly half for patients treated by vascular surgeons compared with other specialties (16.2% vs. 32.3%; p=.04, TABLE 3). By repair type, mortality after reoperation was lower for vascular surgeons for OAR (15.6% vs. 33.3%, p=0.06) and EVAR (17.4% vs. 26.3%, p=0.48, TABLE 3) but did not reach statistical significance.
Age greater than 75 was the only other variable associated with mortality after reoperation (29.2% vs. 11.3%, p = 0.02). Both vascular surgeon specialty (OR 0.33, 95% CI 0.1 – 0.9, p=0.03) and age > 75 (OR 3.77, 95%CI 1.18–12.03, p=0.03) remained independent predictors of re-operative mortality by multivariate analysis (TABLE 4). Mortality after complications was not associated with gender, race, co-morbidity, poverty, hospital procedural volume or type of repair. Likewise, by multivariate analysis the need for re-intervention was predicted by female gender and open repair. Patient age, co-morbidity, poverty, or hospital volume were not associated with the likelihood of re-interventions (TABLE 4).
TABLE 4.
Multivariate analysis of factors predicting mortality after re-intervention and re-intervention after AAA repair.
| Factor | Mortality after re-intervention following AAA repair |
Re-intervention following AAA repair | |||||
|---|---|---|---|---|---|---|---|
| O.R. | 95% CI | p value | O.R. | 95% CI | p value | ||
| Age | 45–64 | 14.76 | 0.43 – 499.4 | .13 | 0.92 | 0.32 – 2.71 | 0.88 |
| 65–74 | Referent | ||||||
| 75–84 | 3.77 | 1.18 – 12.03 | .03 | 1.36 | 0.93 – 1.98 | 0.11 | |
| ≤85 | 65.9 | 5.5 – 801.8 | .001 | 0.69 | 0.30 – 1.57 | 0.38 | |
| % male gender | 1.07 | 0.41 – 2.81 | .89 | 0.61 | 0.42 – 0.88 | 0.008 | |
| Race: | Caucasian | Referent | |||||
| African-American | 2.24 | .29 | 2.66 | 1.39 – 5.10 | 0.003 | ||
| Asian | n/a | n/a | 1.15 | 0.14 – 9.22 | 0.90 | ||
| Hispanic | n/a | n/a | 0.84 | 0.10 – 6.95 | 0.88 | ||
| %Medicaid | 0.12 | 0.01 – 1.26 | .08 | 1.00 | 0.54 – 1.83 | 0.99 | |
| HCC score | 1.31 | 0.74 – 2.35 | .36 | 1.22 | 0.96 – 1.53 | 0.10 | |
| Hosp volume >= 50/yr | 0.67 | 0.23 – 1.94 | .46 | 0.92 | 0.64 – 1.33 | 0.67 | |
| Open vs. EVAR repair | 1.62 | 0.52 – 5.00 | .40 | 3.64 | 2.48 – 5.33 | <0.0001 | |
| Surgeon type: | |||||||
| Vascular surgeon | Referent | ||||||
| Other surgeon | 3.00 | 1.09 – 8.34 | .03 | 0.99 | 0.63 – 1.57 | 0.46 | |
O.R.: odds ratio; CI: confidence interval; n/a: no data available
Mortality rates after reoperation varied depending on the complication type, physician specialty, and patient age. With regard to complication type, mortality was highest after reoperation for bowel obstruction (80%), colon resection (67%), or amputation (50%; TABLE 5). Conversely, no deaths occurred after reoperation for wound complications, or abdominal infection. Differences in mortality based on surgeon specialty and complication type were largely accounted for by significantly lower mortality rates after reoperations for arterial complications for vascular surgeons compared to other specialties (30.8% vs. 52.0%, p=0.04, TABLE 5). This difference was most pronounced after OAR (10.0% vs. 68.8%, p=.005). Lower mortality after arterial re-interventions for EVAR by vascular surgeons did not reach statistical significance when compared to other surgeons (13.3% vs. 30.0%, p=0.36).
TABLE 5.
