Abstract
Uniformly-sized preparations with average microbubble (MB) diameters from 1 µm to 7 µm were produced reliably by sonicating decafluorobutane-saturated solutions of serum albumin and dextrose. Detailed protocols for producing and size-separating the MBs are presented, along with the effects that changing each production parameter (serum albumin concentration, sonication power, sonication time, etc.) had on MB size distribution and acoustic stability. These protocols can be used to produce MBs for experimental applications or serve as templates for developing new protocols that yield MBs with physical and acoustic properties better suited to specific applications. Size stability and ultrasonic performance quality control tests were developed to assure that successive MB preparations perform identically and to distinguish the physical and acoustic properties of identically sized MBs produced with different serum albumin-dextrose formulations and sonication parameters. MBs can be stored at 5°C for protracted periods (2 weeks to one year depending on formulation).
Keywords: microbubble, ultrasound, contrast agent, production, sonication, cavitation
1 INTRODUCTION
Medically relevant microbubbles (MBs) are stabilized gaseous cavities with diameters in the range of 0.1 µm to 10 µm. Stabilization is achieved by encapsulating gas within shells comprised of protein [1, 2], protein plus sugar [3, 4] lipids [5–7], polymers [8, 9], or combinations of these materials. Stability is enhanced by producing MBs using heavier than air gasses [10], with fluorocarbon gasses such as octafluoropentane and n-decafluorobutane amongst those used most commonly. The primary medical use for MBs (only approved medical use in many countries) is as contrast agents for ultrasonic imaging. Commercial MB preparations are available for this purpose, including: Optison (serum albumin and dextrose), Definity (lipid), Sonovue (lipid), and Levovist (sugar); among others.
Additional medical applications for MBs are being investigated, many of which utilize their ability to interact with ultrasound to cavitate and/or produce microstreaming. These include introducing drugs, nucleic acids, nanoparticles etc. transdermally or transvascularly (sonophoresis: [11–13]) or directly into cells (sonoporation: [14–17]), and destruction of clots, thrombi and other vascular occlusions (sonothrombolysis: [18–22]). Using MBs to potentiate ultrasonic breaching of the blood-brain barrier [23–25] is a sonophoresis variation of special interest because delivering effective doses of drugs and other therapeutics into brain parenchyma is difficult. MBs are also being investigated as a means of potentiating ultrasonic tissue ablation [26–28]. Of course, consideration is also being given to safety issues and potential adverse sequelae that may ensue from MB-ultrasound interactions [29–32].
In order to facilitate the investigation and development of medical applications for MBs it is essential to better understand the MB-ultrasound interactions that make them possible. The literature is rife with theoretical [33–39] and experimental studies [40–48] concerning interactions of naturally occurring or man-made MBs with ultrasound. A small selection of these many articles is referenced to illustrate the range of MB-ultrasound topics that have been investigated. Recent review articles on MB-ultrasound interactions [49–53] and MB applications for cardiology [54–56], delivery of drugs, genes, and other therapeutics [57–62], focused ultrasound [63–65], molecular imaging [66–69], application safety [70–72] and sonothrombolysis [73–76] should be consulted for more comprehensive information on these subjects and for direction to research articles of specific interest.
Most investigators utilize commercially available MBs to investigate MB-ultrasound interactions and to study and develop non-contrast medical applications for MBs. Reasons for doing so include availability, ease of use, lot to lot consistency, or a desire to use MBs that are already approved for a clinical use. The latter will presumably facilitate translation of alternative MB-ultrasound procedures into the clinic. However, commercial MBs were developed and are quality tested as ultrasonic imaging contrast agents and may not perform satisfactorily for other MB-ultrasound applications. Consequently, studies of MB-ultrasound interaction mechanisms will be limited to adjusting ultrasound parameters because the commercial MB properties are virtually immutable. Hence, the ability to produce MBs and modify their physical and acoustic properties represents a marked advantage for pursuing MB-ultrasound mechanistic studies. There is also an economic advantage because most studies will require large quantities of MBs and commercial MBs are costly.
Some investigators do produce MBs for their studies, e.g. Keller et al. [1], Porter et al. [18], Chen et al. [77], Weller et al. [78], Borden et al. [6], and Cavalieri et al [9]. Several reports even discuss making MB preparations with narrower size distributions using differential centrifugation for size separation [79] or producing only MBs of a specific size via an extrusion process [80]. However, these studies involved making small volume and relatively low concentration MB preparations. Most reports from groups that produce MBs focus on the experimental results obtained using the MBs rather than on production methodology. Even fewer discuss how to modify production parameters or post production handling to adjust and characterize MB physical and acoustic properties and performance. Thus, it is difficult for other investigators to use these reports as guides for producing and adjusting MBs for their own MB-ultrasound studies.
Producing MBs also provides the potential to modify their properties to achieve optimal performance for: ultrasonic contrast, sonophoresis, sonoporation, sonothrombolysis and other biomedical MB-ultrasound applications. Once MB and MB-ultrasound applications are translated to the clinic both MBs and ultrasound might someday be fine tuned further to diagnose or treat a specific disease or class of patients.
This report presents detailed protocols for producing uniformly sized, n-decafluorobutane-filled, MBs with shells formed from serum albumin and dextrose. Methodologies to: rapidly determine MB concentration and size distribution, quality control test MBs for physical parameters and acoustic response to ensure lot to lot consistency, and store MBs to conserve their size and acoustic performance for at least two weeks are included.
2 MATERIALS AND METHODS
2.1 SERUM ALBUMIN AND DEXTROSE SOLUTIONS
MBs were produced using bovine or human serum albumin (SA). Stock solutions were prepared by adding lyophilized BSA (bovine serum albumin; catalog # A7906, Sigma-Aldrich Co., St. Louis, MO) to 18 MΩ. water such that the final BSA concentration was 5%, 10%, 15%, or 20% (w/V).
Human serum albumin (HSA) was purchased as a sterile 50% solution (Plasbumin-25, Talecris Biotherapeutics, Inc., Research Triangle Park, NC), which was diluted with sterile 18 MΩ. water to make stock solutions containing 5%, 10%, 15%, or 20% HSA (w/V).
