Abstract
Dendrites can be maintained for extended periods of time after they initially establish coverage of their receptive field. The long-term maintenance of dendrites underlies synaptic connectivity, but how neurons establish and then maintain their dendritic arborization patterns throughout development is not well understood. Here, we show that the NAD synthase Nicotinamide mononucleotide adenylyltransferase (Nmnat) is cell-autonomously required for maintaining type-specific dendritic coverage of Drosophila dendritic arborization (da) sensory neurons. In nmnat heterozygous mutants, dendritic arborization patterns of class IV da neurons are properly established before increased retraction and decreased growth of terminal branches lead to progressive defects in dendritic coverage during later stages of development. Although sensory axons are largely intact in nmnat heterozygotes, complete loss of nmnat function causes severe axonal degeneration, demonstrating differential requirements for nmnat dosage in the maintenance of dendritic arborization patterns and axonal integrity. Overexpression of Nmnat suppresses dendrite maintenance defects associated with loss of the tumor suppressor kinase Warts (Wts), providing evidence that Nmnat, in addition to its neuroprotective role in axons, can function as a protective factor against progressive dendritic loss. Moreover, motor neurons deficient for nmnat show progressive defects in both dendrites and axons. Our studies reveal an essential role for endogenous Nmnat function in the maintenance of both axonal and dendritic integrity and present evidence of a broad neuroprotective role for Nmnat in the central nervous system.
Keywords: Nmnat, dendrite, axon, motor neuron, neurodegeneration, Drosophila
Introduction
Dendrite arborization patterns have a direct influence on the types and numbers of synaptic or sensory input a neuron receives, and consequently, the way in which a neuron integrates and processes these impinging signals (Hausser et al., 2000; Magee, 2000). Dendritic abnormalities are the most consistent pathological correlate of cognitive decline in the aging human cortex as the progressive loss of dendritic branches is thought to underlie altered patterns of cortical activity in Alzheimer’s disease (AD) (Coleman and Flood, 1987; Scheibel et al., 1975). Likewise, progressive defects in dendritic arborization have been associated with a number of neurodevelopmental disorders, including Down syndrome (DS) (Kaufmann and Moser, 2000). Although dendrite arborization patterns are often established early in development and are largely maintained throughout the lifetime of a neuron (Lin and Koleske, 2010), the specific mechanisms that are required for the maintenance of dendritic arbors are not well understood.
Drosophila da neurons are multidendritic (md) neurons that have emerged as powerful model neurons for studying establishment and maintenance of type-specific dendritic coverage (Parrish et al., 2007b). Each of the four classes of da neurons (I–IV) has a class-specific dendritic morphology and covers a stereotyped region of the body wall (Grueber et al., 2002). Dendritic arbors of class IV da neurons establish complete and non-redundant coverage of the body wall by the end of the first larval instar and maintain this coverage for the remainder of larval development (Emoto et al., 2006). Recent findings that Polycomb group (PcG) genes and the nuclear Dbf2-related (NDR) family kinase Wts are required for proper maintenance of class IV dendrites provide evidence that intrinsic mechanisms are important for maintaining dendritic coverage of receptive fields throughout development (Emoto et al., 2006; Parrish et al., 2007a).
Nmnat catalyzes a key step of NAD synthesis and is an essential component of the Wallerian degeneration slow (WldS) chimeric protein, a neuroprotective factor that delays axonal degeneration (Conforti et al., 2000; Mack et al., 2001). In mouse superior cervical ganglia (SCG) explants, specific knockdown of Nmnat2, one of three mammalian Nmnat isoforms, is sufficient to induce Wallerian-like degeneration, supporting a role for endogenous Nmnat2 in the maintenance of neuronal integrity (Gilley and Coleman, 2010). Studies from Drosophila suggest that Nmnat plays an evolutionarily conserved role in neuronal maintenance as overexpression of Drosophila Nmnat is sufficient to delay both injury-induced axonal degeneration (MacDonald et al., 2006) and spinocerebellar ataxia 1 (SCA1)-induced neurodegeneration (Zhai et al., 2008). Furthermore, Drosophila Nmnat can function endogenously as a maintenance factor to protect against activity-induced neurodegeneration (Zhai et al., 2006). Despite its well-documented role in protecting against axonal degeneration, whether Nmnat functions in a similar capacity to maintain dendritic integrity remains largely unknown. In this study, we show that Nmnat acts cell-autonomously to maintain dendritic arborization patterns of both da sensory neurons and motor neurons and that Nmnat can serve a neuroprotective role in dendrites as shown by the ability of Nmnat overexpression to suppress dendrite maintenance defects associated with loss of wts. We further show that loss of nmnat causes axonal degeneration and that axons and dendrites of class IV neurons show disparate phenotypes in response to reduction of nmnat copy number. Collectively, these studies support an evolutionarily conserved function for Nmnat in axon maintenance and reveal a novel role for Nmnat as a neuroprotective factor against progressive dendritic loss.
