Abstract
The peripheral growth cones of statoacoustic ganglion (SAG) neurons are presumed to sense molecular cues to navigate to their sensory targets during development. Based on previously reported expression data for Frizzled receptors, Wnt ligands, and Wnt inhibitors, we hypothesized that some members of the Wnt morphogen family may function as repulsive cues for SAG neurites. The responses of SAG neurons to mammalian Wnts −1, −4, −5a, −6, and −7b, and the Wnt inhibitors sFRP −1, −2, and −3, were tested in vitro by growing SAG explants from embryonic day 4 (E4) chicken embryos for two days in 3D collagen gels. Average neurite length and density were quantified to determine effects on neurite outgrowth. SAG neurites were strongly repelled by human Sema3E, demonstrating SAG neurons are responsive under these assay conditions. In contrast, SAG neurons showed no changes in neurite outgrowth when cultured in the presence of Wnts and Wnt inhibitors. As an alternative approach, Wnt4 and Wnt5a were also tested in vivo by injecting retroviruses encoding these genes into the chicken otocyst on E3. On E6, no differences were evident in the peripheral projections of SAG axons terminating in infected sensory organs as compared to uninfected organs on the contralateral side of the same embryo. For all Wnt sources, bioactivity was confirmed on E6 chick spinal cord explants by observing enhanced axon outgrowth, as reported previously in the mouse. These results suggest that the tested Wnts do not play a role in guiding peripheral axons in the chicken inner ear.
Keywords: Axon guidance, Inner ear, Spinal cord, Semaphorin, Wnt, sFRP
1. Introduction
In the chicken inner ear, mechanosensory hair cells are innervated by bipolar neurons located within the statoacoustic ganglion (SAG). During development, SAG peripheral processes navigate to their sensory targets by responding to cues within and surrounding the otic epithelium. It is unclear which cues are involved in the guidance process, but a combination of attractants and repellents is expected (Fekete and Campero, 2007). Neurotrophins promote SAG axon outgrowth towards sensory organs in the mouse (Tessarollo et al., 2004). Furthermore, in addition to their neurotropic activity, neurotrophins also serve as survival factors for SAG neurons (Fritzsch, 2003; Fritzsch et al., 2005). Classic repellents, such as the semaphorins (Gu et al., 2003), ephrins (Bianchi and Gray, 2002) and Slits (Battisti and Fekete, 2008), are candidates for preventing axons from innervating non-sensory regions or for stopping axons once they have reached their targets. Morphogens, another class of axon guidance molecules (Charron and Tessier-Lavigne, 2005), have not been investigated in the context of otic axon guidance.
During nervous system development, members of the Wnt morphogen family signal through Frizzled (Fz) and Ryk/Derailed receptors to guide axons to their targets (Bovolenta et al., 2006). Wnts can function as attractants or repellents, but which role a particular ligand plays is highly dependent on the receptor and system context (Zou and Lyuksyutova, 2007). For example, in the mouse spinal cord, Wnt4 and Wnt7b gradients attract post-crossing commissural axons anteriorly through the Fz3 receptor (Lyuksyutova et al., 2003), whereas Wnt1 and Wnt5a gradients repel corticospinal axons posteriorly through the Ryk receptor (Liu et al., 2005). The receptor or Wnt gradient involved in mediating a particular guidance response is specific to a particular axon population. Additionally, the guidance role for a particular Wnt is not always conserved. For example, the anterior projection of spinal cord commissural axons is influenced by a Wnt5a/7a gradient in the chicken (Domanitskaya et al. 2010) and a Wnt4/7b gradient in the mouse (Lyuksyutova et al., 2003). Therefore, it is still unclear which ligand-receptor binding event leads to a particular guidance response or which downstream components are involved, but the role of Wnts as guidance cues is conserved and applies to several neural pathways (Zou and Lyuksyutova, 2007). The Wnt inhibitor, SFRP1, has been shown to interact with the Fz2 receptor and promote neurite outgrowth of retinal ganglion cells in vitro (Rodriguez et al., 2005). This suggests that Wnt inhibitors may play guidance roles during the development of other neural systems as well.
The expression of multiple Wnt, Fz, and Wnt inhibitor transcripts in the chick inner ear at times corresponding to neurite outgrowth and pathfinding suggests Wnts and their inhibitors may play a role in axon guidance during ear development. Specifically, in situ hybridization data show that Fz transcripts are expressed in SAG neurons while Wnts are variously expressed in sensory or non-sensory regions of the ear, depending upon the gene and stage of development (Sienknecht and Fekete, 2008; 2009). This suggests that SAG neurons may be Wnt-responsive and suggests that Wnts expressed in sensory regions could be attractants or that Wnts expressed in non-sensory regions (that lack innervation) could function as repellents. Moreover, the expression of various Wnt inhibitors in either sensory or non-sensory domains, or both in some cases, complicates predictions as to whether the inhibitors could independently provide attractive or repulsive cues.
In this study, we explored the responses of SAG neurites to the Wnts that are expressed in non-sensory regions during stages of SAG neurite outgrowth to test the hypothesis that these molecules may serve as repulsive cues. Two of the Wnts were tested in vitro and in vivo, whereas 3 other Wnts as well as 3 Wnt inhibitors were tested in vitro. We were unable to find evidence that the candidate molecules influenced otic neurite outgrowth. The bioactivity and species cross-reactivity of the tested Wnt-related molecules was confirmed by showing responsiveness of chicken axons in spinal cord explants. The results of this study leave open the possibility that other Wnt ligands or other morphogen families may serve as potential candidates for axon guidance in the chicken inner ear.
2. Materials and methods
2.1 Explant dissection
Fertilized White Leghorn chicken eggs were obtained from Purdue University Farm, incubated at 38°C and staged (Hamburger et al., 1951) prior to dissection. Embryos were removed from the eggs and dissected in cold Hank's Balanced Salt Solution (HBSS; Sigma). The SAGs of E4 (HH20-25) embryos were dissected from the head using sterile dissection pins. E6 (HH29) chicken spinal cords were dissected under sterile conditions in cold L15 medium (Sigma) supplemented with 10% calf serum (Invitrogen). Open book spinal cord explants were prepared as described (Zou et al., 2000) and cut in half past the midline to leave the floor plate intact (Wolf et al., 2008). Several transverse cuts generated numerous explants from each spinal cord.
