Abstract
Background & Aims
Colonization of gastric mucosa by Helicobacter pylori leads to epithelial hyperproliferation, which increases the risk for gastric adenocarcinoma. One H. pylori virulence locus associated with cancer risk, cag, encodes a secretion system that transports effectors into host cells and leads to aberrant activation of β-catenin and p120-catenin (p120). Peroxisome proliferator-activated receptor (PPAR)δ is a ligand-activated transcription factor that affects oncogenesis in conjunction with β-catenin. We used a carcinogenic H. pylori strain to define the role of microbial virulence constituents and PPARδ in regulating epithelial responses that mediate development of adenocarcinoma.
Methods
Gastric epithelial cells or colonies were co-cultured with the H. pylori cag+ strain 7.13 or cagE−, cagA−, slt−, or cagA−/slt− mutants. Levels of PPARδ and Cyclin E1 were determined by real-time, reverse transcription PCR, immunoblot analysis, or immunofluorescence microscopy; proliferation was measured in 3-dimensional culture. PPARδ and Ki67 expression were determined by immunohistochemical analysis of human biopsies and rodent gastric mucosa.
Results
H. pylori induced β-catenin- and p120-dependent expression and activation of PPARδ in gastric epithelial cells, which were mediated by the cag secretion system substrates CagA and peptidoglycan. H. pylori stimulated proliferation in vitro, which required PPARδ-mediated activation of Cyclin E1; H. pylori did not induce expression of Cyclin E1 in a genetic model of PPARδ deficiency. PPARδ expression and proliferation in rodent and human gastric tissue was selectively induced by cag+ strains and PPARδ levels normalized following eradication of H. pylori.
Conclusions
The H. pylori cag secretion system activates β-catenin, p120, and PPARδ, which promote gastric epithelial cell proliferation via activation of Cyclin E1. PPARδ might contribute to gastric adenocarcinoma development in humans.
Keywords: stomach cancer, Mongolian gerbils, signaling, bacteria
Introduction
Helicobacter pylori significantly increases the risk for adenocarcinoma of the stomach, yet only a fraction of colonized persons ever develop neoplasia1. The cag pathogenicity island is a strain-specific locus that augments cancer risk and which encodes a type IV bacterial secretion system. The product of the terminal gene in the island (CagA) is translocated into host epithelial cells, undergoes tyrosine phosphorylation2,3, and induces morphological changes that are reminiscent of unrestrained stimulation by growth factors. Non-phosphorylated CagA also exerts effects within host cells that mediate carcinogenesis, including aberrant activation of phosphatidylinositol 3-phosphate kinase (PI3K) and β-catenin, disruption of apical-junctional complexes, and a loss of cellular polarity4–7. The cag secretion system also delivers components of H. pylori peptidoglycan into host cells where they are recognized by Nod1, an intracytoplasmic pattern-recognition molecule8. Nod1 sensing of H. pylori peptidoglycan activates NF-κB, as well as PI3K, leading to cellular responses that lower the threshold for cancer8,9.
β-catenin is bound to E-cadherin at the cell membrane and/or sequestered in the cytosol within a multi-protein inhibitory complex containing APC, GSK-3β and axin. In the absence of Wnt, β-catenin is constitutively phosphorylated by GSK-3β and targeted for proteasomal degradation. Binding of Wnt to its receptor Frizzled inhibits β-catenin degradation, leading to its nuclear accumulation and targeted transcriptional up-regulation of genes that influence carcinogenesis. CagA has been demonstrated to physically interact with E-cadherin leading to release of β-catenin from the membrane into the nucleus10.
Another host molecule that may influence gastric carcinogenesis in conjunction with H. pylori and β-catenin is p120-catenin (p120). p120 mediates cell-cell adhesion via regulation of E-cadherin recycling, but can also aberrantly localize to the nucleus where it binds Kaiso, a transcriptional repressor of β-catenin target genes11. H. pylori induces nuclear translocation of p120 in gastric epithelial cells in a cag-dependent manner, which increases expression of the β-catenin target mmp712. In addition to mmp-7, p120 and Kaiso regulate expression of other β-catenin target genes, including peroxisome proliferator-activated receptor δ (pparδ)11. PPARδ is a member of the nuclear hormone receptor superfamily13 and through heterodimer formation with the retinoid X receptor, PPARδ regulates transcription of target genes that mediate fatty acid oxidation and glucose utilization13. Recent evidence, however, suggests that collaboration between β-catenin and PPARδ influences oncogenesis within the gastrointestinal tract14.
Levels of PPARδ are elevated in most human colorectal cancer specimens as well as tumors that develop in murine models of colon cancer. The specific PPARδ agonist GW501516 is proneoplastic in mice15, and deletion of PPARδ decreases intestinal adenoma growth in ApcMin/+ mice and inhibits the tumor promoting effects of GW50151613. A potential mechanism through which PPARδ activation may heighten the risk for cancer is via inducing proliferation. GW501516 stimulates proliferation in human breast, prostate, and hepatocellular carcinoma cells16,17, and, in a murine mammary tumor model, treatment with GW501516 accelerated tumor formation18. Consistent with these findings, Cyclin E1, a cell cycle regulatory protein that mediates the transition of cells from G1 into S phase, has recently been identified as a PPARδ target19. Since H. pylori can activate host effectors that regulate expression of PPARδ, the goal of this study was to define the role of PPARδ in conjunction with H. pylori in regulating gastric epithelial responses with carcinogenic potential in vitro and in vivo.
Methods
Details for in vitro, ex vivo, and human studies are contained in Supplemental Data.
Animals and H. pylori challenge
All procedures were approved by the IACUC committee of Vanderbilt University. Mongolian gerbils, wild-type or ppard+/− C57Bl/6 mice, ages 4 to 8 weeks, were orogastrically challenged with Brucella broth, H. pylori wild-type strain 7.13, or an isogenic 7.13 cagE− mutant strain, and were sacrificed 3 or 14 days, or 8 or 12 weeks post-inoculation. One half of the glandular stomach was fixed for histologic examination, one-quarter was homogenized for protein extraction, and one-quarter was cultured for H. pylori5. Indices of inflammation and presence of dysplasia or adenocarcinoma were evaluated by a single pathologist blinded to treatment groups as previously described5.
Antibiotic therapy
Antibiotic therapy was delivered 4–8 weeks after H. pylori challenge and consisted of lansoprazole (1mg/kg) (TAP Pharmaceuticals Inc), amoxicillin (15mg/kg) (Ranbaxy Pharmaceuticals), and clarithromycin (30mg/kg) (Abbott Laboratories) for 14 days, as previously described20. Sham therapy consisted of 500 μL sterile water delivered daily for 14 days. Infected and uninfected gerbils, treated with antibiotics or water, were euthanized 1 week or 8 weeks post-treatment.
