Abstract
Background & Aims
The cingulate cortex (CC) has been reported to be involved in processing pain of esophageal origin. However, little is known about molecular changes and cortical activation that arise from early-life, esophageal acid reflux. Excitatory neurotransmission via activation of the N-methyl-D-aspartate (NMDA) receptor and its interaction with post-synaptic density protein-95 (PSD-95) at the synapse appears to mediate neuronal development and plasticity. We investigated the effect of early-life esophageal acid exposure on NMDA receptor subunits and PSD-95 expression in the developing CC.
Methods
We assessed NMDA receptor subunits and PSD-95 protein expression in rostral CC (rCC) tissues of rats exposed to esophageal acid or saline (control), either during post-natal days 7–14 (P7–P14) and/or acutely, at adult stage (P60), using immunoblot and immunoprecipitation analyses.
Results
Compared with controls, acid exposure from P7 to P14 significantly increased expression of NR1, NR2A, and PSD-95, measured 6 weeks after exposure. However, acute exposure at P60 caused a transient increase in expression of NMDA receptor subunits. These molecular changes were more robust in animals exposed to acid neonatally and rechallenged, acutely, at P60. Esophageal acid exposure induced calcium calmodulin kinase II-mediated phosphorylation of the subunit NR2B at Ser1303.
Conclusions
Esophageal acid exposure during early stages of life has long-term effects, because of phosphorylation of the NMDA receptor and overexpression in the rCC. This molecular alteration in the rCC might mediate sensitization of patients with acid-induced esophageal disorders.
Keywords: brain, developmental neuroscience, pain processing, CamKII
Introduction
The early neonatal period is a critical time for the development of nociceptive neural pathways, and a single episode of inflammation during this period may be sufficient to alter the development and physiology of the maturing brain 1, 2. Recent animal studies indicate that the brainstem pain modulatory pathway manifests only facilitation up to postnatal day 21 (P21) and the inhibitory pathway develops after postnatal day 28 (P28) 3. This early life facilitatory process may contribute to activity-dependent plastic changes in the central processing following neonatal pain experience 4, 5. Visceral or somatic noxious stimuli in early stage of life also result in chronic hyperalgesia in rats as observed in maternal separation-induced hypersensitivity 6–9. The involvement of cingulate and insular cortices in the processing of pain of esophageal origin have been reported in recent studies 10–12. However, no information is available regarding the molecular changes and cortical activation due to early life esophageal sensitization. Knowledge of molecular changes involved in cortical reorganization is essential for better understanding of esophageal pain mechanism.
Among various mechanisms of neuronal transmission, excitatory neurotransmission via NMDA receptors plays a crucial role in neuronal development and plasticity, as well as variety of other brain functions ranging from memory formation to chronic pain 13–15. Moreover, NMDA receptor mediated enhancement in the synaptic transmission of anterior cingulate cortex (ACC) neurons has been reported to contribute towards allodynia and hyperalgesia in rats with visceral hypersensitivity16. Previous studies also indicate that NMDA receptors activation and phosphorylation are mediated via the interaction between C-terminal ends of NR2 subunits and PDZ domains of PSD-95; an abundant scaffold protein at the postsynaptic sites 17, 18.
The overall objective of the present study was to test the hypothesis that esophageal acid exposure in early life results in long-term modulation of cingulate cortex neuroplasticity involved in chronic hypersensitivity in the adulthood. To test this hypothesis, we determined and compared the expressions of NMDA receptor subunits, PSD-95 and NR2B phosphorylation profile in the rCC under following test conditions- i) chronic acid exposure in neonates (P7–P14) with or without acid rechallenge in the adulthood and ii) acute and chronic acid exposure in the adult rats (P40–P60).
Methods
Experimental procedures
A total of 67 male Sprague-Dawley rats (Harlan, Indianapolis IN, USA) were used for this study. The Institutional Animal care and Use Committee (IACUC) of the Medical College of Wisconsin approved all experimental procedure in accordance to the guidelines of the International Association of Study of Pain.
Acid infusion procedure in adult rats
Adult rats (P60) were anesthetized with sodium pentobarbital (40mg/kg, i.p.) and a 15cm long acid infusion catheter (PE-10) was inserted into the esophagus through an incision made 2cm below the UES. The tip of the catheter was positioned near the mid-thoracic area and the catheter was tied near the incision to prevent backflow of the acid into the pharynx. A second catheter (drainage catheter, 4cm length, PE-160) was placed 1cm rostral to the incision with the tip directed towards the pharynx. This catheter was used to remove accumulated saliva to prevent aspiration.
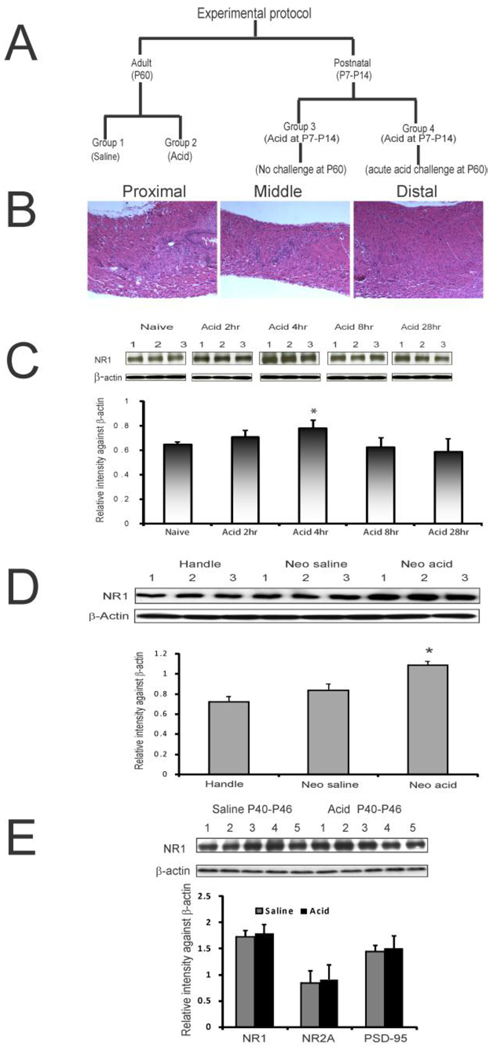
For acute treatment at postnatal day P60, rats received 2 ml of either saline or 0.1N HCl for 20 min and the brains were removed as described in the following section. In our initial study to determine the optimum time point for significant changes in the expression of NMDA receptor subunits, different groups of animals were treated with either acid or saline and rCCs were removed at 2, 4, 8, and 28 hours after the end of infusion and the expression of NMDA receptor subunit NR1 was determined by western blot analysis. Based on the observed expression at different time intervals (fig 1C), tissue procurement for all subsequent studies was done at four hours after esophageal treatment.
Figure 1.