Results
nmnat mutants display progressive defects in terminal dendritic branching
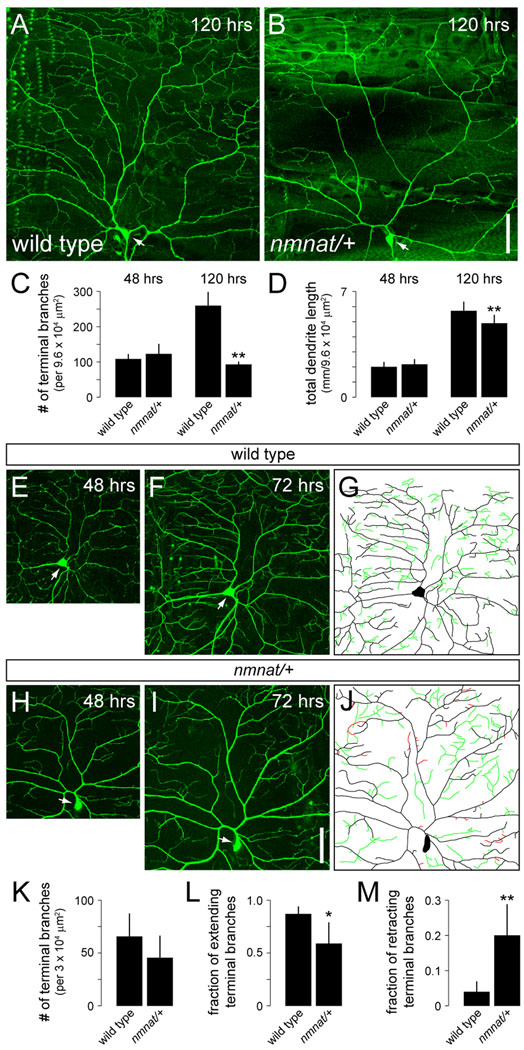
We examined dendrites of class IV neurons in mutants homozygous for the loss-of-function nmnatΔ4792 allele using the class IV-specific pickpocket (ppk)-EGFP reporter (Grueber et al., 2003). At 24–28 hrs after egg laying (AEL), dendrites of the dorsal class IV neuron (ddaC) in homozygous nmnatΔ4792 mutants did not show any patterning defects and were largely indistinguishable from those of wild-type controls (data not shown). Because nmnatΔ4792 mutants do not survive beyond this stage, we next turned to heterozygous mutants to further examine the role of nmnat in dendrite development. Although heterozygosity for nmnatΔ4792 had no effect on dendritic outgrowth or branching of ddaC at 48 hrs AEL, we observed a significant reduction in the total number of terminal dendritic branches and total dendrite length at 120 hrs AEL (Fig. 1A–D). To gain a better understanding of the basis for defects in dendritic coverage in nmnat heterozygotes, we conducted time-lapse imaging of ddaC dendrites at 48 hrs and 72 hrs AEL (Fig. 1E–J). At 48 hrs AEL, ddaC neurons in wild-type and nmnatΔ4792 heterozygotes were indistinguishable (Fig. 1E,H). At 72 hrs AEL, nmnatΔ4792 heterozygotes tended to have fewer terminal dendrites (Fig. 1K), although the average number of terminal dendritic branches was not significantly different from wild-type controls until after 72 hrs AEL. Nevertheless, time-lapse analysis between 48 hrs and 72 hrs AEL revealed significantly fewer branch extension and more terminal branch retraction events in nmnatΔ4792 heterozygotes compared to wild-type controls (Fig. 1L,M). Therefore, it seems likely that the progressive dendrite defects observed in nmnat heterozygotes are a consequence of both increased retraction and reduced growth of terminal dendritic branches.
Figure 1.
nmnat mutants display defects in the maintenance of terminal dendritic branches. (A,B) Dendrites of class IV ddaC neuron in a late third instar larva (120 hrs AEL) for wild type (A) and nmnatΔ4792 heterozygote (nmnat/+) (B). (C,D) Quantification of total number of terminal dendritic branches (C) and total dendrite length (D) per 9.6 ×104 µm2 (mean ± SD) in ddaC neurons for wild type and nmnatΔ4792 heterozygotes at 48 hrs AEL (wild type, n = 8; nmnat/+, n = 13) and 120 hrs AEL (wild type, n = 9; nmnat/+, n = 10). Double asterisk denotes p < 0.001 relative to wild-type controls (Student’s t-test). (E,F) Time-lapse imaging of a representative wild-type ddaC neuron at 48 hrs AEL (E) and at 72 hrs AEL (F). (G) Tracing of a wild-type ddaC neuron indicates new branch growth (green) over the 24 hr interval. (H,I) Time-lapse imaging of a representative ddaC neuron in a nmnatΔ4792 heterozygote at 48 hrs AEL (H) and at 72 hrs AEL (I). (J) Tracing of a ddaC neuron in a nmnatΔ4792 heterozygote indicates instances of dendrite retraction (red) and less new branch growth (green) over the 24 hr interval compared to wild-type (G). Arrows indicate cell body. Anterior is left and dorsal is up. Scale bar, 50 µm. (K–M) Quantification of total number of terminal dendritic branches (mean ± SD) (K), fraction of extending terminal branches (L), and fraction of retracting terminal branches (M) per 3 × 104 µm2 in ddaC neurons for wild-type (n = 7) and nmnatΔ4792 heterozygotes (n = 6) at the 72 hr time point. (Asterisk, p < 0.01; double asterisk, p < 0.001).
