Abstract
Allosteric modulators for the Gi-coupled A3 adenosine receptor (AR) are of considerable interest as therapeutic agents and as pharmacological tools to probe various signaling pathways. In this study, we initially characterized the effects of several imidazoquinolinamine allosteric modulators (LUF5999, LUF6000 and LUF6001) on the human A3 AR stably expressed in CHO cells using a cyclic AMP functional assay. These modulators were found to affect efficacy and potency of the agonist Cl-IB-MECA differently. LUF5999 (2-cyclobutyl derivative) enhanced efficacy but decreased potency. LUF6000 (2-cyclohexyl derivative) enhanced efficacy without affecting potency. LUF6001 (2-H derivative) decreased both efficacy and potency. We further compared the agonist enhancing effects of LUF6000 in several other A3 AR-mediated events. It was shown that although LUF6000 behaved somewhat differently in various signaling pathways, it was more effective in enhancing the effects of low-efficacy than of high-efficacy agonists. In an assay of cyclic AMP accumulation, LUF6000 enhanced the efficacy of all agonists examined, but in the membrane hyperpolarization assay, it only enhanced the efficacy of partial agonists. In calcium mobilization, LUF6000 did not affect the efficacy of the full agonist NECA but was able to switch the nucleoside antagonist MRS542 into a partial agonist. In translocation of β-arrestin2, the agonist-enhancing effect LUF6000 was not pronounced. In an assay of ERK1/2 phosphorylation LUF6000 did not show any effect on the efficacy of Cl-IB-MECA. The differential effects of LUF6000 on the efficacy and potency of the agonist Cl-IB-MECA in various signaling pathway were interpreted quantitatively using a mathematical model.
Keywords: G protein-coupled receptors, purines, positive allosteric modulator, second messenger, nucleoside
1. Introduction
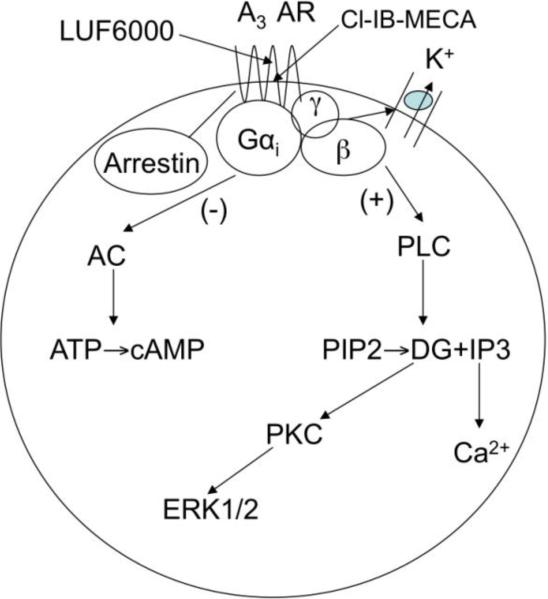
The A3 adenosine receptor (AR) is an attractive G protein-coupled receptor (GPCR) target for development of therapeutic agents for a number of conditions, such as inflammatory, ischemic and autoimmune diseases [1-6]. The principal signaling pathway of the Gi-coupled A3 AR is inhibition of 3’,5’-cyclic adenosine monophosphate (cyclic AMP) production, but a variety of other pathways are also affected by its activation (Fig. 1). Agonists of the A3 AR are currently in clinical trials for psoriasis, rheumatoid arthritis, osteoarthritis, hepatocellular carcinoma and other autoimmune inflammatory conditions [6-10]. Allosteric agonist enhancers of the A3 AR are of considerable interests as therapeutic agents and as pharmacological tools to explore various signaling pathways [11-13].
Fig. 1.
A3 AR-mediated signaling explored in the present study. AC, adenylyl cyclase; PLC, phospholipase C.
The screening of a small compound library identified an allosteric enhancer with weak antagonist activity, DU124183 [14]. Structural modification of DU124183 led to novel allosteric enhancers including LUF6000, which enhanced A3 AR agonist efficacy in a cyclic AMP assay without an effect on agonist potency [15]. Further characterization of the effect of LUF6000 on agonists with variable efficacies using a [35S]GTPγS binding assay demonstrated that LUF6000 was more effective in enhancing low-efficacy agonists and was able to convert a nucleoside antagonist MRS542 into an agonist [16]. A mathematical model simulating allosteric modulation [17] was subsequently used to rationalize the experimental findings [16]. Further modification of LUF6000 and its analogs led to novel classes of enhancers [18,19], but nevertheless LUF6000 is still among the most useful A3 AR enhancers.
In the present study, we have extended the previous studies by further examining the effects of three imidazoquinolinamine allosteric modulators of the A3 AR differing only in the substitution at the 2-position, LUF5999, LUF6000 and LUF6001. In general, the action of allosteric modulators of GPCRs is multifactorial, and each modulator requires characterization of saturability of a given effect, differential effects on affinity vs. efficacy, probe dependence (i.e. effects may differ for different orthosteric ligands), and functional bias (selectivity based on specific signaling pathways), and other properties [20-23]. The effects of these modulators on the efficacy and potency of the agonist Cl-IBMECA were initially measured in a cyclic AMP functional assay using CHO cells expressing the human A3 AR (in the absence of other AR subtypes). We also compared the effect of one of those enhancers, LUF6000, on several other A3 AR-mediated signaling pathways (Fig. 1). We then simulated the experimental curves, rationalized the experimental findings, and particularly analyzed the potential factors leading to the inability of LUF6000 to enhance the efficacy of MRS542 in assays of cyclic AMP accumulation and membrane hyperpolization, as was demonstrated previously in a [35S]GTPγS binding assay [16,24].
2. Materials and Methods
2.1. Materials
The nucleosides NECA (adenosine-5′-N-ethyluronamide), CCPA (2-chloro-N6-cyclopentyladenosine), Cl-IB-MECA (2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide), adenosine and inosine were from Sigma (St. Louis, MO, USA). LUF6000 (N-(3,4-dichloro-phenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine), LUF5999 (N-(3,4-dichloro-phenyl)-2-cyclobutyl-1H-imidazo[4,5-c]quinolin-4-amine) and LUF6001 (N-(3,4-dichloro-phenyl)-1H-imidazo[4,5-c]quinolin-4-amine) were synthesized at Leiden/Amsterdam Center for Drug Research (Leiden, The Netherlands) [15]. MRS541 (N6-(3-iodobenzyl)adenosine) and MRS542 (2-chloro-N6-(3-iodobenzyl)adenosine) were synthesized at NIDDK, National Institutes of Health (Bethesda, MD, USA) [25]. Calcium and membrane potential assay kits were from Molecular Devices (Sunnyvale, CA, USA). Luminescence assay kits for β-arrestin2 translocation and PathHunter CHO cells stably expressing modified versions of both the A3 AR and β-arrestin2 were from DiscoveRx (Fremont, CA, USA). [3H]cyclic AMP (40 Ci/mmol) was purchased from Amersham (Buckinghamshire, UK). AlphaScreen SureFire p-ERK1/2 (Thr202/Tyr204) Assay Kits were from PerkinElmer (Groningen, Netherlands).
