Abstract
Jun N-terminal kinase (JNK) is a multifunctional protein kinase crucial for neuronal apoptosis as well as neurite growth. We have previously shown that JNK activity is correlated with spiral ganglion neuron (SGN) apoptosis following hair cell loss in rats (Alam et al., 2007) implying that JNK inhibition may have therapeutic potential to protect SGNs in deaf individuals. Here we investigated the role of JNK in neurite outgrowth from cultured neonatal rat and mouse SGNs. We show that JNK is required for initial growth of neurites and for continued extension of already established neurites. The effect of JNK inhibition on neurite growth is rapid and is also rapidly reversible after washout of the inhibitor. Using phosphoJNK immunoreactivity as an indicator, we show that JNK is activated in growth cones within 30 min after transfer to medium lacking neurotrophic stimuli (5K medium) but activation in the nucleus and soma requires hours. By transfecting epitope-tagged JNK1, JNK2, or JNK3 isoforms into SGNs, we found that all are present in the nucleus and cytoplasm and that there is no preferential redistribution to the nucleus after transfer to 5K medium. Cotransfection of dominant-negative (dn) JNK1 and JNK2 into SGNs reduced neurite growth, although transfection of dnJNK1 or dnJNK2 alone had no significant effect. SGNs cultured from JNK3−/− mice showed reduced neurite growth that was further reduced by transfection of dnJNK1 and dnJNK2. This indicates that all three JNK isoforms promote SGN neurite growth although there may be functional redundancy between JNK1 and JNK2.
Keywords: spiral ganglion neuron, neurite, JNK, c-Jun N-terminal kinase
Introduction
Spiral ganglion neurons (SGNs) relay auditory information from the cochlea to the brain. Hair cells, the auditory sensory cells, provide the sole afferent input to the SGNs. Loss of hair cells, the primary cause of sensorineural deafness, can result in secondary degeneration of SGNs (Green et al., 2008), presumably because of loss of hair cell-derived neurotrophic support. The sensory function of lost hair cells can be replaced by cochlear implants, which electrically stimulate SGNs, necessitating prevention of SGN degeneration. A number of such neuroprotection strategies are being tested in animal models, typically focusing on restoring neurotrophic factors or preventing SGN apoptosis (Roehm et al., 2005). One potential means of preventing apoptosis is inhibition of c-Jun N-terminal Kinase (JNK). The JNKs are members of the MAP kinase gene family. They are activated by phosphorylation by certain MAP kinase kinases (MKKs) (Davis, 2000); activated JNK can be detected by antibodies specific to the phosphorylated form.
After loss of trophic support, JNK phosphorylates the transcription factor c-Jun and other proteins, events that are necessary for apoptosis after loss of neurotrophic support (Green et al., 2008; Ham et al., 2000; Ip et al., 1998). Thus, inhibitors of JNK or of mixed lineage kinases (MLKs), which are upstream activators of JNK, inhibit neuronal apoptosis (Bogoyevitch et al., 2004; Wang et al., 2004). The death of SGNs following loss of hair cells is correlated with an increase in Jun phosphorylation and JNK activation (Alam et al., 2007), suggesting that JNK inhibition will be protective for SGNs after hair cell loss.
A complexity in JNK signaling is that there are multiple isoforms derived from three genes, JNK1 (Mapk8), JNK2 (Mapk9) and JNK3 (Mapk10) and alternative splicing (Davis, 2000). JNK1 and JNK2 are ubiquitously expressed but JNK3 is largely restricted to the brain, heart and testis (Davis, 2000; Ip et al., 1998; Widmann et al., 1999). JNK1 and JNK2 appear to have, at least partially, overlapping roles. Mice lacking either JNK1 or JNK2 are viable but JNK1−/−; JNK2−/− double knockout results in embryonic lethality (Bogoyevitch, 2006; Chang et al., 2003). JNK3−/− mice are viable and fertile but show reduced neuronal apoptosis in response to a variety of insults including neurotrophic factor deprivation (Bruckner et al., 2001), axotomy (Keramaris et al., 2005), excitotoxicity (Yang et al., 1997), ischemia (Kuan et al., 2003), or toxins (Namgung et al., 2001). While this focuses attention on JNK3 as the crucial mediator of neuronal apoptosis, JNK1/2 can play important roles, for example in apoptosis during development (Ham et al., 2000; Kuan et al., 1999).
JNK has many functions other than apoptosis, for example in stress responses, inflammation and normal development (Ip et al., 1998; Weston et al., 2002). JNK is also involved in regulation of microtubules (Bjorkblom et al., 2005; Chang et al., 2003; Tararuk et al., 2006) and in growth or regeneration of axons and dendrites (Barnat et al., 2010; Eminel et al., 2008; Waetzig et al., 2006). These different actions of JNK are, in part, related to JNK activity in different subcellular locations. Apoptosis requires nuclear JNK while control of dendrite length requires cytoplasmic JNK (Bjorkblom et al., 2008; Bjorkblom et al., 2005; Eminel et al., 2008). In many neurons and neuronal cell lines, inhibition of JNK or of JNK upstream activators inhibits neurite growth (Eminel et al., 2008; Lindwall et al., 2005a; Lindwall et al., 2005b; Oliva et al., 2006; Waetzig et al., 2006). In particular, growth of neurites from cultured SGNs is similarly decreased by inhibition of MLKs, the upstream activators of JNK (Bodmer et al., 2002).
Here we ask whether JNK phosphorylation is spatially and temporally correlated with neurite growth in SGNs and whether JNK inhibition inhibits neurite growth in SGNs? We also ask what JNK isoforms are involved in SGN neurite growth? Because JNK inhibition is a potential therapeutic approach to protecting SGNs in cochlear implant users, it is important to know what other effects JNK inhibition might have on the SGNs to mitigate possible adverse effects on SGN peripheral or central axons.
Materials and methods
Mice and reagents for molecular genetic studies
JNK3 knockout mice (Kuan et al., 2003) were graciously provided by Dr. Chia-Yi (Alex) Kuan (Cincinnati Children’s Hospital Research Foundation, Cincinnati, OH). HA-tagged JNK1, JNK2 and JNK3 were graciously provided by Dr. Roger Davis (UMass Medical School, Amherst, MA). Dominant-negative JNK1 (dnJNK1) and JNK2 (dnJNK2) were also provided by the Roger Davis lab, which has previously established their efficacy as dominant inhibitors of JNK activity (Chow et al., 2000). MKK7-JNK1 was graciously provided by Dr. Lynn Heasley (University of Colorado Health Sciences Center, Denver, CO) who has previously established its efficacy as a constitutively-active JNK (Han et al., 2002). The dnJNK1, dnJNK2 and MKK7-JNK1 plasmids were cotransfected into neurons with eGFP (Clontech, Mountain View, CA) at a 1:4 ratio, i.e., 2 μg/ml eGFP plasmid with 8 μg/ml JNK plasmids for a total DNA concentration of 10 μg/ml. This allowed identification of transfected cells by fluorescence.