A: illustrates esophageal acid exposure protocols for four different groups of animals selected for the study. B: histological examination of the esophageal tissue at P28 after chronic acid exposure during P7–P14. H&E staining reveals no inflammation of the tissues. C: NR1 expression profile in rCCs harvested at different time intervals following esophageal acid exposure at P60. Blots show NR1 and β-actin expression in naïve and acid-treated rats (n=3/group). The bar graph represents relative intensity against β-actin. The asterisk indicates significant difference from naïve controls (p<0.05). D: NR1 expression in rCCs at P60 following neonatal perturbation through P7-P14. The top blots show NR1 and β-actin expression in handled, esophageal saline- and acid-treated groups, respectively. The bar graph shows relative intensity against β-actin. The asterisk indicates significant difference between acid-treated group compared to handled and saline-treated controls (p<0.05). E: NR1, NR2A and PSD-95 expressions in rCCs from rats at P60 following chronic esophageal acid or saline exposure during P40–P46. Top blots show NR1 and β-actin expressions in acid- and saline-treated rats (n=5/group). Bar graphs represent relative intensity against β-actin.
For chronic acid treatment in adult rats (P40–P46) animals were anesthetized in similar fashion as in acute treatment. However, no surgical manipulation (i.e., esophageal ligation) was performed, since these rats were kept alive until P60. Acid or saline was infused intra-esophageally (0.5ml over 5 min) for seven consecutive days and rats were sacrificed at P60.
Acid infusion procedure in neonates (P7–P14)
The rat pups at P7 were removed from the mother and held by hand. An infusion tubing (PE-10) was inserted orally just beyond the pharyngo-esophageal junction. The tubing was attached to a microliter syringe and 20µl of fluid was infused over one minute period. The control pups received saline in similar fashion. The pups were then returned to their mother. The procedure was repeated for 7 days and rCCs from these rats were collected at P60.
To examine whether neonatal (P7–P14) chronic acid exposure produces any esophageal tissues inflammation, a set of rats were sacrificed at P28 and the entire length of esophagus was removed for histological evaluation.
For a detail evaluation of molecular changes in rCC, we selected four different groups as described in figure 1A. The groups are: acute saline P60 (group 1), acute acid P60 (group 2), neonatal chronic acid P7–P14 (group 3) and neonatal chronic acid P7–P14 plus acute acid rechallenge at P60 (group 4). In addition, to confirm the long-term effect of chronic esophageal acid exposure in neonates, we performed another set of experiments in adult rats (P60) that received either acid or saline treatment during P40–P46.
Tissue harvest
In rats, the cingulate cortex is rostro-caudally divided as perigenual anterior cingulate (pACC), midcingulate (MCC), and retrospenial (RSC) cortex. The MCC is again divided as anterior (aMCC) and posterior (pMCC) segments. The RSC in rats is equivalent to posterior cingulate cortex in humans. The rostral cingulate cortex (rCC) in our study included pACC and aMCC covering areas 24, 25, 32, 24’, 25’ and 32’.
The rat brain was removed and placed in Zivic slicing block (model BSRAS 002-2). The slicing slots of the block were 2mm apart. Two slices (2mm thickness) were cut bilaterally from the midline from either side of the hemisphere. The sections were placed on its side on cold plate and subcortical tissues along the corpus callosum were removed. The entire cingulate cortex was cut into half through the MCC. According to rat stereotaxic coordinates the rCC area that we have obtained is comparable to + 2 to 3.8mm from bregma, 0 to 2mm from midline and 1.8–2.5mm dorso-ventral.
Western blot analysis of NMDA receptor subunits (NR1, NR2A and NR2B) and PSD-95 expression in rCC
Crude extracts from various rat tissues were prepared by powderizing the tissues in liquid nitrogen and homogenization in ice cold hypotonic lysis buffer and solubilized as previously described 19. Various antibodies used for western blot analysis were (mouse anti-NMDAR1, 1:1000, BD Biosciences, San Jose, CA), NR2B (rabbit anti-NR2B 1:2000; Alomone Labs, Israel), NR2A (Rabbit anti-NR2A, 1:1000; Cell Signaling, Boston, MA) and anti-PSD-95 (1:2500, Cell Signaling). Serine phosphorylation pattern of NR2B subunit at Ser1303 was examined using anti-SerP1303 NR2B (1:1000; Cell Signaling). Tyrosine phosphorylation pattern of NR2B subunit was evaluated using anti-TyrP1336 NR2B (1:1000, Millipore, Billerica, MA), anti-TyrP1472 NR2B (1:500, EMD Chemicals Inc. Gibbstown, NJ) and anti-TryP1252 NR2B (Cell Signaling 1:500). The intensity of protein expression for experimental and housekeeping gene (mouse anti-β-actin, Sigma) for individual tissue sample was measured by densitometric scanning using Alpha Image software program (Cell Biosciences Inc., Santa Clara, CA)
Microinjection of calcium calmodulin kinase (CaMKII) inhibitor into rCC
A guide cannula (Plastics One Inc., VA, USA) was implanted at CC rostrally (reading: 2.5mm from bregma point, 0.8mm lateral to midline and 2.2mm ventral to the brain surface) for the microinjection of either CaMKII inhibitor (KN-93) or the vehicle (DMSO) before esophageal acid infusion on P60. KN-93 was dissolved in 10% DMSO at a concentration of 0.5mg/ml and 5µl was microinjected into the experimental group. For the vehicle control group, animals received 5µl of 10% DMSO in saline. After 30 mins of the microinjection, animals from both groups received acute esophageal acid exposure for 20 mins at 0.1ml/min and cortical tissues were collected after 4 hrs of acid exposure. The protein extracts were then subjected to western blot analysis using anti-SerP1303NR2B antibody as described above.
Enrichment of synaptic and extrasynaptic membranes from cortical extracts
To examine the distribution pattern of NMDA receptor subunits and PSD-95 proteins in synaptic and extrasynaptic membranes in naïve animals, we carried out a subcellular fractionation to isolate crude membrane preparation 20, 21. The Triton X-100 soluble proteins were defined as extrasynaptic fraction and the insoluble pellet proteins as PSD-associated or synaptic fraction based on differences in their solubility in nonionic detergent Triton X-100. The Triton X-100 insoluble proteins were solubilized in 1% SDS and expression profile of NMDA receptor subunits and PSD-95 in the synaptic and extrasynaptic membrane fractions (20µg each sample) were analyzed in western blots using antibodies against PSD-95, NR1, NR2A, NR2B and SerP1303 NR2B.
Immunoprecipitation of PSD-95 linked NMDA receptor subunits from tissue extract
Cortical tissue extracts (250µg) from both experimental (acute acid-treated on P60) and control rats (acute saline-treated on P60) were mixed with 5µl of polyclonal rabbit anti-PSD-95 antibody (Cell Signaling) overnight and then with protein A-Agarose (Sigma). SDS sample buffer was added to elute proteins from protein A beads. Thereafter, eluents were used for western blot analysis. Because of the differences in sizes of NR2B and NR1 subunits, the same blot was probed first with anti-SerP1303 NR2B antibody and thereafter, reprobed with anti-NR1 antibody.