Results
PPARδ is expressed and is functionally responsive to H. pylori in gastric epithelial cells
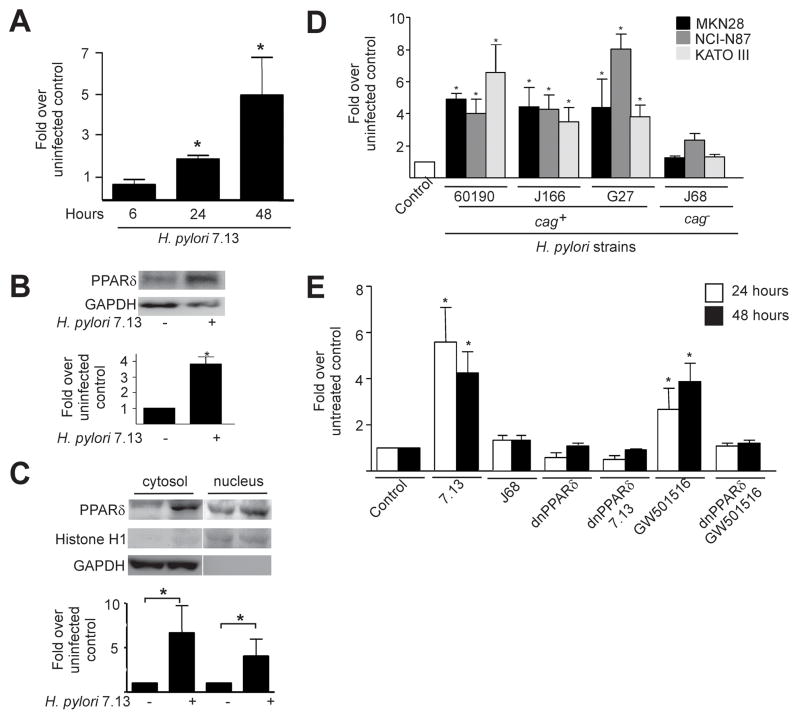
To determine whether H. pylori induces PPARδ expression, MKN28 gastric epithelial cells were co-cultured with a cag+ strain, 7.13, that rapidly induces gastric carcinoma in rodent models of infection5. pparδ mRNA expression significantly increased in cells infected with H. pylori, beginning at 24 hours post-infection (Figure 1A), which was accompanied by increased PPARδ protein levels in whole-cell lysates, and in both the cytosol and nucleus by 48 hours post-infection (Figure 1B, 1C). These results were subsequently extended by demonstrating that levels of pparδ mRNA (Figure 1D) and protein (data not shown) were increased in MKN28 as well as NCI-N87 and KATO III gastric epithelial cells in response to a series of clinical cag+ H. pylori strains (60190, J166, and G27), but not in response to the clinical cag− strain J68.
Figure 1. cag+ Helicobacter pylori induce expression and functional activation of PPARδ in gastric epithelial cells.
(A) MKN28 cells were co-cultured with H. pylori strain 7.13. RNA was analyzed in duplicate by real-time PCR. Data represent fold pparδ induction in infected versus uninfected cells from experiments performed on at least three occasions. Error bars, SEM for all panels. *p< .05 versus uninfected control. (B) MKN28 cells were co-cultured with strain 7.13. Forty-eight hours post-infection, whole cell lysates were analyzed by Western blot using an anti-PPARδ antibody. Anti-GAPDH antibody served as a normalization control. Densitometric analysis of Western blots performed on at least three occasions is shown below representative blots. *p< .05 versus uninfected control. (C) MKN28 cells were co-cultured with strain 7.13. Forty-eight hours post-infection, protein was analyzed by Western blot using an anti-PPARδ antibody. Anti-Histone H1 and anti-GAPDH antibodies served as normalization controls for nuclear and cytosolic fractionations, respectively. Densitometric analysis of Western blots performed on at least three occasions is shown below representative blots. *p< .05 versus uninfected control. (D) MKN28, NCI-N87 or KATO III cells were co-cultured with cag+ strains 60190, J166 or G27, or the cag− strain J68. RNA was extracted 24 hours post-infection and analyzed for pparδ expression. *p< .05 versus uninfected control. (E) MKN28 cells were co-transfected with PPRE3-tk-luciferase and pRL-SV40 with empty vector or dominant-negative PPARδ plasmids followed by treatment with strains 7.13 or J68, or the PPARδ agonist GW501516. Dual luciferase assays were performed as described in Methods. *p< .0004 versus untreated control.
To determine whether endogenous PPARδ was functionally responsive to H. pylori, MKN28 cells were transfected with a PPARδ reporter vector (PPRE3-tk-luc) (Supplemental Methods). Treatment of transfected cells with the PPARδ selective agonist GW501516 increased luciferase activity (Figure 1E). PPRE-transfected cells were then infected with the cag+ strain 7.13, which significantly increased luciferase activity compared to uninfected cells or cells infected with the cag− strain J68 (Figure 1E).
To establish the specificity of this response, MKN28 cells were co-transfected with the reporter PPRE vector and a dominant-negative PPARδ construct (dnPPARδ, Supplemental Figure 1A). PPARδ activation in response to the PPARδ agonist GW501516 or H. pylori was abolished in cells transfected with dnPPARδ, indicating that activation of PPARδ by H. pylori is specific (Figure 1E). Thus, PPARδ is induced and functionally active in gastric cells infected with H. pylori cag+ strains.