Spiral ganglion culture
Our basic culture medium consisted of high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM, 0.1mg/ml penicillin, 0.1mg/ml streptomycin, N2 supplement, 10 μg/ml insulin and is designated as ‘5K’ because the [K+] = 5.4mM. We also used a depolarizing medium ‘25K’ in which Na+ was replaced with equimolar K+ to raise [K+] to 25 mM while maintaining osmolarity. Prior to and during transfections, the cultures were maintained in 25K to which 5% fetal bovine serum (FBS) was added (25K+S). All media and supplements were obtained from Sigma (St. Louis, MO) or Invitrogen (Carlsbad, CA).
Dissociated cultures of spiral ganglion neurons (SGNs) were prepared from post-natal day 5 rat and mice pups and maintained as described previously (Bok et al., 2007). Briefly, the cochleae were removed from the temporal bone and placed in ice-cold phosphate buffered saline (PBS). Each spiral ganglion was isolated from the cochlea by sequential removal of the bony cochlear capsule, the spiral ligament, and the organ of Corti, leaving the spiral ganglion within the modiolus. These were collected in Hank’s Balanced Salt Solution (HBBS) on ice and then transferred to Ca2+/Mg2+-free HBSS with 0.1% trypsin and 0.1% collagenase at 37°C for 20 min to enzymatically dissociate the cells. The enzymatic reaction was quenched by the addition of 10% FBS. After three washes with culture medium, the ganglia were mechanically dissociated by trituration with a mechanical pipettor with 1 ml pipette tips. The dissociated cells were plated in the desired culture medium on 8-well chamber slides (Lab-Tek, Rochester, NY) previously coated with polyornithine (Sigma, 0.1 mg/ml in 10 mM borate buffer, pH 8.4) for 1 h at room temperature followed by laminin (Invitrogen, 20 μg/ml in HBSS) overnight at 4°C. Because of greater ease of culture and transfection, rat cultures were used in all experiments except those of Figure 7.
Figure 7.
A–E: SGNs were cultured either from JNK3+/+ (WT) or JNK3−/− (KO) littermate mice and transfected with either eGFP and empty vector plasmid (1:4 ratio of eGFP:vector) or with eGFP and dnJNK1 and dnJNK2 (1:2:2 ratio). Cotransfection of eGFP identified transfected cells. These combinations resulting in neurons expressing either all three JNK isoforms (JNK1,2,3), or only JNK1 and JNK2 (JNK1,2), or only JNK3, or no JNK (none). A–D: Representative images of cultured mouse SGNs labeled by eGFP fluorescence and transfected with vector (A,B) or dnJNK1 and dnJNK2 (C,D). A and C are from WT mice, B and D are from JNK3 KO mice. Scale bars = 50 μm. E. Neurite growth over a 24 h period was quantified. There is a significant increasing decrement in neurite growth with removal of additional JNK isoforms. F. SGNs were cultured from neonatal rats and transfected with eGFP and empty vector, dnJNK1, dnJNK2, dnJNK1 and dnJNK2 combined, or MKK-JNK1. Neurite lengths were determined after 48 h. There was no significant difference in mean neurite length between vector and a single dnJNK (horizontal bar). Combined dnJNK1 and dnJNK2 significantly reduced neurite length and constitutively-active JNK (MKK-JNK1) significantly increased neurite length. For E and F, error bar shows SEM. The number of independent replicate cultures is given within each bar and statistical significance was determined by ANOVA with a Tukey-Kramer post-hoc test, *p<0.05, **p<0.01, ***p<0.001.
SP600125 (Calbiochem) or AS601245 (Enzo) were added to a final concentration of 20 μM from a 20 mM stock in DMSO so the final DMSO concentration was 0.1% v/v. Control cultures not receiving these drugs had the same concentration of DMSO.
Transfection of spiral ganglion neurons
Transfection was performed only after neurons had firmly attached to the substrate, approximately 6 h after plating. We used a calcium phosphate-based protocol as we have previously described (Bok et al., 2007; Zha et al., 2001). Briefly, plasmids of interest were mixed with 1.25 M CaCl2 solution and then sterile deionized water was added to bring the CaCl2 concentration to 0.25 M. An equal volume of 2X depolarizing HEPES buffer (50 mM HEPES + 220 mM NaCl + 1.5 mM Na2HPO4 + 60 mM KCl, pH 7.0) was added slowly and with agitation. The DNA/Ca2+/PO4 mixture was left for 20 min at RT to allow the precipitate to form and was then added to the culture medium (25K+S) on the cells at a 1:10 (v/v) ratio, the final concentration of DNA being 10 μg/ml. This procedure typically resulted in transfection of 5–10% of the SGNs. Twelve hours after transfection, the wells were washed once with DMEM and replaced with 25K medium to maintain SGN survival and allow initiation of neurite growth. The cells were left to incubate for another 12 h after which they were washed three times with DMEM and the 25K replaced with the desired experimental or control medium.
Lck-GFP (Benediktsson et al., 2005)is a membrane-targeted GFP construct that we designed to brightly label thin membranous structures such as glial processes, neurites, filopodia, or lamellipodia, making it ideal for labeling growth cones. The construct was provided to the University of Iowa Gene Transfer Vector Core (http://www.uiowa.edu/~gene/), which produced FIV lentivirus for efficient gene transfer into cultured SGNs. Lentiviral transduction was performed after dissociated SGNs had been cultured for 24 h in 25K+S. The culture medium was removed and replaced with 100 μl of lentivirus freshly diluted in 25K (dilution is determined empirically to achieve >50% transduction of neurons with few transduced non-neuronal cells). The cells were incubated for 4 h, after which the viral medium was removed, the cells were washed twice with DMEM, and then cultured in 25K for a further 48 h prior to shift to experimental conditions.
Immunocytochemistry
After culture for the time indicated, the cells were fixed for 15 min with 4% paraformaldehyde in PBS, washed with PBS and permeabilized with 0.2–0.8% Triton-X in PBS. Nonspecific binding was reduced by incubation with blocking buffer (PBS + 2% BSA + 5% normal goat serum + 0.1% NaN3) for 1 h at 37°C. Primary antibodies in blocking buffer were then added for 1 h at 37°C or overnight at 4°C, after which cultures were incubated with fluorescently-labeled secondary antibodies in blocking buffer for 1 h at 37°C.