Statistical Analysis
Results are represented as mean ± SD. Statistical analysis was performed using one-way ANOVA to calculate the significance of differences of NMDA receptor subunits and PSD-95 expression among different groups with post hoc Tukey’s multiple comparison test run on Sigma GraphPad prism 5 software (GraphPad Software Inc. LA Jolla, CA). Student’s t-test was used for the comparison between the two groups. Probability value of p<0.05 was considered as significant.
Results
Histological examinations did not reveal any sign of tissue inflammation in rats that receive chronic acid exposure during neonatal stage (P7–P14) when tested at P28 (fig 1B).
To determine the time-point for tissue harvesting after acute acid treatment at P60, the expression of NR1 subunit was examined as described in figure 1C. The highest expression of NR1 subunit was observed in rats after 4 hrs of acid exposure and the difference was statistically significant compared to the expression in naïve rats (fig 1C, p<0.05 vs naive). Saline-treated groups failed to show significant difference in NR1 expression (data not shown). Based on this finding, we selected 4 hrs after acid treatment as the time point for the tissue harvesting. The effect of neonatal treatment and handling at P7–P14 on NR1 expression at P60 is shown in figure 1D. No significant difference in NR1 expression was observed between saline and handle groups. However, P7–P14 acid exposure group exhibited significant increase in NR1 expression at P60 compared to other two groups (p<0.05). The chronic acid exposure in adult (P40–P46) rats failed to show significant differences in NR1, NR2A and PSD-95 protein expressions compared to chronic saline-treated rats (p=0.55, 0.70 and 0.6 for NR1, NR2A and PSD-95, respectively vs saline-treated, fig 1E).
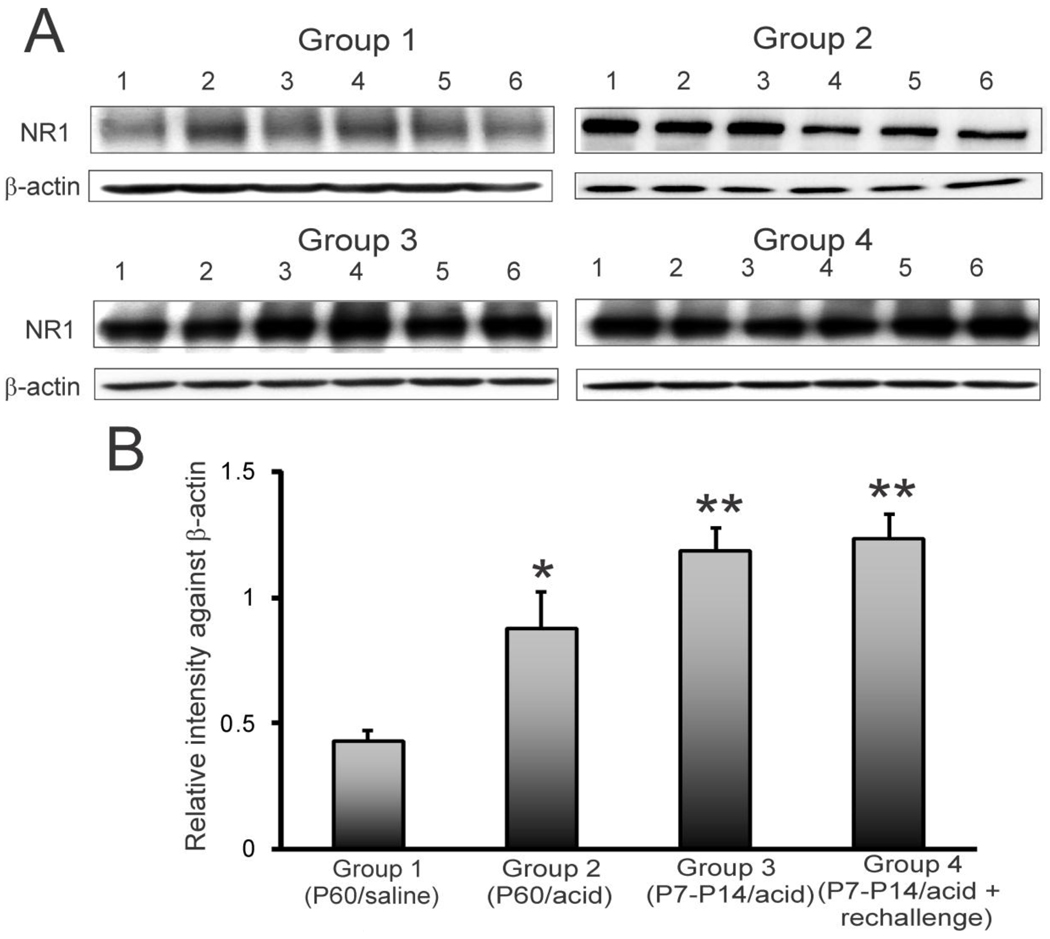
NR1 subunit expression in individual animals in groups 1 through 4 is shown in figure 2A. The densitometric scanning of relative intensity of staining against β-actin indicated a significant increase in NR1 subunit protein expression in group 2, 3 and 4 compared to control group 1 (fig. 2B, *p<0.05, **p<0.001).
Figure 2.
NR1 expression pattern in rCCs at P60 following esophageal acid exposure at different time points of development. The treatment strategies were the same as indicated in fig. 1A. A: blots show NR1 and β-actin expression in individual animals (n=6/group) for 4 different groups. B: bar graphs represent relative intensity of staining against β-actin. Asterisks indicate significant difference compared to group 1 (*p<0.05,**p<0.001).