2.2 Explant culture
High concentration (8-10 mg/ml) rat-tail type I collagen (BD Biosciences) was diluted and neutralized to a concentration of 1.5 mg/ml, according to the manufacturer's protocol. Briefly, collagen was dissolved in 10% phosphate-buffered saline (PBS; Cellgro) in distilled water and adjusted to a pH of 7.4 using 1N NaOH. Explants were placed in 24-well tissue culture dishes (Costar) containing 0.5 ml collagen per well. Plates were incubated at 37°C for 30 min to permit collagen polymerization. All explants were cultured under serum-free conditions as described (Bianchi and Cohan, 1993) in medium consisting of DMEM/F12 (Sigma) supplemented with 10% ITS+ (Insulin, Transferrin, Selenium; Sigma), 15mM Hepes buffer (Sigma) at pH 7.3, 1% L-glutamine (Gibco) and 1% pen-strep (Gibco). To facilitate cell survival and neurite outgrowth, 10 ng/ml ciliary neurotrophic factor (CNTF; Sigma) and 10 ng/ml Neurotrophin-3 (NT-3; Sigma) were added to the medium immediately before 0.5ml was added to each well. Cultures were incubated in 5% CO2 at 37°C.
For purified protein experiments, explants were cultured with or without media supplemented with recombinant proteins purchased from R&D Systems. Human Sema3E, mouse Wnt4 , mouse Wnt5a, human sFRP1, mouse sFRP2 and mouse sFRP3 were added at a range of concentrations centered on levels known to be bioactive on chick tissue (from published studies) and according to the manufacturer's instructions. For co-culture experiments, untransfected or Wnt-transfected cell aggregates were placed 200-500 μm from the edge of explants. SAG and spinal cords explants were cultured for 40h and 24h, respectively.
2.3 Preparation of Wnt-expressing HEK cells for co-culture
Expression constructs containing CMV promoters to drive transcription of myc-tagged Wnt −1, −4, −5a, −6, or −7b were previously published (Liu et al., 2005; Lyuksyutova et al., 2003). HEK 293T cells (ATCC) were grown in medium containing DMEM (Sigma), 10% calf serum, 1% L-glutamine and 1% pen-strep. Cells were seeded at 1-3 × 105 cells per well in 6-well culture plates (Costar) 20-24 hours before transfection. Transfections were performed using Lipofectamine2000 (Invitrogen) according to a modified protocol (Dalby et al., 2004). Briefly, HEK growth medium was replaced with transfection medium consisting of Optimem with Glutamax (Invitrogen) and 12% fetal calf serum (Atlanta Biologicals). 4 μg plasmid DNA and 8 μl Lipofectamine in 0.5 ml Optimem with Glutamax was added to each well and incubated for 7 hours.
HEK cell aggregates were prepared immediately following transfection using the hanging drop method (Kennedy et al., 1994) or plated to evaluate transfection efficiency. Hanging drops were harvested 16-17 hours later into warm L15 (Sigma), supplemented with 10% calf serum, and dissected into 8 equal sized pieces with forceps to create cell aggregates. One piece was used for each co-culture. Control cell aggregates were made from untransfected HEK cells.
2.4 Immunohistochemistry of explants and HEK cells
Explant cultures were fixed in 4% paraformaldehyde for 1 hour at room temperature, rinsed in PBS, and incubated overnight in blocking solution (10% calf serum, 0.05% Triton X-100 in PBS) at 4°C. To visualize neuron cell bodies and neurites, explants were incubated overnight at 4°C with monoclonal anti-β-tubulin (Sigma) diluted 1:500 in blocking solution, rinsed in PBS, and incubated overnight at 4°C with Alexa Fluor 488 goat anti-mouse (Molecular Probes) diluted 1:500 in blocking solution. After rinsing, samples were mounted with Vectashield (Vector Laboratories) for imaging.
HEK cells transfected with myc-tagged Wnt plasmids were immunostained ~24 hours after transfection with anti-myc antibodies to determine the transfection efficiency of cells used in co-cultures. Cells were fixed in 4% paraformaldehyde for 10 min at room temperature and incubated for 1 hour at room temperature in a blocking solution of 10% goat serum (Vector Laboratories) and 0.05% Triton X-100 in PBS. Cells were incubated with mouse α-myc 9E10 (Developmental Studies Hybridoma Bank, University of Iowa; DSHB) collected from hybridoma cell supernatant, for one hour at room temperature. Following PBS washes, cells were incubated with Alexa Fluor 568 goat anti-mouse (Molecular Probes) diluted 1:500 in blocking solution. Samples were mounted with Vectashield for imaging. Transfection efficiencies were 80-90%.
2.5 Neurite outgrowth quantification
Confocal image stacks of 3D cultures were captured using a BioRad MRC-1024 laser. Images were collapsed into 2D projections and measured using NIH ImageJ software. Neurite length and pixel measurements were performed to determine neurite responsiveness.
Neurite length measurements were only performed on SAG cultures. Images were divided into 4 quadrants and the average neurite length for one quadrant was measured. Specifically, the length of each neurite or neurite bundle was measured from the edge of the explant and the average length was calculated. A quadrant with representative outgrowth was chosen for purified protein conditions. The quadrant facing the cell aggregate was used for co-culture experiments.
Pixel measurements were performed to quantify overall neurite outgrowth. Confocal image projections were transformed into black and white images by setting upper and lower threshold values to highlight the neurites extending from the explant's edge in black and the background in white. The number of black pixels (i.e, those that overlapped with neurites) was normalized to explant length to control for variation in explant size. Explant length was determined by tracing a smoothed curve along the explant edge between the boundaries of the measured quadrant. For SAG cultures, pixels were quantified from the same quadrant as neurite length. For spinal cord cultures, the number of pixels occupied by neurites growing from the edge of the floor plate was quantified.
Measurements are given in mean ± standard error (SE). To compare measurements between control and experimental groups, a one-way ANOVA followed by a Tukey's multiple-comparisons test was performed using Statistical Analysis Software 9.2 (SAS Institute Inc, Cary, NC). A p value of less than 0.05 was considered significant.
2.6 TUNEL labeling and cell death quantification
Detection of apoptotic cells was determined by terminal dUTP nick-end labeling (TUNEL) of double-stranded DNA breaks (Gavrieli et al., 1992). Collagen gels were fixed in 4% paraformaldehyde for 1 hour at room temperature, rinsed in PBS and incubated overnight in 20% sucrose in PBS. Gels were embedded in Tissue Freezing Medium (TFM; Triangle Biomedical Sciences), frozen in liquid nitrogen and cryosectioned at 15 μm. TUNEL was performed using a TUNEL kit (In situ Cell Death Detection Kit, TMR Red; Roche Applied Science), according to the manufacturer's instructions. Adjacent sections were used as negative (incubation with dUTP only) and positive (incubation with DNase I for 10 min prior to TdT) controls. Immunostaining was performed after the TUNEL reaction. Sections were rinsed in PBS, incubated for 1 hour in blocking solution at room temperature and incubated with monoclonal anti-Islet1 39.4D5 (DSHB) diluted 1:100 in blocking solution, to specifically label SAG neuron nuclei (Li et al., 2004; Neves et al., 2007). Samples were washed with PBS, incubated with Alexa Fluor 488 goat anti-mouse (Molecular Probes) diluted 1:500, rinsed in PBS and mounted with Vectashield for imaging. Confocal image stacks of the 2 middle sections of each explant (equatorial level) were collapsed into 2D projections and counted using NIH ImageJ. The number of Islet1 and Islet1-TUNEL positive cells (SAG neuron nuclei) was counted. The percentage of TUNEL positive SAG neurons was calculated by dividing the number of Islet1-TUNEL double positive cells by the total number of Islet1 cells. Measurements are given in % TUNEL positive neurons ± standard error. Control and experimental group measurements were compared with a one-way ANOVA using SAS. A p value of less than 0.05 was considered significant.