The primary antibodies used in this study were anti-neurofilament 200 (NF200) monoclonal antibody N52 (1:800; Sigma-Aldrich, St. Louis, MO), anti-hemagglutinin (1:200; Upstate, Lake Placid, NY), anti-phosphoJNK (1:200; Cell Signalling, Beverly, MA). Secondary antibodies used were AlexaFluor®-labeled goat antibodies (Molecular Probes, Eugene, OR).
Imaging and quantitation
Cultures were viewed on a Zeiss Axiovert 200M equipped for fluorescence optics. Digital images were captured with a Hamamatsu Orca ER camera using Improvision Openlab v.5 (Waltham, MA) software. Images were then transferred to Apple Macintosh computers for further analysis using ImageJ (NIH, Bethesda, MD). Neurite length was determined by measuring the entire length of the longest process extending from the SGN soma to the end of the longest branch (for branched neurites) using the line segment tool in ImageJ. For untransfected cultures, ≈50 neurites were measured per well with two wells per experimental condition for each culture. For experiments in which neurons were transfected, all transfected neurons in each well, typically 5–10, were measured. Neurite lengths in all nontransfected neurons in each well were also measured to provide an additional intrawell comparison of the effect of the transfection. Means and SEM are for the indicated number (n) of independent replicate cultures.
ImageJ was also used to quantify phosphoJNK immunofluorescence as we have previously described (Alam et al., 2007). Briefly, for soma measurements, a circular selection, typically 5 μm in diameter, was made just inside the boundary of each neuronal nucleus and the intensity of the phosphoJNK immunofluorescence determined as the average pixel density within the circle. Background fluorescence was determined as the average pixel density within a circle of equal diameter just outside of the cell and this background was subtracted from the value obtained for nuclear fluorescence.
For measurements of phosphoJNK in the growth cone a 1.4 NA 63X oil immersion objective was used. Because of the highly irregular outline of the growth cone, measurements were made using 10–15 single-pixel selections within the growth cone. Lck-GFP was used to accurately label the growth cone. For each growth cone, a Lck-GFP and a phosphoJNK immunofluorescence image were obtained. In ImageJ, the Lck-GFP image was visible and was used to determine the coordinates for the pixel selections, which were redirected (in the Set Measurements dialog) to the phosphJNK image for the density measurements. Thus, the phosphoJNK image itself was not visible to the person obtaining the measurements, preventing subjective choices about the location of the pixel selections. Measurements were made of ≈40 somata and 30–40 growth cones per well with two wells per experimental condition for each culture. Means and SEM are for the indicated number (n) of independent replicate cultures. Statistical significance for differences in neurite length or phosphoJNK immunoreactivity was determined using Graphpad Instat software.
Quantitation of phosphoJNK was performed on original unmodified images. The antibody labeling was performed simultaneously and in parallel on control and experimentally perturbed cultures and the phosphoJNK images were captured using identical light intensity, microscope, and camera settings adjusted so that images were not saturated.
Results
JNK activity is needed for initial outgrowth of neurites and for continued growth of established neurites
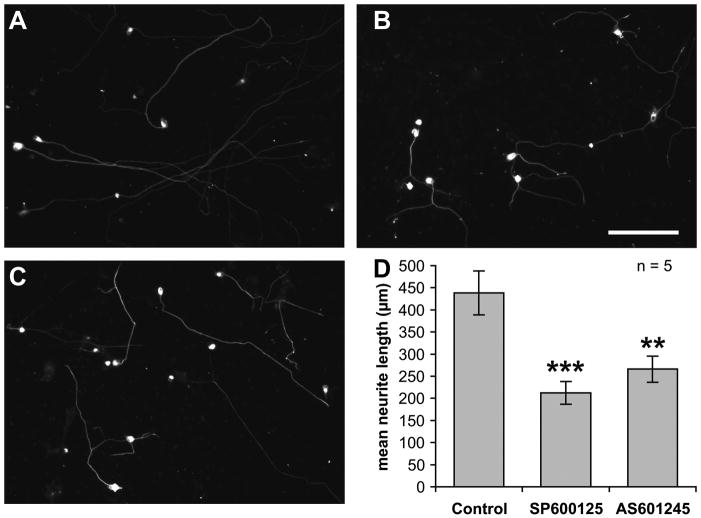
Bodmer et al. (Bodmer et al., 2002) have shown that inhibition of MLKs, upstream activators of JNK, inhibits neurite outgrowth in SGNs. To directly determine whether JNK activity plays a role in neurite outgrowth of SGNs, we inhibited JNK with SP600125 or AS601245 (Bennett et al., 2001; Carboni et al., 2004). We used two different inhibitors to mitigate the possibility that the inhibition is due to nonspecific actions of the compounds. Representative images of SGNs in control conditions or exposed to either inhibitor are shown in Figure 1A–C. Neurites, typically one per cell, are evident in all conditions but addition of either inhibitor to SGNs 24 h after plating resulted in significantly (p<0.01 for AS601245, p<0.001 for SP600125) shorter neurites (Figure 1D), indicating that JNK activity promotes neurite growth.
Figure 1.
JNK inhibitor, SP600125 or AS601245 (both at 20 μM) were added to spiral ganglion cultures after 24 h in vitro. The cultures were fixed after an additional 24 h, labeled with ant-NF200 and imaged. A–C are representative low-magnification (scale bar = 150 μm) images of SGNs cultured in control conditions (A), SP600125 (B), or AS601245 (C). D: quantitation of neurite lengths. Neurite growth was significantly (**p<0.01, ***p<0.001) decreased after the addition of the either JNK inhibitor. Shown are means ± SEM (n = 5 replicate cultures).