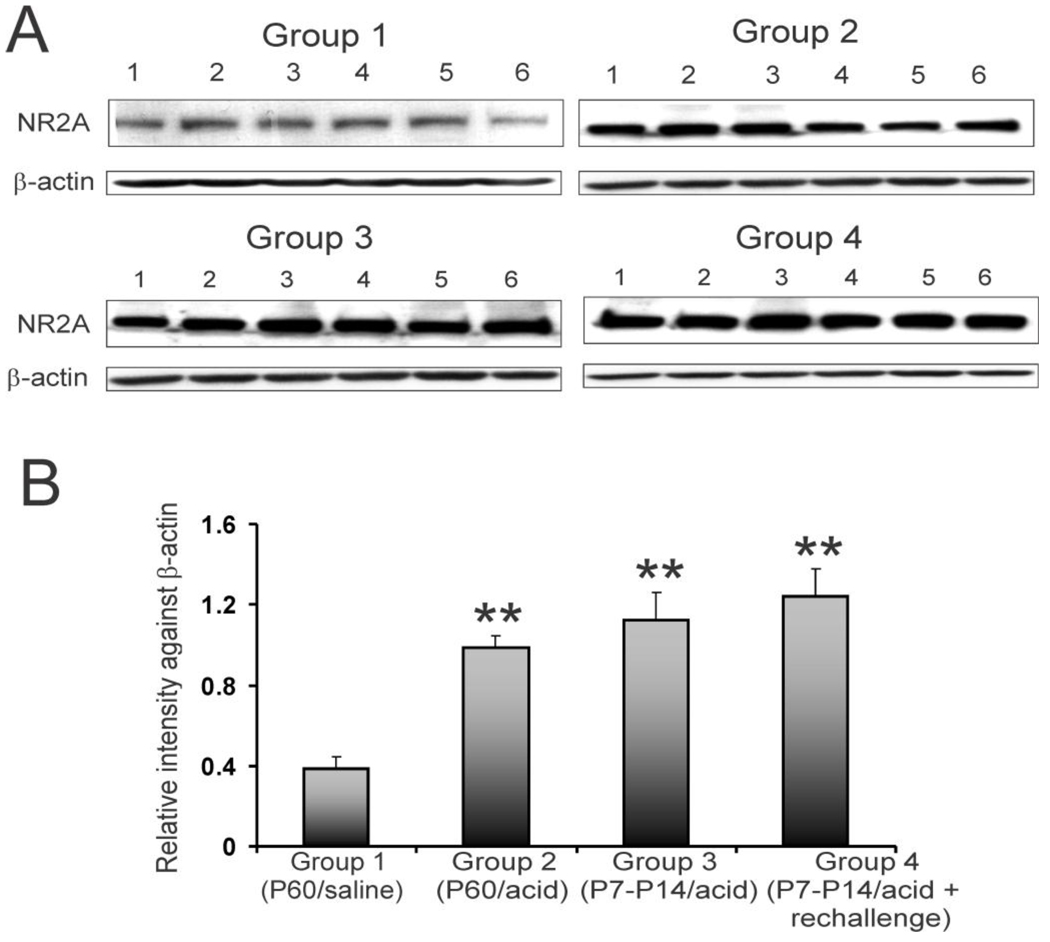
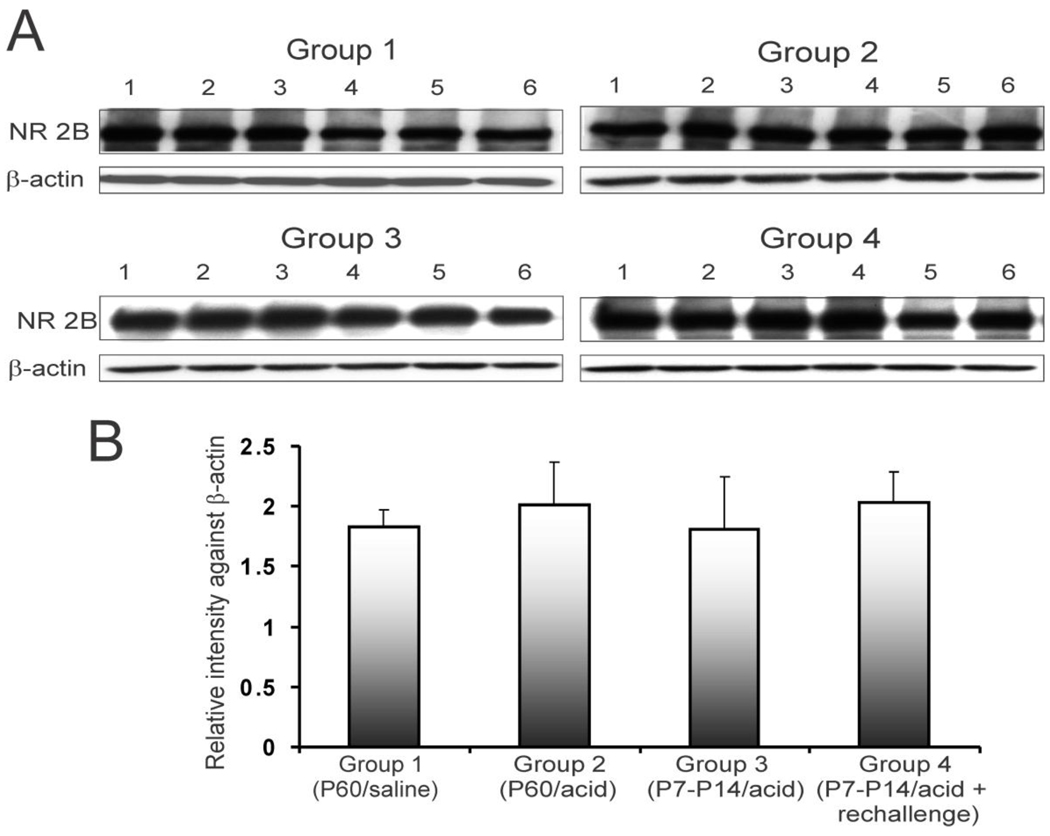
NR2A subunit expression profile in individual animals is shown in figure 3A. The relative intensity of expression indicated a significant increase in expression of NR2A subunit of NMDA receptors in rCC from acid-treated groups (2, 3 and 4) compared to saline-treated group 1 (fig. 3B, **p<0.001). The P7–P14 acid exposed group 3 that did not receive acute acid exposure in adulthood (P60) exhibited a long term increase in NR2A expression. However, rats in group 4 with neonatal acid exposure and acute rechallenge in adulthood failed to show any significant increase in NR2A expression compared to group 3. We also examined the expression of NR2B subunit in the rCC from these groups of rats (fig 4A). Unlike NR2A expression, the relative expression of NR2B subunit in acid-treated rats (groups 2, 3 and 4) was comparable to saline-treated (group 1) rats (fig. 4B).
Figure 3.
NR2A expression in rCCs at P60 following esophageal acid exposure at different time points of development. The treatment strategies were the same as indicated in fig. 1A. A: blots show NR2A and β-actin expression in individual animals (n=6/group) for 4 different groups. B: bar graphs represent relative intensity of staining against β-actin. Asterisks indicate significant difference compared to group 1 (**p<0.001).
Figure 4.
NR2B expression pattern in rCCs at P60 following esophageal acid exposure at different time points of development. The treatment strategies were the same as indicated in fig. 1C. A. blots show NR2B and β-actin expression in individual animals (n=6/group) for 4 different groups. B. bar graphs represent relative intensity of staining against β-actin.
