Abstract
Previously we reported that hydrophobic aryl azides partition into hydrophobic regions of the viral membrane of enveloped viruses and inactivate the virus upon UVA irradiation for 2 minutes. Prolonged irradiation (15 minutes) resulted in viral protein aggregation as visualized via Western blot analysis, due to reactive oxygen species (ROS) formation, with preservation of the surface antigenic epitopes. Herein, we demonstrate that these aggregates show detergent resistance and that this property may be useful towards the creation of a novel orthogonal virus inactivation strategy for use in preparing experimental vaccines. When ROS-modified HIV virus preparations were treated with 1% Triton X-100, there was an increase in the percent of viral proteins (gp41, p24) in the viral pellet after ultracentrifugation through sucrose. Transmission electron microscopy (TEM) of these detergent-resistant pellets show some recognizable virus fragments, and immunoprecipitation studies of the gp41 aggregates suggest the aggregation is covalent in nature, involving short-range interactions.
Keywords: HIV, Detergent, Vaccine, Triton, Hydrophobic, Azide, Reactive Oxygen Species (ROS), Viral Membrane
Introduction
There is a continuing need for safe, effective vaccines against existing and emerging viral threats. Novel vaccine preparation strategies that are broadly applicable to a variety of viruses are desirable. One such method, referred to as an inactivated or “killed” virus vaccine, uses an infectious viral preparation that is rendered non-infectious through chemical, thermal or other means. The advantage of this technique is that after inactivation, the virus is mostly intact and is able to present epitopes similar to that of the infectious virus to the immune system. This strategy is currently used in the United States for the preparation of certain Influenza, Hepatitis A and Polio vaccines. While this method has met with success, there is data supporting that some methods of inactivation damage surface epitopes.(Adler-Storthz et al., 1983; Bachmann et al., 1994; Duque et al., 1989; Grovit-Ferbas et al., 2000; Poon et al., 2005; Sattentau, 1995; Tano et al., 2007) Additionally, there are still reasonable concerns over the safety of such a method(Brown, 1993), especially when it is applied to novel viruses. It is generally accepted that ~15 logs of inactivation are needed to create a vaccine that is reasonably safe.(Schultz, Koff, and Lawrence, 1990; Sheppard, 2005) This requirement for inactivation pushes the limits of detection of most assays and can be difficult to achieve with one inactivation method alone.
A strategy that we are developing for the inactivation of enveloped viruses uses photoactivatable hydrophobic molecules that selectively target the hydrophobic region of the viral membrane. In particular, azidonaphthalene compounds, such as 1,5-iodonaphthyl azide (INA), partition selectively into the hydrophobic region of the viral membrane(Bercovici and Gitler, 1978), and can be photoactivated using UVA irradiation to rapidly (2 minutes of irradiation) inactivate the virus. Photoactivation of INA in purified viral preparations has been shown to result in the inactivation of various enveloped viruses, such as Ebola, Influenza, HIV, and VEEV, with preservation of key surface epitopes, and, in the case of influenza, enhanced immunogenicity.(Belanger et al., 2010; Raviv et al., 2008; Raviv et al., 2005; Sharma et al., 2007; Warfield et al., 2007) In the case of HIV, using a sensitive cell-based p24 assay, it was shown that INA-inactivation resulted in at least 4 logs of inactivation.(Raviv et al., 2005) It was also discovered that prolonged UVA irradiation (15 minutes irradiation time) in the presence of INA or other arylazides resulted in the formation of higher molecular weight viral protein aggregates when viral lysate was characterized via Western blot analysis.(Belanger et al., 2010) These aggregates were caused by reactive oxygen species formation (ROS). It was concluded that viral inactivation occurs through the binding of the azido moiety and that these ROS-induced aggregates, while not needed for viral inactivation, did not result in the damage of surface epitopes and might therefore be advantageous towards the creation of a novel vaccine strategy, described herein. While this INA-inactivation technique has been shown to be rapid and applicable to a variety of enveloped viruses, it still results in an inactivated viral preparation in which 15 logs of inactivation is difficult to prove.
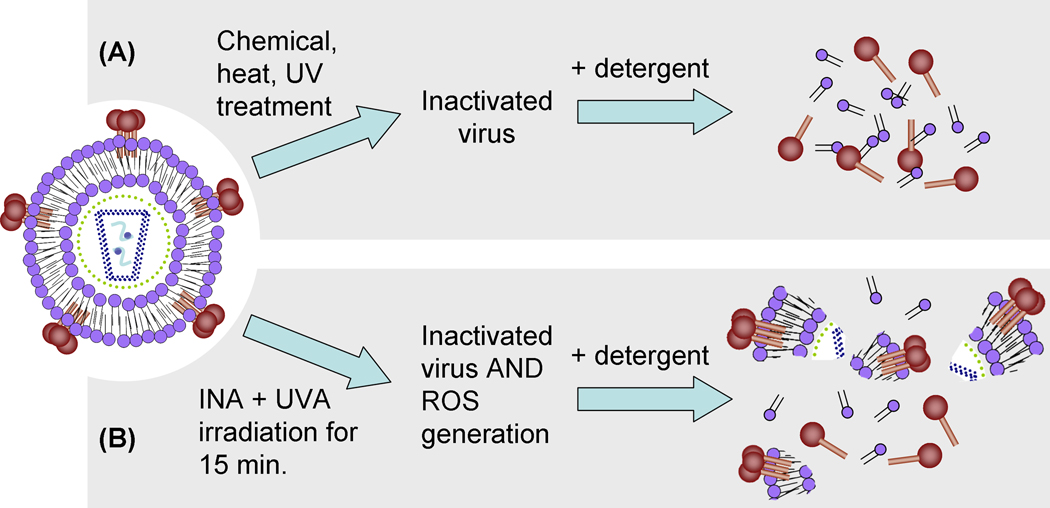
To ensure greater than 15 logs of viral inactivation in inactivated virus preparations, it is generally accepted that multiple methods of inactivation are needed that operate on mechanisms independent of each other, and each produce 6–8 logs of inactivation.(Schultz, Koff, and Lawrence, 1990) Such “orthogonally inactivated” vaccines have the additive effect of each inactivation technique to meet the required 15 logs of inactivation. However, each additional treatment step may result in the destruction or modification of the native virus, and result in the loss of key epitopes needed to elicit an effective immune response. Indeed, when “split” virus vaccines are made, orthogonal inactivation methods are used (viral inactivation typically followed by detergent treatment), but result in vaccine preparations containing soluble viral proteins (see Figure 1a). For example, in some influenza vaccine preparations, virus is inactivated and subsequently treated with a non-ionic detergent to “split” the virus, followed by purification to remove the detergent and isolate the solubilized viral proteins (hemagglutinin, for influenza). While this preparation is “orthogonal” and safe, it no longer consists of intact virus particles which is the form encountered by the host in nature, and undoubtedly many epitopes associated with the native virion are lost.
Figure 1.
A Proposed novel approach for orthogonally inactivated virus vaccine preparation using detergent insoluble viral protein aggregates. A) generalized typical pathway for the preparation of “traditional” inactivated split virus vaccine resulting in solubilized proteins. B) Proposed “novel” orthogonal inactivation strategy using reactive oxygen species (ROS) induced protein aggregation to form detergent resistant viral protein aggregates. Inset of HIV depicts lipids (purple circles with black tails), RNA (light blue squiggles), and various other viral proteins (all other colored circles and sticks).