Mortality after re-interventions by re-intervention and surgeon type
| Failure to Rescue | ||||
|---|---|---|---|---|
| Reason for reoperation (n) |
Overall mortality (%) |
Vascular surgeon mortality (%) |
Other surgeon mortality (%) |
p value |
| ALL (156) | 22.5 | 16.2 | 32.3 | 0.04 |
| Arterial (56) | 39.3 | 30.8 | 52.0 | 0.01 |
| Respiratory (53) | 26.4 | 25.0 | 27.6 | 1.0 |
| Unspecified laparotomy (13) | 23.1 | 25.0 | 40.0 | 1.0 |
| Hemorrhage (7) | 14.3 | 14.3 | 0.0 | 0.52 |
| Abdominal infection (6) | 0.0 | 0.0 | 0.0 | 1.0 |
| Wound (6) | 0.0 | 0.0 | 0.0 | 1.0 |
| Bowel obstruction (5) | 80.0 | 50.0 | 100.0 | 0.40 |
| Repair of organ injury (4) | 0.0 | 0.0 | 0.0 | 1.0 |
| Colon resection (3) | 66.7 | 50.0 | 100.0 | 1.0 |
| Amputation (2) | 0.0 | 0.0 | 50.0 | 1.0 |
| Retained foreign body (1) | 50.0 | 0.0 | 0.0 | 1.0 |
DISCUSSION
Prior studies have emphasized that factors mediating failure to rescue have not yet been elucidated (9). This study helps to fill that knowledge gap by identifying a previously unrecognized relationship between physician level factors and failure to rescue after complications for AAA repair. Mortality differences in this cohort may be explained by differences in outcomes after arterial complications, suggesting that differences in rates of rescue after complications may be attributable in part to differences in specialty training.
Our findings confirm those of others that mortality is increased after complications. Dimick et al (3) demonstrated using Nationwide Inpatient Sample (NIS) that mortality for AAA repair was 10.4% for those with complications compared to 2.9% for those without. Morris et al (8) demonstrated that mortality increased with the number of complications requiring reoperation. The relationship between post-operative complications and mortality is complex. Increased mortality after complications can be due to either increased rates of complications or to alternatively poor management of the complications.
While some researchers have demonstrated an association between complication rates and mortality (10, 11), others have demonstrated that patient demographics are more important determinants of complication risk. Once the complication occurs, other characteristics are associated with the mortality. Thus, variation in mortality may be ascribed to differing complication rates or failure to rescue (9, 12, 13). Our data would support that both processes may be vital in defining quality. For example, mortality was associated with the number of re-interventions, and different physician groups had similar complication rates but variable failure to rescue after complications.
Similarly, Silber et al (14) demonstrated a significant association between failure to rescue and mortality even for procedures with low expected mortality. While surgeons have advocated that using mortality for operations with low risk is not a sensitive measure of quality, our study and others offer that failure to rescue may in fact be an ideal measure of quality for procedures with low expected mortality. Measuring failure to rescue may uncover differences in quality that are otherwise hidden by low overall mortality or complication rates. Although overall mortality rates and rates of arterial complications were no different between vascular surgeons and others, the likelihood of failure to rescue was one-third for vascular surgeons after adjusting for other variables.
Not surprisingly, we found that rescue after reoperation was in part dependent on the reason for reoperation. For example, re-operation for arterial complications was associated with a nearly 40% mortality, whereas re-operation for wound infection or dehiscence carried no additional mortality. These findings are concordant with those of Morris et al who found similar variability after colorectal surgery (8). In addition, in our study patients with an arterial complication experienced significantly lower mortality if managed by a vascular surgeon (30.8% vs. 52.0%, p=0.01, TABLE 3). It is possible that specialized training allows vascular surgeons to have more familiarity with complex arterial disease, more rapidly identify arterial complications, and have a lower threshold for re-intervention before the consequences of the complication become irreversible. . This suggests the importance of having appropriate expertise in managing specific complications.
Other hospital level factors that have been proposed to impact failure to rescue include nurse to patient ratios, hospital size, availability of intensivists and other specialists, high-technology equipment and training, and effective communication between medical personnel responsible for patient care (9). Such resources would aid in rapidly diagnosing post-operative complications and then appropriately and optimally managing them. Examining these factors may be the subject of further research.