Dextrose was purchased from Sigma-Aldrich Co. (G7528) to prepare stock solutions of 5%, 10%, 15%, or 20% dextrose (w/V) that were phosphate buffered (8.2 mM Na2HPO4 and 1.5 mM KH2PO4) at pH 7.4.
Sodium azide (0.1% w/V) was added to non-sterile SA and dextrose stock solutions to inhibit microbial growth.
2.2 PERFLUOROCARBON GAS
MBs were produced using n-decafluorobutane gas (perfluorobutane) (cat# APF-N2HP: FluoroMed, LP, Round Rock, TX).
2.3 SONICATOR
MBs were produced by mixing the SA and dextrose solutions (Table 1), saturating the mixture with perfluorocarbon gas, and then sonication with a 20 kHz Fisher 500 sonic dismembrator (ThermoFisher Scientific, Waltham, MA) using a 1.9 cm diameter sonic horn.
Table 1.
Microbubble Production Protocols
| MB Diameter |
Serum Albumin Solution |
Dextrose Solution |
Sonication Step 1 | Sonication Step 2 | |
|---|---|---|---|---|---|
| 1.0 µm | A | 4 ml 5% | 12 ml 5% | 450 W 70–80 sec | None |
| B | 4 ml 5% | 12 ml 5% | 150 W 20–30 sec | 400–450 W 70 sec | |
| C | 4 ml 5% | 12 ml 15% | 450 W 60–80 sec | None | |
| 2.0 µm | A | 4 ml 5% | 12 ml 5% | 175 W 30 sec | 350 W 40 sec |
| B | 4 ml 15% | 12 ml 15% | 250 W 25 sec | 350 W 40 sec | |
| 3.0 µm | A | 5.3 ml 5% | 10.7 ml 15% | 250 W 30 sec | 450 W 20 sec |
| B | 5.3 ml 15% | 10.7 ml 15% | 250 W 30 sec | 450 W 20 sec | |
| C | 5.3 ml 15% | 10.7 ml 15% | 250 W 40 sec | 450 W 30 sec | |
| 4.0 µm | A | 4 ml 5% | 12 ml 5% | 250 W 30 sec | 350 W 40 sec |
| B | 5.3 ml 15% | 10.7 ml 15% | 300 W 30 sec | 250 W 40 sec | |
| 5–6 µm | A | 4 ml 5% | 12 ml 5% | 250 W 35 sec | 250 W 45 sec |
| B | 5.3 ml 15% | 10.7 ml 15% | 300 W 25 sec | 200 W 30 sec | |
2.3.1 Sonicator Calibration
Experience demonstrated that identical settings of power input to the sonic horn could produce significantly different ultrasonic power output from different Fisher 500 sonicators. This caused difficulty in reproducing results with other Fisher 500 sonicators. Hence, a simple calorimetric test was devised to calibrate other Fisher 500 sonicators to match the power output of the one used to establish the MB protocols in Table 1. This test should also be applicable to other makes of 20 kHz sonicators because the measured endpoint of temperature rise due to ultrasonic energy absorption is directly proportional to the ultrasonic power delivered to the absorbing medium and should be independent of instrumentation.
Several 35 mL capacity polystyrene blood cell counting vials (item #A35473: Beckman Coulter Inc., Fullerton, CA) are filled with 17.76 g (16 mL) of Dow Fluid 710 (silicone oil), capped, and placed in a 25oC water bath for at least 5 min. The 1.9 cm diameter (3/4 inch diameter) sonic horn is inserted into the vial so that its tip is 4 cm from the bottom (position pre-marked on vial) and then activated at a specified power setting (50–500 W) for 5 sec. Immediately following sonication the vial is capped, the oil is mixed by gentle inversion (2–3 times) and then the oil temperature is measured by inserting the tip of a 0.35 mm diameter thermocouple into the oil 9.0 mm below its surface (position pre-marked). This process is repeated three times for each power setting. At least four different power settings are used to establish a calibration curve of achieved temperature (representing power output) versus power setting. The power setting on the sonicator to be used is then adjusted to match the power output of the sonicator used to establish the Table 1 protocols for each sonication step.
2.4 MB PRODUCTION PROTOCOLS
Table 1 lists production parameters for MB protocols that yield high numbers of microbubbles with a desired diameter MB, in the range of 1 µm to 7 µm. Some protocols require one sonication step, others two. Every Table 1 protocol was developed using BSA. The Talecris Biotherapeutics HSA was selected because it performed identically to BSA in all Table 1 protocols. MBs made with HSA are well tolerated in most domestic animals, e.g. mice, rats, and rabbits [81]. Pigs are a noted exception, and their response to HSA must be controlled medically [82]. MBs made with HSA will also be required for any studies performed using human subjects.
MBs for use in longitudinal animal studies must be prepared using sterile-filtered solutions. The sonic horn, all tubes, vials, syringes, etc. must also be sterile.
2.4.1 Mixing SA-Dextrose Solutions and Aeration with Decafluorobutane
SA and dextrose solutions are added to a 50 mL, polypropylene, plug seal cap centrifuge tube (Corning #430290). The tube is purged and filled with n-decafluorobutane gas for 4–6 seconds, capped tightly, and mixed full speed on a Vortex mixer for at least one minute. Foaming during mixing should be minimized, and the gas-mixed solution should appear opalescent. If not, more decafluorobutane is added and vortexing is continued for another 30 sec.
2.4.2 Sonication with the Sonic Dismembrator
One Sonication Step Protocols
Immediately after vortexing the SA-dextrose solution is transferred to a 40 mL ultracentrifuge tube (thin wall) that is purged with decafluorobutane gas. The sonic horn is inserted so that its tip either just touches the surface of the liquid or is 1–2 mm above it, and the sonication step is performed using the specified power setting and duration (Table 1). If necessary, the sonic horn should be moved upwards slowly to prevent overflowing the vessel as the solution’s volume increases as MBs form.
Two Sonication Step Protocols
i) Step One
The vortexed SA-dextrose solution is transferred to a 35 mL polystyrene cell counting vial (Beckman Coulter: item #A35473), the sonic horn tip is immersed midway into the solution and sonication is initiated at the prescribed power setting. If the solution becomes too turbulent and air begins to mix into it, the sonic horn must be lowered deeper into the solution until air mixing ceases. Mixing air into the SA-dextrose solution must be avoided because this yields unstable MBs.