Although traditional “split” vaccines have proven to be effective, it has been shown that whole inactivated influenza vaccines are more immunogenic than split or subunit vaccines.(Cox et al., 2006; Geeraedts et al., 2008) With this in mind, we propose a novel vaccine strategy in which orthogonal methods are used for viral inactivation, with preservation of viral protein aggregates that represent key viral antigens. Herein, infectious purified virus is inactivated through chemical means using photoactivatable aryl azides, which, in addition to inactivation, renders the virus partially detergent resistant. Subsequent treatment with detergent results in a partially split virus vaccine preparation that contains viral aggregates of key viral proteins in the viral lysate (see Figure 1b), unlike traditional split virus vaccines that contain soluble proteins (see Figure 1a). This method is not virus-specific and can be applied to other enveloped viruses as well. Herein, we describe the characterization of such detergent resistance as applied to an enveloped virus, human immunodeficiency virus Type 1 (HIV-1).
Materials and Methods
Safety
All handling of infectious HIV-1 isolates were done under Biosafety Level 2 conditions, following Biosafety level 3 procedures, with the proper personal protective equipment. Synthesis and handling of the azido compounds was performed using the proper precautions due to the potentially explosive nature of these compounds.
Reagents
The azido-containing compound, 1,5-diazidonapthalene (DAN) was made according to previously published procedures.(Belanger et al., 2010) 1,5-iodonaphthylazide (INA) was custom-synthesized by Biotium (Hayward, CA). HIV-1 MN cl. 4 concentrated virus stock (lots #P3592 and #P3602) were prepared by the AIDS Vaccine Program, SAIC.(Chertova et al., 2002) The anti-gp41 monoclonal antibody was purified from the hybridoma for Chessie 8 from Dr. George Lewis, obtained through the ARRRP. The anti-gp120 (ID6) monoclonal antibody used for Western blots, was obtained through the ARRRP from Dr. Kenneth Ugen and Dr. David Weiner. The anti-p24 (183-H12-5C) monoclonal antibody used for Western blot was obtained from the ARRRP, from Dr. Bruce Chesebro and Kathy Wehrly. Secondary antibody used for Western analysis and the quantitation of pelleted proteins was Alexafluor680 goat anti-mouse IgG (Invitrogen) using an Odyssey infrared imaging system and software for lane integration. Secondary antibody of donkey anti-goat Alexafluor 800 (Invitrogen) was used for Western analysis when the polyclonal anti-p17 goat antibody (antisera from the AIDS and Cancer Virus Program, NCI-Frederick) was used. Anti-HIV-1 broadly neutralizing antibodies 2G12 and 4E10 were from Herman Katinger through the ARRRP. Triton X-100 (Surfact-Amps, ampules of 10% solution in water, cat#PI-28314) was used for the detergent treatment of viruses. Sucrose (RNase-free, cat#S9378) was obtained from Sigma-Aldrich.
Treatment of Virus with hydrophobic naphthalene compounds
Treatment of HIV-1 with the compounds was done according to previously published procedures.(Belanger et al., 2010) In brief, each compound was added (from 8 mM stock in DMSO) to a diluted suspension of HIV-1 MN (0.5 mg/mL total protein in PBS) for a final concentration of 100 micromolar of compound. The viral suspension was then irradiated with UVA light for 15 minutes. Sample volumes used for irradiation varied from 0.10 mL to 1.0 mL in clear polypropylene microfuge tubes. For samples that were treated with glutathione prior to irradiation, INA was added to the HIV-1 as described, followed by the addition of glutathione for an end concentration of 20 mM (from a stock solution of 1.0 M glutathione in PBS, pH= 7.4).
Western Blot Analysis for Protein Aggregation
HIV-1 samples treated with azido compound plus UV irradiation and controls were lysed in SDS sample buffer (Laemmli) under reducing conditions for 15–20 minutes at 55–60°C. Aliquots of these were added to a 4–20% Tris-glycine gel. SDS-PAGE was performed and the proteins were transferred to a nitrocellulose membrane and detected using either monoclonal or polyclonal antibodies as specified. Secondary antibodies containing AlexaFluor680 (either goat anti-mouse for the murine primary antibodies or donkey anti-goat for the goat primary antibody) were used for readout on an Odyssey Infrared Imaging System (LiCOR Biosciences). Stripping of the blots was not done; instead, a new blot was done for each antibody probe used.
Detergent Treatment of HIV
Triton X -100 in PBS (10 uL) was added to aliquots of HIV-1 MN (90 uL) that had been treated with aryl azide + UV, or controls, containing 0.5 mg/mL total protein (prepared as described above) for a final Triton concentration of either 0.01%, 0.1% or 1%, and a final protein concentration of 0.45 mg/mL. Mock-treated samples were made with PBS. Samples were briefly mixed by pipette, then allowed to sit at room temperature, in the dark, for 1 hour. Triton was not removed from the samples prior to analysis.
Sucrose cushion and pelleting of detergent-treated virus with Western blot analysis
For the analysis of the detergent insoluble fraction, detergent-treated and control samples were pelleted via ultracentrifugation through 25% sucrose to separate out the soluble and insoluble fractions. 200 uL of 25% sucrose (in PBS) was placed into a polycarbonate ultracentrifuge tube (Beckman #343776, 8 mm × 34 mm). To this, 90 uL of detergent-treated sample (or control) was carefully added (undiluted) on top of the sucrose cushion with caution to maintain the phase boundary. The samples were then ultracentrifuged (Optima TLX tabletop ultracentrifuge) in a fixed-angle rotor (TLA 120.1) at 45,000 rpm for 30 minutes at 15°C. Immediately after centrifugation, the supernatant (290 uL total) was carefully removed and added to SDS sample buffer (Laemmli) under reducing conditions (4×) in a microfuge tube. To the remaining pellet, still in the centrifuge tube, was added 290 uL of PBS and sample buffer. Pellets were very sticky after centrifugation so care was needed to resuspend them in the sample buffer by aspirating gently with a pipette tip and pipettor. The entire centrifuge tube, with pellet solution, was placed into a larger 2 mL microfuge tube with a lid, and stored that way. Both supernatant and pellet solutions were stored at −20°C overnight. The following day, the samples were heated at 60°C for 15–20 minutes and SDS-PAGE was run (Invitrogen, Tris-glycine gels, 10-well, 4–20%). The gels were blotted onto nitrocellulose and probed via Western blot using MAbs for the proteins of interest, and Alexafluor 680 conjugated Mouse IgG secondary antibody for IR readout and integration using an Odyssey imaging system and software. Separate lanes for the pellet (P) solution and supernatant (S) solution were run for each sample on the same gel. Integration of each of these lanes was done using the Odyssey software and each lane was selected to determine the raw integrated intensity of the entire lane. The entire lane was selected to include all the protein aggregates that contained the probed protein of interest, and the entire lanes were integrated in the controls as well for direct comparison. For data labeled as “main band only”, only the main protein band (no aggregates) was integrated. The “percent in pellet” (%P) was then determined using these raw integrated intensities as follows: %P = P / (P+S), where P and S are the respective entire lane integrations of the pellet and supernatant lanes for one sample. Separate gels were run for each protein of interest to be probed via Western blot; Western blots were not reprobed.
TEM of detergent-treated HIV
Detergent-treated HIV samples were prepared as outlined above using a sucrose cushion. The supernatant was removed and to the remaining pellet was added 100 microliters of PBS, followed by ultracentrifugation at 40,000 rpm for 10 min. The PBS was removed and another PBS “wash” followed by centrifugation was performed, to remove residual sucrose. After the second wash, 200 microliters of fixative (2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2 solution) was added to the pellet, allowed to sit at room temperature for 1 hour, then stored at 4°C until TEM analysis was performed. The pellet was post fixed in 1% osmium tetroxide in the same buffer for 1hr. The pellet was dehydrated in ethanol (e.g., 35%, 50%, 75%, 95%, 100%) and 100% propylene oxide. The pellet was infiltrated overnight in 1:1 mixture of propylene oxide and epoxy resin, and embedded in a pure resin and cured for 48hrs in 55°C. The 70nm thin sections were made and mounted on naked copper grids. The thin sections were double stained (uranyl acetate and lead citrate), examined in a Hitachi (Tokyo, Japan) H-7600 transmission electron microscope, and the images were taken using an AMT (Danvers, MA) digital camera.