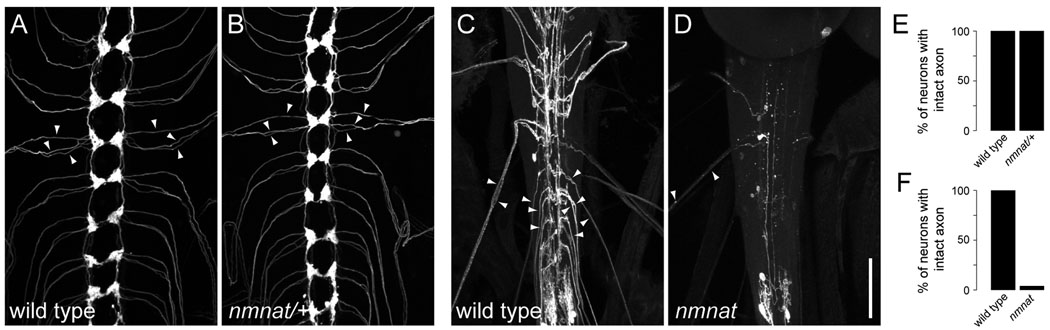
We next examined axons of class IV neurons in nmnatΔ4792 heterozygotes and found that they were largely intact at 120 hrs AEL (Fig. 2A,B,E). Axons entered the CNS appropriately and their projection patterns in the ventral nerve cord (VNC) were morphologically indistinguishable from wild-type controls (Fig. 2A,B). Although it is possible that nmnat is important for maintenance of axon terminal branching, the lack of an axon degeneration phenotype in nmnatΔ4792 heterozygotes suggests that a single copy of nmnat is sufficient to maintain the axonal integrity of class IV neurons.
Figure 2.
Maintenance of da sensory neuron axons in nmnat mutants is dosage-sensitive. (A,B) Axons of class IV neurons in the ventral nerve cord (VNC) were visualized using ppk-EGFP. Class IV axons in wild type (A) or nmnatΔ4792 heterozygotes (nmnat/+) (B) are largely indistinguishable. Arrowheads indicate class IV axons within a single abdominal segment. (C,D) Axons of multiple abdominal sensory neuron clones in the VNC were visualized using MARCM. Whereas axons of wild-type sensory neuron clones project to highly stereotyped regions of the neuropil (C), axons of nmnatΔ4792 sensory neuron clones show extensive beading and fragmentation and loss of terminals (D). Arrowheads indicate axons of identified sensory neuron clones. Anterior is up. Scale bar, 50 µm. (E) Quantification of the total percentage of class IV neurons with intact axons with respect to GFP expression for wild type (n = 148) and nmnatΔ4792 heterozygotes (n = 190). (F) Quantification of the total percentage of sensory neurons with intact axons with respect to α-mCD8 immunolabeling for wild type (n = 99) and nmnatΔ4792 (n = 90) MARCM clones.
nmnat functions cell-autonomously to maintain dendritic coverage
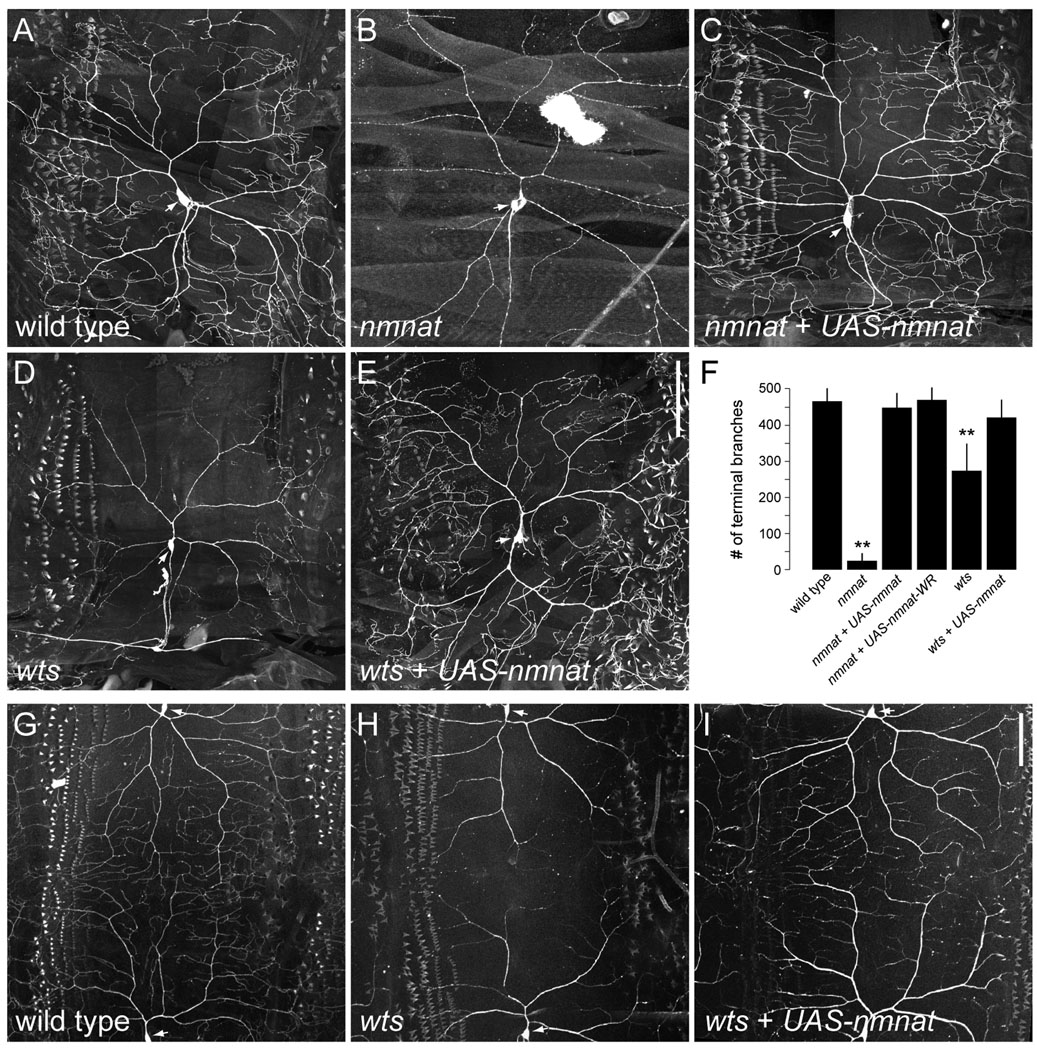
To determine whether the dendrite maintenance defects reflect a cell-autonomous function for Nmnat in da neurons, we used MARCM (mosaic analysis with a repressible cell marker) (Lee and Luo, 1999) to generate mCD8::GFP-labeled single-cell homozygous nmnatΔ4792 sensory neuron clones. Whereas wild-type ddaC clones elaborated highly branched dendrites (Fig. 3A), nmnatΔ4792 ddaC clones showed a significant reduction in the total number of terminal dendritic branches, effectively reducing dendritic field coverage (Fig. 3B,F). Unlike ddaC neurons mutant for some PcG genes (Parrish et al., 2007a), there was no evidence of severed or degenerated dendrites in nmnatΔ4792 clones as dendritic trunks and branches were largely intact. Postmitotic expression of wild-type Nmnat was sufficient to rescue dendritic phenotypes observed in nmnatΔ4792 ddaC clones (Fig. 3C, F), further demonstrating a cell-autonomous function for Nmnat in the proper maintenance of class IV dendrites. Previous studies have shown that an enzymatically inactive form of Nmnat can rescue degeneration phenotypes induced by loss of nmnat or overexpression of ataxin-1 (Zhai et al., 2006; Zhai et al., 2008). We similarly found that overexpression of an enzymatically inactive Nmnat (Nmnat-WR) (Zhai et al., 2006) could rescue terminal branching defects associated with loss of nmnat (Fig. 3F), suggesting that the NAD synthesis activity of Nmnat is dispensable for its function in dendrite maintenance in these neurons. Dendritic phenotypes were not limited to class IV neurons as we found that class III neurons, which normally elaborate actin-rich protrusions along their dendrites, were largely devoid of terminal branches in nmnatΔ4792 clones (Supplementary Fig. 1). On the other hand, we found that class I neurons, which normally elaborate few terminal branches that cover a small receptive field, exhibited a minor, but statistically insignificant decrease in the total number of dendritic branches in nmnatΔ4792 clones (Supplementary Fig. 2). Together with our time-lapse observations, these data suggest that one manner by which nmnat maintains dendritic coverage is by preventing the loss of terminal dendritic branches.