2.7 Retrovirus preparation and injection
Extensive methods for virus preparation, concentration, titering and embryo injections have been published (Morgan and Fekete, 1996). Briefly, plasmids for RCAS(A)/Wnt4 and RCAS(A)/Wnt5a (Hartmann and Tabin, 2000) were provided by Cliff Tabin (Harvard Medical School) and transfected into UMNSAH/DF-1 chicken embryo fibroblasts (called DF-1 cells; ATCC, CRL-12203). Infected cells were passaged for about a week and then grown to confluence for collection of their culture supernatant. For spinal cord cultures, each well received 0.5 ml of conditioned media that contained 10% fetal calf serum and 2% chick serum (Sigma) in DMEM with pen-strep. For virus preparation, fetal calf serum was replaced with half volume of 10% NuSerum (BD Biosciences) supplemented medium the night before collection of supernatant. Supernatant was concentrated by centrifugation. Viral stocks were titered 48 hours after infection of DF-1 cells using immunohistochemistry of the viral 3C2 epitope. The RCAS(A)/Wnt4 titer was 8.5 × 108 infectious units per ml (i.u./ml) and the RCAS(A)/Wnt5a titer was 5 × 108 i.u./ml. Specific-pathogen-free chicken embryos were obtained from Spafas, Inc. and incubated at 37.5°C. On E2, the eggs were windowed in preparation for virus injections into the right otocyst on E3 (HH16-19).
2.8 Histological preparation and immunohistochemistry of virus-infected specimens
On E6, RCAS(A)/Wnt4-injected embryos (n=10) or RCAS(A)/Wnt5a-injected embryos (n=7) were collected, decapitated and fixed in 4% paraformaldehyde in PBS. Specimens were transferred through graded sucrose/PBS solutions, embedded in 15% sucrose/7.5% gelatin in PBS, frozen in liquid nitrogen and sectioned at 15 μm on a Leica cryostat. Adjacent sections were immunostained to detect: (A) the viral gag protein (3C2 mouse monoclonal antibody, DSHB, 1:1 dilution of hybridoma supernatant); (B) axons (3A10 mouse monoclonal antibody, DSHB, 1:20-1:40 dilution of hybridoma supernatant); and (C) prosensory domains of the inner ear (anti-sox2 goat polyclonal antibody, Santa Cruz Biotechnology Inc., 1:250). Biotinylated horse anti-mouse IgG or rabbit anti-goat IgG secondary antibodies (Vector Laboratories, 1:250) were followed by avidin-biotin-HRP complex (ABC kit, Vector Laboratories) and diaminobenzidine (Sigma) histochemistry. Sections were dehydrated, coverslipped, examined on a Nikon Eclipse E800 microscope and photographed with a cooled digital camera (Spot Diagnostics).
2.9 Quantitative analysis of axon outgrowth in vivo
Every third section through the saccular macula was stained with 3A10 to detect the peripheral processes and imaged with a 40x objective for quantitative analysis. Digital photographs of the right sides were photographically reversed to be indistinguishable from the left side and each ear was arbitrarily assigned as either ear 1 or ear 2. An observer, blinded as to the side of origin of each ear, used NIH ImageJ to quantify immunopositive fibers infiltrating the saccular macula. Each 3A10-stained image was converted to grey scale and the threshold was adjusted to convert the image into black (neurites) and white (background). To exclude the nerve bundle, the macula was defined along its basal surface by projecting a smooth line that was continuous with the basal surface of the adjacent non-sensory epithelium. Thus outlined, the image was cropped to isolate the innervated region and the number of black pixels (the neurites) was counted. Pixels were summed across the entire organ (typically about 5-6 sections). To control for variation in age between embryos, we normalized the number of black pixels on the right side (Wnt infected) against the number for the left side (control). The number of pixels for the control side was defined as 1. The average fold increase (above 1) or decrease (below 1) was calculated for each Wnt. Pairwise comparisons of the total innervation (number of pixels) between Control and RCAS/Wnt4 or between Control and RCAS/Wnt5a ears were performed with a paired t-test using SAS. A p value of less than 0.05 was considered significant. Measurements are given in mean ± SE.
3. Results
3.1 Purified Sema3E inhibits SAG neurite outgrowth
SAG explants were grown in serum-free conditions to ensure that effects on neurite outgrowth were exclusively from the presence of purified proteins and because explants displayed poor neurite outgrowth in the presence of serum or chicken embryo extract (not shown). It was necessary to obtain culture conditions that produced robust neurite outgrowth in order to assay for repulsion. Feasibility experiments demonstrated that explants have poor viability under serum-free conditions in the absence of growth factors (not shown). For this reason, it was necessary to add 10 ng/ml CNTF and 10 ng/ml NT-3 to serum-free culture medium to improve neuron survival and neurite outgrowth. Explants consistently displayed dense neurite outgrowth under these control conditions (Fig. 1A).
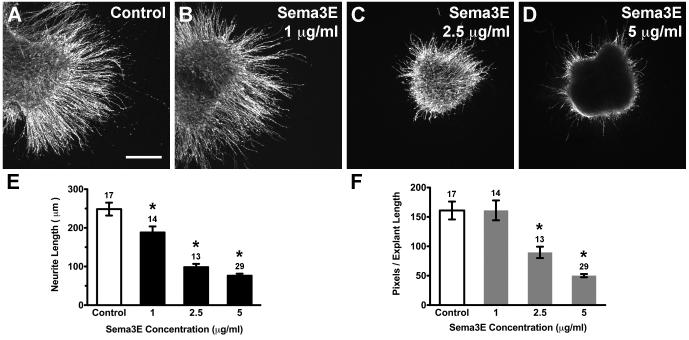
Fig. 1. Purified Sema3E protein inhibited HH20-25 SAG neurite outgrowth.