The solution becomes hot during sonication and the vial must be held near its top (insulating glove optional) or with a holder. During, or shortly after the first sonication, the solution becomes opalescent to milky, depending upon the sonication power. This is due to light scattering by MBs and larger bubbles that have formed.
ii) Step Two
Transfer the solution to a 40 mL ultracentrifuge tube, purge it with decafluorobutane gas, and proceed as described for the single step sonication process.
2.5 MB SIZE SEPARATION
MB preparations must be size-fractionated, even if to just remove foam and very large MBs (≥10 µm diameter) that are also produced. Following the final sonication, the MB preparation tube is placed upright in a rack and 10 mL of room temperature, pH 7.4 phosphate buffered saline (PBS) are added. The tube is sealed with paraffin film, inverted gently several times to mix foam and PBS, and then returned to the rack until the foam rises and separates from the underlying liquid fraction.
A 20 mL plastic, Luer-lock syringe fitted with a blunted 1-1/2 inch long 18 g needle is inserted carefully until the needle tip touches the bottom of the tube. The lower liquid fraction (MB suspension) is drawn slowly into the syringe without taking up any foam, and a small sample is examined microscopically to estimate MB size distribution. Air bubbles are removed by orienting the syringe vertically, plunger end down, tapping it against a solid surface to free bubbles adhering to the syringe walls, and then expelling bubbles by depressing the plunger. An aspirator is used to remove large bubbles and residual foam as they emerge from the syringe tip. The syringe tip is sealed with a Luer-lock syringe cap pre-filled with PBS so that air bubbles are not introduced during capping.
Foam remaining in the 40 mL tube is re-extracted with 5–10 mL of PBS and the liquid MB suspension is examined to see if it contains MBs worth harvesting via size separation.
2.5.1 1 µm Diameter MB Preparations
Capped syringes containing 1 µm MBs made with single sonication protocols are stored plunger-up in racks, at 5°C. Larger MBs dissipate or rise to the plunger within 1 to 6 h to form a distinctly white band. The lower, milky, fraction containing the 1 µm MBs is collected. Both MB fractions must be examined to determine if size-separation was successful (90% or more 1 µm MBs in lower fraction) or if further fractionation is required.
When 1 µm MBs are made using two sonication steps 20% or more of the MBs in the initial extraction are larger than 1 µm and are size-stable. Consequently, size separation must be performed using the scheme described for larger diameter MB preparations (see below).
2.5.2 2 µm and larger Diameter MB Preparations
Syringes containing MBs are placed horizontally on a level surface and larger MBs rise quickly to form a distinctly white band (Fig. 1A). Once this band remains unchanged for a 7–10 min time span the syringe is raised to a 45° angle from the vertical (plunger up) until the white band rises to the rubber plunger (tapping syringe aids process). The syringe is then set vertically and the upper fraction spreads out across the plunger head (Fig. 1B).
FIGURE 1. Size Separation of 3 µm Diameter Microbubbles.
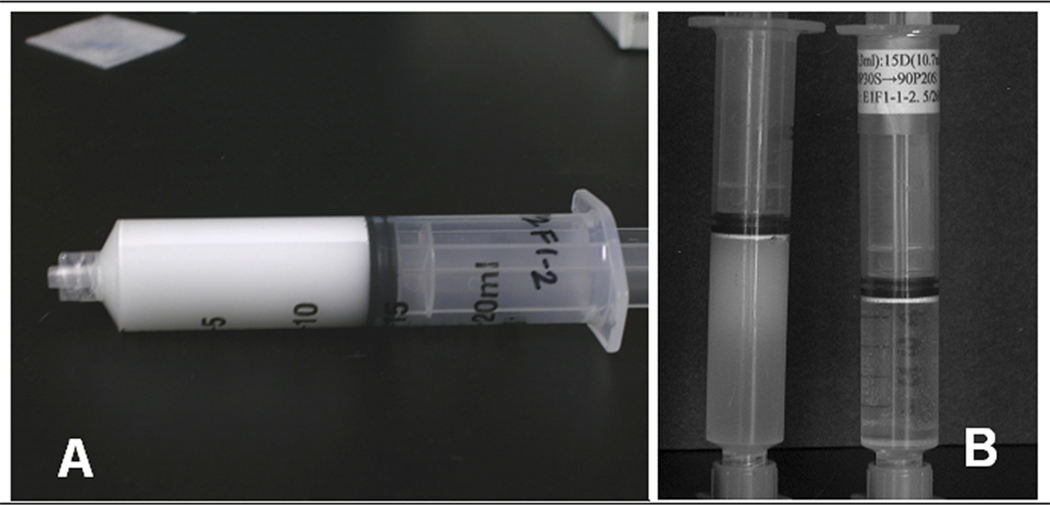
A. The white band visible along the upper surface of the horizontal syringe is formed by 3 µm diameter MBs that separated from the smaller diameter MBs (lower, grayer fraction) due to differential buoyancy during an early step in size separation.
B. The syringe to the left shows the different sized MB fractions at a later stage in size separation. The tight, white band directly adjacent to the black plunger tip is comprised of 3 µm diameter MBs. Below this is a fraction consisting of mostly 2 µm MBs along with some 1 µm and some 3 µm MBs, while the lowest fraction contains 1 µm diameter MBs. The syringe to the right shows the 3 µm diameter MBs after the final size separation step. Note that the liquid under the white band of 3 µm MBs is clear. This clear liquid is expelled and the concentrated MBs are stored in the capped, upright syringe at 5.0°C.
The lower, grayer appearing fraction (Fig. 1B) is expelled gently from the syringe into two separate sub-fractions of essentially equal volume and the upper fraction (whiter band) is re-extracted with 5–10 mL of PBS. Samples of all three fractions are examined microscopically to determine MB size distribution.
Further buoyancy separation and fractionation is generally required to yield final preparations wherein 90% or more of the MBs have diameters that are within 10–20% of a desired mean diameter, e.g. 3 µm.