Figure 3.
Overexpression of Nmnat prevents loss of dendritic branches in wts mutants. (A) Morphology of ddaC dendrites in a wild-type third instar larva MARCM clone. (B,C) ddaC dendrites in nmnatΔ4792 MARCM clones (B) exhibit a significant reduction in terminal branching which can be rescued by inducing expression of Nmnat (nmnat + UAS-nmnat) (C). (D,E) ddaC dendrites in wtslatsX1 MARCM clones (D) show a progressive loss of terminal branches which can be suppressed by overexpressing Nmnat (wts + UAS-nmnat) (E). (F) Quantification of total number of terminal dendritic branches (mean ± SD) in ddaC neurons for wild type (n = 7), nmnatΔ4792 (n = 28), nmnatΔ4792 + UAS-nmnat (n = 11), nmnatΔ4792 + UAS-nmnat-WR (n = 9), wtslatsX1 (n = 16), and wtslatsX1 + UAS-nmnat (n = 11) clones. Double asterisk denotes p < 0.001 relative to wild-type controls (Student’s t-test). (G) Dendrites of wild-type ddaC neurons provide full coverage of the body wall. (H) Dendritic arborization of ddaC neurons is dramatically reduced in a homozygous wtslatsX1 mutant. (I) Overexpression of Nmnat in class IV neurons in a homozygous wtslatsX1 mutant restores dendritic coverage of ddaC neurons. Anterior is left and dorsal is up. Arrows indicate cell body. Scale bar, 100 µm.
Although class IV neurons did not exhibit an axon degeneration phenotype in nmnatΔ4792 heterozygotes (Fig. 2A,B), we found that 96% of homozygous nmnatΔ4792 sensory neuron clones showed extensive fragmentation of axons and a near complete loss of axon terminals in the VNC (Fig. 2C,D,F). Loss of nmnat affected the axons of all classes of da neurons demonstrating its function in maintaining axonal integrity is not restricted to distinct neuronal subtypes. Collectively, these data suggest that neurons with highly branched dendritic arbors (e.g. class IV neurons) have different dosage requirements for nmnat in the maintenance of dendritic coverage and axonal integrity.
Overexpression of Nmnat can suppress dendrite maintenance defects in wts mutants
We investigated the possibility that Nmnat functions as a neuroprotective factor against dendritic loss by examining the effects of Nmnat overexpression in wtslatsX1 mutants, which show a progressive loss of dendritic branches in class IV neurons without affecting axons (Emoto et al., 2006). Whereas wtslatsX1 ddaC MARCM clones showed a simplified dendritic arbor with significantly reduced numbers of terminal branches (Fig. 3D,F), wtslatsX1 clones overexpressing wild-type Nmnat elaborated dendrites with terminal branch numbers that did not differ statistically from wild-type controls (Fig. 3E,F). A similar rescue was observed in class IV neurons overexpressing Nmnat in a homozygous wtslatsX1 mutant background (Fig. 3G–I). Overexpression of Nmnat in wild-type ddaC neurons had no effect on dendrite outgrowth (data not shown), suggesting that the ability of exogenous Nmnat to suppress dendrite maintenance defects in wts mutants is due to its function in preventing dendrite loss rather than initiating new dendrite growth. Finally, although transheterozygous combination of nmnatΔ4792 and wtslatsX1 did not show any synthetic phenotypes in affecting axon or dendrite development of class IV neurons (data not shown), we cannot rule out the possibility that Nmnat functions as a dendrite maintenance factor downstream of the Wts signaling pathway.