Confocal images of SAG explants cultured for 40h with or without (Control) Sema3E purified proteins added to culture medium (A-D). Explants treated with Sema3E display shorter and fewer neurites compared to controls. Scale bar=200 μm. Quantification of neurite length (E) and pixel number (C). Each bar represents the mean (± SE) computed from explants within a treatment group. Sample numbers for each treatment are above the bars. Average neurite length (E) and pixel number (F) are significantly less than control for Sema3E treated explants. *p<0.0001 significantly different from control cultures.
To ensure that SAG neurites could demonstrate a repulsive response to an inhibitory signal even in this outgrowth-promoting environment, explants were cultured in the presence of Semaphorin 3E (Sema3E), a molecule suggested to be an otic axon repellent in mice (Gu et al., 2003). Neurite outgrowth was abrogated in the presence of Sema3E in a dose-dependent manner (Fig. 1B-D). All concentrations of Sema3E significantly reduced neurite length (Fig. 1E; ANOVA, p<0.0001), where the average neurite length was <77±14 and >248±17 μm for Sema3E and control explants, respectively. Furthermore, overall outgrowth in pixels per unit length was significantly less for 2.5 μg/ml (90±10) and 5 μg/ml (50±3) Sema3E concentrations compared to the control (161±15) group (Fig. 1F; ANOVA, p<0.0001). We measured cell death by performing TUNEL assays on the cultured explants to determine if the reduction in neurite outgrowth was due to a reduction in cell survival. The percentage of apoptotic neurons was calculated from cell counts of TUNEL+/Islet1+ divided by the total number of Islet1+ cells in the sample region. There was no significant difference in the percentage of cell death between control (8.79 ± 0.01, n=8), 2.5 μg/ml Sema3E (8.76 ± 0.01, n=8) and 5 μg/ml Sema3E (7.72 ± 0.01, n=8) treated explants (ANOVA, p=0.5286).
3.2 Neither Wnts nor Wnt inhibitors are repulsive for SAG neurite outgrowth
To investigate whether SAG neurites respond to Wnts or Wnt inhibitors, explants were cultured in the presence of purified Wnt −4, −5a, −4+5a and sFRP −1, −2, and −3, within a bioactive concentration range. It was important to test a range of concentrations since Wnts can exist as concentration gradients (Charron and Tessier-Lavigne, 2005) and have been shown to exert concentration-dependent effects during axon guidance (Bovolenta et al., 2006; Domanitskaya et al., 2010; Zou and Lyuksyutova, 2007). Wnt inhibitors were also tested because sFRP1 has been shown to regulate the growth of chick and Xenopus retinal ganglion cell axons (Rodriguez et al., 2005).
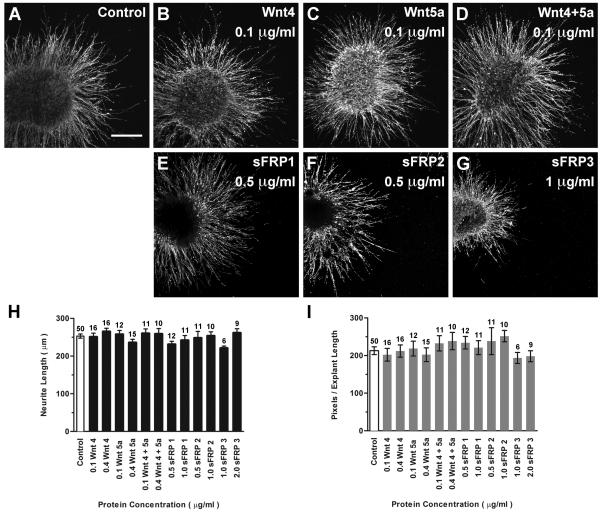
Results show that protein-treated explants displayed a long and dense neurite outgrowth pattern indistinguishable from controls (Fig. 2 A-G). No significant difference in average neurite length (Fig. 2H; ANOVA, p=0.2588) or average pixel number (Fig. 2I; ANOVA, p=0.7126) was found between control and protein-treated SAG explants. These results suggested that SAG neurons were unresponsive to the molecules we tested, that the proteins were not bioactive on chick tissue, or that the proteins were not bioactive in vitro. Spinal cord explants were used to confirm the bioactivity and species cross-reactivity of the Wnt and sFRP purified proteins under these assay conditions. In the mouse, cells expressing Wnt −1, −4, −5a, −6, or −7b promote commissural neurite outgrowth from spinal cord explants when co-cultured in vitro (Lyuksyutova et al., 2003). The current study replicated the neurite promoting effects of Wnt4 and Wnt5a seen in the mouse by culturing E6 chick spinal cord explants in the presence of purified Wnt proteins under the same serum-free conditions as SAG explants (Fig. 3A-D). Neurite outgrowth was quantified using pixel measurements (Fig. 3H). Purified Wnts −4 and −5a significantly promoted the outgrowth of chick spinal cord neurites, compared to controls (Fig. 3H; ANOVA, p<0.0001). Therefore, we conclude that the Wnts used for SAG experiments were bioactive on chick tissue under these assay culture conditions, confirming SAG neurites were indeed unresponsive.
Fig. 2. Purified Wnt and Wnt inhibitor proteins do not affect HH20-25 SAG neurite outgrowth.
Confocal images of SAG explants cultured with or without (Control) Wnt or sFRP proteins added to culture medium (A-G). Control and treated explants display robust neurite outgrowth. Scale bar=200 μm. Quantification of neurite length (H) and pixel number (I). Each bar represents the mean (± SE) computed from explants within a treatment group. Sample numbers are above each bar. There is no significant difference in average neurite length (H; p=0.2588) or pixel number (I; p=0.7126) between control and treated explants.
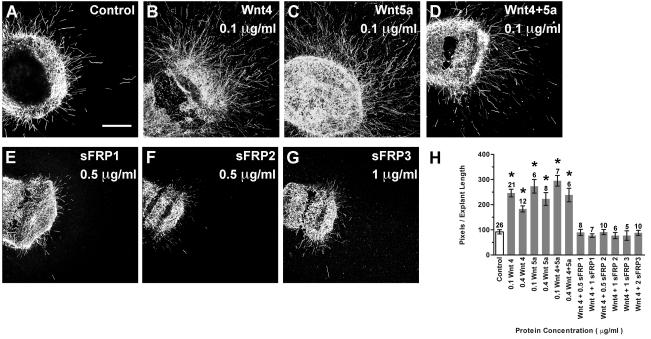
Fig. 3. Purified Wnt and sFRP proteins are bioactive when used in HH29 chick spinal cord neurite outgrowth assays.