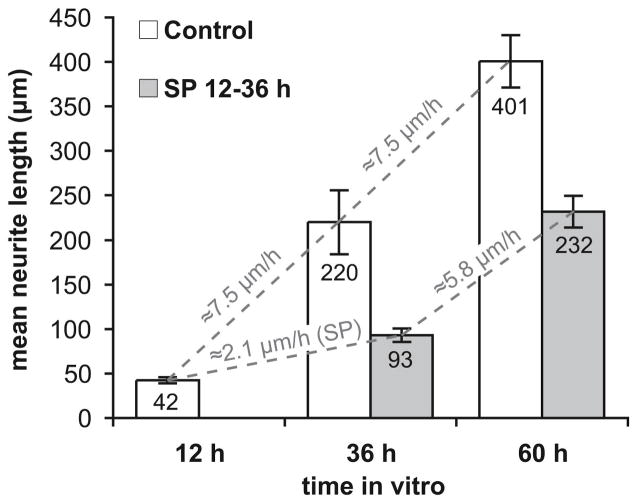
We next asked whether JNK activity is required selectively for initiation of SGN neurites or for their continued elongation (Figure 2). SGNs require several hours to attach to the substrate after plating and neurite outgrowth is just beginning at about twelve hours after plating. Therefore, we assessed the requirement for JNK for early growth of neurites by adding SP600125 for a 24 h period beginning at 12 h post-plating and then fixing the cultures. Mean neurite length in these cultures was compared to mean neurite length in control cultures also fixed at 36 h post-plating. As shown in Figure 2, JNK inhibition significantly (p<0.01) reduced neurite length at 36 h post-plating.
Figure 2.
Spiral ganglion cultures were fixed 36 h or 60 h after plating. SP600125 (20 μM) was present for 24 h – either from 12 h to 36 h in culture or from 36 h to 60 h – and the cultures were fixed immediately after for comparison to control cultures fixed at the same time but not treated with SP600125. Shown are mean neurite lengths ± SEM (n = 5 replicate cultures). JNK inhibition significantly (**p<0.01) inhibited neurite growth whether present 12–36 h after plating, when neurite growth is beginning, or 36–60 hr after plating, when neurites are already established. The approximate mean rate of neurite growth is shown in gray lettering for 24 h time intervals in which SP600125 was either present or absent.
Furthermore, we assessed the requirement for JNK for continued growth of established neurites by adding SP600125 for a 24 h period beginning at 36 h post-plating and then fixing the cultures. Mean neurite length in these cultures was compared to mean neurite length in control cultures also fixed at 60 h post-plating. As shown in Figure 2, JNK inhibition significantly (p<0.01) decreased neurite length at 60 h post-plating. Neurite extension over this 24 h period from 36 to 60 h was also quantified by comparing neurite lengths at 60 h to mean neurite length in control cultures at 36 h post-plating. By this measure, JNK inhibition reduced neurite extension from a rate of ≈12.8 μm/h to a rate of ≈2.6 μm/h. Thus, extension of established neurites and initial growth of neurites both require JNK activity.
Subcellular localization of the three JNK isoforms in the nucleus and cytoplasm of SGNs
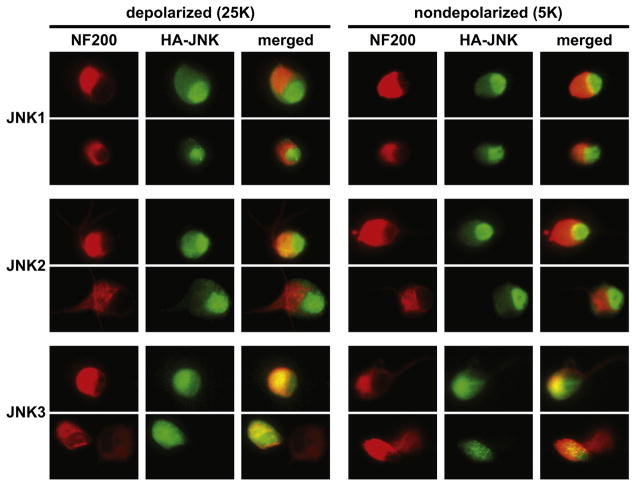
Phosphorylation of the transcription factor Jun by JNK has been implicated in apoptosis following neurotrophic factor withdrawal (Davis, 2000; Eilers et al., 1998; Ip et al., 1998). Consistent with this, is the observation that it is nuclear JNK that is responsible for apoptosis (Bjorkblom et al., 2008). However, a role for JNK in neurite growth implies an extranuclear function. We therefore investigated the subcellular localization in SGNs of the three JNK isoforms. Because isoform-specific anti-JNK antibodies usable for immunofluorescence are unavailable, we instead expressed hemagglutinin (HA)-tagged JNK isoforms in SGNs and used anti-HA antibodies to visualize and localize the expressed protein within SGNs. Localization of each JNK isoform was observed under two culture conditions: with trophic support (25K) and with trophic support removed for 6 hours (5K) prior to fixation.
Representative images are shown in Figure 3. Neuronal cytoplasm is labeled with anti-NF200. All three JNK isoforms are found in the nucleus and in the cytoplasm. However, JNK1 and JNK2, unlike JNK3, appear to be at higher levels in the nucleus relative to the cytoplasm. The pattern of subcellular localization of JNK1–3 is not apparently altered upon the withdrawal of trophic support. In particular, there is no apparent significant increase in nuclear, relative to cytoplasmic, location of any of the JNK isoforms, including JNK3, which remains at relatively lower levels in the nucleus. This is in spite of a clear role for JNK3 in apoptosis (Bruckner et al., 2001; Kuan et al., 2003; Yang et al., 1997)and may imply roles for JNK3 in apoptosis in addition to Jun phosphorylation as has been previously suggested (Keramaris et al., 2005).
Figure 3. Representative images of HA-tagged JNK fusion proteins in cultured SGNs.
Cells were maintained for 24 h in depolarizing (25K) conditions for trophic support and then incubated for an additional 6 h in 25K or without trophic support in nondepolarizing serum-free medium, as indicated. Cultures were then fixed and neurons visualized with anti-NF200 (red) and HA-JNKs with anti-HA (green). Two typical examples are shown for each isoform. The bottom row shows examples of two adjacent neurons of which one was transfected and one not. All JNK isoforms are distributed throughout the cytoplasm and nucleus, although JNK1 and JNK2 are more strongly localized to the nucleus than is JNK3. The subcellular distribution of all JNKs is not apparently affected by withdrawal of trophic support. Scale: individual images are 30 μm × 40 μm.
An important caveat is that in these studies, HA-JNK was expressed in the cell with a CMV promoter and may be at higher levels than the endogenous JNK proteins. The increased amount of protein may affect the intracellular distribution, although the observed difference in the intracellular distribution of JNK3 relative to JNK1 and JNK2 suggests that the expressed proteins are not randomly distributed throughout the cell.