2.5.3 Documenting MB Size Separation Fractions
Size-separation is a simple but involved process that produces numerous fractions from each preparation. Documenting fractionation history is essential for working out and refining protocols and for making final MB preparations with reproducible size distributions. No particular documentation strategy is recommended, but consistency is recommended.
2.6 MICROSCOPE OBSERVATION AND DIAMETER MEASUREMENT
MBs can be observed and photographed quickly using an inverted microscope by placing a drop of MB suspension onto a glass slide and allowing the MBs to rise to the liquid surface. Phase contrast optics produce the best images. Most MBs cannot be observed like this for long because they begin to degas and shrink at the air-liquid interface. Protracted observations require covering the MB suspension with a glass coverslip, using two narrow strips of glass (2 mm × 8 mm; cut from a glass coverslip) as spacers to prevent the coverslip from distorting the MBs. A standard, upright microscope can be used for observing the covered MBs.
2.6.1 Measuring MB Diameter
Good visual estimates of MB diameter can be made by comparing microscopic images to those of latex or polystyrene microsphere size standards (Beckman Coulter, Inc., Fullerton, CA; Bangs Laboratories Inc., Fishers, IN; etc.). More precise diameter measurements are made using an eyepiece micrometer calibrated with a photo etched calibration slide or using calibrated image analysis software, e.g. Image J (NIH).
2.6.2 Measuring MB Size Stability
The length of time that MBs remain at an air-liquid interface before exhibiting significant shrinkage, and the average diameter that MBs shrink to after 15 min at an air-liquid interface, are characteristics of most MB protocols. The exceptions are MBs of 1 µm or smaller because they are very size-stable and exhibit no detectable shrinkage for many hours.
A 75–100 µl aliquot of MBs is placed onto a glass slide and spread out to form a flatter film rather than a rounded droplet. MBs rise to the air-liquid interface within 0.2 to 0.6 min after which they can be imaged clearly. Phase contrast microscopy is optimal but not essential. The time required for at least 25% of the MBs to exhibit a 25–30% diameter reduction is recorded as the size transition time point (STT). After 15 min at the air-liquid interface the average size of the MBs is determined. This can be done quantitatively with image analysis software, but visual estimates are faster and remarkably accurate. It is sometimes necessary to add PBS to prevent sample drying. A 20% or greater change in the STT and/or a 30% or greater change in the 15 min shrinkage diameter indicates that MBs are markedly different than those from earlier preparations and that corrective protocol adjustments are required.
2.7 MEASURING MB CONCENTRATION
MB concentration can be measured using a cell counter [81], hemocytometer, or spectrophotometrically. Initial experiments with a Coulter Counter Z1 or Z2 (Beckman Coulter, Inc., Brea, CA) showed reduced counting accuracy for MBs smaller than 2 µm in diameter, especially with a 100 µm diameter counting orifice. Hemocytometer counts were accurate (hemocytometer inverted so MBs rise to grid) but slow. Consequently, a spectrophotometric method was developed to measure MB concentration accurately and readily (similar to measuring bacterial growth rates). MB suspensions are diluted in PBS to an estimated 530 nm optical density (OD530) between 0.3 and 2.4. OD530 is then measured and the MB concentration is determined using previously established standard curves of OD530 versus MB concentration (measured using a hemocytometer). Calibration curves were established for different diameter MBs because light scattering increases with MB diameter.
2.8 MB ACOUSTIC PROPERTIES
MBs produced from each Table 1 protocol were subjected to two tests to measure benchmark acoustic performance characteristics. These measurements were performed using two different ultrasonic systems. One was a Mettler 730 therapeutic ultrasound system (Mettler Electronics Corporation, Anaheim, CA) that produces continuous or pulsed ultrasound at 1 MHz or 3 MHz. The other used an arbitrary signal generator and 50 dB RF amplifier to drive the Mettler transducers with the driving signal adjusted to match that of the Mettler unit.
A unique aspect of the Mettler system was that it produced a timing pulse at one second intervals. The reasons for this are not intuitive and the company would not respond when asked about the purpose for this pulse.
2.8.1 MB Acoustic Durability Test
This assay measures the MBs’ ability to resist destruction during exposure to continuous wave (CW) ultrasound. A MB preparation is diluted in PBS to a concentration dependent upon MB diameter (Table 2) and 600 µl of the suspension is placed into a 32 mm long, cylindrical, clear, Mylar chamber (5.7 mm outer diameter and 5.22 mm inner diameter). One end of the chamber is sealed with a small plastic rod and the other is closed with a silicone rubber cap that has a 1.7 mm diameter hole through it. The hole serves as a pressure release port so that MBs are not subjected to a disruptive positive pressure when the chamber is capped. A clamp is attached to the plastic rod to position the chamber horizontally within a water tank (degassed water), 6.5 cm from the transducer face, and centered within an approximate plane wave ultrasound field.
Table 2.
Size Stability and Acoustic Performance Characteristics
|
Table 1 Preparation |
Size Transition Size | 15 min Steady State Diameter |
|
|---|---|---|---|
| 1.0 µm | A | N/A | 1.0 µm |
| B | N/A | 1.0 µm | |
| 2.0 µm | A | 4 min 24 sec ± 9 sec | 1.3 µm |
| B | 7 min 14 sec ± 14 sec | 1.6 µm | |
| 3.0 µm | A | 4 min 8 sec ± 15 sec | 2.1 µm |
| B | 9 min 34 sec ± 9 sec | 2.4 µm | |
| C | 14 min 15 sec ± 17 sec | 2.7 µm | |
| 4.0 µm | A | 2 min 38 sec ± 11 sec | 3.0 µm |
| B | 4 min 8 sec ± 12 sec | 3.4 µm | |
The chamber is insonated with 1 MHz sinusoidal CW ultrasound (intensity depending on MB being tested: Table 3) for 20 sec. Afterwards, the percent of MBs remaining is measured. The ultrasound intensities in Table 3 are spatial average, temporal average values.
Table 3.