Nmnat is required for maintaining axonal and dendritic integrity of motor neurons
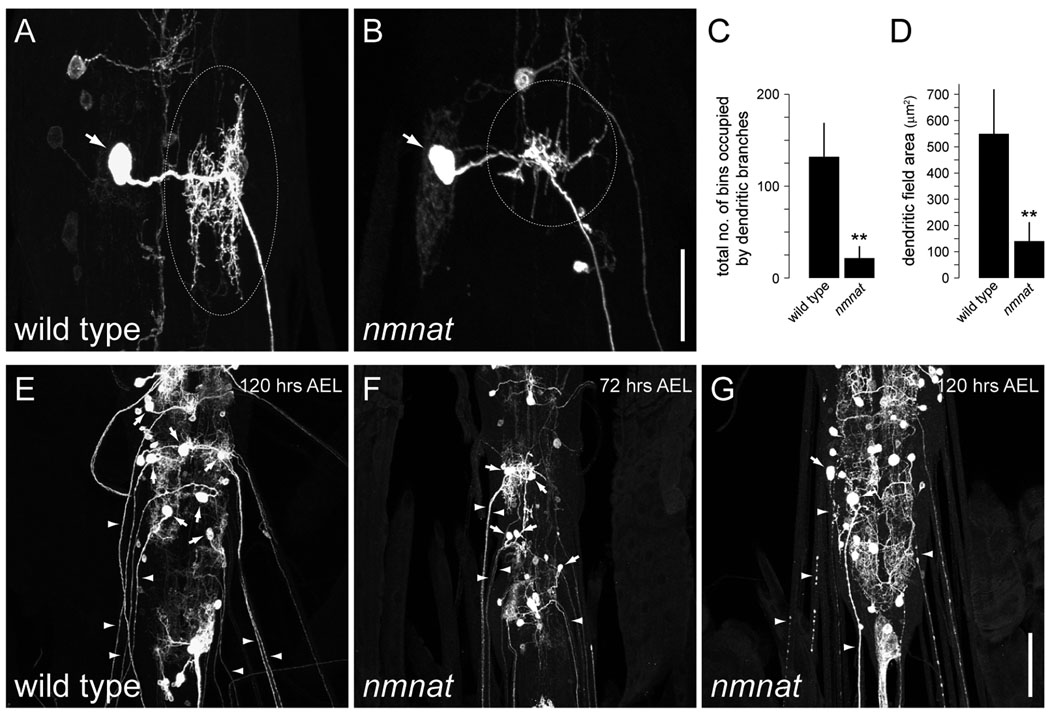
To determine whether Nmnat is required for maintenance of central neurons, we examined nmnat function in a well-defined population of abdominal motor neurons of the larval CNS (Kim et al., 2009). We found that different subclasses of motor neurons displayed both terminal dendritic branching defects (Fig. 4A–D and Supplementary Fig. 3) and extensive axon beading and fragmentation at 120 hrs AEL in nmnatΔ4792 MARCM clones (Fig. 4E,G). We further found that axon terminals of nmnat motor neuron clones were absent in most cases observed (157/179 clones). Phenotypes were more severe with age as axons and dendrites of nmnatΔ4792 motor neuron clones were largely intact at 72 hrs AEL (Fig. 4F). However, given the severity of the axon phenotypes, it is possible that defects in axon maintenance contribute to the progressive loss of dendrites in nmnatΔ4792 motor neuron clones as previous studies have demonstrated that motor neuron axotomy can cause dendritic retraction (Cerf and Chacko, 1958; Sumner and Watson, 1971). Collectively, the data in this study show that Nmnat is required for the maintenance of axonal and dendritic integrity in both central and peripheral neurons.
Figure 4.
nmnat is required for the maintenance of motor neuron dendrites and axons. (A) Dendritic morphology of a wild-type intersegmental nerve b (ISNb) motor neuron. (B) Dendrites of ISNb nmnatΔ4792 MARCM clones exhibit a significant reduction in dendritic branching. Dotted circles indicate dendrites. (C,D) Quantification of terminal dendritic branching (mean ± SD) (C) and total dendritic field area (mean ± SD) (D) for wild-type (n = 14) and nmnatΔ4792 (n = 20) ISNb motor neuron clones. Double asterisk denotes p < 0.001 (Student’s t-test). (E) Axonal and dendritic projections of wild-type motor neuron clones at 120 hrs AEL. (F) Axons and dendrites of nmnatΔ4792 motor neuron clones are largely intact at 72 hrs AEL. (G) nmnatΔ4792 motor neuron clones exhibit severe beading and fragmentation of axons and loss of dendrites by 120 hrs AEL. Arrows indicate cell body, arrowheads indicate axons. Anterior is up. Scale bar represents 25 µm (A,B) and 50 µm (E–G).
Discussion
While the protective effects of Nmnat in axons have been widely reported (for reviews, see Coleman and Freeman, 2010; Zhai et al., 2009), Nmnat’s role in dendrites is not well understood. In this study, we provide the first evidence that Nmnat functions endogenously to maintain dendritic coverage throughout development and that Nmnat can function as a neuroprotective factor against progressive dendritic loss. Our finding that loss of nmnat leads to defects in motor neuron dendrites and progressive degeneration of motor axons is further suggestive of a widespread neuroprotective role for Nmnat in the CNS.