2.2. AR agonists and allosteric modulators used in the present study
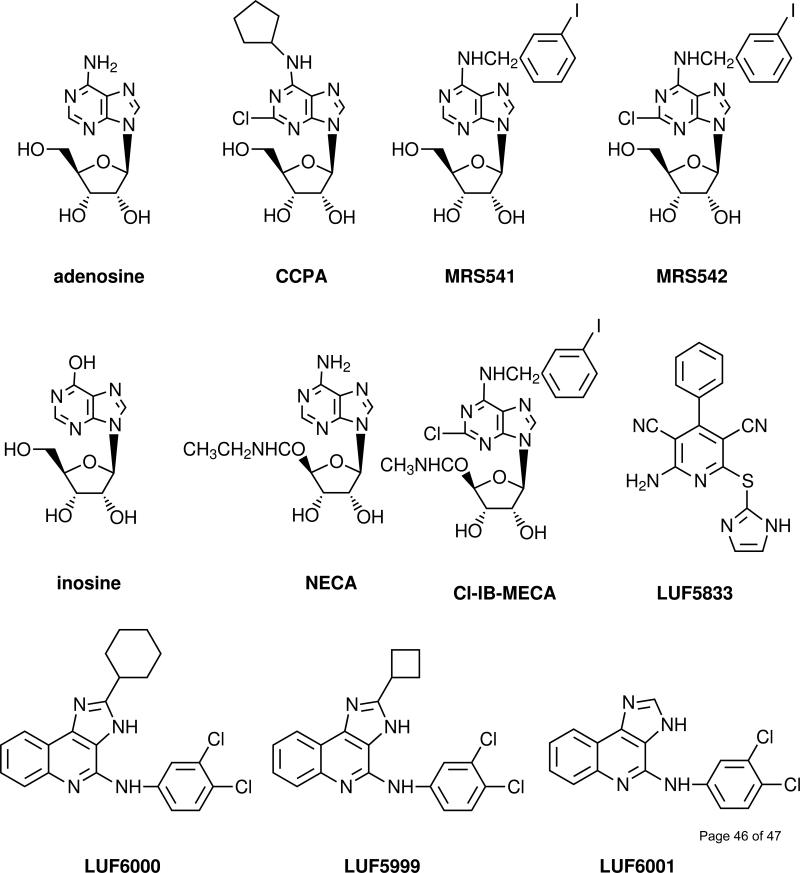
A3 AR agonists (Fig. 2) having a range of relative efficacies were selected for this study, in order to examine if there is a differential effect by the same allosteric modulator. The agonists used included NECA, a high-efficacy and non-selective AR agonist, the N6, 2, 5’-substituted derivative Cl-IB-MECA, which is a potent and selective A3 AR agonist, and a nucleoside A3 AR antagonist MRS542 (Fig. 2) [16,25]. Other agonists of diverse structure include the native agonist adenosine and the following partial A3 AR agonists: inosine, an N6-substituted adenosine derivative MRS541, the N6-substituted 2-chloroadenosine derivative CCPA [25] and a non-nucleoside adenosine agonist, LUF5833 [26]. Inosine was earlier noted to bind to the rat A3 AR [27] and to stimulate mast cell degranulation [28]. In several assays it was found that NECA was either similar in efficacy to or more efficacious than Cl-IB-MECA as an agonist for the A3 AR [16,25,29], although Cl-IB-MECA is a more potent and selective agonist. Thus, the efficacy of NECA was set as 100% in all of the following assays.
Fig. 2.
Chemical structures of the agonists, antagonists, and allosteric modulators of the A3 AR used in the present study.
Allosteric modulators (Fig. 2) used in the present study included LUF5999, LUF6000 and LUF6001, which differ only in the substitution at the 2 position. Each of these compounds was selected based on its potentially characteristic effect on agonist efficacy and binding properties of orthosteric ligands, such as dissociation rate, as demonstrated or indicated by previous [14-16]. LUF6000, at concentrations up to 10 μM, did not bind appreciably at the other subtypes of ARs [15,16]. The enhancement of the endogenous agonist inosine was evident only in the cyclic AMP assay.
2.3. Cyclic AMP accumulation assay
CHO cells stably expressing the recombinant human A3 AR were cultured in DMEM and F12 (1:1) supplemented with 10% fetal bovine serum, 100 Units/ml penicillin, 100 μg/ml streptomycin and 2 μmol/ml glutamine. Cells were plated in 24-well plates in 0.5 ml medium. After 24 h, the medium was removed and cells were washed three times with 1 ml DMEM, containing 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4. Cells were then treated with agonists and/or test compounds in the presence of rolipram (10 μM) and adenosine deaminase (3 units/ml). After 30 min, forskolin (10 μM) was added to the medium, and incubation was continued for an additional 15 min. The reaction was terminated by removing the supernatant, and cells were lysed upon the addition of 200 μl of 0.1 M ice-cold HCl. The cell lysate was resuspended and stored at -20°C. For determination of cyclic AMP production [30], protein kinase A (PKA) was incubated with [3H]cyclic AMP (2 nM) in K2HPO4/EDTA buffer (K2HPO4, 150 mM; EDTA, 10 mM), 20 μl of the cell lysate, and 30 μL 0.1 M HCl or 50 μl of cyclic AMP solution (0-16 pmol/200 μl for standard curve). Bound radioactivity was separated by rapid filtration through Whatman GF/C filters, which were washed once with cold buffer. Bound radioactivity was measured by liquid scintillation spectrometry.
2.4. Intracellular calcium mobilization and membrane hyperpolarization assay
The measurement of intracellular calcium mobilization was essentially as previously described [31]. Briefly, CHO cells stably expressing the human A3 AR were grown overnight in 100 μl of media in 96-well flat bottom plates at 37°C at 5% CO2 or until approx. 90% confluency. The calcium assay kit was used as directed without washing cells, and with probenecid added to the loading dye at a final concentration of 2.5 mM to increase dye retention. Cells were incubated with 50 μl dye/probenecid for 60 min at room temperature. The compound plate was prepared using dilutions of various compounds in Hank's Buffer (pH 7.4). Samples were run in duplicate using a Flexstation (Molecular Devices, Sunnyvale, CA, USA) at room temperature. Cell fluorescence (Excitation = 485 and Emission = 525 nm) was monitored following exposure to the compound. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
For the measurement of A3 AR-mediated hyperpolarization, the experimental procedure was similar to that used in the calcium assay, except that membrane potential dye and different wavelengths of excitation (535 nm) and emission (565 nm) were used. Mechanistically, this measurement is based on a Gi-coupled AR-mediated hyperpolarization and the measurement of membrane potential fluorescence of nicotinic receptor-mediated depolarization [32,33].
2.5. Methods of ERK1/2 activation
CHO-A3 cells (40,000 cells/200 μl) were seeded in a 96 well plate in complete medium. After cell attachment, medium was removed and cells were serum-starved overnight in medium without newborn calf serum (90 μl). Dilutions of LUF6000 and Cl-IB-MECA were prepared in PBS from a 10-2 M DMSO solution of the compounds. LUF6000 (10 μl) or DMSO control (final concentration 0.1% corresponding to the highest concentration of LUF6000 that was used) was added in a 10-fold concentrated solution and cells were pre-incubated for 20 min. Cl-IB-MECA (10 μl) was added in a 11-fold concentrated solution in PBS and cells were stimulated for 5 min. Medium was removed and cells were lysed with 1x Lysis buffer (25 μl) (AlphaScreen SureFire p-ERK1/2 (Thr202/Tyr204) Assay Kit). Lysate (4 μl/well) was transferred to a 384 well ProxiPlate Plus (PerkinElmer, Groningen, Netherlands). Acceptor Beads were diluted 1:100 in a 1:5 mixture of Activation buffer in Reaction Mix and added to the 384 well plate (5 μl/well). The plate was sealed, agitated on a plate shaker (2 min 600 rpm) and incubated for 2 h at 22°C. Next, Donor beads (2 μl) diluted 1:40 in Dilution buffer were added. The plate was again sealed, agitated on a plate shaker (2 min 600 rpm) and incubated for 2 h at 22°C. The plate was measured on an EnVision multilabel reader using standard AlphaScreen settings.
2.6. β-Arrestin2 translocation assay in CHO cells stably expressing the human A3 AR
The β-arrestin2 translocation assay was performed using the PathHunter™ β-arrestin assay kit from DiscoveRx (Fremont, CA, USA) as previously described [24]. Briefly, PathHunter CHO cells expressing the human A3AR were grown in 96- or 384-well plates for 24 hours in Hank's F-12 medium supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 μmol/mL glutamine. Cells were treated with LUF6000 for 20 min before the addition of agonists to be incubated for an additional 60 min. Cells were then treated with detection reagents (mixture of 1 part Galacton Star substrate with 5 parts Emerald II™ Solution, and 19 parts of PathHunter Cell Assay Buffer), and incubated at room temperature for an additional 60 min before luminescence was measured.
2.7. Simulation of concentration-effect curves using MatLab
A mathematical model for allosteric modulation [17] was implemented in MatLab (version 7.1), a software package for technical computing. A graphic user interface was composed to facilitate parameter input and to yield output in the form of simulated curves.
Statistical analysis
Functional parameters were calculated using Prism 5.0 software (GraphPAD, San Diego, CA, USA). Data were expressed as mean ± standard error. Data were analyzed by analysis of variance (ANOVA) followed by post hoc analysis to check the statistical difference among groups with P<0.05 being considered significant.