H. pylori-mediated up-regulation of PPARδ is dependent on β-catenin and p120
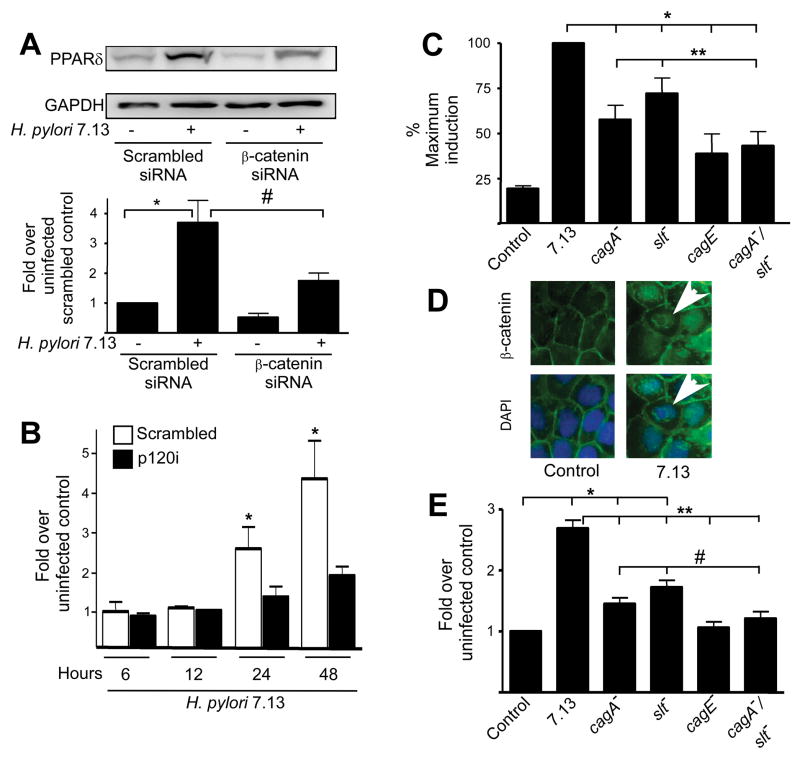
We next investigated the relationship between H. pylori, β-catenin and PPARδ by examining levels of PPARδ in H. pylori-infected MKN28 cells treated with β-catenin-specific siRNA (Supplemental Figure 1B, Methods). Levels of PPARδ expression in response to H. pylori were significantly decreased in infected β-catenin-deficient cells compared to infected control cells (Figure 2A).
Figure 2. PPARδ expression induced by H. pylori requires β-catenin and p120 and cag island substrates. (A).
MKN28 cells transiently transfected with control or β-catenin-specific siRNA were co-cultured with strain 7.13 for 24 hours. Total protein was analyzed by Western blot using an anti-PPARδ antibody. Bar graph represents densitometric analysis of multiple experiments. Error bars, SEM for all panels. *p < .05 versus uninfected scrambled controls. #p < .05 versus 7.13-infected scrambled controls. (B) Scramble control or p120i cells were co-cultured with strain 7.13 or medium alone. Total RNA was analyzed in duplicate by real-time PCR. *p < .05 versus uninfected control. (C) MKN28 cells were transiently transfected with PPRE3-tk-luciferase and pRL-SV40, followed by infection with strain 7.13, or the cagA−, slt−, cagE− or cagA−/slt− mutants. Dual luciferase assays were then performed. *p < .001 versus 7.13-infected samples. **p < .05 versus cagA−/slt−-infected samples. (D) MKN28 cells were co-cultured with strain 7.13 or medium alone for 24 hours. Cells were stained with anti-β-catenin and AlexaFluor-488 antibodies and DAPI nuclear dye and analyzed by immunofluorescence microscopy. Arrowheads, nuclear β-catenin. (E) MKN28 cells were transiently transfected with β-catenin reporter constructs in the absence or presence of wild-type strain 7.13 or mutants lacking cagA, slt, cagE, or cagA/slt. Luciferase activity was determined 24 hours after infection. *p < .05 versus uninfected control. **p < .001 versus 7.13-infected samples. #p < .05 versus cagA−/slt−-infected samples.
Since p120 can regulate expression of β-catenin target genes, we determined if H. pylori-induced upregulation of PPARδ was mediated by p120. MKN28 cells stably transduced with control or p120-specific siRNA (Supplemental Figure 1C, Methods) were co-cultured with H. pylori strain 7.13. pparδ expression was significantly attenuated in H. pylori-infected p120 deficient cells when compared to infected controls, indicating that p120 is also required for H. pylori-mediated transcriptional up-regulation of PPARδ (Figure 2B).
Microbial effectors translocated by the cag island are required for PPARδ activation
Our earlier studies (Figure 1) indicated that induction of PPARδ may be a cag-specific effect; therefore, we next directly examined the effects of cagA, cagE (encoding a structural element of the cag secretion system), or the cag island substrate peptidoglycan on PPARδ activation. To examine the role of peptidoglycan, we generated an isogenic H. pylori mutant lacking a critical enzyme required for peptidoglycan turnover, soluble lytic transglycosylase (slt)8.
MKN28 cells transfected with the PPARδ reporter PPRE were infected with wild-type strain 7.13 or its isogenic cagA−, cagE−, or slt− null mutant derivatives as well as a cagA−/slt− double mutant strain. Loss of cagA or slt alone led to partial attenuation of PPARδ activation (Figure 2C). However, inactivation of cagE, or cagA and slt in combination further reduced the ability of wild-type strain 7.13 or the 7.13 cagA− or slt− single mutants to activate PPARδ (Figure 2C), indicating that a non-functional cag island or loss of both translocated cag effectors similarly attenuate the ability of H. pylori to fully activate PPARδ.
Activation of β-catenin requires CagA and peptidoglycan
β-catenin regulates the expression of PPARδ in intestinal epithelial cells; therefore we determined if the same microbial constituents required for H. pylori-induced PPARδ activation (Figure 2C) also mediated β-catenin activation in gastric cells by using isogenic mutant strains generated within wild-type H. pylori strain 7.13. As expected, infection of MKN28 cells with strain 7.13 induced translocation of β-catenin from the membrane into the cytoplasm and the nucleus (Figure 2D). Staining was punctate, which may represent β-catenin in complex with APC/GSK3β/axin in the cytoplasm or coupled with known transcriptional co-factors such as LEF/TCF in the nucleus. MKN28 cells transfected with a β-catenin reporter construct (Topflash) or a control construct containing mutated LEF/TCF sites (Fopflash) (Supplemental Methods) were then infected with H. pylori wild-type strain 7.13 or its isogenic mutants.
Luciferase activity did not differ in cells transfected with the control construct with or without H. pylori (data not shown); however, activity was significantly higher in H. pylori-infected versus uninfected cells harboring the β-catenin responsive LEF/TCF construct (Figure 2E). Similar to effects on PPARδ (Figure 2C), inactivation of cagE significantly reduced the ability of strain 7.13 to activate β-catenin to levels observed in uninfected control cells (Figure 2E). Inactivation of either cagA or slt alone partially attenuated the increase in β-catenin activation induced by the wild-type 7.13 strain (Figure 2E).