Microbubble Acoustic Performnce Test Results
|
TABLE 1 Preparation |
Durability Test (Mettler) |
Destruction Threshold 1 MHz (Mettler) |
Destruction Threshold 1 MHz |
Destruction Threshold 3 MHz (Mettler) |
Destruction Threshold 3 MHz |
|
|---|---|---|---|---|---|---|
| 1 µm | A | 51% ± 2% (1MHz, 1.0 W/cm2) |
N/A | N/A | N/A | N/A |
| B | 65% ± 2% (1MHz, 1.0 W/cm2) |
N/A | N/A | N/A | N/A | |
| 2 µm | A | 23% ± 1.3% (1MHz, 0.2 W/cm2) |
0.3 W/cm2 | 0.2 W/cm2 | 0.7 W/cm2 | 0.6 W/cm2 |
| B | 36% ± 2% (1MHz, 0.2 W/cm2) |
0.4 W/cm2 | 0.3 W/cm2 | 0.8 W/cm2 | 0.6 W/cm2 | |
| 3 µm | A | 36% ± 2% (1MHz, 0.1 W/cm2) |
Timing pulse | 0.1 W/cm2 | 0.2 W/cm2 | 0.2 W/cm2 |
| B | 43% ± 3% (1MHz, 0.1 W/cm2) |
Timing pulse | 0.2 W/cm2 | 0.4–0.5 W/cm2 | 0.3 W/cm2 | |
| C | 52% ± 3% (1MHz, 0.1 W/cm2) |
0.1 W/cm2 | 0.2 W/cm2 | 0.6–0.7 W/cm2 | 0.4 W/cm2 | |
| 4 µm | A | 45% ± 1.3% (1MHz, 0.1 W/cm2) |
Timing pulse | 0.1 W/cm2 | 0.5 W/cm2 | 0.6 W/cm2 |
| B | 52% ± 0.7% (1MHz, 0.1 W/cm2) |
Timing pulse | 0.1 W/cm2 | 0.6 W/cm2 | 0.6 W/cm2 | |
2.8.2 MB Destruction Threshold Assay
This assay determines the pulsed ultrasound intensity required to completely lyse a MB stream after traversing 5 mm. MBs are suspended in PBS at a concentration of 1.1 × 108 MBs/mL and pumped through a 3 mm diameter orifice at 0.5 mL/min into a vertical, acoustically transparent Mylar tube (5.2 mm I.D., 0.2 mm thick wall) pre-filled with PBS. Sonication is performed in a tank filled with degassed water at room temperature. Sinusoidal, pulsed ultrasound (1 or 3 MHz) is delivered using a pulse duration of 2 msec and a pulse repeat frequency of 100 Hz (20% duty factor). The temporal average, spatial average intensity is increased until the MB stream remains visible to the human eye as it travels across a distance of 5mm, but then becomes undetectable (by eye) immediately thereafter. An acoustic absorber is used to shield the MBs prior to entering the Mylar tube to prevent premature lysis.
2.9 MB STORAGE
Following size-separation MBs are permitted to rise within a capped syringe (room temperature or 5°C) to form a tight concentrated band against the plunger (Fig. 1B). With the syringe vertical (tip down) the plunger is depressed to move the MB band towards the syringe tip expelling almost all underlying PBS. The syringe is capped and stored vertically at 5°C.
MBs larger than 1 µm rise quickly (0.5–4 h). The 1 µm diameter MBs require 9–16 h to rise, but centrifugation can hasten the process (see below).
Nonsterile MB preparations have been stored at 5°C for more than 6 months without bacterial contamination. Concentrated sodium azide (1%–5% in PBS) can be added to a MB suspension for a final azide concentration of 0.05–0.1% prior to concentrating the MBs to insure against microbial infection.
2.9.1 Concentrating 1 µm MBs by Centrifugation
MBs are drawn up into a syringe with the upper 3/4ths of the plunger shaft cut off, enabling the syringe to spin in a centrifuge with a swinging bucket rotor. The plunger shaft need not be cut for a fixed-angle rotor. The syringe is spun at 800–1,200 rpm (110–250 rcf) for 12–20 min: The exact centrifugation speed and duration need to be determined empirically. After the MBs have risen the PBS is expelled and the concentrated MBs are stored. Needle-nose pliers are used to move cut plunge shafts along the syringe barrel.
3 RESULTS and DISCUSSION
Table 1 protocols consistently produced preparations enriched with the desired diameter MBs that were then isolated successfully by size fractionation. More than 97% of the preparations met the quality control test benchmarks for MB size stability and acoustic properties characteristic of each protocol in Table 1 Those that did not were discarded.
Experiences with selected MB protocols are presented to illustrate how changing each production parameter affects MB size, size stability, and/or acoustic performance. This information will enable investigators to use Table 1 protocols as templates to develop new protocols to achieve desired changes in MB size and/or performance or to correct for variations in MB characteristics and performance caused by changing SA or decafluorobutane lots, etc.
3.1 SONICATOR POWER PERFORMANCE
Power test results for the Fisher 500 sonicator used to establish Table 1 protocols are presented in Fig. 2. If power test results for another sonicator differ significantly from that of Fig. 2 then its power settings must be adjusted to deliver the same power output (temperature increase) as the sonicator used to produce Fig. 2 for each sonication step in Table 1. Otherwise, MBs produced using the Table 1 protocols will have different properties than those presented herein. All Fisher 500 sonicators subjected to the power test (four) displayed a linear temperature rise versus power setting relationship. Hence, any power adjustment factor is simply the ratio of the slope of the power test for the sonicator being tested to that of the power test graph in Fig. 2.
FIGURE 2. Temperature Increase in Silicone Oil 710 Induced by Sonication.