JNK phosphorylation is upregulated in the soma and growth cone when SGNs are deprived of trophic support
Phosphorylated JNK has been shown to be enriched in developing axons (Oliva et al., 2006) and in the growth cones of dorsal root ganglion (DRG) sensory neurons undergoing plastic changes in vivo (Barnat et al., 2010). We asked whether JNK is phosphorylated in SGN growth cones. Because removal of trophic support is an effective means of stimulating JNK phosphorylation (Davis, 2000; Ip et al., 1998), we changed culture medium in the spiral ganglion cultures from 25K to 5K and subsequently labeled the cells with an antibody to phosphorylated JNK (phosphoJNK). The antibody does not distinguish among JNK isoforms but detects all phosphoJNK; however, the data in Figure 3 already suggest that all three JNK isoforms are ubiquitously distributed in the neurons. Therefore, these experiments can show the location and spatial pattern of JNK phosphorylation in the neurons but can’t determine whether different isoforms are preferentially phosphorylated in those locations.
Representative images of phosphoJNK are shown in Figure 4. In the cell soma (Figure 4A–F), JNK phosphorylation is evident in the cytoplasm and, to a lesser degree, also in the nucleus after 6 h exposure to 5K medium lacking neurotrophic factors. We imaged growth cones at high resolution using a 1.4 NA (63X) oil-immersion objective (Figure 4G–L). Because high molecular weight neurofilament (NF200) does not extend throughout the growth cone, we visualized the growth cone by expressing a membrane-targeted GFP construct, Lck-GFP, that is especially effective at labeling thin membranous structures (Benediktsson et al., 2005). PhosphoJNK is elevated in growth cones within 30 min of exposure to 5K medium. As shown in Figure 4, phosphoJNK immunoreactivity is in a punctate pattern in the growth cone.
Figure 4. Representative images of JNK phosphorylation in SGN somata and growth cones.
A–F: JNK is phosphorylated in the soma and nucleus of a SGN in vitro, but not in adjacent glia, six hours after transfer to medium lacking neurotrophic support (5K, A–C) from depolarizing (25K, D–F) medium. Scale bar = 10 μm. A,D: NF200 (green) and bisbenzamide nuclear label (blue). B,E: pJNK immunofluorescence (red). C,F: merged NF200, nuclear and pJNK labeling. G–L: JNK is phosphorylated in a punctate pattern in the growth cone 30 minutes after transfer to medium lacking neurotrophic support (5K, G–I) from depolarizing (25K, J–L) medium. Scale bar = 5 μm. G,J: Lck-GFP (green). H,K: pJNK immunofluorescence (red). I,L: merged Lck-GFP and pJNK labeling.
We quantified phosphoJNK immunofluorescence intensity using ImageJ (Figure 5) as described in Methods. Because of the small size and highly irregular shape of growth cones, phosphoJNK immunofluorescence in growth cones was measured in multiple single pixel selections and was done in such a way that the observer could see only the Lck-GFP labeling and not the phosphoJNK labeling while making the selections (see Methods). A significant (p<0.05) increase in phosphoJNK immunofluorescence can be detected 30 min after changing culture medium from 25K to 5K (Figure 5A). However, changing from 25K to NT-3, also an effective neurotrophic factor for SGNs (Green et al., 2008), does not cause increased JNK phosphorylation. Also, SP600125 prevents the increase in JNK phosphorylation.
Figure 5.
JNK phosphorylation was assayed by measuring phosphoJNK immunofluorescence (IF) in growth cones (A) or somata (B,C), at 30 min (A,B) or 6 h (C) after shift from 25K to one of the following experimental conditions: 25K control; 5K DMEM lacking neurotrophic factors; 5K with 100 ng/ml NT-3; 5K with 20 μM SP600125 (SP). The data were normalized to phosphoJNK IF in the 5K condition in each experiment. JNK phosphorylation is significantly elevated in growth cones within 30 min of trophic factor deprivation by shift to 5K but the increase is prevented by NT-3 or by SP600125. In contrast, JNK phosphorylation is not elevated in somata 30 min after shift to 5K but is elevated 6 h after shift. Statistical significance of differences relative to 5K was determined by ANOVA with Tukey-Kramer correction (*p<0.05, **p<0.01, ***p<0.001).
As shown in Figure 5B, there is no significant change in JNK phosphorylation in the soma in 30 min after change of culture medium to 5K. JNK phosphorylation in the soma occurs over a much longer timecourse than in the growth cone but is evident after 6 h. By 6 h after changing to 5K, there is a significant (p<0.001) increase in JNK phosphorylation (Figure 5C). This increase in JNK phosphorylation is again blocked by SP600125.
The effect of JNK inhibition on neurite growth is rapidly reversible
We next asked whether JNK activity has acute effects on neurite growth or whether inhibition of JNK results in a long-lasting reduction of neurite growth. SGNs were maintained for 24 h in SP600125 to inhibit JNK and the rate of neurite growth after washout of the SP600125 was compared to the rate of neurite growth in SP600125 and that in cultures never exposed to SP600125. As shown in Figure 6, during exposure to SP600125, the growth rate of SGN neurites is reduced. However, the rate of neurite elongation following washout is close to that in control cultures not exposed SP600125. Assuming that the rate of growth after washout is ultimately equal to the rate in control cultures, it would imply recovery within a few hours. Because JNK activity in the nucleus responds more slowly, this argues against a mechanism by which JNK affects neurite growth by regulating gene expression. It is consistent with our observation of rapid JNK phosphorylation in the growth cone and implies that in the growth cone there is a relatively rapid local JNK-dependent response to changes in trophic conditions, which regulates neurite elongation. In contrast, there is a relatively slow response to changes in trophic conditions, presumably associated with apoptosis.
Figure 6. Inhibition of SGN neurite growth by JNK inhibitor is reversible.
Cultures were fixed 12, 36 or 60 hours after plating. Control cultures were maintained in 25K/0.1% DMSO. SP600125-treated cultures had SP600125 present from 12 to 36 h after plating. Shown are means ± SEM (n = 5 replicate cultures) with the mean neurite length printed in each bar. The approximate mean rate of neurite growth is shown in gray for each 24 h interval, 12–36 h and 36–60 h post-plating. This was determined by dividing the difference in mean neurite length by 24 h. SP600125 reduces the neurite growth rate when present in the 12–36 h interval but growth returns to a nearly control rate in the 36–60 h during which SP600125-treated SGNs had 24 h to extend neurites after SP600125 withdrawal.