Based on our previously published data using H. pylori strain 7.139 and other studies linking PI3K activation to phosphorylation-mediated inhibition of GSK3β21, we postulated that peptidoglycan may induce β-catenin-dependent PPARδ activation via PI3K-mediated inhibition of GSK3β. In support of this, we found that pretreatment of MKN28 cells with a chemical inhibitor of PI3K reduced H. pylori strain 7.13-induced GSK3β phosphorylation, β-catenin translocation to the nucleus, and PPARδ activation (Supplemental Figure 2). Of interest, although the reduction in PPARδ activation induced by PI3K inhibition was statistically significant, it did not fall to baseline levels that were seen in uninfected control cells, which closely mirrors our results in Figure 2C demonstrating that a partial reduction in PPARδ activation occurs following co-culture with the H. pylori strain 7.13 peptidoglycan deficient slt− mutant.
To determine if the cag type IV secretion system substrates CagA and peptidoglycan exerted synergistic effects on β-catenin activation as was observed for PPARδ activation (Figure 2C), cells were infected with the 7.13 cagA−/slt− double mutant. Loss of both CagA and Slt significantly decreased β-catenin activation when compared to reductions induced by loss of either bacterial constituent alone (Figure 2E). These findings indicate that multiple H. pylori proteins can mediate β-catenin activation and are concordant with results demonstrating that the same bacterial mediators are required for H. pylori-induced PPARδ activation (Figure 2C).
Activation of PPARδ by H. pylori promotes epithelial cell proliferation
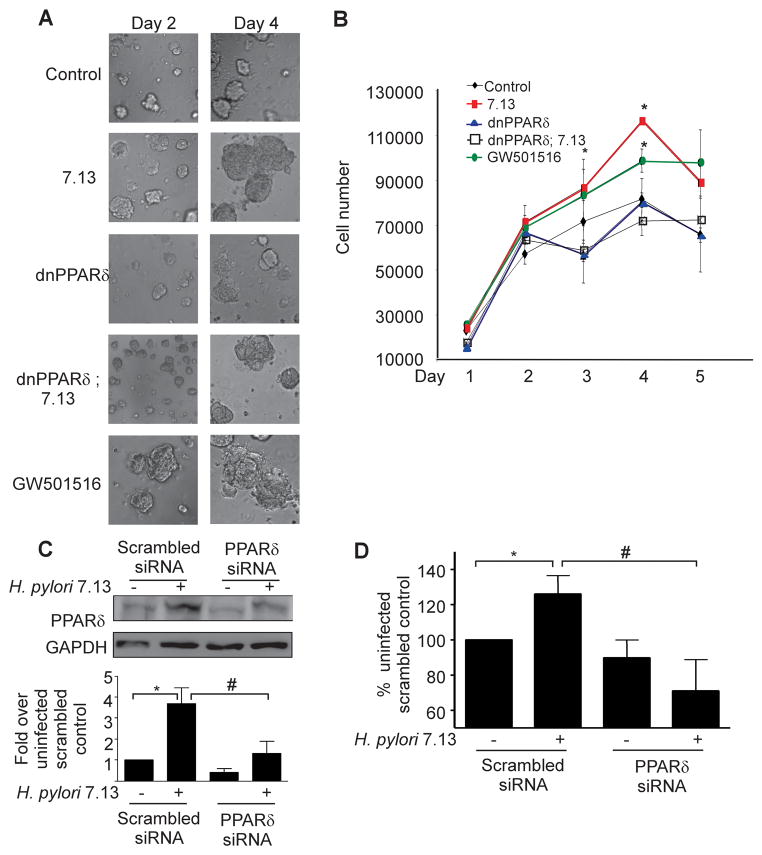
H. pylori increases gastric epithelial cell proliferation in colonized mucosa, and PPARδ stimulates proliferation of human cells17,19; therefore, we next defined the role of PPARδ in regulation of this carcinogenic response. Proliferation was assayed using a three-dimensional model system, which provides interactions between cells, growth factors, and an extracellular matrix (Figure 3A). MKN28 cells treated with the PPARδ agonist GW501516 proliferated at a significantly higher rate than untreated cells (Figure 3A, 3B).
Figure 3. H. pylori stimulates proliferation in a PPARδ-dependent manner. (A).
MKN28 cells were transfected with control vector or dominant-negative (dn) PPARδ constructs, seeded in a three-dimensional culture system, and then infected with strain 7.13, or treated with the PPARδ agonist GW501516. Representative images are shown. (B) Following infection with strain 7.13, GW501516 or medium alone, cells were removed from Matrigel at 24-hour intervals and enumerated using Trypan blue staining. Error bars = SEM for experiments performed on at least 3 occasions. *p < .05 7.13-infected or GW501516-treated samples versus uninfected control. (C) MKN28 cells transfected with scrambled or PPARδ-specific siRNA were co-cultured with strain 7.13 for 48 hours. Total protein was analyzed by Western blot using an anti-PPARδ antibody. Bar graph represents densitometric analysis of multiple experiments. Error bars, SEM for all panels. *p < .05 versus uninfected scrambled control. #p < .05 versus 7.13-infected scrambled controls. (D) MKN28 cells transfected with scrambled or PPARδ-specific siRNA were seeded in Matrigel and infected with strain 7.13 or medium alone for 72 hours. BrdU was added to culture medium and ELISA for BrdU incorporation was performed. Error bars = SEM for experiments performed on at least 3 occasions. *p < .05 versus uninfected scrambled control. #p < .03 vs 7.13-infected scrambled control.
Treatment of MKN28 cells with strain 7.13 significantly increased proliferation compared to cells treated with medium alone, but dual treatment of cells with GW501516 and strain 7.13 did not significantly enhance proliferation compared to either treatment alone (Figure 3A, 3B, Supplemental Figure 3). In addition, proliferation in H. pylori-infected cells expressing dnPPARδ was significantly decreased compared to H. pylori-infected control cells (Figure 3B).
These results were confirmed using an independent assay for proliferation. MKN28 cells were treated with control or PPARδ-specific siRNA (Figure 3C) and were then seeded into Matrigel. After 72 hours of H. pylori co-culture, cells were incubated with BrdU and results were quantified by ELISA. BrdU incorporation in H. pylori-infected control cells was significantly higher than in either uninfected cells or H. pylori-infected cells harboring PPARδ-specific siRNA (Figure 3D), confirming that activation of PPARδ by strain 7.13 promotes gastric epithelial proliferation.