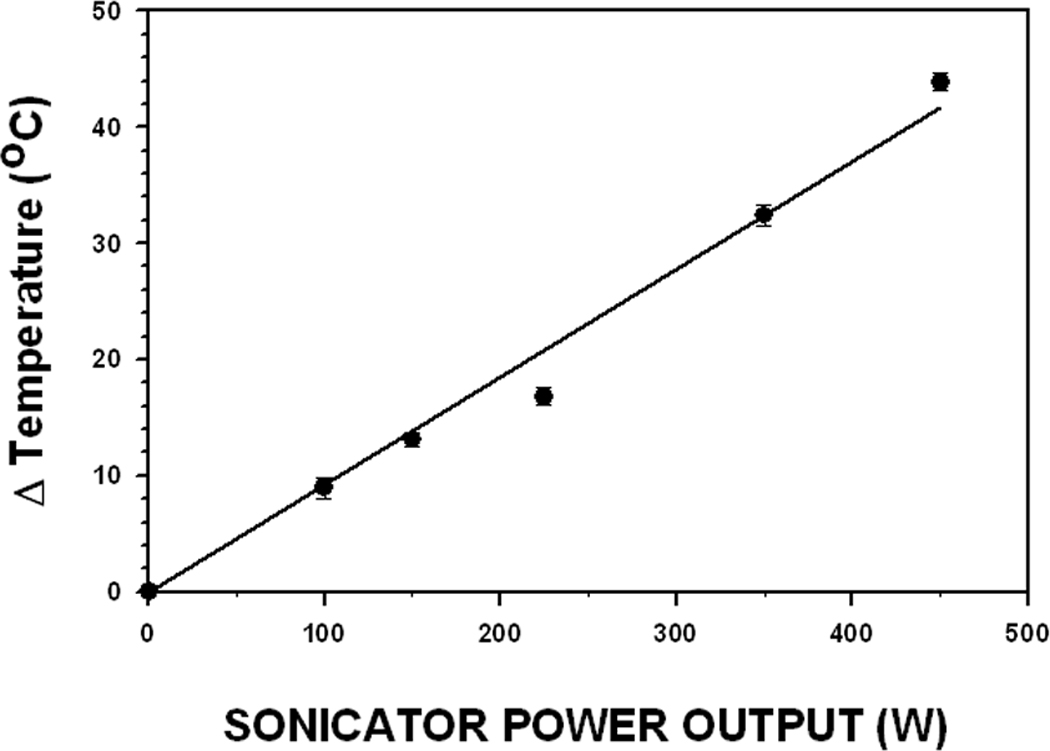
The 1.9 cm diameter sonicator horn was inserted into the tube of Dow Corning Silicone fluid (oil) 710 as described in the Materials and Methods section. The temperature increase induced by the 5 sec sonication treatment is plotted as a function of the sonicator’s power setting, which indicates electrical power input to the sonic horn. The recorded temperature increase within the silicone oil is indicative of the resultant ultrasonic power output from the sonic horn. Each data point represents the mean value of three measurements.
3.2 EFFECTS OF MATERIALS AND ENVIRONMENT ON MB PRODUCTION
Using a new lot of SA or decafluorobutane typically resulted in a slight change in size distribution, size stability, and/or acoustic performance for MBs produced using most Table 1 protocols. No variations were ever observed following a change in dextrose lot or after making new dextrose stock solutions.
3.2.1 Serum Albumin-Related Variability
Changing SA lots usually required adjusting protocol parameters slightly to achieve MB production results comparable to those obtained using earlier SA lots. The reasons for this are unknown, but are likely related to variations in SA production and/or lot to lot variations in contaminants.
If MBs produced with a new SA lot are smaller, less size stable (shorter STT), and/or acoustically less durable, one or more of the following steps rectified the problem: 1) increasing the final SA concentration by 3–6% (the most commonly effective correction) 2) increasing the first sonication step power by 5–15%, 3) decreasing the second sonication step power by 10–20%, or 4) increasing the duration of the first or second sonication step by 5–15%. The magnitude of each adjustment was determined empirically. Once production of acceptable MBs was re-established the adjusted protocols remained effective throughout subsequent use of the new SA lot.
Converse adjustments were made when MBs were larger and/or stronger than those made with an earlier SA lot.
3.2.2 Decafluorobutane Gas-Related Variability
Changing decafluorobutane gas lots sometimes affected MB size distribution, stability, and acoustic performance. Protocol adjustment strategies to re-achieve prior results are the same as those discussed for new SA lots; however, smaller adjustments were required. It is recommended strongly that SA and decafluorobutane lots are never changed concomitantly!
3.3 MB SIZE SEPARATION AND SIZE STABILITY
Figure 3 illustrates size-separated MBs with mean diameters of 1.0 µm (Fig. 3A), 2.0 µm (Fig. 3B), 3.0 µm (Fig. 3C), 4.5 µm (Fig. 3D), and 6.5 µm (Fig. 3E). Combining several preparations before size separation markedly reduced both MB loss and the variance from the mean MB diameter to yield preparations with narrower size distributions.
FIGURE 3. MB Preparations with Different Mean Diameters.