Nmnat is neuroprotective in dendrites
Although our understanding of the molecular mechanisms that regulate the growth and patterning of dendritic arbors has significantly progressed (Jan and Jan, 2010), the mechanisms by which dendritic arborization patterns are effectively maintained during development are still relatively unknown. Studies using Drosophila da neurons have provided initial insight into the genetic programs that are specifically required for the continued maintenance of receptive field coverage by sensory neuron dendrites (Emoto et al., 2006; Parrish et al., 2007a). Class IV neurons follow a stereotyped course of development whereby their dendritic fields are initially established by homotypic repulsion then continue to maintain complete and non-redundant coverage of the body wall throughout larval development (Emoto et al., 2006; Grueber et al., 2003; Sugimura et al., 2003). Although class IV dendritic fields are properly established in nmnat mutants, reduced growth and a progressive loss of terminal dendritic branches effectively reduces dendritic coverage of the body wall during later stages of development, consistent with a role for Nmnat in maintenance of dendritic coverage. It is likely that the fraction of extending and retracting terminal branches in nmnat heterozygotes is an underestimation as quantification of our time-lapse data does not take into account new dendritic branches that may have extended and completely retracted within the 24 hr time period. This would point to a greater role for Nmnat in protection against dendritic loss. Nevertheless, the increased retraction and decreased late-stage growth of terminal branches observed in nmnat mutants strongly resemble mutants of other genes that are specifically required for maintaining dendritic coverage (Parrish et al., 2007a).
Nmnat’s function as a dendrite maintenance factor is further supported by our finding that overexpression of Nmnat can suppress progressive defects in dendritic branching caused by loss of wts. Axons of class IV neurons are unaffected in wts mutants (Emoto et al., 2006), suggesting that the protective function of Nmnat in dendrites is not a secondary consequence of its role in axonal maintenance. Although overexpression of Nmnat does not induce new dendritic growth in wild-type da neurons, we cannot completely exclude the possibility that overexpression of Nmnat promotes branch formation as the growth of wild-type class IV dendrites may be self-limiting due to self-avoidance and tiling (Grueber et al., 2003; Sugimura et al., 2003). However, based on our observations that reduced nmnat dosage leads to more terminal branch retraction and fewer branch extension events, we favor a model whereby Nmnat selectively stabilizes existing or newly formed dendritic branches to maintain coverage of the receptive field.
Expression of WldS has been previously shown to protect against pruning of class IV dendrites during metamorphosis in a NAD+-sensitive manner (Schoenmann et al., 2010). However, we found that enzymatically inactive Nmnat is sufficient to rescue dendrite maintenance defects induced by loss of nmnat. This apparent discrepancy may be attributed to the fact that the molecular programs that control dendrite maintenance are mechanistically distinct from those of dendrite pruning (Parrish et al., 2007b). Furthermore, although numerous studies suggest that the enzymatic activity of Nmnat is required for axonal protection (Avery et al., 2009; Conforti et al., 2009; Jia et al., 2007; Sasaki et al., 2009; Yahata et al., 2009; Yan et al., 2010), these studies have not directly addressed a neuroprotective role for Nmnat specifically in dendrites. Our data support a model whereby the NAD synthesis function of Nmnat is not required for dendrite maintenance and that a separate chaperone function of Nmnat (Zhai et al., 2008) may be important for its role in dendrites. As molecular chaperones are localized at postsynaptic sites and have been implicated to play a role in synaptic plasticity (Ohtsuka and Suzuki, 2000; Suzuki et al., 1999), our studies raise the possibility that chaperones such as Nmnat may further play a role in maintaining postsynaptic stability.
Our finding that heterozygous nmnat mutants have progressive dendrite defects in class IV neurons suggests that a critical threshold of endogenous Nmnat is likely required in some neurons to maintain dendritic coverage. Notably, this dosage-sensitivity is specific to nmnat as heterozygosity for wts or PcG genes, which are also cell-autonomously required for maintaining dendritic coverage of class IV neurons, does not have an adverse effect on either axon or dendrite maintenance (Emoto et al., 2006; Parrish et al., 2007a). Although our data showing that dendrites of class IV neurons are selectively affected in nmnat heterozygotes suggest that dendrites are more sensitive than axons to reduced levels of Nmnat, we cannot completely rule out the possibility that reduction of nmnat dosage causes specific defects in axon terminal morphology. However, it is likely that dendrites require higher levels of Nmnat in order to maintain the expansive growth in dendritic coverage that occurs during larval development, where dendritic fields of dorsal class IV neurons expand by more than 6-fold between 48–120 hrs AEL to achieve full coverage of the growing larval body wall (Parrish et al., 2009). In contrast, class I neurons cover their receptive territories by 24 hrs AEL and exhibit minimal growth of new terminal branches between 24–120 hrs AEL (Parrish et al., 2009), which may explain why nmnat is not overtly important for the maintenance of class I dendrites.
Dendritic regression and the loss of dendritic spines are associated with normal human aging as well as neurodegenerative diseases such as AD. Changes in synaptic connectivity resulting from reduced dendrite arborization are thought to underlie memory loss and other cognitive deficits that characterize this and other pathological conditions. Although a number of studies have examined the protective effects of Nmnat after axonal injury (for reviews, see Coleman and Freeman, 2010; Zhai et al., 2009), our studies raise the intriguing possibility that Nmnat could serve as a neuroprotective factor against dendritic regression associated with aging and disease.