H. pylori induces accumulation and nuclear localization of PPARδ in gastric colonies
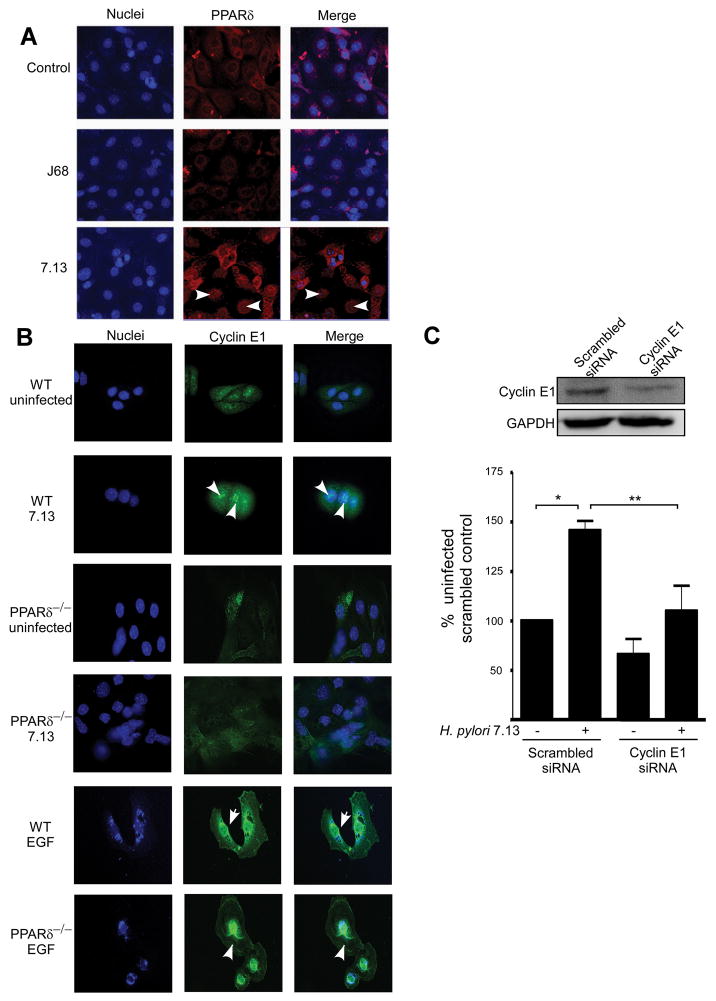
To extend our in vitro results, we capitalized upon a model that more closely recapitulates cellular organization in the stomach. Gastric cell colonies were isolated from C57Bl/6 mice (Supplemental Methods), co-cultured with the cag+ strain 7.13, the cag− strain J68, or medium alone for 24 hours, and PPARδ localization was assessed by immunofluorescence microscopy. Consistent with our in vitro results (Figure 1), cytoplasmic accumulation and/or nuclear translocation of PPARδ was observed in 7.13-infected cells, but not in cells infected with the cag− strain J68 or in uninfected cells (Figure 4A). Differences in the sub-cellular distribution of PPARδ among individual cells infected with strain 7.13 may reflect differences in cell cycle phase. To quantify these effects and determine the role of cag island substrates on PPARδ expression ex vivo, we performed immunoblot analyses which demonstrated that levels of PPARδ were significantly increased in primary gastric cell colonies co-cultured with wild-type, but not cagA− or slt− mutant H. pylori strains (Supplemental Figure 4).
Figure 4. H. pylori induces aberrant PPARδ and cyclin E1 localization to the nucleus in gastric colonies.
(A) Primary murine gastric colonies were co-cultured with medium alone or strains 7.13 or J68 for 24 hours. Cells were incubated with an anti-PPARδ antibody, followed by an anti-goat AlexaFluor-546 antibody and TO-PRO3 nucleic acid dye and visualized by immunofluorescence microscopy. PPARδ, red; nuclei, blue. 400x magnification. Arrowheads, nuclear PPARδ. (B) Gastric colonies isolated from wild-type or PPARδ−/− mice were co-cultured with strain 7.13, EGF (10 ng/ml) or medium alone for 24 hours. Cells were incubated with an anti-Cyclin E1 antibody, followed by an anti-goat AlexaFluor-488 antibody and DAPI nucleic acid dye. Cyclin E1, green; nuclei, blue. 400x magnification. Arrowheads, nuclear Cyclin E1. (C) MKN28 cells transfected with scrambled or Cyclin E1-specific siRNA were seeded in Matrigel and infected with strain 7.13 or medium alone for 72 hours. BrdU was added to culture medium and ELISA was performed. Error bars = SEM for experiments performed on at least 3 occasions. *p < .05 versus uninfected scrambled control. #p < .04 versus 7.13-infected scrambled control.
H. pylori-induced expression of the cell-cycle regulator Cyclin E1 is PPARδ-dependent
Having demonstrated that PPARδ regulates H. pylori-induced proliferation, we next defined the relationship between H. pylori, PPARδ and Cyclin E1 in a genetic model of PPARδ deficiency by using gastric glands obtained from wild-type or PPARδ−/− mice. H. pylori increased Cyclin E1 levels in the nuclei of wild-type glands but not in glands isolated from PPARδ−/− mice (Figure 4B). Conversely, Cyclin E1 nuclear translocation developed in glands isolated from either wild-type or PPARδ−/− mice in response to mitogenic stimulation with EGF (Figure 4B). These results indicate that PPARδ−/− gastric glands maintain replicative potential and that Cyclin E1 activation by H. pylori, but not EGF, is dependent on PPARδ.
To establish a role for Cyclin E1 in H. pylori-induced proliferation, we examined BrdU incorporation in gastric cells expressing control or Cyclin E1-specific siRNA (Figure 4C). MKN28 cells harboring reduced levels of Cyclin E1 exhibited a significant attenuation in proliferation in response to H. pylori (Figure 4C), demonstrating that H. pylori-induced proliferation requires the PPARδ target, Cyclin E1.
PPARδ is increased within human gastric mucosa colonized by H. pylori cag+ strains
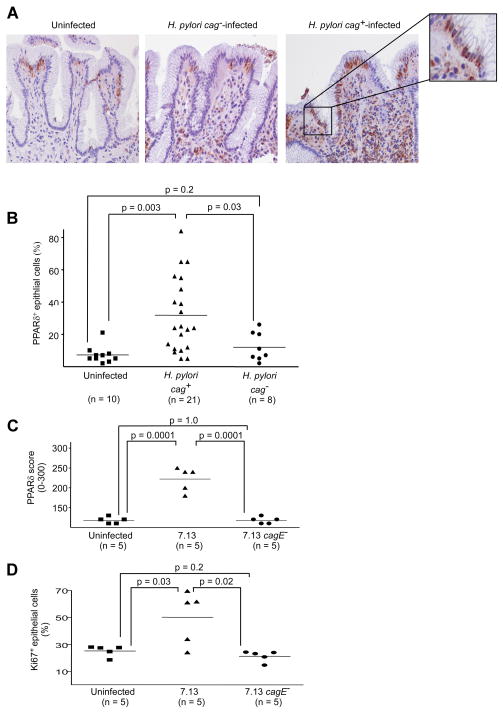
To extend our results into the natural niche of H. pylori, we examined PPARδ expression in human gastric biopsies by immunohistochemistry (n=39) and genetically characterized infecting H. pylori isolates. Cytoplasmic and nuclear PPARδ was detected significantly more frequently in gastric epithelial cells from specimens harvested from cag+-colonized persons compared with either cag−-infected or uninfected persons (Figure 5A, 5B). The inflammatory infiltrate present within H. pylori-infected subjects also displayed marked PPARδ staining, which is consistent with the role exerted by PPARδ in modulating macrophage activation22.