Immunoprecipitation of HIV in RIPA + SDS buffer
Samples of HIV (0.5 mL of 0.5 mg/mL) were treated with 100 micromolar INA followed by UVA irradiation for 15 minutes (as outlined above). Control samples with DMSO + UVA for 15 minutes were also prepared. To these samples, after irradiation, was added protease inhibitor (Complete Mini Protease Inhibitor, Roche) and RIPA lysis buffer (Upstate Cell Signaling Solutions, Cat#20-188) for a final 1× concentration of each, plus SDS (BioRad 20% solution, cat#161-0418) was added for a final concentration of 0.1% SDS. Samples were briefly vortexed, then placed on a rotator at room temperature for 2 hours to lyse the samples. During this time, protein G Dynabeads (Invitrogen, cat#100.03D) with immobilized antibody were prepared. To a clean microfuge tube was added 50 uL of dynabeads, a magnet was applied and the liquid removed. To this was added 20 microliters of anti-gp41 antibody diluted to 200 microliters in PBS. The beads were resuspended and incubated at room temperature with rotation for 30 minutes. Subsequently, the beads were washed with PBS three times and resuspended in a 25 mM dimethyl pimelimidate (DMP, ThermoFisher, cat#21667) solution containing triethanolamine, pH = 8.0, to crosslink the antibodies to the beads. Beads were incubated at room temperature with rotation for one hour, then washed 3 times with 200 uL of 0.2 M glycine-HCl, pH = 2.5, to remove any non-crosslinked antibody. The beads were then washed with PBS until the supernatant was pH= 7.4, followed by washing 2 times with the same buffer used to lyse the HIV (RIPA 1×, Protease Inhibitor 1×, 0.1% SDS). After the 2 hour incubation of the lysed HIV above, 250 uL of the lysed HIV sample was added to the beads. Another 250 uL of the lysed sample was added to beads that had been mock-treated (through all the same steps above), without the addition of the anti-gp41 antibody, to assess background binding of HIV to the beads. In total, 6 samples were prepared; HIV+INA+UV 15 minutes + beads +/− antibody; HIV+DMSO+UV 15 minutes + beads +/− antibody; beads mock-treated without addition of HIV +/− antibody. The last two controls were to account for any non-specific binding of either the primary or secondary antibodies upon subsequent Western blot analysis. After addition of the lysed (or mock) samples to the beads, the beads were incubated for 30 minutes, washed three times with lysis buffer, beads and sup were transferred to a new microfuge tube, and washed one more time with plain PBS. The beads were then resuspended in 100 microliters of 1× Laemmli reducing sample buffer and heated at 60 °C for 15 minutes to recover the immunoprecipitated proteins. A magnet was applied to remove the beads, and Western blot analysis was performed on the sample for the HIV proteins of interest. Antibodies used for the Western blot were as follows: Cleanblot HRP (Thermoscientific, cat#21230) (used as secondary when primary Ab of anti-p17 was used), Trueblot HRP (eBioscience, cat#18-8877-33) (used as secondary when primary Ab of anti-gp120 or anti-gp41 were used). HRP-probed blots were developed using an ECL Western Blotting substrate (Pierce, cat#32209) and film.
Immunoprecipitation of HIV using neutralizing antibodies
HIV (0.30 mL of 0.5 mg/mL) was treated with 100 micromolar INA followed by UVA irradiation for 15 minutes (as outlined above). HIV control sample without DMSO or irradiation were also prepared. To these samples, was added Triton X-100 (from 10× stock containing protease inhibitor (Complete Mini Protease Inhibitor, Roche)) for a final Triton X-100 concentration of 1%. Samples were briefly vortexed, then incubated at room temperature for one hour. After one hour, the sample was divided into 100 uL aliquots and treated with one of the following: 10 microliters PBS (mock), 10 microliters of 1 mg/mL 2g12 antibody, 10 microliters of 4E10 antibody. All six samples were incubated overnight at room temperature with rotation. The following day, Dynabeads (washed 2 times in equal volume of 1% Triton X-100 with protease inhibitor) were added to each sample (1 mg beads per 100 microliter sample) and rotated for one hour. After this incubation, a magnet was applied, supernatant was removed and the beads were washed with 1% Triton X-100 + protease inhibitor (twice) and PBS + protease inhibitor (twice), with gentle resuspension and application of magnet between washings. After the final washing step, the beads were resuspended in PBS and transferred to a new tube. A magnet was applied, supernatant was removed, and to each sample 60 microliters Laemmli reducing sample buffer (1×) was added. Samples were heated at 70°C for 15–20 min. before running SDS-PAGE and subsequent Western. Blots were probed with either anti-gp41 (Chessie 8) or anti-gp120 (ID6) followed by Trueblot Biotin secondary antibody (ebioscience, cat#13-88717-82) and Streptavidin Alexafluor 800 (Odyssey IRdye, cat#926-32210), with readout performed on an Odyssey Infrared Imaging system.
Results and Discussion
It has been shown that treatment of HIV-1, and other enveloped viruses, with hydrophobic aryl azides plus UVA irradiation resulted in inactivation of the virus within two minutes, without damage to the surface epitopes, making such a preparation a viable method for the generation of inactivated virus vaccines.(Belanger et al., 2010; Raviv et al., 2008; Raviv et al., 2005; Sharma et al., 2007; Warfield et al., 2007) Additionally, it was found that treatment of HIV-1 with aryl azides and prolonged UVA irradiation (15 minutes) resulted in the formation of higher molecular weight aggregates as seen on Western blot analysis which were a result of reactive oxygen species (ROS) formation.(Belanger et al., 2010) The formation of these protein aggregates was eliminated by using methods to scavenge the radicals formed or to oxygen deplete the system during treatment, resulting in an infectious viral preparation free of detectable aggregates. Interestingly, these protein aggregates are recognized by the antibodies used for Western blot detection and can be captured using neutralizing antibodies.(Belanger et al., 2010) These protein aggregates were not dissociated under the conditions of SDS-PAGE, which uses SDS (an ionic detergent) to solubilize proteins. Herein, this “detergent resistance” was characterized using Western blot, ultracentrifugation, immunoprecipitation and electron microscopy to further understand the mechanism of this detergent resistance and it’s applicability towards the generation of a novel orthogonally inactivated virus vaccine strategy.