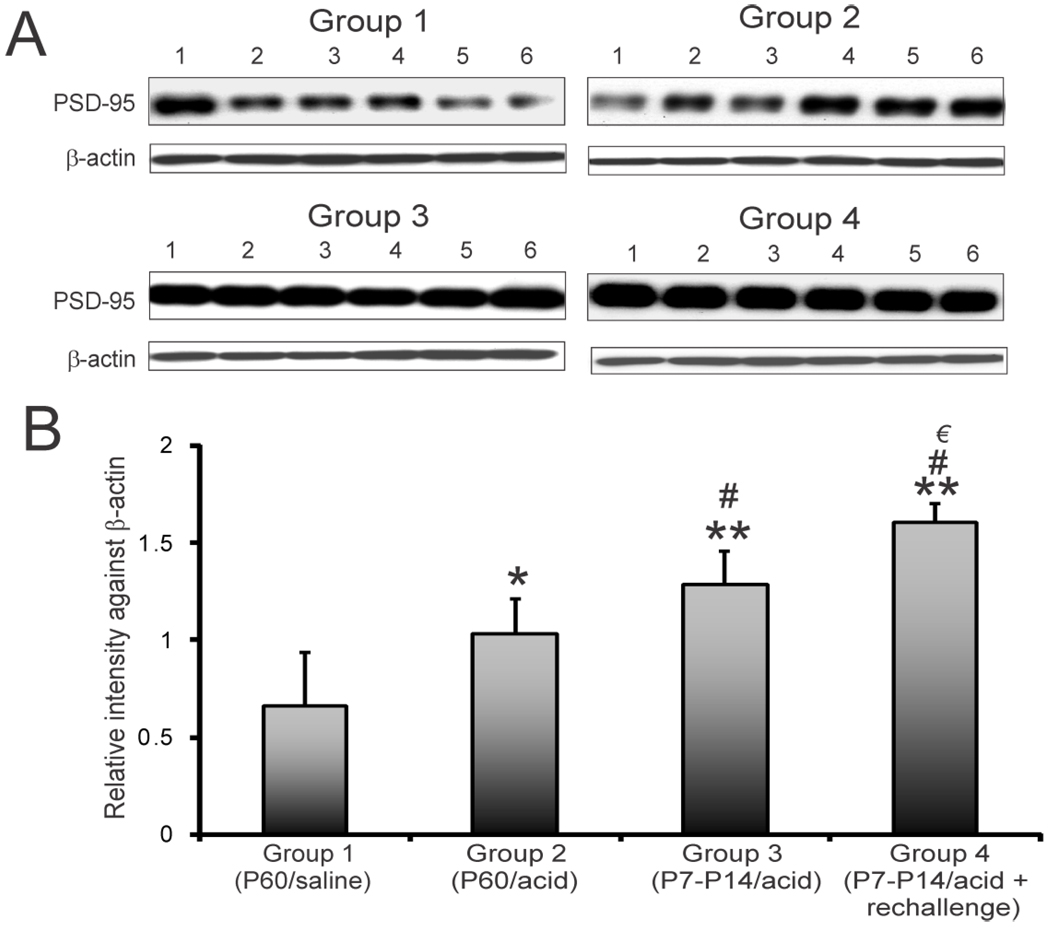
The PSD-95 expression profile for individual animals in 4 groups is shown in figure 5A. The relative expression of PSD-95 against β-actin in individual animals in both experimental and control groups is shown in figure 5B. A significant increase in expression was observed in groups 2, 3 and 4 compared to in group 1 (*p<0.05, **p<0.001 vs. saline controls). The highest expression of PSD-95 was observed in group 4 that received acid at P7–P14 with a re-challenge at P60. The expression in group 4 was significantly higher than that in groups 2 (#p<0.001) and 3 (€p<0.05).
Figure 5.
PSD-95 expression pattern in rCCs at P60 following esophageal acid exposure at different time points of development. The treatment strategies were the same as indicated in fig. 1A. A: blots show PSD-95 and β-actin expression in individual animals (n=6/group) for 4 different groups. B: bar graphs represent relative intensity of staining against β-actin. *p<0.05 vs group 1, **p<0.001 vs group1, #p<0.05 vs group 2, € p<0.05 vs group 3.
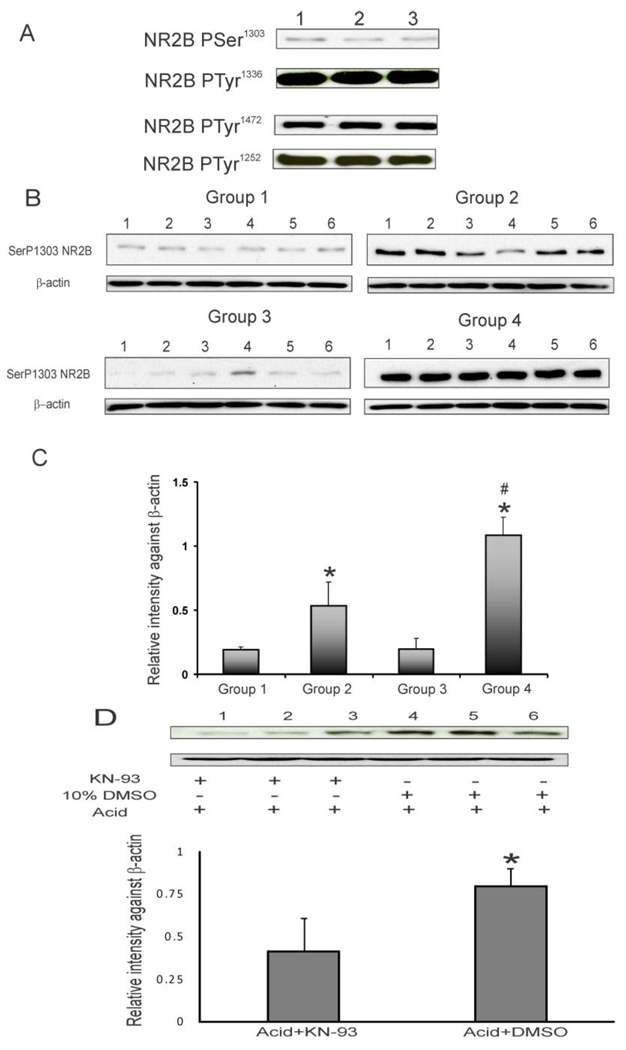
The phosphorylation of NR2B subunit in rCC from naïve animals is shown in figure 6A. The analysis of phosphorylated NR2B revealed a high level of constitutively expressed TyrP1252 NR2B, TyrP1336 NR2B and TyrP1472 NR2B in the rCC, whereas, the level of constitutively expressed SerP1303NR2B is significantly less compared to that of tyrosine phosphorylated NR2B subunits. Following acute esophageal acid treatment, SerP1303 NR2B subunit expression significantly increased in rCCs from groups 2 and 4 rats compared to group 1 and as well as neonatal acid group 3 (fig. 6B & C, *p<0.001). The maximum expression of SerP1303NR2B was observed in group 4 rats with neonatal acid treatment and acute rechallenge at P60, which is significantly higher than that in group 2 (*p<0.001). None of the tyrosine phosphorylated NR2B subunits examined in this study demonstrated significant differences in expression between control and acid-treated animals (data not shown).
Figure 6.