Figure 5. PPARδ expression is increased within human and rodent antral gastric mucosa colonized by H. pylori cag+ strains.
(A) Human gastric epithelial cells with PPARδ staining were quantified by an observer unaware of H. pylori status. Representative staining is shown for uninfected or H. pylori cag+ or cag−-infected persons. 400x magnification. (B) Results reflect percentage of surface epithelial cells with detectable PPARδ staining. At least 100 cells in at least three different fields were counted. (C) Gerbil gastric epithelial cells with PPARδ staining were scored by an observer unaware of H. pylori status as described in Supplemental Methods. (D) Proliferation in gerbil gastric epithelium was assessed by Ki67 immunohistochemistry. The percentage of cells positive for Ki67 was calculated for 10 high-power fields for each gerbil.
H. pylori increases expression of epithelial PPARδ in a rodent model of gastric cancer
We next extended the results obtained from human samples into a rodent model of H. pylori-induced gastric carcinogenesis, Mongolian gerbils. Based on our in vitro data indicating that a functional cag secretion system is required for full activation of PPARδ (Figure 2C), immunohistochemistry for PPARδ was performed on gastric mucosa harvested from gerbils infected with wild-type H. pylori strain 7.13, an isogenic 7.13 cagE− mutant, or broth alone, 12 weeks post-inoculation. Twelve weeks was selected for this analysis based on previous studies from our laboratory demonstrating that dysplasia and carcinoma are present in the majority of gerbils infected with strain 7.13 at this time point5. All gerbils infected with strain 7.13 or its cagE− mutant were successfully colonized and gerbils infected with wild-type strain 7.13 developed more severe gastric inflammation when compared with uninfected controls or cagE−-infected gerbils (data not shown). Compared with either uninfected or cagE−-infected gerbils, gastric epithelial PPARδ staining was detected significantly more frequently in gerbils infected with wild-type strain 7.13 (Figure 5C, Figure 6A). Mucosal inflammatory cells also contained increased levels of PPARδ (Figure 6A), similar to findings in infected human gastric mucosa (Figure 5A).
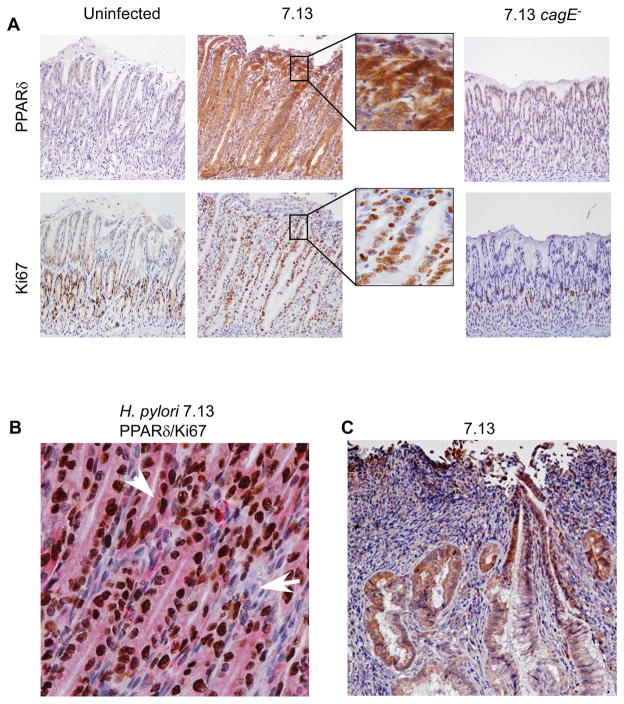
Figure 6. PPARδ and Ki67-positive epithelial cells are increased within antral gastric mucosa of Mongolian gerbils infected with wild-type H. pylori strain 7.13.
(A) Representative staining in serial sections for PPARδ and Ki67 is shown for uninfected, 7.13-infected, or 7.13 cagE−-infected gerbils. 200x magnification. (B) Dual staining for PPARδ (pink) and Ki67 (brown) is shown for H. pylori 7.13-infected gerbil. 400x magnification. Arrowhead, epithelial cell positive for PPARδ and Ki67; Arrow, stromal cell negative for PPARδ and Ki67. (C) Representative staining for PPARδ is shown in an invasive adenocarcinoma in an H. pylori 7.13-infected gerbil. 200x magnification.
Having demonstrated that PPARδ mediates H. pylori-induced proliferation in vitro, we also quantified epithelial proliferation in challenged gerbils. Similar to PPARδ levels, epithelial proliferation was significantly enhanced in gerbils infected with wild-type strain 7.13 compared to either uninfected or 7.13 cagE−-infected gerbils (Figure 5D, Figure 6A). In uninfected controls or gerbils infected with the 7.13 cagE− mutant, Ki67+ epithelial cells were tightly clustered within the neck region of the glands; however, staining in gerbils infected with wild-type strain 7.13 extended bidirectionally from the isthmus (Figure 6A). Ki67 staining also co-localized with PPARδ staining in epithelial cells from gerbils infected with H. pylori strain 7.13 (Figure 6B).
Gastric dysplasia and adenocarcinoma developed in 3 gerbils challenged with wild-type strain 7.13 by 12 weeks. In contrast, dysplasia or adenocarcinoma was not observed in any gerbils infected with the cagE− mutant. Among gerbils infected with wild-type strain 7.13, the intensity of PPARδ staining was enhanced in foci of dysplasia or adenocarcinoma (Figure 6C) when compared with adjacent non-dysplastic epithelium, indicating that the gradient of PPARδ expression parallels the severity of lesions in this model of gastric carcinogenesis.