UV-activated aryl azide treatment of HIV-1 causes detergent insoluble fragments
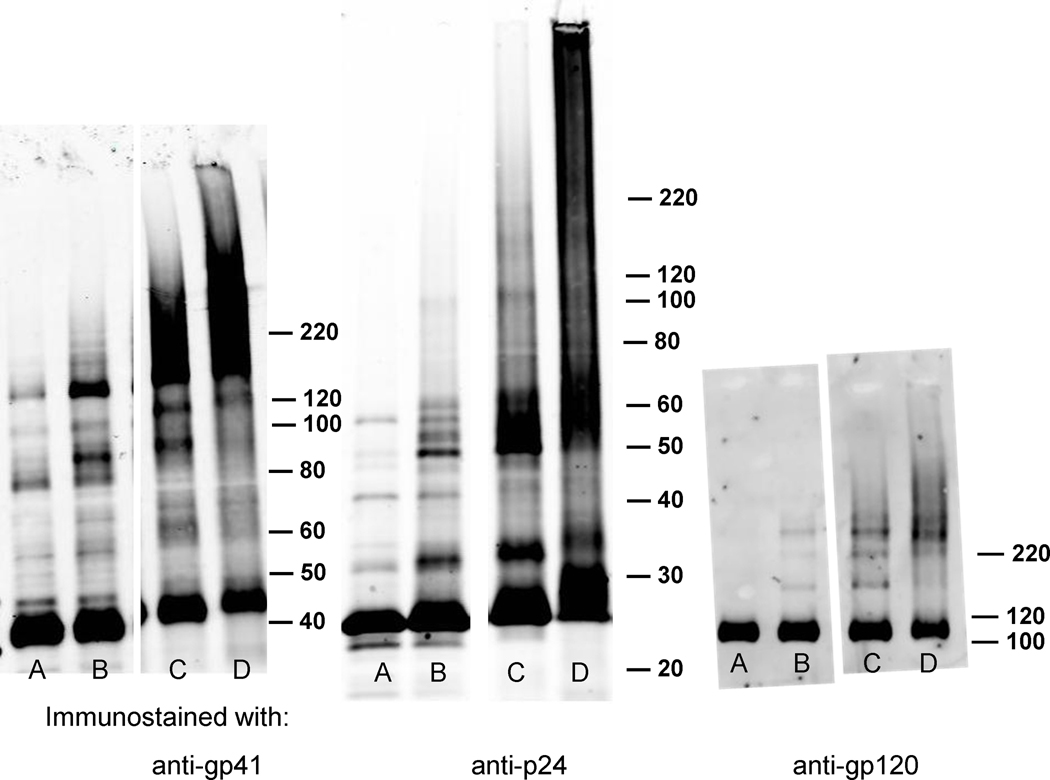
Using either INA or DAN followed by UVA irradiation for 15 minutes resulted in protein aggregates that persisted under the denaturing and reducing conditions of SDS-PAGE. Western blotting indicated that these aggregates contained key viral proteins that although aggregated, were still recognized by the antibodies used for immunostaining (see Figure 2, and reference (Belanger et al., 2010)). These detergent insoluble aggregates contained the transmembrane proteins (gp41), as well as the capsid protein(p24), and matrix protein (p17). The surface protein (gp120), which is hydrophilic and heavily glycosylated, does not appear in this higher molecular weight smear. This lack of aggregation in gp120 can possibly be attributed to the protection of the protein against ROS damage due to heavy glycosylation.(Uchida et al., 1997)
Figure 2.
Viral proteins from HIV-1 MN exhibit protein aggregates for gp41 and p24 proteins, but not for surface protein gp120, after treatment with aryl azides + UVA for 15 minutes when analyzed by Western blot for the protein of interest. Lanes: A) HIV, B) HIV + UV, C) HIV + UV + DAN, D), HIV + UV + INA. Westerns are immunostained for the proteins, as labeled; gp41, p24, gp120.(Belanger et al., 2010)
The exact mechanism of this protein aggregation remains unclear, although it is directly related to ROS that are induced from UVA irradiation of the azido compounds. These aggregated proteins are only produced in systems where aryl azides are used, suggesting that a side-product of the chemical inactivation is the production of chemical species that act as photosensitizers to produce ROS under the wavelengths studied. Protein aggregation has been seen in other systems involving oxidation of proteins and has been attributed to the chemical modification of the proteins by oxygen, and their association via crosslinking into multimers of homoproteins.(Greilberger and Jurgens, 1998; Shoukry, Gong, and Nichols, 1994)
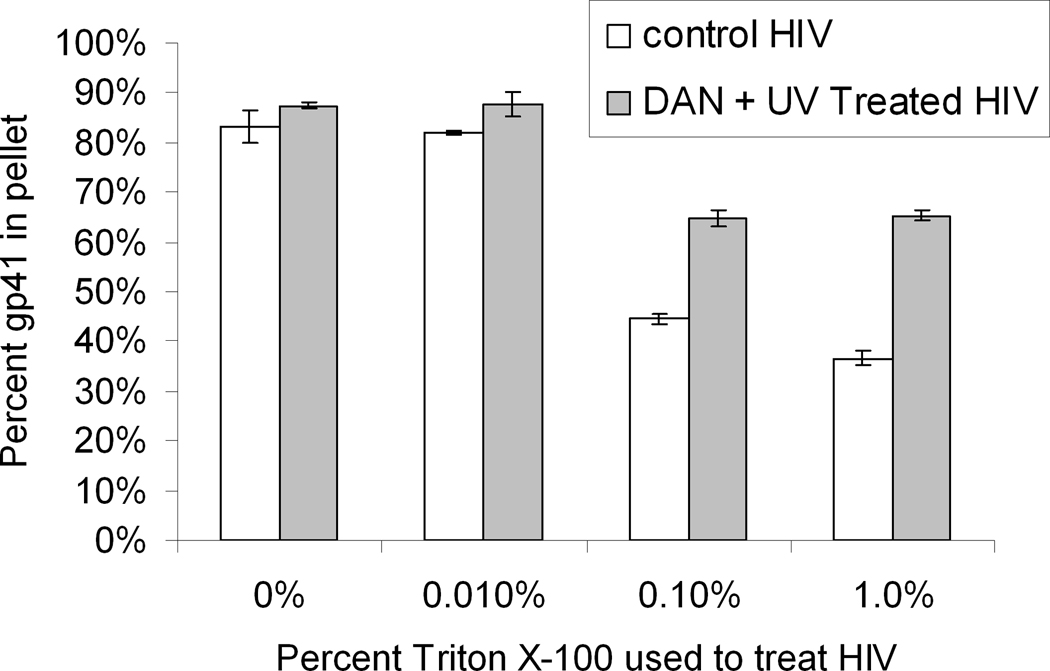
To further characterize the aggregation seen in Western blot analysis, viral protein aggregation and resistance to solubilization with detergent (“detergent-resistance”) was analyzed using ultracentrifugation. Conditions were chosen where intact virus and aggregates of similar density would pellet, whereas soluble proteins would not penetrate the 25% sucrose cushion and thus remain in the supernatant. When HIV-1 was treated with Triton X-100 (a non-ionic detergent) at various concentrations, viral protein solubilization occurred when the Triton concentrations were above the CMC (see Figure 3). These data showed that the optimal conditions for solubilization of the virus were above the CMC for Triton X-100 (CMC = 0.021% w/v).(Coligan, 2001; Jones, Earnest, and McNamee, 1987) It was also noted that the amount of gp41 viral protein that was pelleted after solubilization in Triton was increased when the virus was pretreated with the aryl-azido compound, 1,5-diazidonaphthalene (DAN) and UVA irradiated for 15 minutes (Figure 3). The maximal difference in the amount of pelletable gp41 protein between untreated and DAN-treated samples, under the conditions tested, occurred for 1% Triton X-100. This percentage was therefore chosen as the percentage to test in subsequent experiments.
Figure 3.
Treatment of HIV-1 above the CMC is needed for viral membrane solubilization. HIV-1 control (no UV) samples were compared with HIV-1 treated with DAN (100 uM with UV for 15 minutes) followed by Triton X-100 (0%, 0.01%, 0.1% and 1%) treatment. Detergent-treated samples were then passed through a 25% sucrose cushion via ultracentrifugation. The percent gp41 in the pellet was determined. Data averaged from two separate independent experiments using HIV lot#3592. Error bars represent the difference between these experiments.