3. Results
3.1. Pharmacological characterization
3.1.1. Distinct effects of LUF6000, LUF5999 and LUF6001 on the potency and efficacy of the selective A3 AR agonist Cl-IB-MECA in a cyclic AMP functional assay using CHO cells stably expressing the human A3 AR
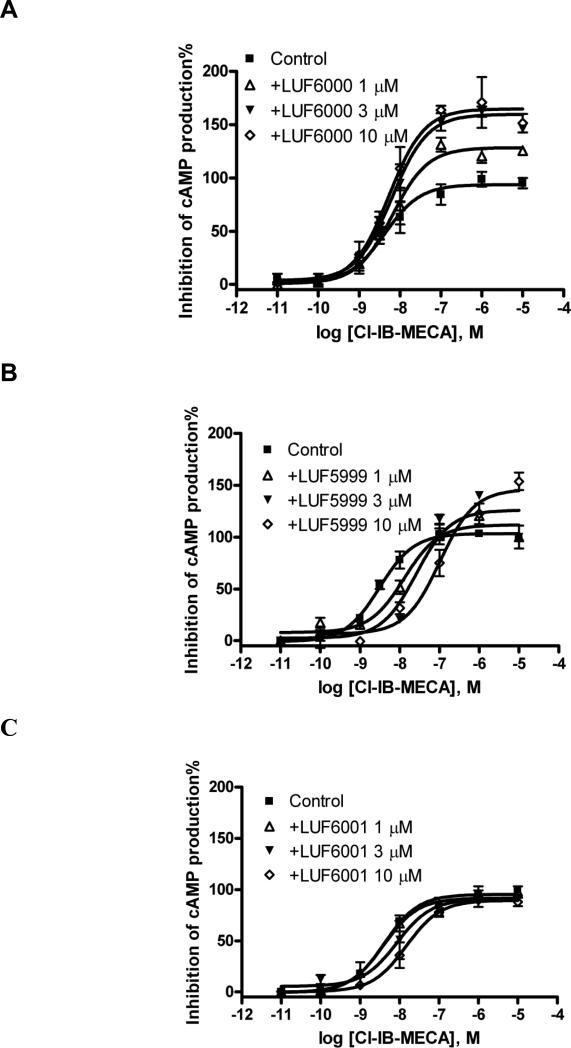
LUF6000 enhanced the efficacy of Cl-IB-MECA to inhibit forskolin-stimulated cyclic AMP levels in CHO cells stably expressing the human A3 AR (Fig. 3A). The enhancing effect was concentration dependent, starting from ≤ 1 μM and reaching a maximum at approx. 10 μM. A higher concentration (30 μM) of LUF6000 did not cause further enhancement (data not shown), thus suggesting a saturation effect of this modulator. At a concentration of 10 μM or lower, LUF6000 did not significantly affect the agonist potency. Unlike LUF6000, LUF5999 has mixed effects on agonist action, i.e. concentration-dependently increasing efficacy while decreasing potency (Fig. 3B). LUF6001 slightly decreased both the efficacy and potency of the A3 AR agonist (Fig. 3C). The efficacy and potency of Cl-IB-MECA in the absence and presence of allosteric modulators are listed in Table 1.
Fig. 3.
Effect of LUF6000 and its analogues on the potency and efficacy of the A3 AR agonist, Cl-IB-MECA, measured in a cyclic AMP accumulation assay using intact CHO cells stably expressing the human A3 AR. Agonist effect on the y-axis represents percent inhibition of cyclic AMP production. Cells were pretreated with LUF6000 (A) or its analogues (B, C), 20 min before the addition of an agonist. Data were representative of 2-4 separate experiments providing similar results performed in duplicate.
Table 1.
Differential effects of LUF6000, LUF5999 and LUF6001 on the efficacy and potency of the agonist Cl-IB-MECA determined in a cyclic AMP functional assay using CHO cells stably expressing the human A3 AR.
| Compound(s) | C1-IB-MECA, cyclic AMP assay | |
|---|---|---|
| Emax | -logEC50 | |
| Control | 95.8 ± 4.8 | 8.38 ± 0.15 |
| +LUF6000 1.0 μM | 121 ± 5.3 | 8.26 ± 0.13 |
| +LUF6000 3.0 μM | 141 ± 7.8* | 8.32 ± 0.16 |
| +LUF6000 10 μM | 148 ± 9.4* | 8.36 ± 0.18 |
| +LUF5999 1.0 μM | 112 ± 4.4 | 7.89 ± 0.13 |
| +LUF5999 3.0 μM | 126 ± 8.6 | 7.59 ± 0.21 |
| +LUF5999 10 μM | 146 ± 7.6* | 6.99 ± 0.14 |
| +LUF6001 1.0 μM | 93.7 ± 2.8 | 8.40 ± 0.11 |
| +LUF6001 3.0 μM | 90.6 ± 4.2 | 8.07 ± 0.15 |
| +LUF6001 10 μM | 89.5 ± 3.4 | 7.83 ± 0.12 |
Significantly different from control group (P<0.05).
3.1.2. Effects of LUF6000 on the potency and efficacy of structurally diverse agonists in the cyclic AMP assay using CHO cells expressing the human A3 AR
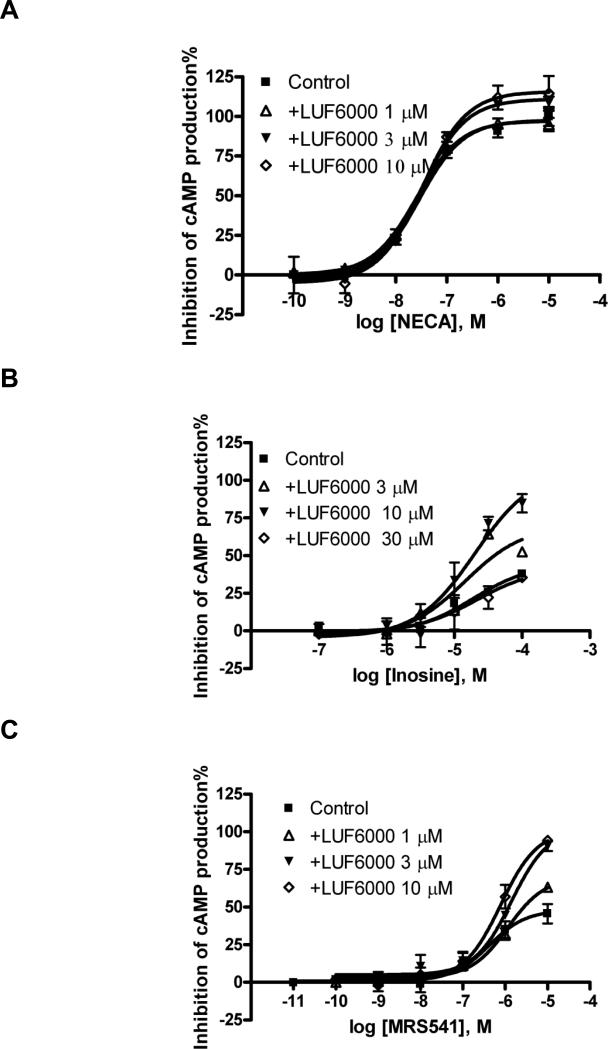
The efficacy-enhancing effect of LUF6000 was further tested with other structurally diverse agonists (Table 2). The maximum enhancement of the efficacy of NECA (Fig. 4A) by 10 μM LUF6000 was about 16%, which was lower than the relative enhancement of the efficacy of Cl-IB-MECA (50%). However, LUF6000 (10 μM) augmented the efficacy of two partial agonists, MRS541 (Fig. 4C) and LUF5833 (Fig. 4D), to over 200% of the control level (agonist alone). Interestingly, LUF6000 (10 μM) increased the efficacy of inosine (about 300% of control; Fig. 4B) to nearly full agonism, i.e. 85% of the level of NECA. However, LUF6000 had no effect on the efficacy of two nucleoside antagonists of the A3 AR, MRS542 (Fig. 4E) and CCPA (Fig. 4F). Thus, LUF6000 had little or no effect on full agonists such as NECA, and antagonists such as MRS542 and CCPA, but had a much larger enhancing ability for the partial agonists with low to medium efficacy. LUF6000 also did not show any effect on the inability of nonnucleoside A3 AR antagonists MRS1220 and MRS1191 [34] to inhibit forskolin-stimulated cyclic AMP levels in A3 AR-expressing CHO cells (data not shown).