To more directly determine whether the host inflammatory response to H. pylori modifies up-regulation of gastric epithelial cell PPARδ, expression of PPARδ was examined in gerbils infected for short periods of time (3 and 14 days), prior to the development of mucosal inflammation. Mucosa from gerbils infected with strain 7.13 at these time points did not harbor inflammatory cells; however, significantly enhanced levels of gastric epithelial PPARδ were present in infected compared to uninfected gerbils (Figure 7A, 7B). H. pylori were frequently closely associated with epithelial cells that contained the most intense PPARδ staining (Figure 7A). These results demonstrate that PPARδ expression increases in response to H. pylori prior to the development of inflammation.
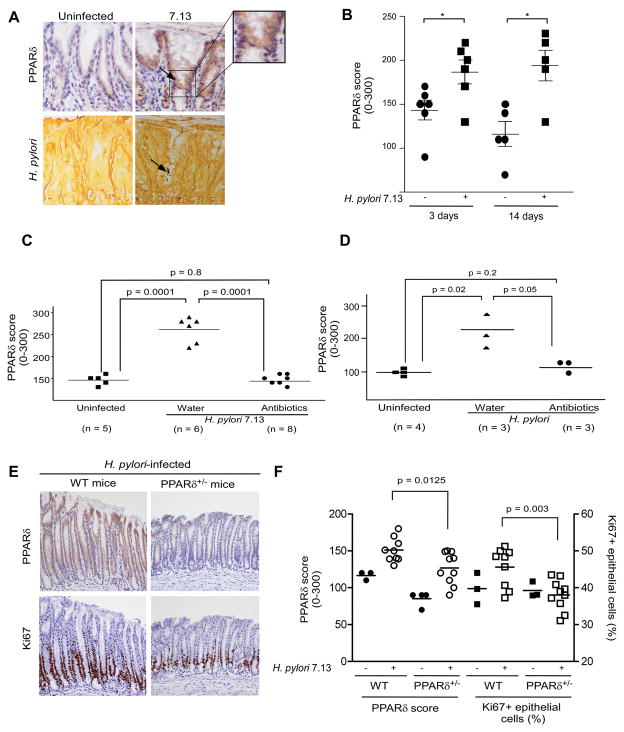
Figure 7. PPARδ expression within gastric mucosa of Mongolian gerbils, wild-type mice or PPARδ+/− mice in the presence or absence of H. pylori.
(A) Representative staining for PPARδ and H. pylori is shown for uninfected or H. pylori 7.13-infected gerbils, 3 days post-infection. 400x magnification. Arrows, cells with increased PPARδ staining adjacent to H. pylori organisms (Steiner silver stain). (B) PPARδ expression was evaluated in infected and uninfected gerbils. *, p < .05 versus uninfected samples. (C, D) Gerbils were infected with broth alone or with strain 7.13. Four weeks after H. pylori challenge, gerbils were treated with antibiotics or water and then sacrificed eight (C) or one (D) week later. (E) Representative staining for PPARδ and Ki67 is shown for H. pylori 7.13-infected wild-type and PPARδ+/− mice. 200x magnification. (F) PPARδ or Ki67 staining in gastric epithelial cells was evaluated by an observer unaware of H. pylori status or mouse genotype.
Previous studies in our laboratory have demonstrated that eradication of H. pylori in Mongolian gerbils significantly attenuates inflammation associated with infection20. Therefore, we examined whether interventional therapy would reduce PPARδ expression. Compared with gerbils that remained persistently infected (no antibiotic treatment), H. pylori-challenged gerbils treated with antibiotics 4 weeks post-infection and sacrificed 8 weeks post-treatment, had significantly decreased levels of PPARδ expression (Figure 7C). However, gastric mucosa from antibiotic-treated gerbils displayed no inflammation at this time-point.
To more clearly demonstrate that PPARδ upregulation is a direct result of H. pylori interactions with epithelial cells, we next examined PPARδ levels in H. pylori 7.13-infected gerbils that were either persistently infected (no antibiotic treatment) or treated with antibiotics and then sacrificed 1 week post-treatment, when mucosal inflammation was still present. There was no evidence of H. pylori in antibiotic-treated gerbils at this time-point as assessed by quantitative culture. Levels of inflammation in antibiotic-treated gerbils decreased by approximately 40–50% 1 week post-treatment compared to levels seen in persistently infected gerbils; however, gastric epithelial PPARδ levels were reduced to levels similar to uninfected control gerbils (Figure 7D). Collectively, these results indicate that infection with cag+ strains induces expression of PPARδ in gastric epithelial cells in vivo, which is concordant with our in vitro data focused on PPARδ activation in response to H. pylori.
Finally, we used a genetic approach to more firmly implicate PPARδ in the proliferative response induced by H. pylori in vivo. The absolute numbers of PPARδ−/− homozygous offspring are small in our mouse breeding litters; therefore, we infected PPARδ+/− heterozygous mice with strain 7.13 and assessed PPARδ expression and Ki67 staining. PPARδ expression was significantly increased in gastric epithelial cells (p=0.0047, Figure 7F) in uninfected wild-type mice compared to uninfected PPARδ+/− mice. Western blotting studies (Supplemental Figure 4B) for PPARδ using gastric mucosal lysates harvested from wild-type (n=3) and PPARδ+/− mice (n=3) similarly demonstrated that PPARδ levels were significantly (p=0.048) increased in gastric tissue from uninfected wild-type mice, confirming haploinsufficiency of the pparδ gene. H. pylori strain 7.13 induced gastric epithelial cell PPARδ expression to significantly higher levels in wild-type mice versus PPARδ+/− mice (Figure 7E, 7F). Importantly, proliferation was significantly increased in wild-type mice infected with strain 7.13 versus H. pylori-infected PPARδ deficient mice (Figure 7E, 7F), providing additional support that PPARδ mediates hyperproliferation that develops in response to H. pylori.
Discussion
Translocation of p120 to the nucleus relieves transcriptional repression that is exerted by Kaiso on β-catenin target genes possessing oncogenic properties11. Using stable reductions of p120 in gastric epithelial cells, we identified pparδ as a p120 target that may influence carcinogenesis within the context of H. pylori infection. We have previously shown that H. pylori strain 7.13 induces alterations in the cellular localization of p120 which is required for regulation of mmp-7 expression12, leading us to speculate that increased pparδ expression in response to H. pylori may be regulated in a similar manner, although definitive proof will require more in-depth analyses.