All JNK isoforms contribute to neurite growth
JNK isoforms have both distinctive as well as redundant functions (Bogoyevitch, 2006). The localization results of Figure 3 raise the possibility that all three JNK isoforms contribute to neurite growth. To determine whether this is the case, we used a combination of genetic and molecular techniques to selectively inhibit different JNK isoforms. SGNs were cultured from mice lacking JNK3 or from wild-type litter mates. These SGNs were transfected with a combination of catalytically inactive dominant negative JNK1 and JNK2 or with empty vector. These experimental manipulations generated neurons possessing all three JNK isoforms, neurons lacking all three JNK isoforms, neurons expressing only JNK3 or neurons expressing JNK1 and JNK2 but lacking JNK3. This was designed to allow us to distinguish between the role of JNK1/2 in neurite growth and the role of JNK3 alone. Because JNK3 has been associated with apoptosis due to neurotrophic factor withdrawal (Bruckner et al., 2001; Kuan et al., 2003; Yang et al., 1997) we focused on the possibility that selective inhibition of JNK3 might prevent SGN apoptosis without affecting neurite growth.
Successive removal of each JNK isoform results in a further decrement in mean neurite length (Figure 7A), indicating that each JNK isoform makes a contribution to normal neurite growth. Removal of JNK3 alone does cause a small but significant decrement in neurite length, implying that targeting JNK3 selectively might be a way to suppress apoptosis while minimizing adverse effects on neurite regeneration but can’t completely eliminate inhibition of neurite growth. Blockade of JNK1 and JNK2 activity causes a significantly greater reduction in neurite lengths than removal of JNK3 activity alone. As might be expected, genetic and molecular deletion of all three JNK isoforms results in an even greater inhibition: neurite growth comparable to that in SGNs treated with a JNK inhibitor that inhibits all JNK isoforms nonselectively.
To determine whether JNK1 and JNK2 have redundant functions, we also inhibited each of these individually by expressing only dnJNK1 or only dnJNK2 in SGNs. As shown in Figure 7B, inhibition of either one of these JNK isoforms alone did not cause a significant decrease in neurite growth even though, in this experiment, the cells were cultured for an additional 24 h in an attempt to reveal a more subtle effect on neurite growth. This result might imply that JNK1 and JNK2 have entirely overlapping or redundant functions in neurite growth, consistent with the viability of single JNK1 or JNK2 knockouts, in contrast to lethality of the double knockout (Bogoyevitch, 2006; Chang et al., 2003). Alternatively, silencing only a single isoform simply doesn’t cause a sufficient decrease in total JNK activity to affect neurite growth.
Finally, we tested the possibility that increased JNK activity would result in more rapid neurite growth. To do so, we expressed a constitutively-active MKK7-JNK1 fusion protein (Han et al., 2002) in SGNs and compared mean neurite length to control SGNs. As shown in Figure 7B, constitutively-active JNK caused a significant (p<0.001) increase in neurite length. This further links JNK with neurite growth by showing that depressing or elevating JNK activity causes a corresponding change in neurite growth rate.
As described in Methods, cells in these experiments were cultured in a strongly neurotrophic condition, 25K with 5% serum, to prevent apoptosis. Consequently, survival of MKK7-JNK1-transfected neurons was 126.9% ± 18.8% (SD) of control while survival of dnJNK1,2-transfected SGNs was 93.2% ± 13.6% (SD) of control. These numbers are not significantly different from each other nor from control. Therefore, it is unlikely that the observed increase in neurite length in MKK7-JNK1-transfected SGNs is due to selective apoptosis of SGNs with shorter neurites.
Discussion
Inhibition of JNK typically inhibits neurite growth in neurons and neuronal cell lines (Barnat et al., 2010; Eminel et al., 2008; Lindwall et al., 2005a; Lindwall et al., 2005b; Oliva et al., 2006; Waetzig et al., 2006), although this is not the case for all types of neurons (Coffey et al., 2000; Eminel et al., 2008). Here we have used molecular, genetic, and pharmacological tools to investigate the requirement for JNK in neurite growth from cultured neonatal rat or mouse SGNs. We show that the level of JNK activity is correlated with neurite length: inhibition of JNK – using either pharmacological means or by means of transfection of catalytically-inactive dominant-negative JNK mutants into SGNs – results in inhibition of neurite growth and decreased neurite length. Conversely, expression of constitutively-active JNK – a fusion of JNK1 and its activator MKK7 – causes accelerated neurite growth and increased neurite length. While we and others have noted that pharmacological or molecular genetic inhibition of JNK results in a reduced rate of neurite extension, at least one study (Barnat et al., 2010) has observed neurite retraction in mouse DRG sensory neurons following pharmacological JNK inhibition. This may reflect differences among different types of neurons as not all studies report neurite retraction and some report enhancement of neurite growth by JNK inhibitors under some circumstances (Coffey et al., 2000; Eminel et al., 2008).
Role of MAP kinase kinase kinases (MAPKKKs) upstream of JNK
A previous report (Bodmer et al., 2002) showing that the MLK inhibitor CEP-11004 reduces SGN neurite growth in vitro is consistent with our results as MLKs phosphorylate and activate the MKKs that, in turn, phosphorylate and activate JNKs (Davis, 2000). However, MAPKKKs other than MLKs have been shown to contribute to JNK activation in apoptosis. One of these, dual leucine zipper kinase (DLK) is a more significant activator of JNK in at least one instance of neuronal apoptosis: a neurotoxin Parkinson disease model (Chen et al., 2008). Relevant to the data presented here is that knockout of the gene encoding DLK in mice results in reduced phosphorylation of JNK substrates and reduction of axonal projections (Hirai et al., 2006).
Kinetics of JNK activation suggests that JNK acts in the growth cone to promote neurite growth
We observed that JNK activation (assessed as phosphoJNK immunoreactivity) in response to neurotrophic factor withdrawal requires hours in the soma and nucleus but is much faster in the growth cone where it is detectable within 30 min. This is consistent with a role for nuclear JNK in apoptosis (Bjorkblom et al., 2008). Presumably, this reflects the transcriptional requirement for neuronal apoptosis, involving the transcription factor c-Jun (Ham et al., 1995). In contrast, the timing of the effects of JNK inhibitors on neurite growth suggests a local role, in the growth cone, for JNK in neurite growth. This is supported by the observation that phosphoJNK immunofluorescence responds rapidly in the growth cone and that the effect of addition or removal of JNK on neurite growth is also rapid relative to JNK phosphorylation in the soma.
Local actions of JNK in the growth cone can include regulation of the microtubule cytoskeleton. Genetic deletion of JNK1 in mice results in decreased phosphorylation of high-molecular-weight microtubule-associated proteins, MAP1B and MAP2, resulting in decreased microtubule assembly, loss of axonal and dendritic microtubules, and disrupted formation of some axon tracts and of dendritic arbors (Bjorkblom et al., 2005; Chang et al., 2003). JNK1 has also been shown to stabilize microtubules by phosphorylating SCG10 and other stathmins (Tararuk et al., 2006). Stathmins destabilize microtubules but are negatively regulated by JNK so JNK inhibition results in microtubule destabilization. While not necessarily affecting cell motility directly, inhibition of microtubule polymerization would limit the rate neurite extension by slowing stabilization of the growing neurite at the trailing edge of the growth cone.