Table 2.
Effect of LUF6000 on the potency and efficacy of structurally diverse agonists measured at different signaling pathways mediated via the A3 AR.a
| Compound(s) | cyclic AMP | Ca2+ | hyperpolarization | β-arrestin2 | p-ERK | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emax | -logEC50 | Emax | -logEC50 | Emax | -logEC50 | Emax | -logEC50 | Emax | -logEC50 | |
| NECA | 100 | 7.55 ± 0.10 | 100 | 7.24 ± 0.14 | 100 | 7.02 ± 0.09 | 100 | 7.11 ± 0.12 | ||
| +LUF 1.0 μM | 101 ± 1.9 | 7.59 ± 0.06 | ||||||||
| +LUF 3.0 μM | 111 ± 2.0* | 7.49 ± 0.05 | ||||||||
| +LUF 10 μM | 116 ± 4.3* | 7.48 ± 0.11 | 106 ± 6 | 7.05 ± 0.16 | 103 ± 4 | 6.92 ± 0.10 | 106 ± 2 | 6.82 ± 0.08 | ||
| Cl-IB-MECA | 95.8 ± 4.8 | 8.38 ± 0.15 | 55.2 ± 3.4 | 7.53 ± 0.17 | 58.3 ± 3.6 | 7.27 ± 0.17 | 104 ± 5 | 7.95 ± 0.14 | 99 ± 6 | 7.42 ± 0.30 |
| +LUF 1.0 μM | 121 ± 5.3 | 8.26 ± 0.13 | 63.8 ± 6.5 | 7.42 ± 0.24 | 86.3 ± 5.6# | 7.44 ± 0.19 | 101 ± 10 | 7.10 ± 0.43 | ||
| +LUF 3.0 μM | 141 ± 7.8# | 8.32 ± 0.16 | 73.8 ± 6.4# | 7.38 ± 0.22 | 97.6 ± 5.2# | 7.23 ± 0.15 | 102 ± 7 | 7.74 ± 0.38 | ||
| +LUF 10 μM | 148 ± 9.4# | 8.36 ± 0.18 | 67.7 ± 3.4 | 7.06 ± 0.13 | 103 ± 5# | 7.25 ± 0.12 | 114 ± 4 | 7.64 ± 0.13 | 98 ± 6 | 7.77 ± 0.33 |
| MRS542 | NA | NA | 7.5 ± 2.5 | NA | -3.8 ± 5.6 | NA | 41.1 ± 3.2 | 7.36 ± 0.24 | ||
| +LUF 1.0 μM | NA | NA | 14.8 ± 4.1 | 6.60 ± 0.84 | NA | NA | 51.3 ± 3.6 | 7.28 ± 0.22 | ||
| +LUF 3.0 μM | NA | NA | 31.8 ± 3.0** | 7.00 ± 0.24 | NA | NA | ||||
| +LUF 10 μM | NA | NA | 43.4 ± 3.5** | 6.74 ± 0.19 | -4.9 ± 4.7 | NA | 59.6 ± 2.2** | 6.94 ± 0.09 | ||
LUF, LUF6000; Results are expressed as mean ± SE. The Emax of NECA was set as 100% in all assays. The efficacies of other agonists (1 or 10 μM where appropriate) in the presence and absence of LUF were normalized based on the efficacy of NECA. MRS542 did not show any significant effect in inhibiting forskolin-stimulated cyclic AMP accumulation or inducing membrane potential changes. NA, no activity or not applicable.
Significant from NECA control group (P<0.05).
Significantly different from Cl-IB-MECA control group (P<0.05).
Significantly different from MRS542 control group (P<0.05).
Fig. 4.
Functional enhancing effect of LUF6000 in a cyclic AMP functional assay on a full agonist NECA (A), partial agonists, inosine (B) and MRS541 (C) (nucleoside) and LUF5833 (D, non-nucleoside), and nucleoside antagonists MRS542 (E) and CCPA (F) in a cyclic AMP functional assay of the A3AR. Agonist effect on the y-axis represents percent inhibition of cyclic AMP production. Cells were pretreated with LUF6000 20 min before the addition of an agonist. Results were expressed as mean ± SEM from three separate experiments providing similar results performed in duplicate.
3.1.3. Effects of LUF6000 on A3 AR agonist-mediated membrane hyperpolarization in CHO cells expressing the human A3 AR
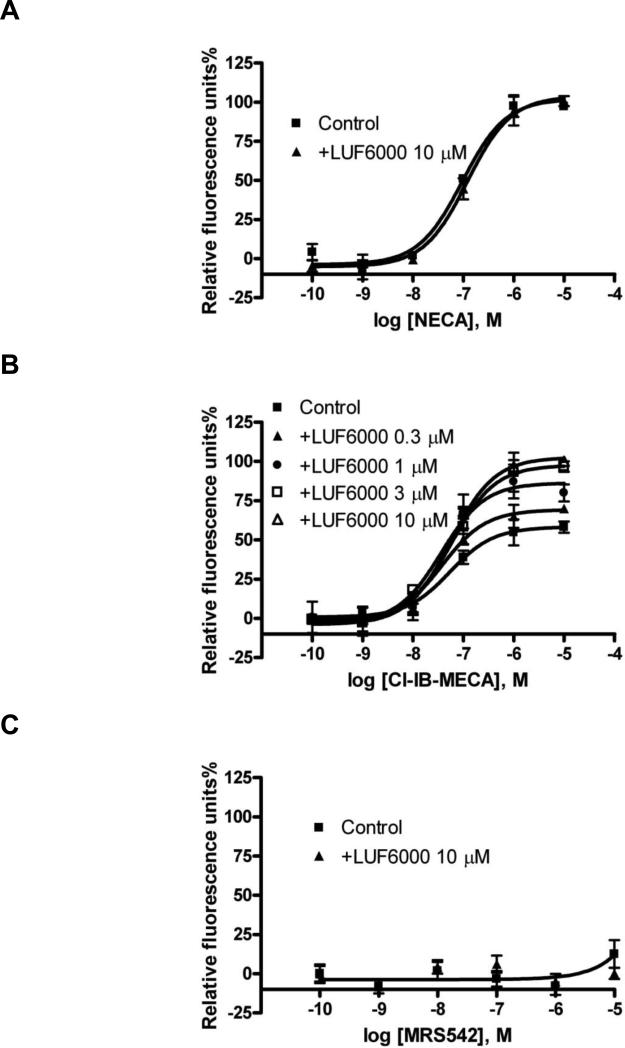
AR agonists can induce hyperpolarization of membranes, which is related to K+ channel activation [33]. In an assay of A3 AR-mediated membrane hyperpolarization, as detected by a fluorescence change of membrane potential, NECA was shown to have a much higher efficacy than Cl-IB-MECA (Fig. 5). LUF6000 alone did not show any effect. We compared the effect of LUF6000 on the efficacy of various agonists in this assay. LUF6000 showed a more pronounced effect on the efficacy of the partial agonist Cl-IBMECA and but did not have any effect on the full agonist NECA (Fig. 5A). The endogenous agonist adenosine was found to be equally efficacious as NECA, and LUF6000 also did not show any effect on its efficacy (data not shown). Interestingly, LUF6000 (10 μM) raised the efficacy of Cl-IB-MECA to almost exactly the level of NECA (Fig. 5B). A higher concentration of LUF6000 (30 μM) did not induce a further increase of the efficacy. The nucleoside antagonist MRS542 did not show any agonist activity in the assay and LUF6000 did not have any enhancing effect (Fig. 5C). Also, other agonists characterized as having low efficacy in the cyclic AMP functional assay, such as inosine, MRS541 and the non-nucleoside LUF5833, did not show any significant activation of the A3 AR in the membrane hyperpolarization assay, and LUF6000 did not show any enhancement of the residual activity of these compounds.
Fig. 5.