In vivo, H. pylori consistently induces epithelial hyperproliferation, and crosstalk between gastric epithelial cells and cellular elements within the lamina propria can influence proliferative responses to H. pylori. For example, myofibroblasts are a major cellular component of the extracellular matrix, and when treated with MMP-7, which we have shown to be up-regulated by H. pylori in a cag island-dependent manner12, myofibroblasts stimulate proliferation of gastric epithelial cells via release of insulin growth factor II23. This prompted us to utilize a three dimensional model, which contains insulin-like growth factor, and primary gastric gland cultures that contain elements present within gastric mucosa, and these systems identified a mechanism through which H. pylori stimulates proliferation; namely that H. pylori induces cyclin E1 expression in a PPARδ-dependent manner. Cyclin E1 deregulation is a common event in oncogenesis, and the majority of gastric tumors express high levels of Cyclin E1, which is strongly linked with tumor invasion24. Consistent with our ex vivo studies, Yao et al. demonstrated an overexpression of Cyclin E1 in Mongolian gerbils infected with H. pylori25.
Our results also indicate that full PPARδ activation by H. pylori requires a functional cag secretion system and both of the cag effectors CagA and peptidoglycan, consistent with findings from human studies that cag+ strains more robustly alter rates of epithelial cell turnover26. Of interest, we observed that the sizes of immunoreactive PPARδ bands differed in H. pylori-infected versus uninfected samples. We speculate, based on previous data, that this may reflect post-translational modification of PPARδ27.
In conclusion, H. pylori induces the expression and activation of PPARδ in vitro and in vivo, which is mediated by a signaling axis consisting of p120 and β-catenin. Activation of PPARδ requires the cag secretion system effectors, CagA and peptidoglycan, which in turn, promotes epithelial proliferation via the PPARδ target Cyclin E1. Since PPARδ regulates a multitude of host responses, activation of this receptor may not only contribute to varying levels of cellular turnover within gastric tissue, but also to the diverse pathologic outcomes associated with H. pylori infection.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants CA 77955 and DK 58587 (to RMP), CA 028842 (to PC), AI 039657, AI 068009, and the Department of Veterans Affairs (to TLC), CA 116087 (to RMP and TLC), and DK 058404 (to the Vanderbilt Digestive Disease Research Center).
Footnotes
Conflict of interest: All of the authors declare that there are no conflicts of interest
Author contributions: Toni Nagy: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis
Dingzhi Wang, M. Blanca Piazuelo, Alberto Delgado, Judith Romero-Gallo, Lydia Wroblewski, Jennifer Noto, Seth Ogden: acquisition of data
Dawn Israel, Pelayo Correa, Timothy Cover: material support, critical revision of manuscript for intellectual content
Richard Peek: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of manuscript for important intellectual content, statistical analysis, obtained funding, study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odenbreit S, Puls J, Sedlmaier B, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 3.Selbach M, Moese S, Hauck CR, et al. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J Biol Chem. 2002;277:6775–6778. doi: 10.1074/jbc.C100754200. [DOI] [PubMed] [Google Scholar]
- 4.Amieva MR, Vogelmann R, Covacci A, et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco AT, Israel DA, Washington MK, et al. Activation of β-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saadat I, Higashi H, Obuse C, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki M, Mimuro H, Kiga K, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Viala J, Chaput C, Boneca IG, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 9.Nagy TA, Frey MR, Yan F, et al. Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J Infect Dis. 2009;199:641–651. doi: 10.1086/596660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murata-Kamiya N, Kurashima Y, Teishikata Y, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 11.Park JI, Kim SW, Lyons JP, et al. Kaiso/p120-catenin and TCF/beta-catenin complexes coordinately regulate canonical Wnt gene targets. Dev Cell. 2005;8:843–854. doi: 10.1016/j.devcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Ogden SR, Wroblewski LE, Weydig C, et al. p120 and Kaiso regulate Helicobacter pylori-induced expression of matrix metalloproteinase-7. Mol Biol Cell. 2008;19:4110–4121. doi: 10.1091/mbc.E08-03-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Wang H, Guo Y, et al. Crosstalk between peroxisome proliferator-activated receptor delta and VEGF stimulates cancer progression. Proc Natl Acad Sci U S A. 2006;103:19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulholland DJ, Dedhar S, Coetzee GA, et al. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26:898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RA, Tan J, Krause WF, et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci U S A. 2000;97:13275–13280. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glinghammar B, Skogsberg J, Hamsten A, et al. PPARdelta activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2003;308:361–368. doi: 10.1016/s0006-291x(03)01384-6. [DOI] [PubMed] [Google Scholar]
- 17.Stephen RL, Gustafsson MC, Jarvis M, et al. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004;64:3162–3170. doi: 10.1158/0008-5472.can-03-2760. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Russell RG, Dettin LE, et al. Peroxisome proliferator-activated receptor delta and gamma agonists differentially alter tumor differentiation and progression during mammary carcinogenesis. Cancer Res. 2005;65:3950–3957. doi: 10.1158/0008-5472.CAN-04-3990. [DOI] [PubMed] [Google Scholar]
- 19.Zeng L, Geng Y, Tretiakova M, et al. Peroxisome proliferator-activated receptor-delta induces cell proliferation by a cyclin E1-dependent mechanism and is up-regulated in thyroid tumors. Cancer Res. 2008;68:6578–6586. doi: 10.1158/0008-5472.CAN-08-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Gallo J, Harris EJ, Krishna U, et al. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab Invest. 2008;88:328–336. doi: 10.1038/labinvest.3700719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokolova O, Bozko PM, Naumann M. Helicobacter pylori suppresses glycogen synthase kinase 3beta to promote beta-catenin activity. J Biol Chem. 2008;283:29367–29374. doi: 10.1074/jbc.M801818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang K, Reilly SM, Karabacak V, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCaig C, Duval C, Hemers E, et al. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology. 2006;130:1754–1763. doi: 10.1053/j.gastro.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Bani-Hani KE, Almasri NM, Khader YS, et al. Combined evaluation of expressions of cyclin E and p53 proteins as prognostic factors for patients with gastric cancer. Clin Cancer Res. 2005;11:1447–1453. doi: 10.1158/1078-0432.CCR-04-1730. [DOI] [PubMed] [Google Scholar]
- 25.Yao YL, Xu B, Song YG, et al. Overexpression of cyclin E in Mongolian gerbil with Helicobacter pylori-induced gastric precancerosis. World J Gastroenterol. 2002;8:60–63. doi: 10.3748/wjg.v8.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peek RM, Jr, Moss SF, Tham KT, et al. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 27.Blanquart C, Barbier O, Fruchart JC, et al. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. J Steroid Biochem Mol Biol. 2003;85:267–273. doi: 10.1016/s0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.