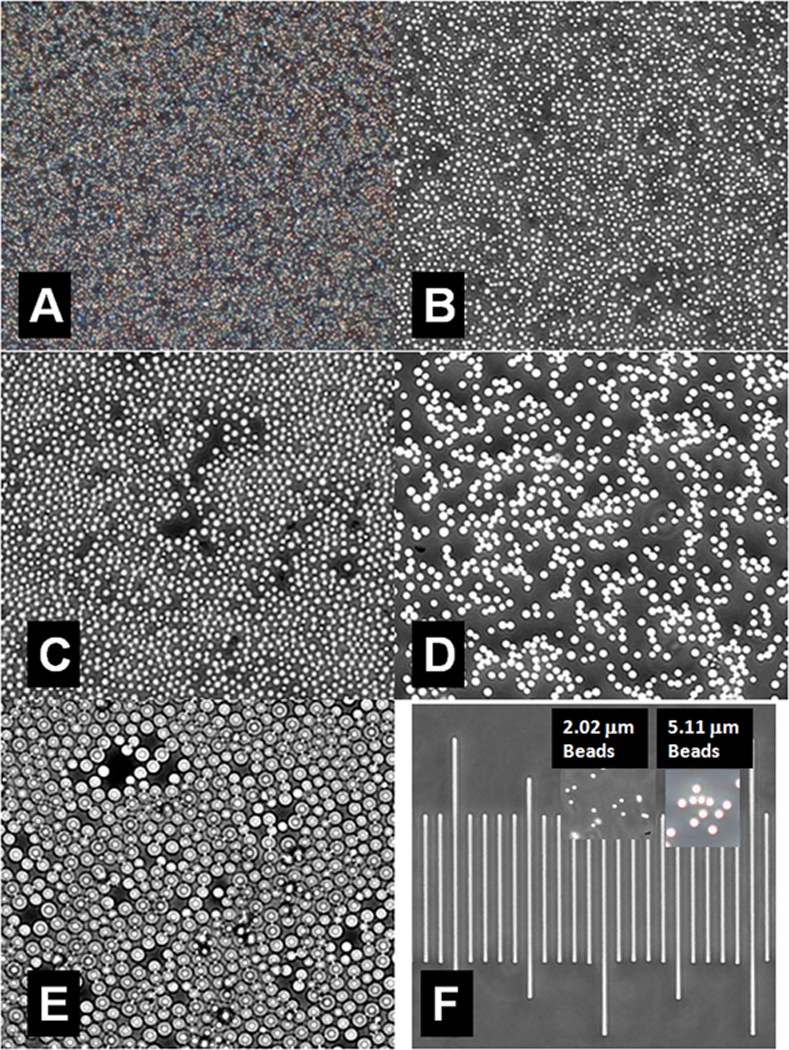
A. 1.0 µm Average Diameter.
B. 2.0 µm Average Diameter.
C. 3.0 µm Average Diameter
D. 4.5 µm Average Diameter
E. 6.5 µm Average Diameter
F. Microbead standards and calibration scale (distance between shortest lines is 10.0 µm)
Typical size distributions for 1 µm and 3 µm diameter MB preparations are presented, respectively, in Fig 4A and 4B. Preparations can be produced with smaller standard deviations if necessary. However, obtaining more homogeneous size distributions required more size separation steps and involved greater MB loss.
FIGURE 4. Size Distributions for 1 µm diameter (A) and 3 µm (B) diameter Microbubbles.
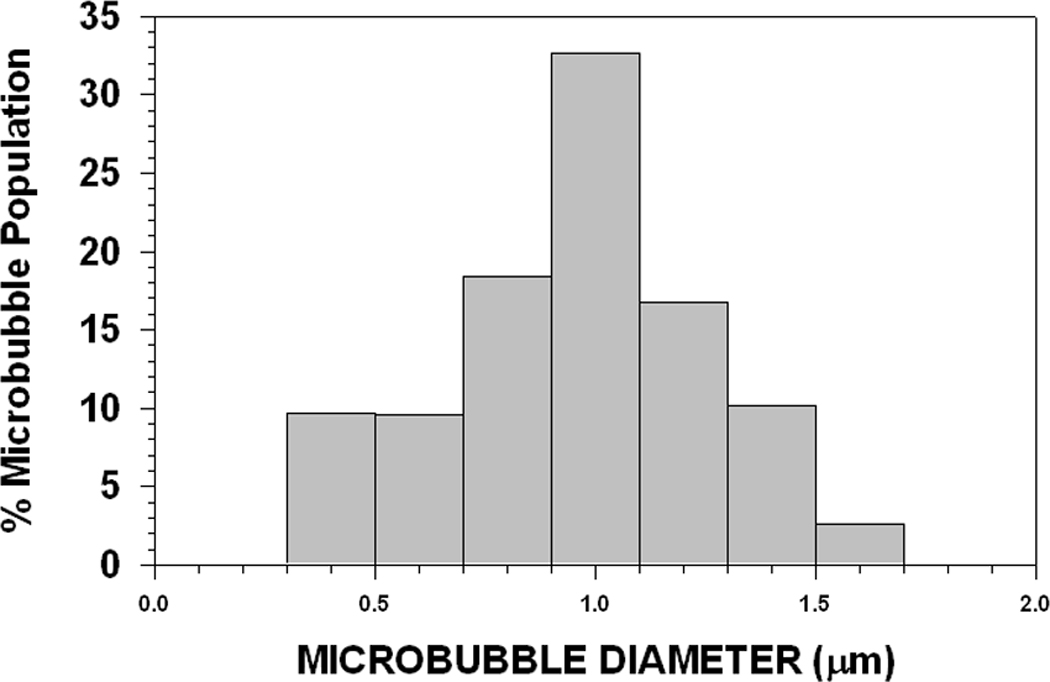
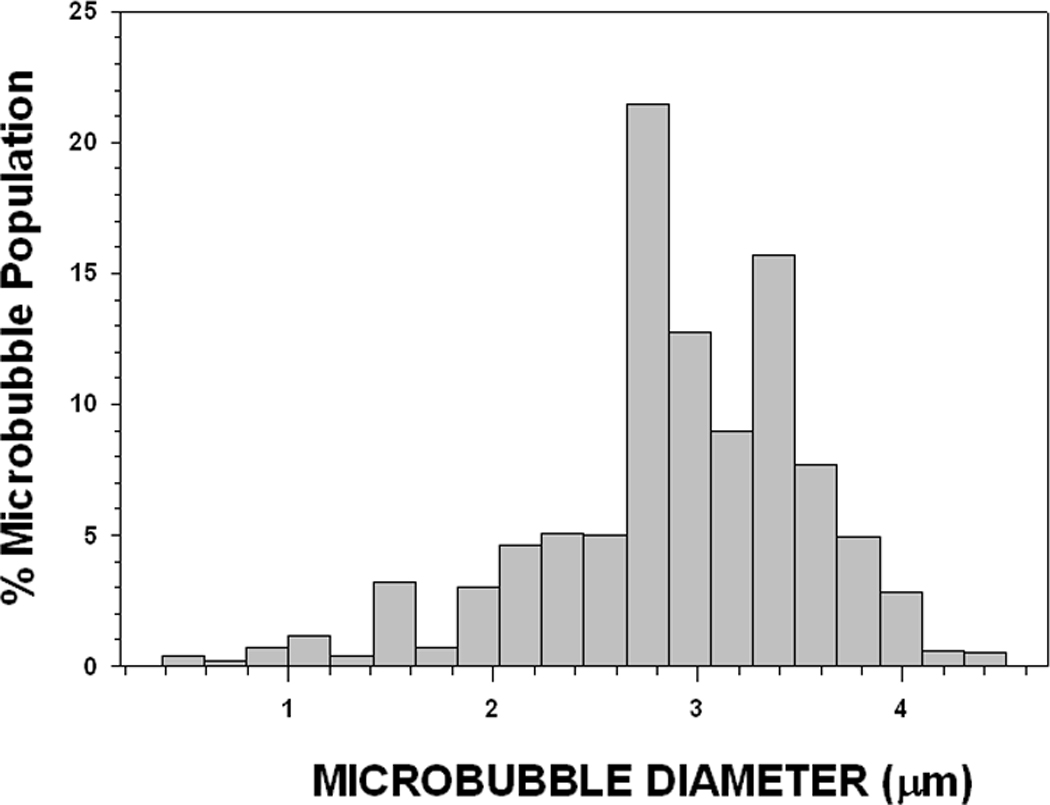
These are typical size distributions for 1 µm diameter (A) (1.0 µm ± 0.3 µm: 9,313 counts) and 3 µm (B) diameter (3.0 µm ± 0.6 µm: 1,680 counts) MB preparations following size separation. Narrower size distributions are attainable, but require additional size fractionation steps and result in a significant reduction in MB yield.