This study has certain limitations. Clinical data such as aneurysm extent, aneurysm diameter, and severity of co-morbid conditions may be important in determining treatment and outcomes but is not obtainable from administrative claims. The absence of clinical correlative data precludes comparing outcomes of all patients with aneurysms, as for example we could not correlate the risk of complications or mortality to aneurysm diameter or other clinical parameters. In addition, errors in coding may lead to inaccuracies. Miscoding of diagnosis or procedure codes may result in under- or over-reporting the condition. This is less likely for serious conditions requiring major procedures such as AAA (15). Although inaccuracy in coding is a well-described phenomenon, previous studies have concluded that these data are accurate when patients are undergoing surgical procedures, procedures with risk, procedures requiring anesthesia, or procedures requiring specialized training (15–17). Failure to code complications may result from variations in physician charting or lack of precise definitions for ICD-9-CM codes. However, specifically focusing on complications that require secondary procedures and by identifying them with both ICD-9-CM and CPT codes reduces the underestimation of complications significantly (8). These procedures are generally performed to treat complications after the index operation (18), and may therefore be a more valid measure of surgical technical care (8) and more appropriate for measuring surgical quality than medical or non-operative complications.
The greatest utility of identifying and measuring operative complications and variations in failure to rescue may be in surgical outcomes research and health policy (8,9). Accurately measuring complications and failure to rescue with administrative data may provide a valuable tool for assessing surgical quality, especially for low-risk procedures. While national policy efforts have been proposed to improve quality by minimizing complications, an equally important quality measure is the care patients receive once a complication has occurred. Identifying organizational, resource, and provider factors that impact the timely recognition and subsequent management of such complications is of critical importance in measuring and optimizing surgical quality.
In summary, complications requiring re-intervention dramatically increase mortality after AAA repair. Despite similar complication rates, vascular surgeons demonstrated lower mortality following re-intervention. Further work is necessary to more clearly define the role of specialty vascular care for improving rescue rates.
Appendix - Discussion
Paper #17
WVS discussion
Failure to Rescue: Physician Specialty and Mortality after Reoperation for Abdominal Aortic Aneurysm (AAA) Repair
Matthew W. Mell, MD, Amy Kind, MD, Christie M. Bartels, MD and Maureen A. Smith, MD, MPH, PhD
Division of Vascular Surgery, Stanford University, Stanford, CA and University of Wisconsin, Madison, WI
Discussant: James W. Holcroft, MD
The authors found that the 30-day mortality rate after elective abdominal aneurysmectomy in Medicare beneficiaries was 2.5% when the operation was done by a vascular surgeon and 2.7% when done by a general or cardiac surgeon. If, however, the patient had a post operative complication that required reoperation, the death rate skyrocketed, and a large gap in the mortality rates emerged when comparing the vascular surgeons with the cardiac and general surgeons.
This study has implications. I have two questions.
Can you glean from the data why some patients went to vascular surgeons for their operations while others went to general or cardiac surgeons? In particular, were patients who lived in rural areas more likely to have their operations done by a local general or cardiac surgeon? Did it seem to be mostly a matter of convenience for the patients and families, not wanting to drive long distances for the preoperative evaluation and the post-operative follow up, not to mention the need for the families to find lodging near the hospital in the big city during the stay for the operation itself?
And second, what should we as a profession do with this information? The death rate in the patients with complications was 16%, when the operation was done by a vascular surgeon, which is uncomfortably high, but the death rate with the nonvascular surgeons, of 32%, is flat out distressing. We frequently talk about number needed to benefit when talking about a potentially beneficial intervention. In this case, one could talk about the number killed with an intervention. For every 6.25 patients with a post operative complication requiring a re-operation, one will die if the surgeon taking care of the patient is a general or cardiac surgeon, compared with a vascular surgeon. That should make any patient think twice. After all, one doesn’t know going into an operation if a complication is going to develop.
I’m sure that there are many general and cardiac surgeons who do a good job with aortic surgery. And I assume that there must be some vascular surgeons who do a poor job. Thus it wouldn’t seem fair to single out all general and cardiac surgeons and make it difficult for them to do these procedures.
On the other hand, I don’t think that we can stand idly by and do nothing. One way to get at this problem might be to mandate participation in the National Surgery Quality Improvement Program if a hospital is going to be re-imbursed for aortic surgery. We should all be participating anyway, and it wouldn’t be asking too much to set the bar a little higher when dealing with an operation that has the potential for having such disastrous results. The information from the NSQIP findings would allow a hospital to deal with problems, if they were present, and it would make the process fair. No one would be shut out of doing a procedure that he or she did well; and no one would be given carte blanch approval to do these procedures without scrutiny of his or her results.