Effects of LUF6000 on agonist-induced membrane potential changes in CHO cells stably expressing the human A3 AR. Cells were pretreated with LUF6000 20 min before the addition of agonists. Data were from three separate experiments providing similar results performed in duplicate. Cells were incubated with 50 μl membrane potential dye/probenecid for 60 min at room temperature. The compound plate was prepared using dilutions of various compounds in Hank's Buffer (pH 7.4). Cell fluorescence (Excitation = 535 and Emission = 565 nm) was monitored following exposure to the compound. Cell fluorescence changes are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
3.1.4. Effects of LUF6000 on A3 AR agonist-mediated intracellular calcium mobilization in CHO cells expressing the human A3 AR
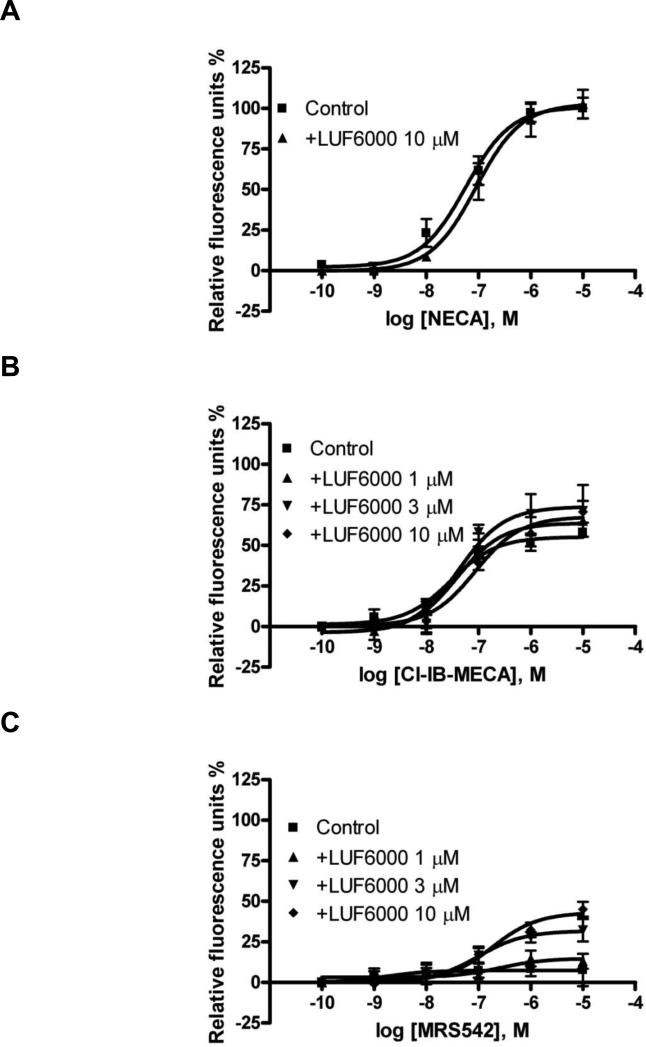
Next, we studied if LUF6000 could affect A3 AR-mediated intracellular calcium mobilization. As is shown in Fig. 6, similar to A3 AR agonist-induced hyperpolarization, NECA was more efficacious, albeit less potent, than Cl-IB-MECA in mediating intracellular calcium mobilization in CHO cells expressing the human A3 AR. LUF6000 (10 μM) caused a modest but significant decrease of the potency of NECA without significantly affecting its efficacy. Fig. 6B shows that LUF6000 induced a slight but significant enhancement of the efficacy of Cl-IB-MECA. MRS542 has been shown to be to be an A3 AR antagonist in an assay of cyclic AMP [21]. However, MRS542 showed some residual efficacy in this assay of calcium mobilization. In the presence of 3 and 10 μM of LUF6000, the efficacy of MRS542 was significantly elevated (Fig. 6C). LUF6000 alone had no effect on the A3 AR-mediated intracellular calcium mobilization in CHO cells.
Fig. 6.
Effect of LUF6000 (10 μM) on agonist-induced calcium mobilization in CHO cells stably expressing the human A3 AR. Cells were pretreated with LUF6000 20 min before the addition of agonists. Data were from 3-4 separate experiments providing similar results performed in duplicate.
3.1.5. Effects of LUF6000 on A3 AR agonist-mediated β-arrestin2 translocation in CHO cells expressing the human A3 AR
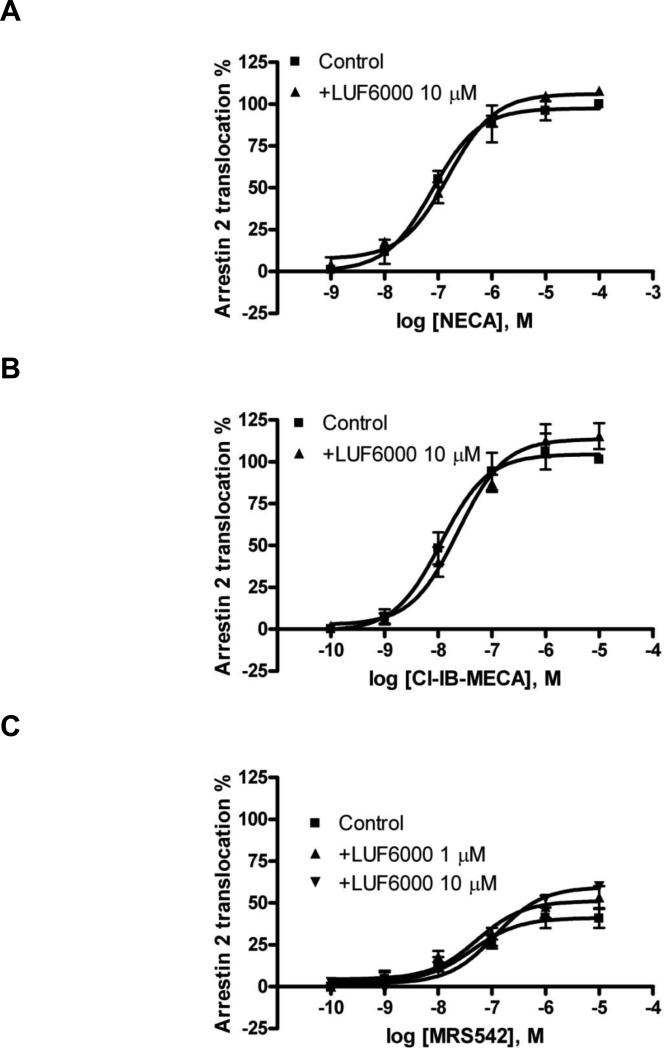
NECA and Cl-IB-MECA were both shown to be full agonists in inducing β-arrestin2 translocation mediated by the human A3 AR [24], and MRS542 was shown to be a partial agonist. LUF6000 (10 μM) caused a less than 10% enhancement of both the effects of NECA and Cl-IB-MECA, and about 40% enhancement of the efficacy of MRS542 (Fig. 7).
Fig. 7.
Effect of LUF6000 (10 μM) on agonist-induced translocation of β-arrestin2 in CHO cells stably expressing the human A3 AR. Cells were pretreated with LUF6000 20 min before the addition of agonists. Data were from 3-4 separate experiments providing similar results performed in duplicate.
3.1.6. Effect of LUF6000 on agonist Cl-IB-MECA-induced ERK1/2 phosphorylation in CHO cells expressing the human A3 AR
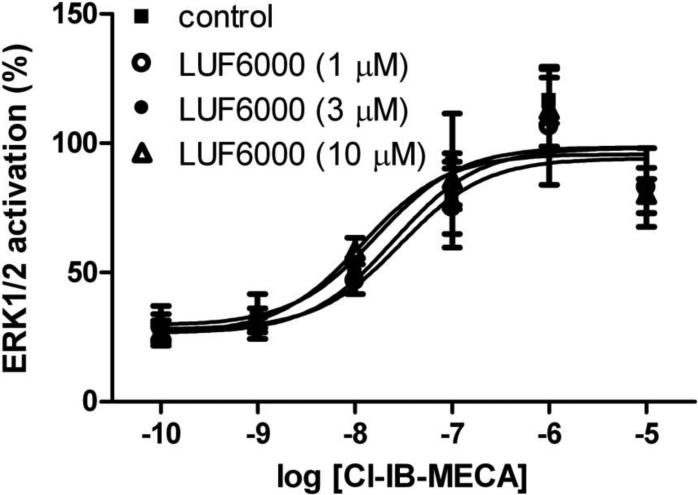
We further tested the potential effect of LUF6000 on the efficacy of Cl-IB-MECA in inducing ERK1/2 activation. Fig. 8 shows that LUF6000 at concentrations of 1, 3, and 10 μM, unlike its behavior in other signaling pathways, had no effect on the efficacy of Cl-IB-MECA.
Fig. 8.