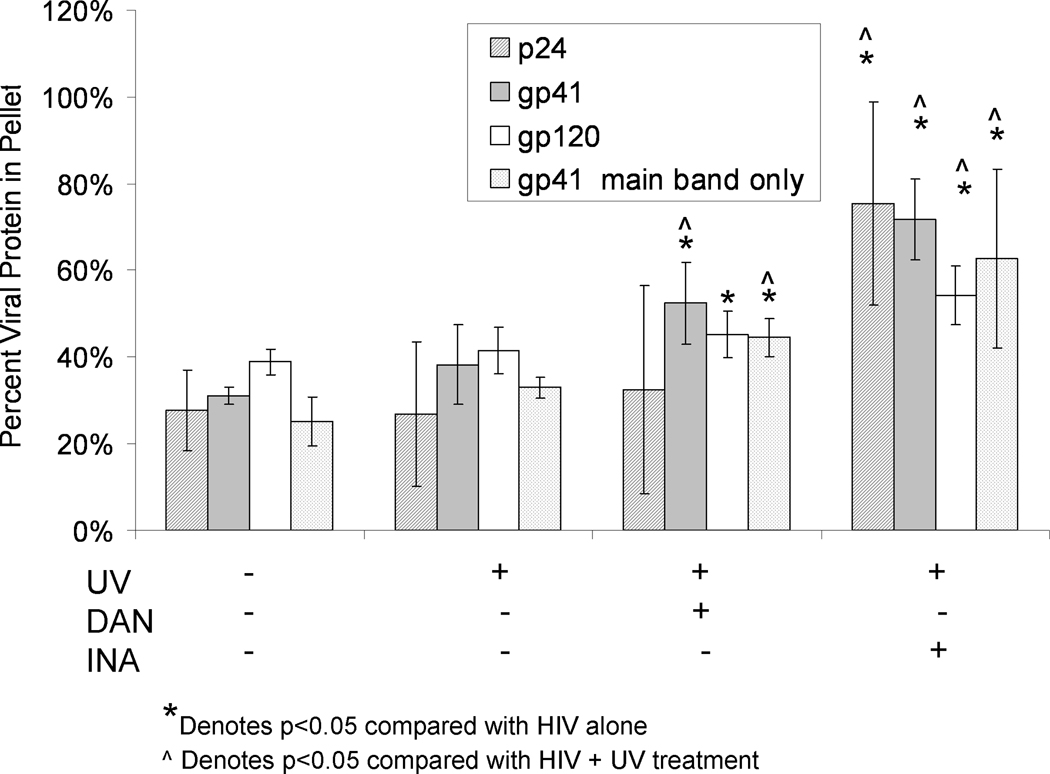
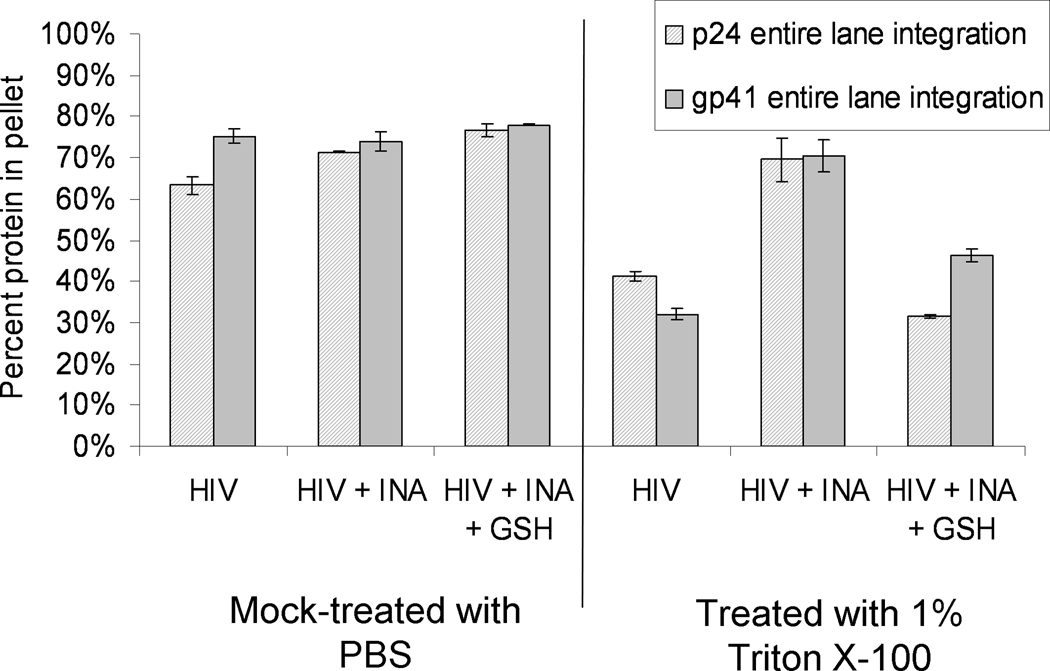
When 1% Triton was used, and the pellets and supernatants were assayed for the viral proteins p24, gp41 and gp120, there was an increase in the percent of some of these viral proteins in the pelleted fraction of INA or DAN-treated samples, versus non-treated controls (Figure 4). This increase of pelletable material was measured using the integration of the entire lanes for both the supernatant and pellet (as described in Materials and Methods) in the Western blot analysis to include the integration for the aggregated and non-aggregated (“main band”) regions for each protein. Analysis of these data for their significance (p<0.05) is given in Figure 4. In the DAN-treated samples, the percentage of gp41 and gp120 recovered in the pellet was significantly increased over the untreated control, whereas the percent of p24 was not. In the INA-treated samples, however, all of the proteins tested were significantly increased in the pelleted fraction, with p24 and gp41 approaching the value recovered from the non-solubilized HIV control (Figure 3). Additionally, the pelletable amount of all three proteins tested was significantly increased (p<0.05) with INA treatment compared to DAN treatment. This increase of pelletable material in the INA versus DAN treated samples correlates with the extent of protein aggregation seen in the Western blot analysis. There is an increase in the intensity of the aggregated protein region when INA is used versus other azido compounds.(Figure 2 and reference (Belanger et al., 2010)) This implies that the amount of aggregation as seen on the Western blot analysis may be correlated with the amount of pelletable material via sucrose cushion analysis.
Figure 4.
Detergent treatment of virus after treatment with aryl-azido compounds+UVA shows an increase in detergent-resistant pelletable material. The percent viral protein (either gp41, p24 or gp120) in the pellet was determined after treatment with 1.0 % Triton X-100. Prior to detergent treatment, the samples were either treated with aryl azides or were controls, as denoted below the graph. All hydrophobic compounds were used at a final concentration of 100 micromolar and irradiated with UV for 15 minutes. Data for each protein was averaged from separate independent experiments using fresh HIV lot#3602. All data is from integration of the entire lane in Western blot, except for the gp41 “main band only” data, which represents data obtained from the integration of the main gp41 band alone (as outlined in Materials and Methods). Error bars represent plus and minus two standard deviations. P-values were calculated between data sets using a two-tailed student’s T-test with unequal variances, with significant values (p<0.05) as denoted.
Interestingly, in the case of gp41, when the main band alone was integrated and compared between samples there was also a significant increase in the pelleted non-aggregated proteins (main band proteins) indicating that the aggregated proteins are associating with, or otherwise promoting the pelleting of, their non-aggregated viral protein counterparts.(Figure 4) In other words, after prolonged UV-irradiation in the presence of the aryl azide, non-aggregated monomeric (main band) protein is pelleted with the aggregated proteins. This is of important note, because the non-aggregated proteins would be expected to most closely represent the native viral proteins which would be advantageous to have present in a vaccine preparation. Also, since the non-aggregated proteins are increased in the detergent insoluble (pelleted) fraction, these proteins would not be excluded from a preparation where ultracentrifugation was used to purify or isolate the detergent insoluble fraction for use in vaccine preparation.
Detergent resistance is reversed when glutathione is used during UVA irradiation
To explore this correlation of detergent resistance as seen in the ultracentrifugation experiments with the aggregation seen in Western blot, experiments were performed where glutathione was added prior to UVA irradiation. As previously shown, the addition of glutathione and the enzymatic depletion of oxygen prior to the UVA irradiation step, result in the elimination of the higher molecular weight aggregates as seen by Western blot.(Belanger et al., 2010) When samples were INA-treated and UVA-irradiated in the presence of glutathione, they no longer maintained their detergent resistance (Figure 5). Similar results were seen when enzymatic depletion of oxygen was used prior to UVA irradiation (data not shown). This result shows that the presence of the detergent resistant fraction depends on the formation of ROS.