Confocal images of spinal cord explants cultured for 24h with or without (Control) 0.1 μg/ml Wnt proteins (A-D) or with 0.1 μg/ml Wnt4 and sFRPs added to culture medium (E-G). Scale bar=200 μm. Quantification of pixel number (H). Each bar represents the mean (± SE) computed from explants within a treatment group. Sample numbers for each treatment are above the bars. Explants cultured with purified Wnts display significantly greater neurite outgrowth compared to controls (p<0.0001), demonstrating that the Wnts used in the culture assays were bioactive. Spinal cord explants cultured with 0.1 μg/ml Wnt4 and any one of the sFRPs do not display neurite outgrowth significantly different from the control group (p=0.3707), demonstrating that sFRPs can counteract the neurite promoting effects of Wnt4 and are therefore bioactive. *p<0.0001 significantly different from controls.
Exogenous sFRPs were previously shown to antagonize Wnt4 activity on mouse spinal cord explants (Lyuksyutova et al., 2003). In the current study, sFRP bioactivity was confirmed by its ability to abrogate the neurite-promoting effect of 0.1 μg/ml Wnt4 on E6 chick spinal cord explants (Fig. 3E-G). Explants displayed significantly greater neurite outgrowth in the presence of Wnt4 compared to controls lacking Wnt4 (Fig. 3H; ANOVA, p<0.0001). In contrast, in the presence of any one of the sFRP proteins, the neurite promoting effects of Wnt4 were reduced to the level of outgrowth seen under control conditions (Fig. 3H; ANOVA, p=0.3707). When added alone, sFRP proteins did not exert effects on neurite outgrowth (data not shown), consistent with results previously reported for chick spinal cord explants (Domanitskaya et al., 2010). This demonstrates that the sFRP purified proteins are also bioactive on chicken tissue, under these culture conditions.
3.3 SAG neurons do not respond to Wnt-secreting HEK cells
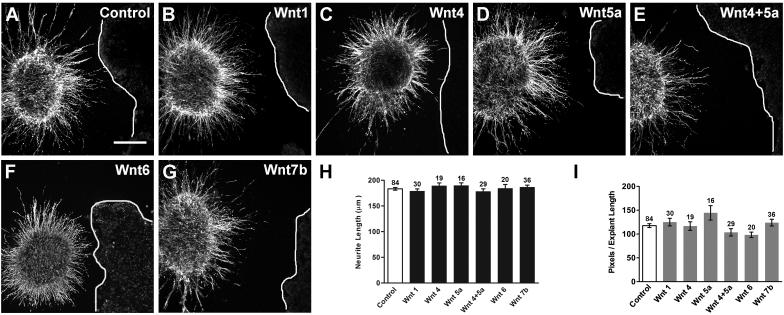
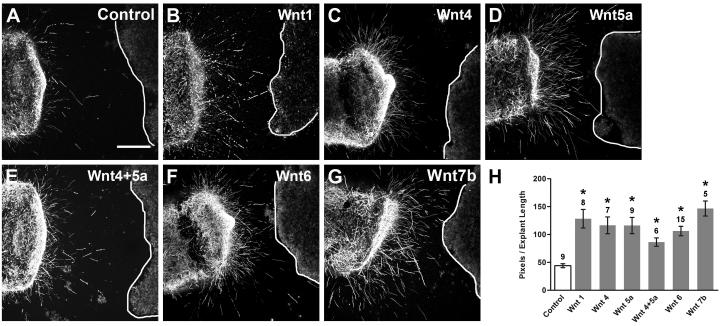
Although neither Wnt nor sFRP purified proteins affected the overall neurite outgrowth of SAG explants when uniformly added to the medium, we considered whether a Wnt gradient might influence the direction of neurite outgrowth. For this we used a co-culture assay to provide an asymmetric source of Wnt. SAG explants were co-cultured with HEK cells transfected with Wnt −1, −4, −5a, −4+5a, −6, or −7b or untransfected (control) HEK cells (Fig. 4). Effects on neurite outgrowth were evaluated by comparing neurite outgrowth on the side of the explant facing the cells between untransfected (control) and Wnt-transfected co-cultures. If Wnts are repulsive to SAG neurons, we expected neurites either to avoid growing in the direction of Wnt expressing cells or to turn away from such cells as they approach some defined distance. If Wnts are attractive, neurites might be expected to turn towards Wnt-transfected cells. None of these patterns was observed qualitatively. Explants displayed similar neurite outgrowth patterns in the presence of control and Wnt-expressing cells (Fig. 4A-G), suggesting there was no effect on neurite outgrowth. This was shown quantitatively as no significant difference in average neurite length (Fig. 4H; ANOVA, p=0.6142) or pixel number (Fig. 4I; ANOVA, p=0.1112) between control and Wnt treatment groups.
Fig. 4. Wnt-expressing HEK cells do not affect HH20-25 SAG neurite outgrowth in a co-culture assay.
Confocal images of SAG explants co-cultured for 40h with control (Untransfected) or Wnt-transfected HEK cells (A-G). Lines indicate the edge of HEK cell aggregates (rightmost in each image). Explants cultured with control and Wnt-transfected cells display similar neurite outgrowth patterns. Scale bar=200 μm. Quantification of neurite length (H) and pixel number (I) for quadrants facing the cell aggregate. Each bar represents the mean (± SE) computed from SAG explants within a treatment group. Sample numbers for each treatment are above the bars. There is no significant difference in average neurite length (H; p=0.6142) or pixel number (I; p=0.1112) between explants grown with control or Wnt-transfected cells.
Wnt bioactivity was confirmed by co-culturing E6 chick spinal cord explants with Wnt-expressing cells, under the same conditions as SAG co-cultures. All Wnt expressing HEK cells significantly promoted spinal cord neurite outgrowth as compared to untransfected HEKs (Fig. 5; ANOVA, p<0.0001). These results demonstrated that the HEK cells used for co-cultures were secreting bioactive Wnts that can affect neurite outgrowth from chicken tissue.
Fig. 5. Wnt-expressing HEK cells promote HH29 chick spinal cord neurite outgrowth in a co-culture assay.
Confocal images of spinal cord explants co-cultured for 24h with control or Wnt-transfected HEK cells (A-G). Lines indicate the edge of HEK cell aggregates (rightmost in each image). Scale bar=200 μm. Quantification of pixel number for quadrants facing the cell aggregate (H). Each bar represents the mean (± SE) computed from explants within a treatment group. Sample numbers for each treatment are above the bars. Explants cultured with Wnt-expressing cells display significantly greater neurite outgrowth compared to control cultures (H; p<0.0001), confirming that bioactive Wnts were secreted from HEK cells used in the co-culture assay. *p<0.0001 significantly different from control cultures.
3.4. SAG unresponsiveness is not age-dependent
On E4, the SAG contains populations of vestibular and auditory neurons. The vestibular component of the SAG is the first to differentiate and send out peripheral processes (D'Amico-Martel, 1982; Hemond and Morest, 1991b) and pioneer vestibular fibers are the first to enter the wall of the otocyst at HH20 (Sanchez Calderon et al., 2004). Auditory fibers begin contacting the basilar papilla on HH24 (Hemond and Morest, 1991a). Due to the time-sensitive nature of SAG development, we divided SAG cultures from each treatment group into HH20-23 and HH24-25 age groups, to see if there was an age-dependent SAG response. We found no significant difference in average neurite length or overall outgrowth for soluble protein and co-culture treatments for either developmental time point (data not shown). These results suggest that both populations of neurons responded similarly at the time points we examined.