A: blots represent phosphorylation pattern of NR2B subunit in rCCs from naïve rats. B: blots show SerP1303 NR2B and β-actin expression in 4 groups of animals. The treatment strategies were the same as described in fig. 1A. C: bar graphs represent relative intensity of staining against β-actin, *p<0.001 vs groups 1 & 3 and #p<0.05 vs group 2. D: blots show the effect of KN-93 (CaMKII kinase inhibitor) microinjection on esophageal acid-induced SerP1303 NR2B upregulation in rCCs. The lower panel represents relative intensity of expression against β-actin with *p<0.05 vs vehicle control.
To further examine the involvement of CaMKII in this phosphorylation, KN-93, the specific inhibitor of CaMKII was microinjected into rCC in a group of rats immediately before treating them acutely with acid at P60. For vehicle control group, DMSO was microinjected into rCC before esophageal acid exposure. The effect of KN-93 on Ser1303 phosphorylation of NR2B subunit is shown in figure 6D. A significant downregulation of SerP1303 NR2B was observed in rCCs of animals receiving KN-93 treatment (*p<0.05 vs vehicle).
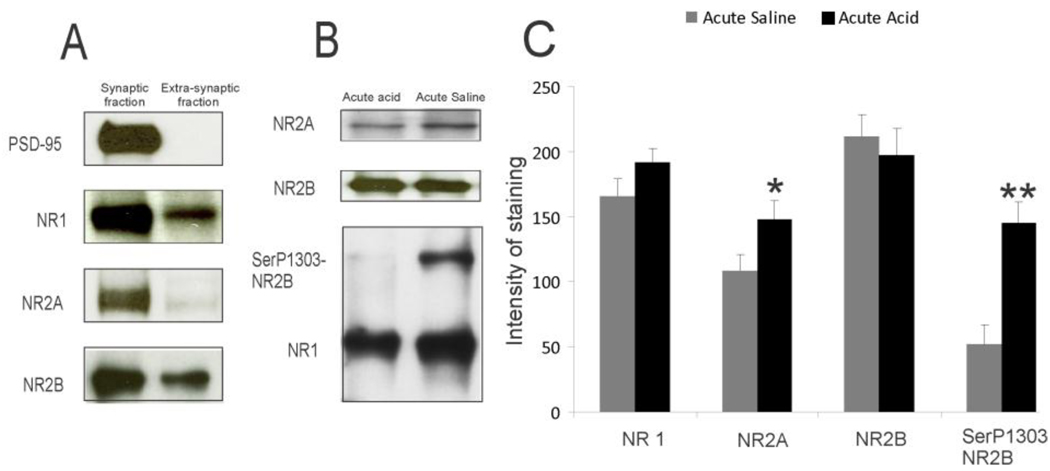
The PSD-95 protein was exclusively enriched in the synaptic fraction as shown in figure 7A. The expression of NR1, NR2A and NR2B subunits of NMDA receptors were also significantly higher in synaptic membrane preparation compared to the levels in extrasynaptic membrane. The western blot analysis of NR1, NR2A, NR2B and SerP1303 NR2B expression in PSD-95 immunoprecipitated rCC from rats with either acute acid or saline treatment is shown in figure 7B. The SerP1303 NR2B subunit in PSD-95 immunoprecipitated complex from acid-treated group exhibited a significant increase compared to controls (**p<0.001). There was also a significant increase in NR2A subunits in PSD-95 immunoprecipitated rCC samples from acute acid-treated rats compared to saline controls (*p<0.05). The total NR2B and NR1 subunits in PSD-95 immunoprecipitated complex were comparable between acute acid and saline control groups (fig. 7C).
Figure 7.
A: isolation of synaptic and extrasynaptic membrane fractions from cortical tissue extracts. Representative western blots with equal amount of proteins (10 µg) from both fractions were probed with antibodies as indicated in the figure. B and C: immunoprecipitation of NMDA receptor subunits in the cortical extracts from rats either receiving esophageal saline or acid on P60. Proteins were immunoprecipitated with PSD-95 antibodies and probed with various antibodies as indicated in the figure,*p<0.05, **p<0.001 vs acute saline.
Discussion
The present study provides the first evidence for long-term changes in NMDA receptor subunits composition and postsynaptic membrane protein expressions in rCCs from rats receiving esophageal acid exposure during critical periods of development. Interestingly, adult rats receiving chronic esophageal acid exposure failed to exhibit significant changes in the receptor subunit expression compare to saline-treated control. Moreover, molecular changes in rCCs from neonatal acid-treated rats were significantly higher that with neonatally handled and saline-treated, emphasizing thereby the specificity of the response. These findings indicate that the long lasting effect of acid when given early during development may be sufficient to induce discernible molecular changes in adulthood. In humans, both clinical and experimental data support early childhood as a critical time period in which trauma can induce visceral hypersensitivity and manifestation of functional disorders 22.
In the brain, glutamate receptors including NMDA mediate most of the excitatory neuronal transmission and play essential role in the regulation of synaptic activity 23, 24. The unique feature of NMDA receptors is that the receptor activation requires the binding of a co-agonist glycine in addition to glutamate 25. Therefore, a functional NMDA receptor requires both an NR1 subunit, which has the glycine binding site, and an NR2 subunit for binding to glutamate. Among NR2 subunits, the expression patterns of NR2A and NR2B are relatively broad and both are developmentally regulated, with concurrent increase in NR2A expression and decrease in NR2B expression as neuron matures. With this rationale, in the present study we examined the expression patterns of NR1, NR2A and NR2B after infusion of acid at early stage of life as well as in the adulthood. Acute esophageal acid exposure in adult rats resulted in a transient increase in NR1 and NR2A expression in the rCC, whereas, neonatally acid expose rats receiving acute rechallenge in adulthood (P60) exhibited the highest level of NR1 and NR2A expressions indicating their enhanced susceptibility to esophageal acid exposure. Our present findings are in agreement with the previous study that reported a long-term alteration in NDMA receptor subunit mRNA from the hippocampus and cortex of rats treated with a single exposure of LPS1.
We did not observe any change in NR2B following acid exposure. This result is in contrast with most of the previous studies that report upregulation of NR2B subunit in supraspinal regions under various pathological conditions 16, 26, 27. The differential subunit expression pattern of NMDA receptor is an important factor in regulating NMDAR-dependent function and neuronal plasticity. For example, rapid calcium-mediated signaling through NR1/NR2A in contrast to slower signaling through NR1/NR2B may activate different downstream signaling and gene expression pattern. Moreover, synaptic NMDA receptor levels are not only regulated by the type of NR2 subunits, but also by lateral movement of extrasynaptic receptors in and out of the synapses 28, 29. In this context, NR2A-subunit receptors are fairly stable in the synapse, whereas, NR2B-subunit receptors are highly mobile, 30. Therefore, increase in cortical NR2A subunit in the present study may result in faster neuronal transmission and stable NMDA receptor expression in the synapses with a distinct downstream signaling pathway during esophageal acid- induced hypersensitivity.