Figure 5.
Reactive oxygen species are needed to promote viral protein aggregation and detergent resistance. Sucrose cushion data showing the elimination of detergent resistance by the addition of glutathione prior to irradiation. This data uses HIV-1 MN lot #3602.
Immunoprecipitation with gp41 shows no co-immunoprecipitation of viral proteins within detergent-resistant aggregates
Immunoprecipitation of samples that were treated with INA plus UVA irradiation for 15 minutes was performed using RIPA buffer + 0.1% SDS and anti-gp41 MAb, to assess the nature of the “detergent resistant” aggregation. The same gp41 antibody was used for immunoprecipitation as was used for the primary antibody probe via Western blot analysis. When treated samples were immunoprecipitated using the anti-gp41 antibody, there was no co-immunoprecipitation of gp120, p17 or p24, although the gp41 protein aggregates remained (Figure 6a, p24 not shown). This suggests that the higher molecular weight smear of gp41 is due to gp41-gp41 interactions and possibly other protein-gp41 interactions involving other proteins on the virus that were not tested. Furthermore, since these aggregates could not be removed with 0.1% SDS and RIPA buffer (which contains 0.25% deoxycholic acid and 1% NP-40), it is possible that these interactions are covalent in nature.
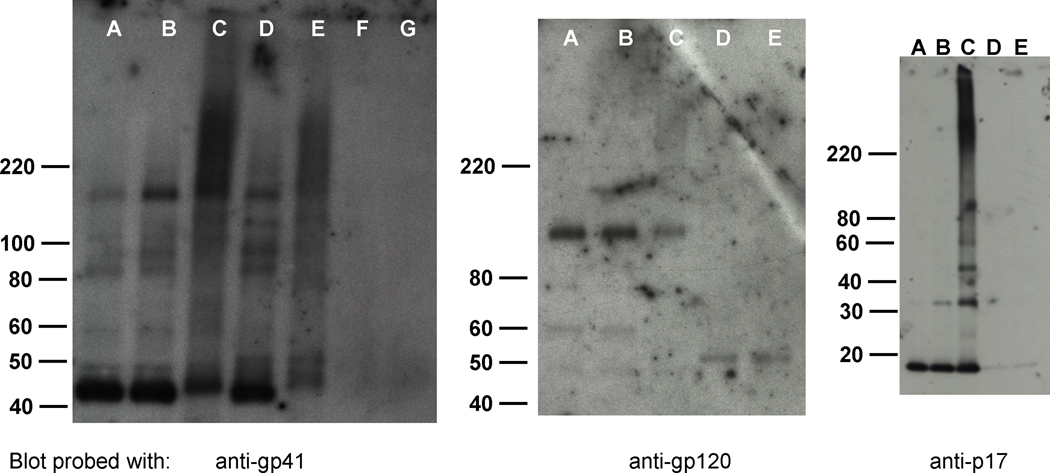
Figure 6.
Immunoprecipitation using anti-gp41 in RIPA buffer + 0.1% SDS, shows stable aggregation for gp41. Gp41 aggregates remain after IP using anti-gp41 but do not contain gp120, p17, or p24 (not shown). A,B, and C are non-immunoprecipitated controls, where A= HIV control, B= HIV + DMSO + UV 15 min., C= HIV + INA + UV irradiation 15 min.; D, E are immunoprecipitated samples using anti-gp41 and dynal beads with samples where, D= HIV + DMSO + UV irradiation 15 min., E= HIV + INA + UV irradiation 15 min.; and F and G are mock immunoprecipitated samples, using treated HIV samples with Dynal beads and no antibody. Westerns probed using antibodies noted below the blot image, with secondary antibodies described in Materials and Methods. Blots shown are representative samples of experiments done in duplicate.
Immunoprecipitation using neutralizing antibodies shows preservation of neutralization epitopes after triton-treatment
Previous studies have shown that treatment of HIV-1 with INA or DAN plus UV for 15 minutes does not damage key neutralizing epitopes.(Belanger et al., 2010) Herein, an additional immunoprecipitation experiment was performed using broadly neutralizing antibodies (NAbs) to assess whether or not these antibodies could still recognize the neutralization epitopes after the additional step of triton treatment, of the aryl-azide ROS-modified-virus. For this experiment, 1% Triton X-100 was used to lyse the virus after treatment with aryl azide, followed by binding of either an anti-gp41 NAb (“4E10”) or anti-gp120 NAb (“2g12”) and subsequent capture by protein G dynabeads. Attempts were made to first pellet the Triton-treated virus prior to NAb binding, but resuspension of the aryl-azide + triton treated viral pellet was difficult (as could be seen by eye) and led to irreproducible results. The data obtained for the triton-treated suspensions, without pelleting, is shown in Figure 7. In the HIV control (without UV irradiation), 1% triton treatment does not disrupt recognition of gp120 by 2g12, nor of gp41 by 4E10 (Figure 7, lanes C and D, respectively). Furthermore, triton treatment of the control does not disrupt the gp41–gp120 association. Treatment with INA + UV for 15 minutes, followed by 1% triton shows that there is some preservation of both 4E10 and 2g12 epitopes. Quantitative analysis of this data was not performed, however, it can be seen that the intensity of the bands from the INA-treated virus are diminished compared to the controls, suggesting that some damage to the epitopes may have taken place during this combined treatment of aryl azide + triton. Although damage to the epitopes may have occurred, 4E10 appears to immunoprecipitate the gp41 aggregates, suggesting that either the epitope is still present in the aggregated region or that the aggregated region is otherwise associated with a fraction containing the epitope. Interestingly, in the case of the INA-treated virus plus triton, it is seen that immunoprecipitation with 4E10 does not co-IP gp120, as it did in the control. This may be due to the INA-treated virus being more prone to gp120 shedding upon binding of 4E10. In support of this hypothesis, it has been previously noted that binding of 4E10 and similar antibodies promotes gp120 shedding, the extent of which may be virus strain dependent.(Ruprecht et al., 2011) These data provide evidence that although some damage of epitopes may have occurred, there is still some recognition by 2g12 and 4E10 in the treated preparations, which is advantageous for the use of this method towards vaccine preparation. Further optimization and characterization is still needed to assess preservation or destruction of additional epitopes, including those that may be novel to the aryl-azide + triton treated virus preparation.
Figure 7.
Immunoprecipitation (IP) of HIV-1 treated with INA plus UV irradiation, followed by triton treatment partially maintains two key neutralizing epitopes. Virus was either treated with INA plus UVA for 15 min., or left untreated, followed by treatment with 1% Triton X-100 for 1 hour at room temperature prior to IP using neutralizing antibodies 2g12 and 4E10. Western blotting using anti-gp41 and anti-gp120 (ran on separate blots) as shown. A) HIV-1 before IP (no triton), B) HIV + beads only, C) HIV + 2g12, D) HIV + 4E10, E) HIV+INA+UV 15 min+beads only, F) HIV+INA+UV 15 min.+2g12, G)HIV+INA+UV 15 min.+4E10. Heavy (H) chain from the immunoprecipitating antibody is also present.