Effect of LUF6000 on Cl-IB-MECA-induced ERK1/2 phosphorylation in CHO cells expressing the human A3 ARs. Results are from 2 separate experiments performed in duplicate. Cells were pre-incubated with various concentrations of LUF6000 for 20 min before addition of agonists.
3.2. Mathematical modeling
The experimental curves representing the effects of LUF6000 on the efficacy of various agonists in stimulating [35S]GTPγS binding have been simulated previously [16]. In the present study, we extended this mathematical modeling by simulating the effects of LUF6000 on the action of agonist Cl-IB-MECA in various signaling pathways. The equations from Hall [17] and experimental curves from the measurement of various pathways were used as a basis for simulation of experimental curves and to derive conditions in which efficacy and potency vary.
The fraction of receptors in the active state of the total pool of receptors can be expressed as: [R]active / [R]T = ([R*] + [AR*] + [R*B] + [AR*B]) / RT, which can be re-stated as:
L is the receptor isomerization constant (the ratio of receptor in the active state over the inactive state). K is the equilibrium association constant of the orthosteric ligand A. M is the equilibrium association constant of ligand B (allosteric modulator). γ is the intrinsic efficacy of ligand A. β is the intrinsic efficacy of ligand B. γ is the binding coöperativity between A and B. δ is the activation coöperativity between A and B. L is only related to the receptor. The allosteric modulator brings three properties, β, γ and δ, to the system.
We simulated the concentration-response curves for Cl-IB-MECA ion the absence and presence of various concentrations of LUF6000. Parameter settings are listed in Table 3.
Table 3.
Parameters used in MATLAB simulations.
| Pathway | L | K | M | α | β | γ | δ | [R] |
|---|---|---|---|---|---|---|---|---|
| Cyclic AMP | 0.015 | 4E+7 | 7E+5 | 150 | 1 | 0.156 | 15 | 1 |
| Hyperpolar. | 0.015 | 4E+7 | 7E+5 | 50 | 1 | 0.156 | 7 | 1 |
| Calcium mobil. | 0.015 | 4E+7 | 7E+5 | 30 | 1 | 0.156 | 2 | 1 |
| β-Arrestin-2 | 0.015 | 4E+7 | 7E+5 | 100 | 1 | 0.156 | 1.5 | 1 |
| p-ERK | 0.015 | 4E+7 | 7E+5 | 1500 | 1 | 0.156 | 10 | 1 |
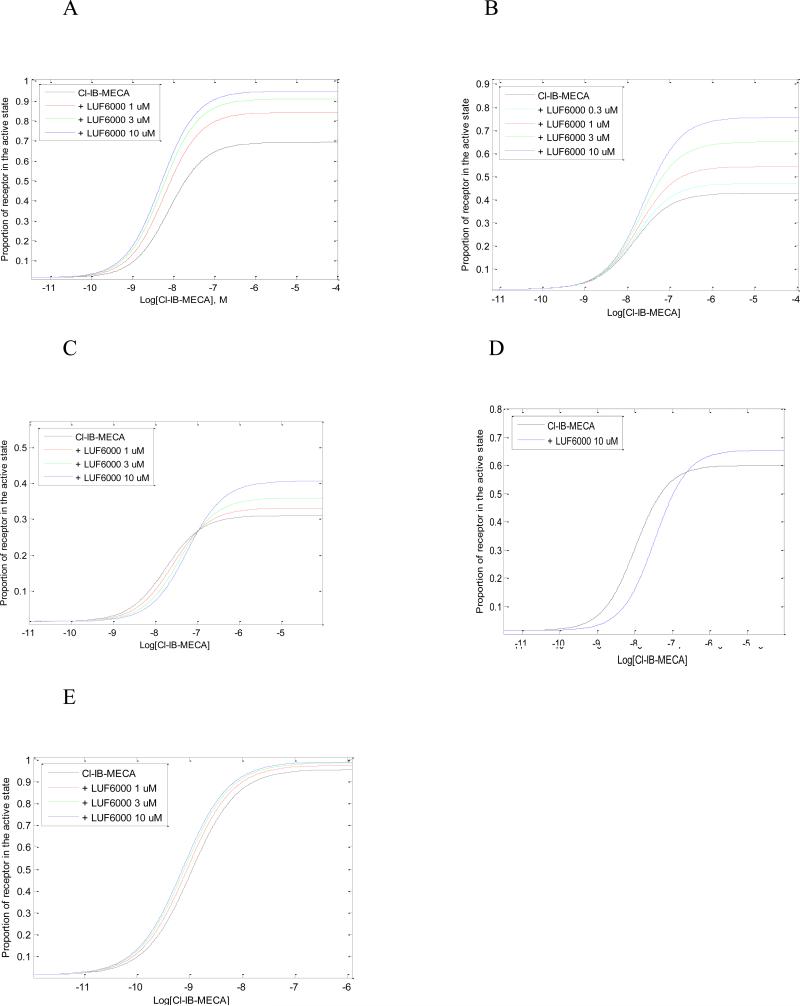
In all instances we assumed an identical fraction of active receptors (L), identical equilibrium dissociation constants for both orthosteric (K) and allosteric ligand (M), identical intrinsic activity of the allosteric modulator (β) and identical binding cooperativity between orthosteric and allosteric ligand (γ). The experimental curves of the agonist Cl-IB-MECA in the absence and presence of LUF6000 at various signaling pathways were closely simulated by varying values of α and δ only (Fig. 9).
Fig. 9.
Simulation of pharmacological curves with MatLab. The equations from Hall (2000) were used to derive conditions that vary efficacy and potency. Parameters in these equations are listed in the text. A. cyclic AMP; B, membrane hyper polarization; C, calcium mobilization; D, arrestin translocation; E, ERK1/2 activation.
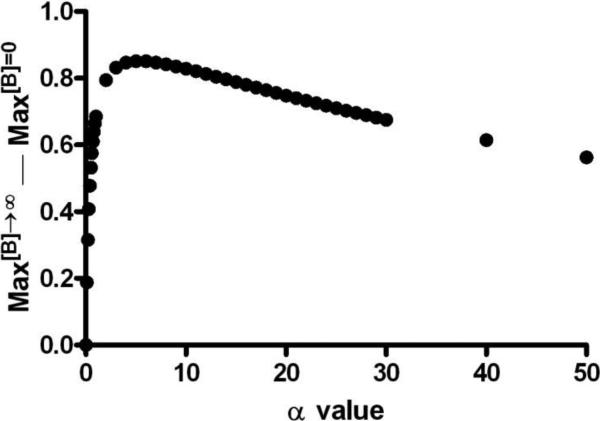
In order to explain mechanistically the results that LUF6000 is particularly effective in enhancing the action of low-efficacy agonists, and that MRS542 cannot be converted to an agonist in the cyclic AMP pathway as demonstrated previously in a [35S]GTPγS binding assay [16], we further explored the role of α in the enhancement.
Based on equations used for simulation, the difference of maximal agonist effect in the presence and absence of a maximally active concentration of an allosteric modulator (B) can be stated as: Max[B]→∞ — Max[B]=0 = αβδγ/(1+ αβδγ) — αL/(1+αL).
Using the parameters selected for simulation of Fig. 4A (β=1; δ=15; γ=0.156) and leaving α as a variable, the equation can be restated as: Max[B]→∞ — Max[B]=0 = 2.34α/(1+ 2.34α) — 0.015α/(1+0.015α). Fig. 10 shows the relationship between Max[B]→∞ — Max[B]=0 and α, which suggests enhancement is maximal when α is not too high or too low. This may explain why LUF6000 is particularly effective for low-efficacious agonists. However, if α = 0, then Max[B]→∞ — Max[B]=0 = 0, which indicates LUF6000 cannot convert a neutral antagonist into an agonist. As a consequence residual efficacy of an orthosteric ligand is needed for the enhancing capability of LUF6000.
Fig. 10.
Analysis of the maximum agonist efficacy in the presence and absence of LUF6000. By using the parameters selected for simulation of Figure 2A (β=1; δ=15; γ=0.156) and leaving α as a variable, the equation can be restated as: Max[B]→∞ — Max[B]=0 = 2.34α/(1+ 2.34α) — 0.015α/(1+0.015α).