An evolutionarily conserved role for Nmnat in axon maintenance
In mammals, specific knockdown of Nmnat2 can induce Wallerian-like degeneration of uninjured axons while endogenous Nmnat2 protein is rapidly degraded prior to the initiation of degeneration in transected axons, providing direct support for Nmnat2 as an endogenous maintenance factor in axons (Gilley and Coleman, 2010). Although loss of Drosophila nmnat was previously shown to cause photoreceptor degeneration (Zhai et al., 2006), whether nmnat is important for maintaining axon integrity in Drosophila had not been directly demonstrated. Our data showing that loss of nmnat causes degeneration of sensory and motor axons in Drosophila provides further evidence of an evolutionarily conserved role for endogenous Nmnat in axon survival. Although it is possible that axonal phenotypes are a secondary consequence of dendrite maintenance defects in nmnat mutants, that neither wts nor PcG gene mutants exhibit any axon degeneration phenotypes (Emoto et al., 2006; Parrish et al., 2007a) suggests that the proper maintenance of axons and dendrites is achieved by mechanistically distinct processes. The loss of axon terminals in motor neurons deficient for nmnat suggests a “dying-back” axonopathy characteristic of Wallerian degeneration or retrograde forms of degeneration in diseases like Amyotrophic lateral sclerosis (ALS) (Fischer et al., 2004). Nmnat is expressed at the NMJ and its importance in maintaining synaptic integrity and function (Zhai et al., 2006) could explain the “dying-back” phenotype observed in motor neurons deficient for nmnat. The recent report that Nmnat can act as a chaperone (Zhai et al., 2008) is consistent with a model whereby chaperones maintain continued synaptic function to prevent the degeneration of presynaptic nerve terminals (Fernandez-Chacon et al., 2004). Understanding how Nmnat functions as a chaperone to maintain the integrity of motor axon terminals will undoubtedly shed light on the mechanisms underlying ALS and other neurodegenerative disorders.
Experimental methods
Live imaging
To visualize class IV dendrites in nmnat and wts mutants, we recombined either the ppk-EGFP (Grueber et al., 2003) or ppk-Gal4, UAS-mCD8::GFP (Kuo et al., 2005) reporter with nmnatΔ4792 (Zhai et al., 2006) and wtslatsX1 (Xu et al., 1995) mutant alleles. For staging, embryos were collected for 2 hrs on grape agar plates at 25°C and allowed to develop at 25°C to the appropriate stage for live imaging analysis. Larvae were mounted in 90% glycerol and visualized using an Olympus FluoView FV1000 confocal microscope (Olympus, Tokyo, Japan).
MARCM labeling and immunocytochemistry
To generate da neuron clones, 1) FRT82B, nmnatΔ4792 /TM6B, 2) UAS-nmnat; FRT82B, nmnatΔ4792 /TM6B, 3) UAS-nmnat-WR; FRT82B, nmnatΔ4792/TM6B, 4) FRT82B, wtslatsX1/TM6B, 5) UAS-nmnat; FRT82B, wtslatsX1/TM6B, or 6) w; FRT82B (for control clones) males were mated with yw, hsFLP; Gal4217, UAS-mCD8::GFP; FRT82B, tub-Gal80 virgin females. In brief, embryos were collected for 2 hrs and allowed to develop for 3–5 hrs at 25°C before heat shock. Embryos were heat-shocked for 1 hr at 38°C and allowed to develop at 25°C to the third instar larval stage. Larvae with da neuron clones were then dissected, fixed, and co-stained with rat α-mCD8 antibody (Invitrogen, Carlsbad, CA) at 1:250 dilution and monoclonal α-22C10 antibody (Zipursky et al., 1984) [Developmental Studies Hybridoma Bank (DSHB)] at 1:500 dilution. To generate motor neuron clones, FRT82B, nmnatΔ4792/TM6B or w; FRT82B (for control clones) males were mated with elav-Gal4, UAS-mCD8::GFP, hsFLP; FRT82B, tub-Gal80 virgin females. Embryos were collected and heat shocked as described (Kim et al., 2009). Appropriately staged larvae with motor neuron clones were then dissected, fixed, and co-stained with rat α-mCD8 antibody and monoclonal α-Fasciclin II antibody (Lin et al., 1994) (1D4; DSHB) at 1:500 dilution. Confocal image stacks were obtained using an Olympus FluoView FV1000 microscope and reconstructed using ImageJ software (NIH, Bethesda, MD).
Quantitative analysis
For quantitative analysis of time-lapse images, we assessed the dynamic behavior of individual terminal dendritic branches within a defined 150 × 200 µm area spanning the cell body and dorsal dendritic region at the 72 hr time point. Only branches with a measured difference of ≥ 4 µm between the 48 and 72 hr time points were considered to be either extending or retracting.
For quantitative analysis of motor neuron dendrites, we first identified individual motor neuron clones as described in Kim et al., 2009. Total dendritic branching of motor neurons was then quantified according to Singh et al. (Singh et al., 2010) by counting the total number of bins of a defined size (5 µm2) occupied by dendritic branches in reconstructed 2D images of individual MARCM clones using ImageJ software.