TEM analysis of pelleted viral protein indicates overall virus structure is no longer intact
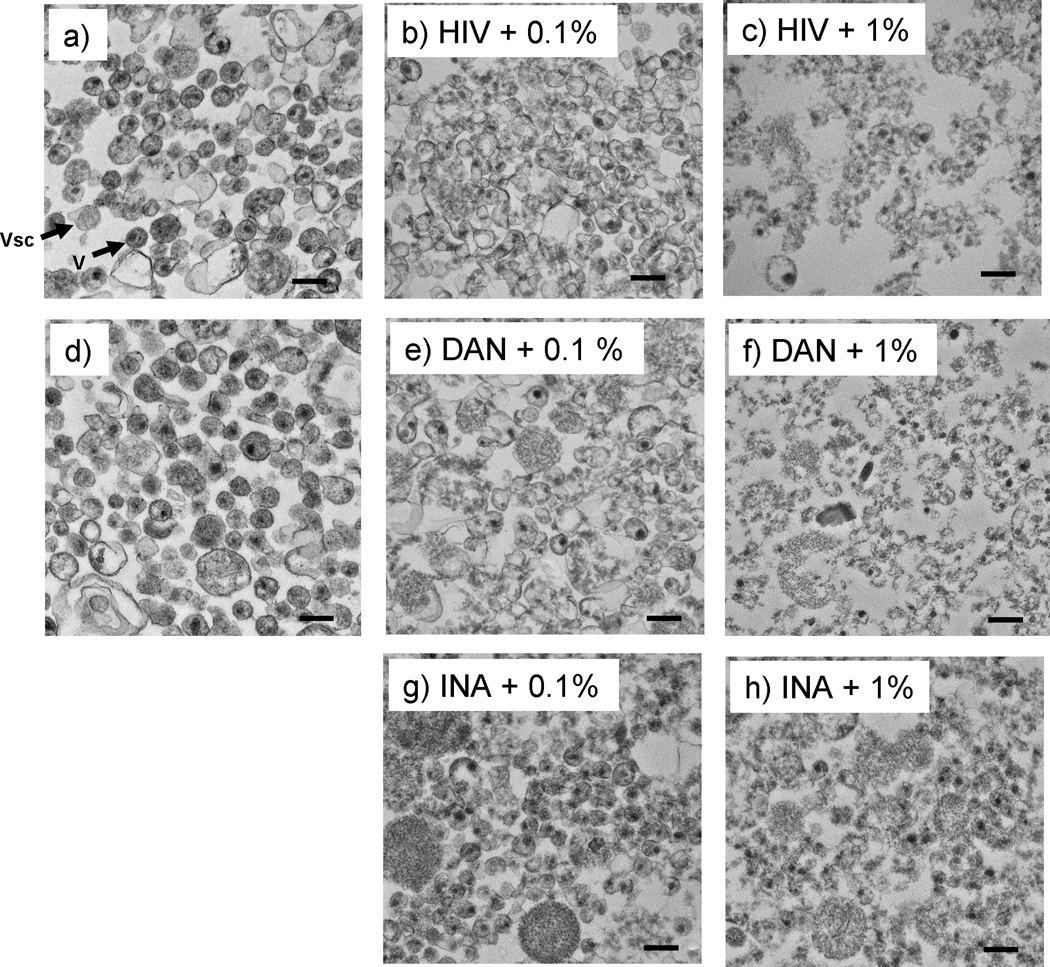
TEM analysis was performed on samples treated with aryl azides under conditions where ROS-induced viral protein aggregation occurred, followed by detergent treatment, and pelleting of the viral lysates. TEM studies of similar preparations and controls indicate that the overall virus structure after triton treatment is damaged, but fragments of virus are still intact (see Figure 8). HIV virions with damaged envelope that are reminiscent of intact virions can be seen, even after detergent treatment.
Figure 8.
Transmission electron microscopy (TEM) images of pelleted HIV after triton treatment show virions and viral protein aggregates remain after Triton treatment. Samples were treated as specified with either DAN or INA then treated with 1% Triton X-100 at room temperature for 1 hour before pelleting through 25% sucrose, and TEM analysis. a) and d) are controls of HIV and HIV+DAN+ UVA for 15 minutes, respectively; b and c are HIV controls + triton treatment with either 0.1% or 1% triton as specified; e-h are images of virus treated with DAN +UVA for 15 minutes, or INA +UVA for 15 minutes, with either 0.1% or 1% triton treatment, as specified. Arrows indicate: Vsc = microvesicle, V= HIV virion. Scale bars are 200 nm.
Similar studies using Vesicular Stomatitis and Sindbis viruses show that treatment with formaldehyde or gluteraldehyde (common fixation techniques) followed by detergent (SDS) also resulted in an increase in the pelletable fraction of viral proteins, with the resulting virus appearing as a shell of viral proteins in TEM.(Brown, Smale, and Horzinek, 1974) However, fixation can cause damage to surface epitopes in treated viruses. Our strategy, using ROS-induced protein aggregation promoted via aryl azide reactions, has been shown to preserve surface epitopes and also has the unique advantage that the reaction to generate the aggregates is dependent on the external influence of UVA irradiation.(Belanger et al., 2010) Conversely, treatment with fixatives usually requires additional chemical treatment and purification steps to scavenge any unreacted aldehydes. Although TEM indicates that the whole virons are damaged, it does support that there are intact viral fragments remaining. This TEM data combined with the aforementioned pelleting data (Figure 3), supports the hypothesis that the pelleted viral lysate of the aryl-azido treated virus contains more viral proteins than the pellet from the lysate of the infectious control.
Towards the generation of a Novel Orthogonally Inactivated Virus Vaccine Strategy
Using aryl-azides and UVA irradiation to inactivate enveloped viruses under conditions to create detergent-resistant viral protein aggregates through reactive oxygen species, provides a novel means for the production of an orthogonally inactivated virus preparation. This preparation has the unique advantage that the inactivated virions are partially detergent resistant, while any “missed” virions (such as those present in clumps or on walls of the treatment vessel) that may still be infectious are detergent sensitive. Thus, combining aryl-azide + UVA treatment, with a subsequent detergent treatment step, provides a means for the orthogonal inactivation of enveloped viruses towards the 15 logs of inactivation proposed(Schultz, Koff, and Lawrence, 1990) for an inactivated virus vaccine to be safely considered. Furthermore, the generation of detergent-resistant viral fragments that contain key viral proteins may provide an advantage over traditional split-virus vaccine strategies (see Figure 1), by potentially presenting unique epitopes preserved from the native virion structure to the immune system. It has been shown in vitro that some neutralizing epitopes are at least partially in tact in the treated HIV-1 virus, which is desirable towards the application of this method for the generation of vaccines. Further development and optimization of this method, including in vivo characterization against known vaccines is still needed, but this study provides a promising starting point for this novel strategy.
Conclusions
Preparation of inactivated virus vaccines using photoactivatable aryl azido compounds can be used not only for the preparation of inactivated whole virus vaccines, but can also be used towards the generation of a novel class of orthogonally inactivated virus vaccines. Prolonged UVA irradiation in the presence of aryl azides, such as 1,5-iodonaphthylazide (INA) resulted in ROS-induced viral protein aggregation, without damage to surface epitopes, which persisted even in the presence of detergent. This can be used towards an orthogonal inactivation technique whereby infectious material is rendered non-infectious through photoactivation of aryl azides, with further irradiation to induce ROS species, followed by detergent-treatment. The detergent treatment step provides a “mop-up” to remove any residual untreated infectious virus, while the treated virus, although no longer whole, maintains pelletable detergent-resistant aggregates, hypothesized to represent epitopes similar to those found in the native intact virus. Further studies are ongoing to characterize this effect in other viruses, such as influenza, and to compare this novel orthogonal inactivation strategy versus conventional chemical inactivation techniques for vaccine preparation, in animal studies.