NMDA receptors are reported to anchor in the postsynaptic membrane by interactions between cytoplasmic C-terminal ends of their NR2 subunits and PDZ domains of PSD-95, an abundant scaffold protein that assembles a specific set of signaling proteins around NMDA receptors 31, 32. Recent study indicates that NMDA receptor and PSD-95 interaction may have a role in the processing of spinal nociceptive information 33. Furthermore, PSD-95 knockdown shows a delay in the onset of mechanical and thermal hyperalgesia in chronic neuropathic pain model, indicating an involvement of this protein in NMDA receptors-mediated thermal hyperalgesia 34. In the present study, although acute acid treatment showed an increase in PSD-95 protein expression in the rCC, the maximum expression was observed in neonatal acid and rechallenge group followed by only neonatally treated group. Therefore, the long-term effect and memory of neonatal treatment on PSD-95 expression in the rCC indicates effective surface expression and clustering of NMDA receptors at the synapses that eventually may play an important role in NMDA receptor-dependent function and neuronal plasticity.
The phosphorylation of NMDA receptor subunit regulates many cellular processes including surface expression and protein activity resulting in changes in synaptic strength underlying many forms of synaptic plasticity 35, 36. Several kinases such as protein kinase C (PKC), calcium/calmodulin kinase II (CaMKII), protein tyrosine kinases are reported to phosphorylate various serine/threonine and tyrosine residues at the C-terminal ends of NR2 subunits of NMDA receptors 37.
In order to study the effect of esophageal acid exposure on cortical NMDA receptor activation, we examined the phosphorylation pattern of C-terminal amino acids of NR2B subunits at Ser1303, Tyr1336, Tyr1472 and Tyr1252. The effect of esophageal acid exposure on NMDA receptor activation is evident in our present findings, as a significant upregulation of Ser1303 NR2B phosphorylation is observed in rCCs from rats receiving acute esophageal acid treatment at P60 and also the group with neonatal acid treatment followed by an acute exposure at P60. Interestingly, rats with only neonatal acid treatment failed to exhibit upregulation of SerP1303 NR2B, indicating this specific phosphorylation is an immediate effect of esophageal acid exposure.
We further confirmed the involvement of CaMKII in acid-induced Ser1303 NR2B phosphorylation in the rCC as microinjection of CaMKII inhibitor KN-93 in the rCC resulted in a significant reduction of SerP1303 NR2B in animals receiving acute esophageal acids exposure. KN-93 is reported to bind specifically to the CaM binding site of CAMKII and prevents its activation 38. Recent study indicates that the phosphorylation of NMDA receptors by CaMKII enhances influx of Ca2+ through the channels 39. CaMKII up-regulation has also been reported in the superficial laminae of the dorsal horn and DRGs after inflammation or injuries to peripheral tissues 40–42 Moreover, phosphorylation of Ser1303 NR2B by CaMKII also promotes slow dissociation of preformed CaMKII-NR2B complexes and stabilizes the receptor-enzyme in the membrane 43.
Given that after Ca2+ influx through NMDA receptors, activation of CaMKII results in long-term potentiation in the hippocampus 44, 45, our results indicate that central sensitization through cortical activation and phoshorylation of NMDA receptors could initiate a variety of intracellular processes leading to neuronal changes by activating second/third messenger systems. CaMKII being a major component of PSDs, phosphorylation and stabilization of NMDA receptor-CaMKII complex in the PSD may in turn activate multiple proteins and enzymes, such as neuronal proteins, Ca2+-ATPase and tyrosine hydroxylate and transcription factor cAMP-responsive-element-binding protein (CREB) 46–49.
We examined the distribution pattern of NMDA receptor subunits and PSD-95 in synaptic and extrasynaptic membranes in the rCCs from naïve rat. The enrichment of NMDA receptor subunits along with PSD-95 protein in the synaptic membrane preparation indicates that NMDA receptors anchoring in the cortical synapses probably is mediated by interaction between the C-terminal end of NR2 subunit with PDZ domain of PSD-95 protein of the postsynaptic membranes. We further investigated whether the acid-induced molecular changes in the rCC is mainly due to increase in NMDA receptors in the post synaptic membrane via its binding with PSD-95. The cortical membrane extracts from saline and acid-treated rats were immunoprecipitated using PSD-95 antibody. PSD-95 immunoprecipitated fractions from acid-treated rats exhibited a significantly higher expression of NR2A, and importantly SerP1303 phosphorylated NR2B subunit compared to controls. These findings clearly indicate that the NMDA receptor upregulation and phosphorylation are predominantly occurring at the postsynaptic membranes in the rCC and may be involved in synaptic transmission and increased neuronal activity in acid-induced esophageal hypersensitivity.
In conclusion, results demonstrate a long lasting change in NMDA receptors expression in neurons of the rCC following neonatal esophageal acid exposure. Further study of the complex interaction between various downstream signaling pathways may provide a better understanding for the neuronal plasticity during the development and its influence on esophageal pain mechanism of NERD and NCCP patients. We acknowledge that NMDA receptors in multifunctional brain region like CC can mediate many different functions including chronic pain. Currently, there is no quantifiable, reliable and reproducible behavioral model in experimental animals to complement the results of the present study. Therefore, in the absence of concurrent behavioral study our findings can only suggest that such molecular changes play a possible role in chronic esophageal pain.
Acknowledgement
The study has been supported by NIH Grant 5R01 DK025731 awarded to Reza Shaker and NIH 1R56DK089493-01 awarded to J.N. Sengupta and Banani Banerjee.
Grant support: The work was supported by NIH Grant 5R01 DK025731 and 1R56DK089493-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors agreed to participate in this study without any potential conflict of interest.
Author’s contributions:
BB: Study design, data acquisition & analysis,
BKM: Study design,
JS: Data acquisition,
IML: Critical revision of the manuscript,
JNS: Critical revision of the manuscript,
RS: Concept, critical revision of the manuscript, funding.