3.5 Wnt4 and Wnt5a over-expression does not affect sensory organ innervation in vivo
A set of in vivo experiments was conducted to ask whether ectopic Wnt4 or Wnt5a expression could influence the innervation of the inner ear. Retrovirus-mediated gene transfer was the method of choice, after first confirming that the RCAS(A)/Wnt4 and RCAS(A)/Wnt5a viral stocks were bioactive based on their ability to enhance neurite outgrowth of E6 spinal cord explants (Fig. 6A-D). Neurite outgrowth was quantified by counting pixels occupied by neurites per unit length of the explant (Mean ± SE). Spinal cord neurites grew rather poorly in conditioned media collected from DF-1 fibroblasts, regardless of whether the cells were uninfected (17.9 ± 0.6, n = 7) or infected with RCAS parent virus (20.0 ± 1.0, n=26). In contrast, neurites grew more robustly in media collected from cells infected with RCAS/Wnt4 (58.0 ± 4.6, n=9) or RCAS/Wnt5a (42.2 ± 2.2, n=18). The enhancement of outgrowth in the presence of Wnts is statistically significant (ANOVA, p<0.0001).
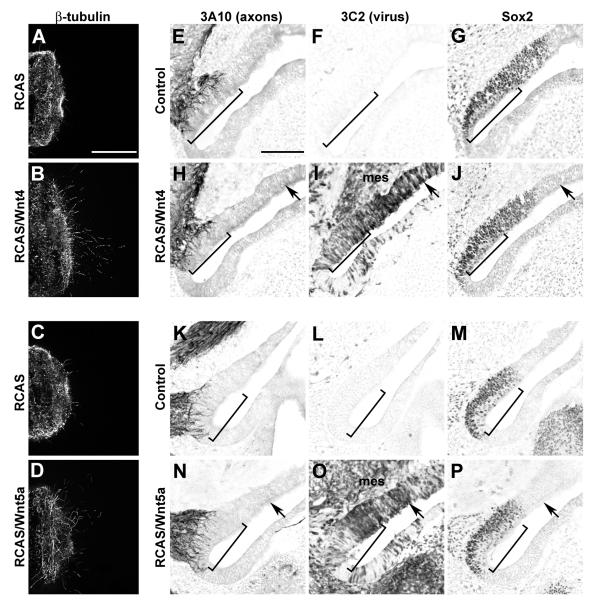
Fig. 6. Spinal cord axons but not inner ear SAG axons are responsive to Wnt4 and Wnt5a delivered via RCAS retroviral vectors.
Confocal images of E6 spinal cord explants cultured for 24h in the presence of supernatant collected from virus-infected cells (A-C). Scale bar = 200 μm. More axons emerge from spinal cord explants grown in culture supernatant collected from fibroblasts infected with RCAS/Wnt4 (A) or RCAS/Wnt5a (B) as compared to RCAS (C) or supernatant from uninfected fibroblasts (not shown). Infection with Wnt4 or Wnt5a viruses on E3 does not affect innervation to the saccular macula on E6 (E-P). Serial horizontal sections through inner ears were immunostained with antibodies to detect axons (3A10), virus (3C2) or prosensory domains (Sox2; E-P). Images are oriented with medial to the left and anterior down. Images of the right ears were flipped to mimic the orientation of the left ears to faciliate comparisons. Scale bar=100 μm. Virus-mediated misexpression of Wnt4 (H-J) or Wnt5a (N-P) does not alter the trajectory of SAG axons into the prosensory domains of the E6 inner ear when compared with the contralateral uninfected control sides (E-G and K-M, respectively). The innervation density (brackets) of Sox2-positive prosensory maculae is similar on control and experimental sides, while nearby virus-infected non-sensory patches (black arrows) remain free of axons. Furthermore, axons are not attracted into infected mesenchyme (mes).
High-titer RCAS/Wnt viruses were injected into the right otocyst at HH16-19, prior to the establishment of definitive prosensory domains. Detectable virus-mediated protein expression is expected to begin within ~10h and to be robust by 24h (Homburger and Fekete, 1996). At this time, pioneer SAG afferents will have reached the prosensory domains of the anterior and posterior cristae, with the innervation of other organs following a day or two later. We reasoned that if either of these Wnts was acting as a repellant to the SAG afferents, then Wnt misexpression within the prosensory domains should reduce or eliminate the propensity of afferents to penetrate into the epithelial layer of the sensory primordia. Alternatively, should either Wnt4 or Wnt5a provide an attractive cue, Wnt-infected territories might show more robust innervation than normal. Innervation was assayed on E6, when axons would normally have penetrated the primordia of all three cristae, the utricular, saccular and lagenar maculae and the basilar papilla.
On E6, Sox2 immunostaining was used to identify prosensory domains, whose size and spatial distribution were similar on the infected and uninfected sides of each embryo. Immunopositive staining of the p19 viral gag epitope using the 3C2 antibody was used to determine which sensory organs were infected. Although virus can spread into the periotic mesenchyme and beyond, the left ear ectoderm and mesoderm were rarely immunopositive for 3C2 and thus were used as a matched control for each individual. The degree of viral infection was judged qualitatively based on the percent of 3C2+ cells within the Sox2+ domains as follows: (−) fewer than 5%, (+) 5-15%, (++) 20-50%, (+++) >50%. Moderately-infected sensory organs (++ or +++) on the right side were matched to the corresponding uninfected organs on the left side and their innervation patterns compared using an axon-associated monoclonal antibody, 3A10. From 10 Wnt4-infected embryos, pair-wise comparisons were available for the basilar papilla (n=9), saccular macula (n=10) and utricular macula (n=9). In contrast, the three cristae were often poorly infected, probably because they are the first organs to become postmitotic, although a few were found to be moderately infected and useful for pairwise comparisons for Wnt4 (2 anterior, 1 posterior and 5 lateral cristae). Wnt5a infection was comparable to Wnt4, allowing us to include the following number of samples from a total of 7 processed Wnt5a-infected embryos: basilar papilla (n=6), saccular macula (n=7), utricular macula (n=5) and cristae (1 anterior, 2 posterior and 4 lateral cristae). Across all 6 organs, we found that infection with either Wnt4- or Wnt5a-expressing retroviruses neither enhanced nor reduced prosensory innervation on E6 when compared with the contralateral sides. Fig. 6E-P, (brackets) shows representative images from left and right saccular maculae as examples. Quantification of neurite outgrowth into the saccular macula of the infected side normalized to the control side showed no change for both Wnt4 (1.08 ± 0.11, n=4) and Wnt5a (0.99 ± 0.06, n=3). There was no significant difference in the total innervation (number of pixels) between control and Wnt4 ears (t-test, p=0.873) or between control and Wnt5a ears (t-test, p=0.375). Furthermore, we found no evidence that Wnt expression in non-sensory regions was attractive to axons, as these regions remained free of innervation, even when well infected (Fig. 6H-J and N-P, arrows).