Supplementary Material
Acknowledgements
We thank the Bloomington Stock Center for fly stocks, the Developmental Studies Hybridoma Bank for antibodies, and the members of our laboratories for critical reading of the manuscript and helpful discussions. This work was supported by a postdoctoral research fellowship from the James & Esther King Biomedical Research Program (Y.W.), NIH MH084277 (J.Z.P.), NIH NS64269 and the Pew Charitable Trust (R.G.Z.), and NIH NS072588 (M.D.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avery MA, Sheehan AE, Kerr KS, Wang J, Freeman MR. WldS requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J Cell Biol. 2009;184:501–513. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf JA, Chacko LW. Retrograde reaction in motoneuron dendrites following ventral root section in the frog. J Comp Neurol. 1958;109:205–219. doi: 10.1002/cne.901090205. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR. Wallerian degeneration, Wld(s), and Nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging. 1987;8:521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, Wagner D, Perry VH, Coleman MP. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci U S A. 2000;97:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Wilbrey A, Morreale G, Janeckova L, Beirowski B, Adalbert R, Mazzola F, Di Stefano M, Hartley R, Babetto E, Smith T, Gilley J, Billington RA, Genazzani AA, Ribchester RR, Magni G, Coleman M. WldS protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J Cell Biol. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto K, Parrish JZ, Jan LY, Jan YN. The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature. 2006;443:210–213. doi: 10.1038/nature05090. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Wolfel M, Nishimune H, Tabares L, Schmitz F, Castellano-Munoz M, Rosenmund C, Montesinos ML, Sanes JR, Schneggenburger R, Sudhof TC. The synaptic vesicle protein CSP alpha prevents presynaptic degeneration. Neuron. 2004;42:237–251. doi: 10.1016/s0896-6273(04)00190-4. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Yan T, Feng Y, Zeng C, Shi X, Zhai Q. Identification of a critical site in Wld(s): essential for Nmnat enzyme activity and axon-protective function. Neurosci Lett. 2007;413:46–51. doi: 10.1016/j.neulet.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kim MD, Wen Y, Jan YN. Patterning and organization of motor neuron dendrites in the Drosophila larva. Dev Biol. 2009;336:213–221. doi: 10.1016/j.ydbio.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Jan LY, Jan YN. Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc Natl Acad Sci U S A. 2005;102:15230–15235. doi: 10.1073/pnas.0507393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lin DM, Fetter RD, Kopczynski C, Grenningloh G, Goodman CS. Genetic analysis of Fasciclin II in Drosophila: defasciculation, refasciculation, and altered fasciculation. Neuron. 1994;13:1055–1069. doi: 10.1016/0896-6273(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, Fernando FS, Tarlton A, Andressen C, Addicks K, Magni G, Ribchester RR, Perry VH, Coleman MP. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Suzuki T. Roles of molecular chaperones in the nervous system. Brain Res Bull. 2000;53:141–146. doi: 10.1016/s0361-9230(00)00325-7. [DOI] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Jan LY, Jan YN. Polycomb genes interact with the tumor suppressor genes hippo and warts in the maintenance of Drosophila sensory neuron dendrites. Genes Dev. 2007a;21:956–972. doi: 10.1101/gad.1514507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci. 2007b;30:399–423. doi: 10.1146/annurev.neuro.29.051605.112907. [DOI] [PubMed] [Google Scholar]
- Parrish JZ, Xu P, Kim CC, Jan LY, Jan YN. The microRNA bantam functions in epithelial cells to regulate scaling growth of dendrite arbors in Drosophila sensory neurons. Neuron. 2009;63:788–802. doi: 10.1016/j.neuron.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Lund FE, Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Exp Neurol. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, Yaron A. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J Neurosci. 2010;30:6375–6386. doi: 10.1523/JNEUROSCI.0922-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, VijayRaghavan K, Rodrigues V. Dendritic refinement of an identified neuron in the Drosophila CNS is regulated by neuronal activity and Wnt signaling. Development. 2010;137:1351–1360. doi: 10.1242/dev.044131. [DOI] [PubMed] [Google Scholar]
- Sugimura K, Yamamoto M, Niwa R, Satoh D, Goto S, Taniguchi M, Hayashi S, Uemura T. Distinct developmental modes and lesion-induced reactions of dendrites of two classes of Drosophila sensory neurons. J Neurosci. 2003;23:3752–3760. doi: 10.1523/JNEUROSCI.23-09-03752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner BE, Watson WE. Retraction and expansion of the dendritic tree of motor neurones of adult rats induced in vivo. Nature. 1971;233:273–275. doi: 10.1038/233273a0. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Usuda N, Murata S, Nakazawa A, Ohtsuka K, Takagi H. Presence of molecular chaperones, heat shock cognate (Hsc) 70 and heat shock proteins (Hsp) 40, in the postsynaptic structures of rat brain. Brain Res. 1999;816:99–110. doi: 10.1016/s0006-8993(98)01083-x. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yahata N, Yuasa S, Araki T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J Neurosci. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Feng Y, Zheng J, Ge X, Zhang Y, Wu D, Zhao J, Zhai Q. Nmnat2 delays axon degeneration in superior cervical ganglia dependent on its NAD synthesis activity. Neurochem Int. 2010;56:101–106. doi: 10.1016/j.neuint.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Cao Y, Hiesinger PR, Zhou Y, Mehta SQ, Schulze KL, Verstreken P, Bellen HJ. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 2006;4:2336–2348. doi: 10.1371/journal.pbio.0040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Rizzi M, Garavaglia S. Nicotinamide/nicotinic acid mononucleotide adenylyltransferase, new insights into an ancient enzyme. Cell Mol Life Sci. 2009;66:2805–2818. doi: 10.1007/s00018-009-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Zhang F, Hiesinger PR, Cao Y, Haueter CM, Bellen HJ. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature. 2008;452:887–891. doi: 10.1038/nature06721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky SL, Venkatesh TR, Teplow DB, Benzer S. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell. 1984;36:15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.