4. Discussion
In the present study, we have shown that the enhancement of agonist action by LUF6000 at the A3 AR depends on which signaling pathway is measured. Thus, the emerging receptor concept of functional selectivity or ligand-biased signaling may also apply to allosteric modulators. For example, the efficacy of the nucleoside antagonist MRS542 was enhanced in the Ca2+ assay and to a lesser extent in the β-arrestin assay, but not in assays of the cyclic AMP and membrane polarization. Enhancement of MRS542 had been seen previously when the functional endpoint was guanine nucleotide binding [16]. The enhancement of the endogenous agonist inosine was evident only in the cyclic AMP assay. Another aspect of allosteric modulation of GPCRs is probe dependence with respect to the agonist, i.e. the pharmacological properties of a particular allosteric modulator may vary depending on which orthosteric ligand is used in the experiment [22]. We have observed this phenomenon in the present studies in that the efficacy of Cl-IB-MECA was generally enhanced to a larger extent than the efficacy of NECA.
The findings from the present study and an earlier study [16] that LUF6000 can enhance the efficacies of both NECA and Cl-IB-MECA in both the GTPγS binding and cyclic AMP assays, suggest that none of the agonists studied are truly full agonists by these criteria. However, it seemed that NECA was a full agonist as demonstrated by its effect on intracellular calcium mobilization and membrane hyperpolarization, while Cl-IBMECA was a partial agonist in the same pathways. The lack of effect of LUF6000 on Cl-IB-MECA-induced ERK1/2 phosphorylation may indicate that Cl-IB-MECA is a full agonist in this pathway. The smaller effect of LUF6000 on β-arrestin2 translocation may predict that allosteric enhancers produce a lesser degree of receptor desensitization compared with full agonists, although further experiments are needed to confirm this. The variable agonist efficacy of agonists and nonequivalent enhancing effects of LUF6000 in different events mediated via the A3 AR is indicative for both biased agonism and biased allosteric enhancement. A growing number of examples of biased agonism have recently been reported for various GPCRs [20,21,35,36], including for ARs specifically [23,24].
The strong enhancing effect of LUF6000 on the action of inosine, which is also an endogenous AR agonist in addition to adenosine, may be therapeutically relevant. The resting levels of inosine in the extracellular space in the brain and heart are estimated to be as high as 0.1-10 μM [37,38]. A >30-fold increase in concentrations of both nucleosides could occur under ischemic conditions [38]. Inosine was found to reduce ischemic brain injury in rats by an A3 AR-dependent mechanism [39].
We have previously investigated the effects of LUF6000 on the binding of [35S]GTPγS to membranes from CHO cells stably expressing the human A3 AR stimulated by agonists of diverse structures and variable efficacies [16]. In the present study, we further explored the effects of several allosteric modulators (LUF5999, LUF6000 and LUF6001) on agonist Cl-IB-MECA-induced inhibition of cyclic AMP accumulation stimulated by forskolin in intact CHO cells stably expressing the human A3 AR. It was found that LUF5999, LUF6000 and LUF6001 affected agonist efficacy and potency differently; LUF6000 was more effective in enhancing low-efficacy agonists, although behaving somewhat differently in different events mediated by the A3 AR. We also compared the effects of LUF6000 on several other A3 AR-mediated events, i.e. membrane hyperpolarization, intracellular calcium mobilization, ERK1/2 phosphorylation and the translocation of β-arrestin2.
The allosteric enhancing effects of LUF6000 on the agonist Cl-IB-MECA in various signaling pathways were closely simulated by varying the intrinsic agonist activity α and the cooperativity parameter δ. By analyzing the conditions in which an allosteric modulator converts an antagonist into agonist (difference in Emax in the absence and presence of the modulator), it was found that the efficacy of the orthosteric ligand is critical for the enhancement by LUF6000. Thus, the lack of effect on the efficacy of Cl-IB-MECA in the ERK1/2 pathway may indicate that Cl-IB-MECA is a full agonist in this pathway or the δ value is low or both.
To analyze the potency change of Cl-IB-MECA in the presence of various modulators, the ratio [A]50B=0 / [A]50B=∞ was used as a measure of the degree of allosteric interaction between A, the orthosteric ligand, and B, the allosteric modulator. This ratio is equal to γ(1+αδL) / (1+αL), when β=1. As α is a fixed number for Cl-IB-MECA, and L is fixed number in the current assay system, the ratio of [A]50B=0 / [A]50B=∞ is determined by γδ. Although, in theory, the potency change in the presence of these allosteric modulators can be analyzed quantitatively, the possibility of these three modulators (especially LUF5999 and LUF6001) to affect both orthosteric (based on the partial inhibition of [125I]AB-MECA binding [15]) and allosteric sites cannot be excluded. Thus, the quantitative analysis of potency change is potentially less conclusive compared with that of the efficacy enhancement. Dualsteric or hybrid allosteric/orthosteric ligands have been reported for a number of GPCRs [40-42]. Thus, the explanation for potency change may be only qualitative due to the fact that some of these allosteric modulators may have antagonist activity (in addition to their binding cooperativity) at a higher concentration, which will also affect agonist potency.
In summary, the present study extended our earlier studies and demonstrated the robustness of enhancement and exquisite control of efficacy by LUF6000 in several A3 AR-mediated signaling pathways. The mathematical modeling and analysis of the experimental data suggest that only two parameters define the differential shifts observed for the concentration-effect curves; that knowledge should be useful for the fine-tuning of efficacy by these and other allosteric modulators.
Acknowledgements
This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA. The financial contribution of the Dutch Top Institute Pharma is also gratefully acknowledged (project D1-105).
Abbreviations
- CCPA
2-chloro-N6-cyclopentyladenosine
- Cl-IB-MECA
2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide
- ERK
extracellular signal-regulated kinase
- GPCR
G protein-coupled receptor
- LUF5833
2-aminophenyl-6-(1H-imidazol-2-ylmethylsulfanyl)-pyridine-3,5-dicarbonitrile
- LUF6000 (N-(3,4-dichloro-phenyl)-2-cyclohexyl-1H-imidazo[4,5-c]quinolin-4-amine)
LUF5999 (N-(3,4-dichloro-phenyl)-2-cyclobutyl-1H-imidazo[4,5-c]quinolin-4-amine) and LUF6001 (N-(3,4-dichloro-phenyl)-1H-imidazo[4,5-c]quinolin-4-amine
- MRS541
N6-(3-iodobenzyl)adenosine
- MRS542
2-chloro-N6-(3-iodobenzyl)adenosine
- NECA
adenosine-5′-N-ethyluronamide
- PKA
protein kinase A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller C. Nomenclature and classification of adenosine receptors – An update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan L, Burbiel JC, Maass A, Müller CE. Adenosine receptor agonists: from basic medicinal chemistry to clinical development. Expert Opin Emerg Drugs. 2003;8:537–76. doi: 10.1517/14728214.8.2.537. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nature Rev Drug Disc. 2006;5:247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao ZG, Jacobson KA. Allosterism in membrane receptors. Drug Discov Today. 2006;11:191–202. doi: 10.1016/S1359-6446(05)03689-5. (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borea PA, Gessi S, Bar-Yehuda S, Fishman P. A3 adenosine receptor: pharmacology and role in disease. Handb Exp Pharmacol. 2009:297–327. doi: 10.1007/978-3-540-89615-9_10. [DOI] [PubMed] [Google Scholar]
- 6.Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. BBA Biomembranes. 2011;1808:1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Silverman MH, Strand V, Markovits D, Nahir M, Reitblat T, Molad Y, Rosner I, Rozenbaum M, Mader R, Adawi M, Caspi D, Tishler M, Langevitz P, Rubinow A, Friedman J, Green L, Tanay A, Ochaion A, Cohen S, Kerns WD, Cohn I, Fishman-Furman S, Farbstein M, Bar Yehuda S, Fishman P. Clinical Evidence for Utilization of the A3 Adenosine Receptor as a Target to Treat Rheumatoid Arthritis: Data from a Phase II Clinical Trial. J Rheumatol. 2008;35:1–7. [PubMed] [Google Scholar]
- 8.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, Del Valle L, Fishman P. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-kappaB signal transduction pathways. Int J Oncol. 2008;33:287–95. [PubMed] [Google Scholar]