Acknowledgments
We thank ARRRP for reagents, and Jeff Lifson and the AIDS and Cancer Virus Program for generously providing purified virus and anti-p17 antibody. We thank Randall Johnson for his assistance with the statistics. We also thank Julian W. Bess, Jr for many helpful discussions and his critical reading of the manuscript. This research was supported [in part] by federal funds from the Intramural AIDS Targeted Antiviral Program and the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler-Storthz K, Sehulster LM, Dreesman GR, Hollinger FB, Melnick JL. Effect of alkaline glutaraldehyde on hepatitis B virus antigens. Eur. J. Clin. Microbiol. 1983;2(4):316–320. doi: 10.1007/BF02019460. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Bast C, Hengartner H, Zinkernagel RM. Immunogenicity of a viral model vaccine after different inactivation procedures. Med. Microbiol. Immunol. 1994;183:95–104. doi: 10.1007/BF00277160. [DOI] [PubMed] [Google Scholar]
- Belanger JM, Raviv Y, Viard M, de la Cruz JM, Nagashima K, Blumenthal R. Characterization of the Effects of Aryl-azido Compounds and UVA Irradiation on the Viral Proteins and Infectivity of Human Immunodeficiency Virus Type 1. Photochem. Photobiol. 2010 doi: 10.1111/j.1751-1097.2010.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovici T, Gitler C. 5-[125I]Iodonaphthyl azide, a reagent to determine the penetration of proteins into the lipid bilayer of biological membranes. Biochemistry. 1978;17(8):1484–1489. doi: 10.1021/bi00601a020. [DOI] [PubMed] [Google Scholar]
- Brown F. Review of Accidents Caused by Incomplete Inactivation of Viruses. In: Brown F, editor. Virological Safety Aspects of Plasma Derivatives; Dev. Biol. Stand. Vol. 81. Basel: Karger; 1993. pp. 103–107. [PubMed] [Google Scholar]
- Brown F, Smale CJ, Horzinek MC. Lipid and Protein Organization in Vesicular Stomatitis and Sindbis Viruses. J. Gen. Virol. 1974;22:455–458. doi: 10.1099/0022-1317-22-3-455. [DOI] [PubMed] [Google Scholar]
- Chertova E, Bess JW, Jr, Crise BJ, Sowder RC, II, Schaden TM, Hilburn JM, Hoxie JA, Benveniste RE, Lifson JD, Henderson LE, Arthur LO. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type I and simian immunodeficiency virus. J. Virol. 2002;76(11):5315–5325. doi: 10.1128/JVI.76.11.5315-5325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coligan JE. Commonly Used Detergents. Curr. Prot. Immunol. 2001:A.1D.1–A.1D.3. doi: 10.1002/0471142735.ima01ds17. [DOI] [PubMed] [Google Scholar]
- Cox RJ, Hovden A-O, Brokstad KA, Szysko E, Madhum AS, Haaheim LR. The humoral immune response and protective efficacy of vaccination with inactivated split and whole influenza virus vaccines in BALB/c mice. Vaccine. 2006;24:6585–6587. doi: 10.1016/j.vaccine.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Duque H, Marshall RL, Israel BA, Letchworth GJ. Effects of formalin inactivation on bovine herpes virus-1 glycoprotein and antibody response elicited by formalin-inactivated vaccines in rabbits. Vaccine. 1989;7:513–520. doi: 10.1016/0264-410x(89)90275-2. [DOI] [PubMed] [Google Scholar]
- Geeraedts F, Bungener L, Pool J, ter Veer W, Wilschut J, Huckriede A. Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs. Influenza and Other Respiratory Viruses. 2008;2(2):41–51. doi: 10.1111/j.1750-2659.2008.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilberger J, Jurgens G. Oxidation of high-density lipoprotein HDL3 leads to exposure of apo-AI and apo-AII epitopes and to formation of aldehyde protein adducts, and influences binding of oxidized low-density lipoprotein to type I and type III collagen in vitro. Biochem. J. 1998;331:185–191. doi: 10.1042/bj3310185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grovit-Ferbas K, Hsu JF, Ferbas J, Gudeman V, Chen ISY. Enhanced binding of antibodies to neutralization epitopes following thermal and chemical inactivation of human immunodeficiency virus type 1. J. Virol. 2000;74(13):5802–5809. doi: 10.1128/jvi.74.13.5802-5809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones OT, Earnest JP, McNamee MG. Solubilization and Reconstitution of Membrane Proteins. In: Findlay J, Evans WH, editors. Biological Membranes: A Practical Approach. Oxford: IRL Press; 1987. [Google Scholar]
- Poon B, Safrit JT, McClure H, Kitchen C, Hsu JF, Gudeman V, Petropolous C, Wrin T, Chen ISY, Grovit-Ferbas K. Induction of humoral immune response following vaccination with envelope-containing, formaldehyde-treated, thermally inactivated human immunodeficiency virus type 1. J. Virol. 2005;79(8):4927–4935. doi: 10.1128/JVI.79.8.4927-4935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv Y, Blumenthal R, Tompkins SM, Humberd J, Hogan RJ, Viard M. Hydrophobic inactivation of influenza viruses confers preservation of viral structure with enhanced immunogenicity. J. Virol. 2008;82(9):4612–4619. doi: 10.1128/JVI.02233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv Y, Viard M, Bess JW, Jr, Chertova E, Blumenthal R. Inactivation of retroviruses with preservation of structural integrity by targeting the hydrophobic domain of the viral envelope. J. Virol. 2005;79(19):12394–12400. doi: 10.1128/JVI.79.19.12394-12400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht CR, Krarup A, Reynell L, Mann AM, Brandenburg OF, Berlinger L, Abela IA, Regoes RR, Gunthard HF, Rusert P, Trkola A. MPER-specific antibodies induce gp120 shedding and irreversibly neutralize HIV-1. J. Exp. Med. 2011;208(3):439–454. doi: 10.1084/jem.20101907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau OJ. Conservation of HIV-1 gp120 neutralizing epitopes after formalin inactivation. AIDS. 1995;9:1383–1385. doi: 10.1097/00002030-199512000-00017. [DOI] [PubMed] [Google Scholar]
- Schultz AM, Koff CW, Lawrence DN. Conference Reports: Workshop on HIV inactivated vaccines. Vaccine. 1990;8:516–517. [Google Scholar]
- Sharma A, Raviv Y, Puri A, Viard M, Blumenthal R, Maheshwari RK. Complete inactivation of venezuelan equine encephalitis virus by 1,5-iodonaphthylazide. Biochem. Biophys. Res. Commun. 2007;358:392–398. doi: 10.1016/j.bbrc.2007.04.115. [DOI] [PubMed] [Google Scholar]
- Sheppard HW. Inactivated- or Killed-Virus HIV/AIDS Vaccines. Curr. Drug Targets - Infect. Disord. 2005;5:131–141. doi: 10.2174/1568005054201599. [DOI] [PubMed] [Google Scholar]
- Shoukry MI, Gong EL, Nichols AV. Apolipoprotein-lipid association in oxidatively modified HDL and LDL. Biochim. Biophys. Acta. 1994;1210:355–360. doi: 10.1016/0005-2760(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Tano Y, Shimizu H, Martin J, Nishimura Y, Simizu B, Miyamura T. Antigenic characterization of a formalin-inactivated poliovirus vaccine derived from live-attenuated sabin strains. Vaccine. 2007;25:7041–7046. doi: 10.1016/j.vaccine.2007.07.060. [DOI] [PubMed] [Google Scholar]
- Uchida E, Morimoto K, Kawasaki N, Izaki Y, Said AA, Hayakawa T. Effect of Active Oxygen Radicals on Protein and Carbohydrate Moieties of Recombinant Human Erythropoietin. Free Rad. Res. 1997;27(3):311–323. doi: 10.3109/10715769709065769. [DOI] [PubMed] [Google Scholar]
- Warfield KL, Swenson DL, Olinger GG, Kalina WV, Viard M, Aitichou M, Chi X, Ibrahim S, Blumenthal R, Raviv Y, Bavari S, Aman MJ. Ebola virus inactivation with preservation of antigenic and structural integrity by a photoinducible alkylating agent. J. Infect. Dis. 2007;196(2):S276–S283. doi: 10.1086/520605. [DOI] [PubMed] [Google Scholar]