In some embryos, viral infection spread through the periotic mesoderm (Fig. 6 H-I and N-O) as well as the SAG itself on the right side; neither of these conditions generated obvious misrouting of axons either peripherally or as they bundled together to form the eighth cranial nerve to enter the brainstem (data not shown). In summary, there was no evidence that SAG axons were responsive to Wnt4 or Wnt5a in vivo.
4. Discussion
During vertebrate inner ear development, each bipolar SAG neuron extends a peripheral process towards the sensory organs and a central process to nuclei within the hindbrain. The peripheral processes are thought to use multiple attractive and repulsive cues as they navigate towards their sensory targets, based on mouse and chick studies (Fritzsch, 2003). It is unclear how many cues are involved in this process and several molecules still remain to be tested. This is the first study to describe the response of SAG neurons to members of the Wnt morphogen family.
Two in vitro assays were used to test the hypothesis that either Wnts or Wnt inhibitors (sFRPs) could influence the outgrowth of SAG neurites. Both attractive and repulsive guidance responses were considered. E4 SAG explants did not respond to a concentration range of purified proteins and did not show directional responses to cells secreting Wnt ligands. Quantitatively, there was no significant difference in neurite length or density between control and Wnt-treated explants, demonstrating SAG neurons did not respond to the molecules that were tested. This contrasted with reductions in SAG outgrowth in the presence of Sema3E. These in vitro results suggest that chick otic axons are unlikely to use Wnts −1, −4, −5a, −6, −7b or sFRPs as guidance cues in vivo during the first week of development. Several Wnts that we did not test are also expressed during this developmental time window and remain as candidates for guiding SAG axons.
Wnt-treated spinal cords and Sema3E-treated SAG controls confirmed that the molecules we tested were bioactive on chicken tissue and that the SAG explants were responsive under these assay conditions. Neurite length measurements suggested that neither neurite stalling nor the promotion of outgrowth occurred in this system. Pixel counts were used as a quantitative measure for the density of outgrowth and to indicate if there were effects on neurites other than length. For example, Wnts have also been shown to promote neurite branching (Hall et al., 2000). If there were effects on neurite density, branching, or bundling, this should have been reflected in the pixel measurements. Furthermore, we tested a large concentration range of purified molecules, as well as presumed concentration gradients using transfected HEK co-cultures, all of which yielded negative results.
It is possible that only a subset of neurons or axons showed a response in our in vitro system, but we failed to notice it due to the developmental ages that we examined. On E4, the SAG contains two populations of neurons (vestibular and auditory) and two populations of axons (peripheral and central branches). The vestibular and auditory ganglia develop from the SAG when the cell bodies comprising each ganglion separate (D'Amico-Martel, 1982). This results in the vestibular ganglion innervating the vestibular sensory organs and the auditory (cochleolagenar) ganglion innervating the basilar papilla and lagena macula in the bird. There may be multiple guidance cues expressed in the ear in order to selectively guide vestibular or auditory neurites to their respective targets. Each population of SAG neurites may only use a particular set of cues during development and therefore could respond differently to the same guidance molecule. We separated cultures from each treatment group into two age groups: HH20-23 and HH24-25 (data not shown). Since vestibular fibers begin pathfinding before auditory fibers, we reasoned that the vestibular neurons from HH20-23 explants should be more advanced than the auditory, and may dominate neurite outgrowth at those stages. We did not observe an age-dependent response across treatment groups (data not shown), suggesting auditory and vestibular axons are both equally unresponsive to the Wnts we tested. SAG responsiveness was not investigated beyond E5 because at these stages the majority of axons have already reached their targets (Whitehead and Morest, 1985) and may no longer be responding to pathfinding cues. On the other hand, peripheral and central processes may be differentially responsive to Wnts, but due to the technical limitations of our in vitro system we were unable to distinguish between these two axon populations. The results from the in vivo experiments suggest that auditory and vestibular axons respond similarly (unresponsive) to Wnts −4 and −5a. These Wnts were overexpressed in both sensory and non-sensory regions of the chicken inner ear without influencing axon trajectories or the density of innervation in the infected otic epithelia on E6.
These negative results may be due to the in vitro culture conditions we used to test SAG neurite responsiveness. We recognize the limitations of in vitro models in that they are not as complex as in vivo systems, and may not represent a true physiological environment. The Wnts that were solely tested in vitro may have effects in vivo, where the environment is more complex. One example of complexity is the extracellular matrix (ECM), which represents the microenvironment of a cell. ECM components, such as the proteins laminin and fibronectin, are able to modify axon outgrowth during development. In the chick, laminin and fibronectin are distributed within a matrix that surrounds SAG neurons and sensory epithelia during stages of neurite outgrowth and sensory organ innervation (Hemond and Morest, 1991a; Whitehead and Morest, 1985). These molecules are able to provide guidance cues to P5 rat spiral ganglion neurites when presented in an in vitro stripe assay (Evans et al., 2007), suggesting these ECM molecules may modify responses to guidance molecules in vivo. P1-2 mouse spiral ganglion neurites growing in 2-dimensional cultures have also been shown to preferentially grow on non-neuronal cells that secrete ECM components (Whitlon et al., 2009), suggesting that the cell types SAG neurites encounter may also influence their responses. In our in vitro system, collagen is the only substrate that SAG neurites are growing on and this may oversimplify the micro-environment that they encounter in vivo. It is possible that if cultured in the presence of ECM molecules or other cell types, in addition to collagen, SAG neurites may express different receptors than those expressed when on a collagen substrate and that could influence their responsiveness to Wnts. However, in the case of Wnts −4 and −5a, both in vitro (Fig. 2, 4) and in vivo (Fig. 6) data are consistent in failing to reveal guidance effects, suggesting that the lack of a response to these Wnts is unlikely to result from to the simplicity of the in vivo environment.
Our results in chicken may not extend to other species, such as the mouse. For example, during spinal cord development, post-crossing commissural axons use different guidance cues in the mouse and chicken. In the mouse, a high anterior to posterior gradient of Wnt4 activity attracts post-crossing commissural axons anteriorly (Lyuksyutova et al., 2003), whereas in the chick it is a Wnt5a/7a activity gradient (Domanitskaya et al., 2010). Therefore, Wnts different from or the same as the ones we tested could play guidance roles in the mouse.