- 9.Bar-Yehuda S, Rath-Wolfson L, Del Valle L, Ochaion A, Cohen S, Patoka R, Zozulya G, Barer F, Atar E, Piña-Oviedo S, Perez-Liz G, Castel D, Fishman P. Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum. 2009;60:3061–71. doi: 10.1002/art.24817. [DOI] [PubMed] [Google Scholar]
- 10.Ochaion A, Bar-Yehuda S, Cohen S, Barer F, Patoka R, Amital H, Reitblat T, Reitblat A, Ophir J, Konfino I, Chowers Y, Ben-Horin S, Fishman P. The anti-inflammatory target A3 adenosine receptor is overexpressed in rheumatoid arthritis, psoriasis and Crohn's disease. Cell Immunol. 2009;258:115–22. doi: 10.1016/j.cellimm.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Gao ZG, Kim SK, IJzerman AP, Jacobson KA. Allosteric modulation of the adenosine family of receptors. Mini Rev Med Chem. 2005;5:545–53. doi: 10.2174/1389557054023242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Göblyös A, IJzerman AP. Allosteric modulation of adenosine receptors. Biochim Biophys Acta Biomembranes. 2011;1808:1309–18. doi: 10.1016/j.bbamem.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson KA, Gao ZG, Göblyös A, IJzerman AP. Allosteric modulators of purine and pyrimidine receptors. Adv Pharmacol. 2011;61:187–220. doi: 10.1016/B978-0-12-385526-8.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao ZG, Kim SG, Soltysiak KA, Melman N, IJzerman AP, Jacobson KA. Selective allosteric enhancement of agonist binding and function at human A3 adenosine receptors by a series of imidazoquinoline derivatives. Mol Pharmacol. 2002;62:81–89. doi: 10.1124/mol.62.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Göblyös A, Gao ZG, Brussee J, Connestari R, Santiago SN, Ye K, IJzerman AP, Jacobson KA. Structure-activity relationships of 1H-imidazo-[4,5-c]quinolin-4-amine derivatives as allosteric modulators of the A3 adenosine receptor. J Med Chem. 2006;49:3354–61. doi: 10.1021/jm060086s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao ZG, Ye K, Göblyös A, IJzerman AP, Jacobson KA. Flexible modulation of agonist efficacy at the human A3 adenosine receptor by the imidazoquinoline allosteric enhancer LUF6000. BMC Pharmacol. 2008;8:20. doi: 10.1186/1471-2210-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall DA. Modeling the functional effects of allosteric modulators at pharmacological receptors: an extension of the two-state model of receptor activation. Mol Pharmacol. 2000;58:1412–23. doi: 10.1124/mol.58.6.1412. [DOI] [PubMed] [Google Scholar]
- 18.Heitman LH, Göblyös A, Zweemer AM, Bakker R, Mulder-Krieger T, van Veldhoven JP, de Vries H, Brussee J, IJzerman AP. A series of 2,4-disubstituted quinolines as a new class of allosteric enhancers of the adenosine A3 receptor. J Med Chem. 2009;52:926–31. doi: 10.1021/jm8014052. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, de Castro S, Gao ZG, IJzerman AP, Jacobson KA. Novel 2- and 4-substituted 1H-imidazo[4,5-c]quinolin-4-amine derivatives as allosteric modulators of the A3 adenosine receptor. J Med Chem. 2009;52:2098–108. doi: 10.1021/jm801659w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336:296–302. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- 21.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–39. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keov P, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors: A pharmacological perspective. Neuropharmacology. 2011;60:24–35. doi: 10.1016/j.neuropharm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Verzijl D, IJzerman AP. Functional selectivity of adenosine receptor ligands. Purinergic Signal. 2011 May 5; doi: 10.1007/s11302-011-9232-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao ZG, Jacobson KA. Translocation of arrestin induced by human A3 adenosine receptor ligands in an engineered cell line: Comparison with G protein-dependent pathways. Pharmacol Res. 2008;57:303–11. doi: 10.1016/j.phrs.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao ZG, Kim SK, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. Structural determinants of A3 adenosine receptor activation: Nucleoside ligands at the agonist/antagonist boundary. J Med Chem. 2002;45:4471–4484. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beukers MW, Chang LCW, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Spanjersberg RF, Brussee J, IJzerman AP. New, Non-Adenosine, High-Potency Agonists for the Human Adenosine A2B Receptor with an Improved Selectivity Profile Compared to the Reference Agonist N-Ethylcarboxamidoadenosine. J Med Chem. 2004;47:3707–9. doi: 10.1021/jm049947s. [DOI] [PubMed] [Google Scholar]
- 27.van Galen PJ, van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL, Jacobson KA. A binding site model and structure-activity relationships for the rat A3 adenosine receptor. Mol Pharmacol. 1994;45:1101–11. [PMC free article] [PubMed] [Google Scholar]
- 28.Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–57. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fossetta J, Jackson J, Deno G, Fan X, Du XK, Bober L, Soude-Bermejo A, de Bouteiller O, Caux C, Lunn C, Lundell D, Palmer RK. Pharmacological analysis of calcium responses mediated by the human A3 adenosine receptor in monocyte-derived dendritic cells and recombinant cells. Mol Pharmacol. 2003;63:342–50. doi: 10.1124/mol.63.2.342. [DOI] [PubMed] [Google Scholar]
- 30.Nordstedt C, Fredholm BB. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Anal Biochem. 1990;189:231–4. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 31.Gao ZG, Hechler B, Besada P, Gachet C, Jacobson KA. Caged agonist of P2Y1 and P2Y12 receptors for light-directed facilitation of platelet aggregation. Biochem Pharmacol. 2008;75:1341–47. doi: 10.1016/j.bcp.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitch RW, Xiao Y, Kellar KJ, Daly JW. Membrane potential fluorescence: a rapid and highly sensitive assay for nicotinic receptor channel function. Proc Natl Acad Sci U S A. 2003;100:4909–14. doi: 10.1073/pnas.0630641100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark BD, Kurth-Nelson ZL, Newman EA. Adenosine-evoked hyperpolarization of retinal ganglion cells is mediated by G-protein-coupled inwardly rectifying K+ and small conductance Ca2+-activated K+ channel activation. J Neurosci. 2009;29:11237–45. doi: 10.1523/JNEUROSCI.2836-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson KA, Park KS, Jiang JL, Kim YC, Olah ME, Stiles GL, Ji Xd. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology. 1997;36:1157–65. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rakesh K, Yoo B, Kim IM, Salazar N, Kim KS, Rockman HA. beta-Arrestin-biased agonism of the angiotensin receptor induced by mechanical stress. Sci Signal. 2010;3:ra46. doi: 10.1126/scisignal.2000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo A, Soh UJ, Trejo J. Proteases display biased agonism at protease-activated receptors: location matters! Mol Interv. 2009;9:87–96. doi: 10.1124/mi.9.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballarin M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand. 1991;142:97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 38.Backstrom T, Goiny M, Lockowandt U, Liska J, Franco-Cereceda A. Cardiac outflow of amino acids and purines during myocardial ischemia and reperfusion. J Appl Physiol. 2003;94:1122–8. doi: 10.1152/japplphysiol.00138.2002. [DOI] [PubMed] [Google Scholar]
- 39.Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y. Inosine reduces ischemic brain injury in rats. Stroke. 2005;36:654–9. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- 40.Antony J, Kellershohn K, Mohr-Andrä M, Kebig A, Prilla S, Muth M, Heller E, Disingrini T, Dallanoce C, Bertoni S, Schrobang J, Tränkle C, Kostenis E, Christopoulos A, Höltje HD, Barocelli E, De Amici M, Holzgrabe U, Mohr K. Dualsteric GPCR targeting: a novel route to binding and signaling pathway selectivity. FASEB J. 2009;23:442–50. doi: 10.1096/fj.08-114751. [DOI] [PubMed] [Google Scholar]
- 41.Aurelio L, Valant C, Figler H, Flynn BL, Linden J, Sexton PM, Christopoulos A, Scammells PJ. 3- and 6-Substituted 2-amino-4,5,6,7-tetrahydrothieno[2,3-c]pyridines as A1 adenosine receptor allosteric modulators and antagonists. Bioorg Med Chem. 2009;17:7353–61. doi: 10.1016/j.bmc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narlawar R, Lane JR, Doddareddy M, Lin J, Brussee J, IJzerman AP. Hybrid ortho/allosteric ligands for the adenosine A1 receptor. J Med Chem. 2010;53:3028–37. doi: 10.1021/jm901252a. [DOI] [PubMed] [Google Scholar]