Reference List
- 1.Harre EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ. Neonatal inflammation produces selective behavioural deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain. Eur J Neurosci. 2008;27:644–653. doi: 10.1111/j.1460-9568.2008.06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer SJ, Heida JG, Pittman QJ. Early life immune challenge--effects on behavioural indices of adult rat fear and anxiety. Behav Brain Res. 2005;164:231–238. doi: 10.1016/j.bbr.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol. 2009;587:2927–2935. doi: 10.1113/jphysiol.2008.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermann C, Hohmeister J, Demirakca S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125:278–285. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Hohmeister J, Kroll A, Wollgarten-Hadamek I, Zohsel K, Demirakca S, Flor H, Hermann C. Cerebral processing of pain in school-aged children with neonatal nociceptive input: an exploratory fMRI study. Pain. 2010;150:257–267. doi: 10.1016/j.pain.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 8.Miranda A, Peles S, Shaker R, Rudolph C, Sengupta JN. Neonatal nociceptive somatic stimulation differentially modifies the activity of spinal neurons in rats and results in altered somatic and visceral sensation. J Physiol. 2006;572:775–787. doi: 10.1113/jphysiol.2006.108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7:469–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- 10.Hobson AR, Furlong PL, Worthen SF, Hillebrand A, Barnes GR, Singh KD, Aziz Q. Real-time imaging of human cortical activity evoked by painful esophageal stimulation. Gastroenterology. 2005;128:610–619. doi: 10.1053/j.gastro.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Lawal A, Kern M, Sanjeevi A, Antonik S, Mepani R, Rittmann T, Hussaini S, Hofmann C, Tatro L, Jesmanowicz A, Verber M, Shaker R. Neurocognitive processing of esophageal central sensitization in the insula and cingulate gyrus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G787–G794. doi: 10.1152/ajpgi.00421.2007. [DOI] [PubMed] [Google Scholar]
- 12.Kern MK, Birn RM, Jaradeh S, Jesmanowicz A, Cox RW, Hyde JS, Shaker R. Identification and characterization of cerebral cortical response to esophageal mucosal acid exposure and distention. Gastroenterology. 1998;115:1353–1362. doi: 10.1016/s0016-5085(98)70013-7. [DOI] [PubMed] [Google Scholar]
- 13.Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- 14.Zhao MG, Ko SW, Wu LJ, Toyoda H, Xu H, Quan J, Li J, Jia Y, Ren M, Xu ZC, Zhuo M. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci. 2006;26:8923–8930. doi: 10.1523/JNEUROSCI.2103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology. 2004;126:683–692. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Cao Z, Wu X, Chen S, Fan J, Zhang R, Owyang C, Li Y. Anterior cingulate cortex modulates visceral pain as measured by visceromotor responses in viscerally hypersensitive rats. Gastroenterology. 2008;134:535–543. doi: 10.1053/j.gastro.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 17.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 18.Sheng M, Pak DT. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Annu Rev Physiol. 2000;62:755–778. doi: 10.1146/annurev.physiol.62.1.755. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee B, Medda BK, Schmidt J, Zheng Y, Zhang Z, Shaker R, Sengupta JN. Altered expression of P2X3 in vagal and spinal afferents following esophagitis in rats. Histochem Cell Biol. 2009;132:585–597. doi: 10.1007/s00418-009-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies KD, Alvestad RM, Coultrap SJ, Browning MD. alphaCaMKII autophosphorylation levels differ depending on subcellular localization. Brain Res. 2007;1158:39–49. doi: 10.1016/j.brainres.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goebel SM, Alvestad RM, Coultrap SJ, Browning MD. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor is enhanced in synaptic membrane fractions of the adult rat hippocampus. Brain Res Mol Brain Res. 2005;142:65–79. doi: 10.1016/j.molbrainres.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- 24.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 25.Ulbrich MH, Isacoff EY. Rules of engagement for NMDA receptor subunits. Proc Natl Acad Sci U S A. 2008;105:14163–14168. doi: 10.1073/pnas.0802075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan J, Wu X, Cao Z, Chen S, Owyang C, Li Y. Up-regulation of anterior cingulate cortex NR2B receptors contributes to visceral pain responses in rats. Gastroenterology. 2009;136:1732–1740. doi: 10.1053/j.gastro.2009.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Wu X, Gao J, Yan J, Fan J, Owyang C, Li Y. Role for NMDA receptors in visceral nociceptive transmission in the anterior cingulate cortex of viscerally hypersensitive rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G918–G927. doi: 10.1152/ajpgi.00452.2007. [DOI] [PubMed] [Google Scholar]
- 28.Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Peng Y, Xu Z, Chen RQ, Gu QH, Chen Z, Lu W. Synaptic metaplasticity through NMDA receptor lateral diffusion. J Neurosci. 2008;28:3060–3070. doi: 10.1523/JNEUROSCI.5450-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardoni F, Marcello E, Di LM. Postsynaptic density-membrane associated guanylate kinase proteins (PSD-MAGUKs) and their role in CNS disorders. Neuroscience. 2009;158:324–333. doi: 10.1016/j.neuroscience.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 32.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 33.Tao YX, Huang YZ, Mei L, Johns RA. Expression of PSD-95/SAP90 is critical for N-methyl-D-aspartate receptor-mediated thermal hyperalgesia in the spinal cord. Neuroscience. 2000;98:201–206. doi: 10.1016/s0306-4522(00)00193-7. [DOI] [PubMed] [Google Scholar]
- 34.Tao F, Tao YX, Gonzalez JA, Fang M, Mao P, Johns RA. Knockdown of PSD-95/SAP90 delays the development of neuropathic pain in rats. Neuroreport. 2001;12:3251–3255. doi: 10.1097/00001756-200110290-00022. [DOI] [PubMed] [Google Scholar]
- 35.Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao GY, Wagner DA, Hsu MH, Leonard JP. Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol Pharmacol. 2001;59:960–964. doi: 10.1124/mol.59.5.960. [DOI] [PubMed] [Google Scholar]
- 37.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- 39.Kitamura Y, Miyazaki A, Yamanaka Y, Nomura Y. Stimulatory effects of protein kinase C and calmodulin kinase II on N-methyl-D-aspartate receptor/channels in the postsynaptic density of rat brain. J Neurochem. 1993;61:100–109. doi: 10.1111/j.1471-4159.1993.tb03542.x. [DOI] [PubMed] [Google Scholar]
- 40.Carlton SM. Localization of CaMKIIalpha in rat primary sensory neurons: increase in inflammation. Brain Res. 2002;947:252–259. doi: 10.1016/s0006-8993(02)02932-3. [DOI] [PubMed] [Google Scholar]
- 41.Carlton SM, Hargett GL. Stereological analysis of Ca(2+)/calmodulin-dependent protein kinase II alpha -containing dorsal root ganglion neurons in the rat: colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol. 2002;448:102–110. doi: 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- 42.Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci. 2002;22:4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 44.Fukunaga K, Muller D, Miyamoto E. CaM kinase II in long-term potentiation. Neurochem Int. 1996;28:343–358. doi: 10.1016/0197-0186(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 45.Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23:75–80. doi: 10.1016/s0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- 46.Hanson PI, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- 47.Colbran RJ, Soderling TR. Calcium/calmodulin-dependent protein kinase II. Curr Top Cell Regul. 1990;31:181–221. doi: 10.1016/b978-0-12-152831-7.50007-x. [DOI] [PubMed] [Google Scholar]
- 48.Schulman H, Hanson PI. Multifunctional Ca2+/calmodulin-dependent protein kinase. Neurochem Res. 1993;18:65–77. doi: 10.1007/BF00966924. [DOI] [PubMed] [Google Scholar]
- 49.Yang E, Schulman H. Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J Biol Chem. 1999;274:26199–26208. doi: 10.1074/jbc.274.37.26199. [DOI] [PubMed] [Google Scholar]