In summary, the present study tested the response of chick SAG neurites to Wnts and Wnt inhibitors and found no effect on neurite outgrowth. This suggests that the Wnts and sFRPs that were tested do not influence axon guidance in the chicken inner ear, that their effects are restricted to a specific axon subpopulation, or that they have activity that was not observable with our experimental conditions. Although these results are negative, they are still significant as this is the first study to investigate Wnt morphogens as axon guidance cues in the inner ear. Additionally, we have successfully established a culture system that we can now use to screen other candidate molecules, including other morphogens, for their effects on the peripheral sensory neurons of the inner ear.
Acknowledgements
This work was funded by National Institutes of Health Grant RO1DC002756 and the Purdue Research Foundation. We thank Kirsten Luethy for assistance with histology and data analysis, Cliff Tabin for providing the RCAS viruses, and Sherry Harbin, Joanne Kuske, and Seth Kreger for advice and technical assistance.
Abbreviations
- CNTF
Ciliary neurotrophic factor
- E
Embryonic day
- HBSS
Hank's Balanced Salt Solution
- HH
Hamburger and Hamilton stage
- NT-3
Neurotrophin-3
- PBS
Phosphate-buffered saline
- SAG
Statoacoustic ganglion
- Sema3E
Semaphorin 3E
- sFRP
Secreted frizzled related protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Battisti AC, Fekete DM. Slits and robos in the developing chicken inner ear. Dev. Dyn. 2008;237:476–84. doi: 10.1002/dvdy.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi LM, Cohan CS. Effects of the neurotrophins and CNTF on developing statoacoustic neurons: comparison with an otocyst-derived factor. Dev. Biol. 1993;159:353–65. doi: 10.1006/dbio.1993.1247. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Gray NA. EphB receptors influence growth of ephrin-B1-positive statoacoustic nerve fibers. Eur. J. Neurosci. 2002;16:1499–506. doi: 10.1046/j.1460-9568.2002.02248.x. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Rodriguez J, Esteve P. Frizzled/RYK mediated signalling in axon guidance. Development. 2006;133:4399–408. doi: 10.1242/dev.02592. [DOI] [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–62. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- D'Amico-Martel A. Temporal patterns of neurogenesis in avian cranial sensory and autonomic ganglia. Am. J. Anat. 1982;163:351–372. doi: 10.1002/aja.1001630407. [DOI] [PubMed] [Google Scholar]
- Dalby B, Cates S, Harris A, Ohki EC, Tilkins ML, Price PJ, Ciccarone VC. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Domanitskaya E, Wacker A, Mauti O, Baeriswyl T, Esteve P, Bovolenta P, Stoeckli ET. Sonic hedgehog guides post-crossing commissural axons both directly and indirectly by regulating Wnt activity. J. Neurosci. 2010;30:11167–76. doi: 10.1523/JNEUROSCI.1488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Euteneuer S, Chavez E, Mullen LM, Hui EE, Bhatia SN, Ryan AF. Laminin and fibronectin modulate inner ear spiral ganglion neurite outgrowth in an in vitro alternate choice assay. Dev Neurobiol. 2007;67:1721–30. doi: 10.1002/dneu.20540. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Campero AM. Axon guidance in the inner ear. Int. J. Dev. Biol. 2007;51:549–56. doi: 10.1387/ijdb.072341df. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain Res. Bull. 2003;60:423–33. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. Hear. Res. 2005;206:52–63. doi: 10.1016/j.heares.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–35. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–91. [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–59. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- Hemond SG, Morest DK. Formation of the cochlea in the chicken embryo: sequence of innervation and localization of basal lamina-associated molecules. Brain Res. Dev. Brain Res. 1991a;61:87–96. doi: 10.1016/0165-3806(91)90117-2. [DOI] [PubMed] [Google Scholar]
- Hemond SG, Morest DK. Ganglion formation from the otic placode and the otic crest in the chick embryo: mitosis, migration, and the basal lamina. Anat Embryol (Berl) 1991b;184:1–13. doi: 10.1007/BF01744256. [DOI] [PubMed] [Google Scholar]
- Homburger SA, Fekete DM. High efficiency gene transfer into the embryonic chicken CNS using B- subgroup retroviruses. Dev. Dyn. 1996;206:112–20. doi: 10.1002/(SICI)1097-0177(199605)206:1<112::AID-AJA10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–35. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Li H, Liu H, Sage C, Huang M, Chen ZY, Heller S. Islet-1 expression in the developing chicken inner ear. J. Comp. Neurol. 2004;477:1–10. doi: 10.1002/cne.20190. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ, Zou Y. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–9. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–8. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Fekete DM. Manipulating gene expression with replication-competent retroviruses. Methods Cell Biol. 1996;51:185–218. doi: 10.1016/s0091-679x(08)60629-9. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J. Comp. Neurol. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Esteve P, Weinl C, Ruiz J, Fermin Y, Trousse F, Dwivedy A, Holt C, Bovolenta P. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nature Neurosci. 2005;8:1301–9. doi: 10.1038/nn1547. [DOI] [PubMed] [Google Scholar]
- Sanchez-Calderon H, Martin-Partido G, Hidalgo-Sanchez M. Otx2, Gbx2, and Fgf8 expression patterns in the chick developing inner ear and their possible roles in otic specification and early innervation. Gene Expr Patterns. 2004;4:659–69. doi: 10.1016/j.modgep.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Sienknecht UJ, Fekete DM. Comprehensive Wnt-related gene expression during cochlear duct development in chicken. J. Comp. Neurol. 2008;510:378–95. doi: 10.1002/cne.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienknecht UJ, Fekete DM. Mapping of Wnt, frizzled, and Wnt inhibitor gene expression domains in the avian otic primordium. J. Comp. Neurol. 2009;517:751–64. doi: 10.1002/cne.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J. Neurosci. 2004;24:2575–84. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MC, Morest DK. The development of innervation patterns in the avian cochlea. Neuroscience. 1985;14:255–276. doi: 10.1016/0306-4522(85)90177-0. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Tieu D, Grover M, Reilly B, Coulson MT. Spontaneous association of glial cells with regrowing neurites in mixed cultures of dissociated spiral ganglia. Neuroscience. 2009;161:227–35. doi: 10.1016/j.neuroscience.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AM, Lyuksyutova AI, Fenstermaker AG, Shafer B, Lo CG, Zou Y. Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J. Neurosci. 2008;28:3456–67. doi: 10.1523/JNEUROSCI.0029-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Lyuksyutova AI. Morphogens as conserved axon guidance cues. Curr. Opin. Neurobiol. 2007;17:22–8. doi: 10.1016/j.conb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–75. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]