Alternatively, or perhaps in parallel, JNK may promote growth cone motility directly. Indeed, JNK is enriched at the leading edge of motile cells (Amagasaki et al., 2006)and has been implicated as a positive regulator of cell migration in a variety of systems (Huang et al., 2004b). Paxillin is an adaptor protein enriched at focal adhesions and involved in regulation of signal transduction, cell adhesion, and the actin cytoskeleton. Paxillin is phosphorylated in a JNK-dependent manner to regulate cell adhesion and motility (Huang et al., 2004a), in part by regulating assembly and disassembly of focal adhesions (Amagasaki et al., 2006; Smadja-Lamere et al., 2008). Finally, JIP family scaffold proteins are necessary for recruitment and activation of JNK but are also important regulators of axon growth (Barnat et al., 2010; Dajas-Bailador et al., 2008; Morrison et al., 2003)and JIP1 is enriched in neuronal growth cones (Dajas-Bailador et al., 2008; Morrison et al., 2003).
Roles of JNK isoforms in neurite growth
We also asked whether all three JNK isoforms, JNK1–3, are involved in SGN neurite growth. We find that there is little difference between neonatal mouse SGNs transfected with dnJNK1 and those transfected with dnJNK2. Rather, silencing of either alone has no significant effect on SGN neurite outgrowth. Silencing both JNK1 and JNK2 simultaneously does significantly inhibit SGN neurite growth, an inhibition additive with that caused by JNK3. Deletion of JNK3 alone does significantly inhibit neurite growth, although the inhibition is small. Barnat et al. (2010) also used genetic and pharmacological means to inhibit JNK and to delete individual isoforms in mouse DRG neurons. While their results are similar to ours, they reported that neurite initiation is delayed in JNK2−/− or JNK3−/− mice but not in JNK1−/− mice (Barnat et al., 2010), suggesting that JNK1 is distinctly not required for neurite initiation. This difference between our study and that of Barnat et al. regarding the requirement for JNK1 may be due to differences between the neurons. Other studies have shown deficits in axonal growth in vivo in JNK1−/− mice (Bjorkblom et al., 2005; Chang et al., 2003; Tararuk et al., 2006).
JNK as a therapeutic target
We have previously shown (Alam et al., 2007) that following hair cell loss SGNs undergo apoptosis in a two-stage process: an early phase is marked by decreased phosphorylation of the transcription factor CREB, indicative of reduced neurotrophic input; subsequently, beginning 2–3 weeks post-deafening, JNK and Jun phosphorylation appears and most apoptotic SGNs have phosphoJun immunoreactivity. This correlation between JNK activity and apoptosis suggests that JNK is necessary for apoptosis in SGNs as is the case for other neurons. This is supported by our preliminary studies (J. Huang and S.H. Green, unpublished observations) showing that SGNs in vitro can be rescued by pharmacological inhibition of JNK with SP600125 or by genetic deletion of JNK3 in mice.
JNK inhibition has been suggested as a therapeutic approach to neurodegenerative disease in general (Bogoyevitch et al., 2004). JNK inhibition may have therapeutic potential as an approach to protecting SGNs in cochlear implant users. Such an approach may be complicated by the fact that JNK is a multifunctional protein with roles in apoptosis, cell motility, and neurite growth. While inhibiting JNK may prevent SGN apoptosis, it may also inhibit regeneration of peripheral processes or, worse, cause neurite retraction (Lindwall et al., 2005a). Our studies show that JNK inhibition in vitro slows neurite growth but does not completely prevent it nor cause neurite retraction. We also show that, although all JNK isoforms contribute to neurite growth, targeting JNK3 selectively can inhibit apoptosis while having only a small effect on neurite growth. Further study of the cell biology of JNK can suggest additional means to dissociate proapoptotic actions of JNK from those related to cell motility.
Acknowledgments
Support for this study was from NIH grants R01 DC02961 (S.H.G.) and KO8 DC006211-01A1 (M.R.H.) and P30 DC010362. We thank Catherine Kane and Simrit Sodhi for technical assistance.
Literature Cited
- Alam SA, Robinson BK, Huang J, Green SH. Prosurvival and proapoptotic intracellular signaling in rat spiral ganglion neurons in vivo after the loss of hair cells. J Comp Neurol. 2007;503:832–852. doi: 10.1002/cne.21430. [DOI] [PubMed] [Google Scholar]
- Amagasaki K, Kaneto H, Heldin CH, Lennartsson J. c-Jun N-terminal kinase is necessary for platelet-derived growth factor-mediated chemotaxis in primary fibroblasts. J Biol Chem. 2006;281:22173–9. doi: 10.1074/jbc.M513307200. [DOI] [PubMed] [Google Scholar]
- Barnat M, Enslen H, Propst F, Davis RJ, Soares S, Nothias F. Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J Neurosci. 2010;30:7804–16. doi: 10.1523/JNEUROSCI.0372-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141:41–53. doi: 10.1016/j.jneumeth.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkblom B, Vainio JC, Hongisto V, Herdegen T, Courtney MJ, Coffey ET. All JNKs can kill, but nuclear localization is critical for neuronal death. J Biol Chem. 2008;283:19704–13. doi: 10.1074/jbc.M707744200. [DOI] [PubMed] [Google Scholar]
- Bjorkblom B, Ostman N, Hongisto V, Komarovski V, Filen JJ, Nyman TA, Kallunki T, Courtney MJ, Coffey ET. Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J Neurosci. 2005;25:6350–61. doi: 10.1523/JNEUROSCI.1517-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer D, Gloddek B, Ryan AF, Huverstuhl J, Brors D. Inhibition of the c-Jun N-terminal kinase signaling pathway influences neurite outgrowth of spiral ganglion neurons in vitro. Laryngoscope. 2002;112:2057–61. doi: 10.1097/00005537-200211000-00028. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. BioEssays. 2006;28:923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim Biophys Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Bok J, Wang Q, Huang J, Green SH. CaMKII and CaMKIV mediate distinct prosurvival signaling pathways in response to depolarization in neurons. Mol Cell Neurosci. 2007;36:13–26. doi: 10.1016/j.mcn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner SR, Tammariello SP, Kuan CY, Flavell RA, Rakic P, Estus S. JNK3 contributes to c-Jun activation and apoptosis but not oxidative stress in nerve growth factor-deprived sympathetic neurons. J Neurochem. 2001;78:298–303. doi: 10.1046/j.1471-4159.2001.00400.x. [DOI] [PubMed] [Google Scholar]