In general, I don’t like having the government impose standards on physicians. Better than having others do it, we, as members of the profession, could take the initiative. In either case, I don’t think that these findings can be ignored.
Thank you Dr. Holcroft for your comments. Our data demonstrated that patients greater than age 85 were 55% more likely to be treated by vascular surgeons. Patient co-morbidity was not a factor. The impact of rural residence is the subject of another manuscript. To summarize, 15% of the cohort resided in rural areas and 15% resided in small towns. Regardless of residence, 93.9% of repairs were performed in urban centers. Although type of residence had no impact on the liklihood of being treated by a vascular surgeon (rural 48% vs. urban 50%; p=.82), rural patients were more likely to be treated in high-volume centers (rural 52% vs. urban 42%, p<.001). These results would suggest that for complex conditions such as abdominal aortic aneurysms, patients are willing to travel to receive quality care. Clinical factors such as the severity of co-morbid conditions or anatomic information were not available for analysis from this administrative dataset.
With regard to your second question, it remains important to have salient quality measures for aneurysm repair as new technology alters the skill sets required to perform a safe procedure. Setting standards becomes appropriate only after acceptable metrics have been defined. Recent improvements in mortality and complication rates make these measurements more difficult to use as benchmarks after AAA repair since many procedures would need to be performed before accurately measuring differences between hospitals or physicians. Our study adds to the body of research that failure to rescue after complications is an important quality measure. Differences in outcomes after AAA were explained by not the frequency but by the management of complications, most specifically vascular complications. Improved rescue after arterial complications highlights the importance of specialty vascular training when treating vascular conditions with potential vascular complications, and suggests that available vascular expertise is an important metric in defining quality AAA care.
References
- 1.Hallin A, Bergqvist D, Holmberg L. Literature review of surgical management of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2001;22:197–205. doi: 10.1053/ejvs.2001.1422. [DOI] [PubMed] [Google Scholar]
- 2.Heller J, Weinberg A, Arons R, et al. Two decades of abdominal aortic aneurysm repair: Have we made any progress? J Vasc Surg. 2000;32:1091–1098. doi: 10.1067/mva.2000.111691. [DOI] [PubMed] [Google Scholar]
- 3.Dimick JB, Pronovost PJ, Cowan JA, Lipsett PA, Stanley JC, Upchurch GR. Variation in postoperative complication rates after high-risk surgery in the United States. Surgery. 2003;134:534–541. doi: 10.1016/s0039-6060(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 5.Dimick JB, Upchurch GR. Endovascular technology, hospital volume, and mortality with abdominal aortic aneurysm surgery. J Vasc Surg. 2008;47:1150–1154. doi: 10.1016/j.jvs.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 6.Brooke BS, Perler BA, Dominici F, et al. Reduction of in-hospital mortality among California hospitals meeting Leapfrog evidence-based standards for abdominal aortic aneurysm repair. J Vasc Surg. 2008;47:1155–1164. doi: 10.1016/j.jvs.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 8.Morris AM, Baldwin LM, Matthews B, Dominitz J, Barlow WE, Dobie SA, et al. Reopoeration as a quality indicator in colorectal surgery: A population-based analysis. Ann Surg. 2007;245:73–79. doi: 10.1097/01.sla.0000231797.37743.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, Failure to Rescue, and mortality with major inpatient surgery in Medicare patients. Ann Surg. 2009;250:1029–1034. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- 10.Silber JH, Rosenbaum PR, Trudeau ME, et al. Changes in prognosis after the first postoperative complication. Med Care. 2005;43:122–131. doi: 10.1097/00005650-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Houghton A. Variation in outcome of surgical procedures. Br J Surg. 1994;81:653–660. doi: 10.1002/bjs.1800810508. [DOI] [PubMed] [Google Scholar]
- 12.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 13.Friese CR, Lake ET, Aiken LH, et al. Hospital nurse practice environments and outcomes for surgical oncology patients. Health Serv Res. 2008;43:1145–1163. doi: 10.1111/j.1475-6773.2007.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silber JH, Williams SV, Krakauer H, et al. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30:615–629. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Wilchesky M, Tamblyn R, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidem. 2004;57:131–141. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 16.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Medical Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 18.Donabedian A. The end results of health care: Ernest Codman's contribution to quality assessment and beyond. Milbank Q. 1989;67:233–256. [PubMed] [Google Scholar]