- Carboni S, Hiver A, Szyndralewiez C, Gaillard P, Gotteland JP, Vitte PA. AS601245 (1,3-benzothiazol-2-yl (2-[[2-(3-pyridinyl) ethyl] amino]-4 pyrimidinyl) acetonitrile): a c-Jun NH2-terminal protein kinase inhibitor with neuroprotective properties. J Pharmacol Exp Ther. 2004;310:25–32. doi: 10.1124/jpet.103.064246. [DOI] [PubMed] [Google Scholar]
- Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell. 2003;4:521–533. doi: 10.1016/s1534-5807(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Chen X, Rzhetskaya M, Kareva T, Bland R, During MJ, Tank AW, Kholodilov N, Burke RE. Antiapoptotic and trophic effects of dominant-negative forms of dual leucine zipper kinase in dopamine neurons of the substantia nigra in vivo. J Neurosci. 2008;28:672–80. doi: 10.1523/JNEUROSCI.2132-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CW, Dong C, Flavell RA, Davis RJ. c-Jun NH2-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1. Molecular and cellular biology. 2000;20:5227–34. doi: 10.1128/mcb.20.14.5227-5234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey ET, Hongisto V, Dickens M, Davis RJ, Courtney MJ. Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J Neurosci. 2000;20:7602–7613. doi: 10.1523/JNEUROSCI.20-20-07602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F, Jones EV, Whitmarsh AJ. The JIP1 scaffold protein regulates axonal development in cortical neurons. Curr Biol. 2008;18:221–226. doi: 10.1016/j.cub.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Eilers A, Whitfield J, Babij C, Rubin LL, Ham J. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci. 1998;18:1713–24. doi: 10.1523/JNEUROSCI.18-05-01713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eminel S, Roemer L, Waetzig V, Herdegen T. c-Jun N-terminal kinases trigger both degeneration and neurite outgrowth in primary hippocampal and cortical neurons. J Neurochem. 2008;104:957–969. doi: 10.1111/j.1471-4159.2007.05101.x. [DOI] [PubMed] [Google Scholar]
- Green SH, Altschuler RA, Miller JM. Cell Death and Cochlear Protection. In: Schacht J, Popper AN, Fay RR, editors. Auditory Trauma, Protection and Repair. Springer-Verlag; New York: 2008. [Google Scholar]
- Ham J, Eilers A, Whitfield J, Neame SJ, Shah B. c-Jun and the transcriptional control of neuronal apoptosis. Biochem Pharmacol. 2000;60:1015–21. doi: 10.1016/s0006-2952(00)00372-5. [DOI] [PubMed] [Google Scholar]
- Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M, Rubin LL. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14:927–39. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Han SY, Kim SH, Heasley LE. Differential gene regulation by specific gain-of-function JNK1 proteins expressed in Swiss 3T3 fibroblasts. J Biol Chem. 2002;277:47167–74. doi: 10.1074/jbc.M204270200. [DOI] [PubMed] [Google Scholar]
- Hirai S, Cui de F, Miyata T, Ogawa M, Kiyonari H, Suda Y, Aizawa S, Banba Y, Ohno S. The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J Neurosci. 2006;26:11992–2002. doi: 10.1523/JNEUROSCI.2272-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. A role for JNK-paxillin signaling in cell migration. Cell Cycle. 2004a;3:4–6. [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004b;117:4619–28. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK) -- from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Keramaris E, Vanderluit JL, Bahadori M, Mousavi K, Davis RJ, Flavell R, Slack RS, Park DS. c-Jun N-terminal kinase 3 deficiency protects neurons from axotomy-induced death in vivo through mechanisms independent of c-Jun phosphorylation. J Biol Chem. 2005;280:1132–41. doi: 10.1074/jbc.M410127200. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Yang DD, Roy DRS, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, Davis RJ, Rakic P. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–9. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall C, Kanje M. The Janus role of c-Jun: cell death versus survival and regeneration of neonatal sympathetic and sensory neurons. Exp Neurol. 2005a;196:184–94. doi: 10.1016/j.expneurol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Lindwall C, Kanje M. The role of p-c-Jun in survival and outgrowth of developing sensory neurons. Neuroreport. 2005b;16:1655–9. doi: 10.1097/01.wnr.0000183324.75499.fc. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annual Review of Cell and Developmental Biology. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Namgung U, Xia Z. Arsenic induces apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinases. Toxicol Appl Pharmacol. 2001;174:130–8. doi: 10.1006/taap.2001.9200. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Atkins CM, Copenagle L, Banker GA. Activated c-Jun N-terminal kinase is required for axon formation. J Neurosci. 2006;26:9462–9470. doi: 10.1523/JNEUROSCI.2625-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Curr Opin Otolaryngol Head Neck Surg. 2005;13:294–300. doi: 10.1097/01.moo.0000180919.68812.b9. [DOI] [PubMed] [Google Scholar]
- Smadja-Lamere N, Boulanger MC, Champagne C, Branton PE, Lavoie JN. JNK-mediated phosphorylation of paxillin in adhesion assembly and tension-induced cell death by the adenovirus death factor E4orf4. J Biol Chem. 2008;283:34352–34364. doi: 10.1074/jbc.M803364200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tararuk T, Ostman N, Li W, Bjorkblom B, Padzik A, Zdrojewska J, Hongisto V, Herdegen T, Konopka W, Courtney MJ, Coffey ET. JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. J Cell Biol. 2006;173:265–77. doi: 10.1083/jcb.200511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waetzig V, Zhao Y, Herdegen T. The bright side of JNKs – multitalented mediators in neuronal sprouting, brain development and nerve fiber regeneration. Prog Neurobiol. 2006;80:84–97. doi: 10.1016/j.pneurobio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Wang LH, Besirli CG, Johnson EM., Jr Mixed-lineage kinases: a target for the prevention of neurodegeneration. Annu Rev Pharmacol Toxicol. 2004;44:451–74. doi: 10.1146/annurev.pharmtox.44.101802.121840. [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Devel. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the JNK3 gene. Nature. 1997;389:865–70. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Zha XM, Bishop JF, Hansen MR, Victoria L, Abbas PJ, Mouradian MM, Green SH. BDNF synthesis in spiral ganglion neurons is constitutive and CREB-dependent. Hear Res. 2001;156:53–68. doi: 10.1016/s0378-5955(01)00267-2. [DOI] [PubMed] [Google Scholar]