3.3.1 1µm Diameter MBs
The 1 µm diameter MBs were easiest to produce and were extremely size stable during more than one year of storage. They could even be air dried on a glass slide, stored for up to two years, and rehydrated to yield still intact and functional MBs.
Some 1 µm diameter MBs were present in all preparations, but single sonication step protocols using lower SA concentrations (1.0% to 2% final concentration: Table 1, Table 1 µm preparation A) gave the highest yields (6–7×1010 MBs per 16 mL preparation). Larger MBs in these preparations separated out rapidly due to their greater buoyancy and then lysed shortly after production because their shells were weak and unstable. More extensive size separation was required to isolate 1 µm MBs from preparations made with higher SA concentrations or with two sonication steps because larger diameter MBs were now more size stable.
3.3.2 Larger Diameter MBs
Preparations of larger diameter MB preparations yielded fewer MBs (3–6 × 109 MBs for 3 µm diameter preparations). A general rule of thumb was that the number of MBs produced relative to the yield of 1 µm MBs was inversely proportional to the cube of the MB radius.
MBs with diameters in the range of 2 µm to 3 µm were the most difficult to produce, size separate, and were the least stable with regard to maintaining size during protracted storage at 5.0oC. MBs in the range of 4–7 µm in diameter were the easiest to size separate (fast rising) and were surprisingly stable with respect to maintaining size during protracted storage.
3.4 SIZE STABILITY RESULTS
Table 2 lists the size stability test results for selected MB preparations. Values for the STT and the average MB diameter following 15 min at an air-liquid interface serve as first indicators that a MB preparation was comparable to earlier preparations made using the same protocol. More importantly, a significant deviation from these values was a strong, early indicator that something was different with the new MB preparation.
3.5 MEASURING MB CONCENTRATION BY OD530
Counting MBs smaller than 2 µm in diameter was inaccurate using a Coulter Counter model Z1 or Z2 with either a 100 µm or 50 µm diameter counting orifice. Hemocytometer counts were accurate for all MB diameters that could be observed with a microscope, but this process was slow and laborious.
Measuring MB concentration spectrophotometrically was very fast, accurate, and reproducible. The relationship between OD530 and MB concentration was linear and the same whether MBs were counted with a cell counter or hemocytometer. Figures 5 and 6 show how the OD530 versus MB concentration curves varied significantly with MB diameter.
FIGURE 5. Optical Density at 530 nm vs Concentration for 1µm Diameter Microbubbles made using Different Preparation Protocols.
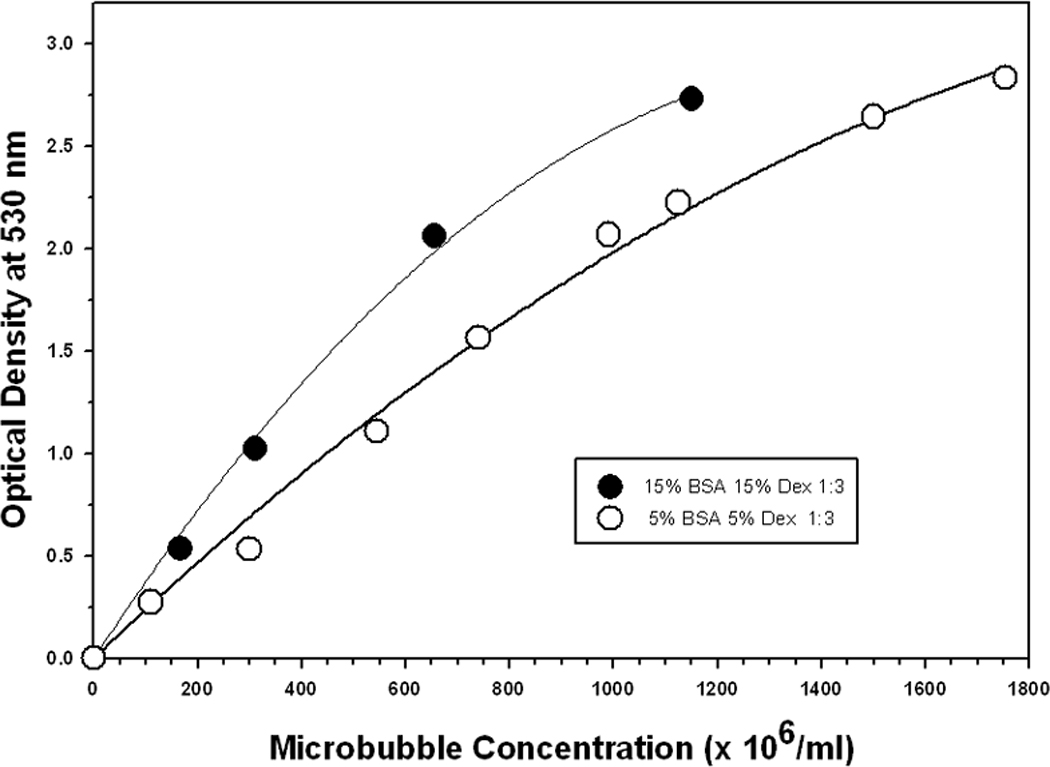
FIGURE 6. Optical Density at 530 nm vs Concentration for Larger Diameter Microbubbles (error bars are smaller than the symbols).
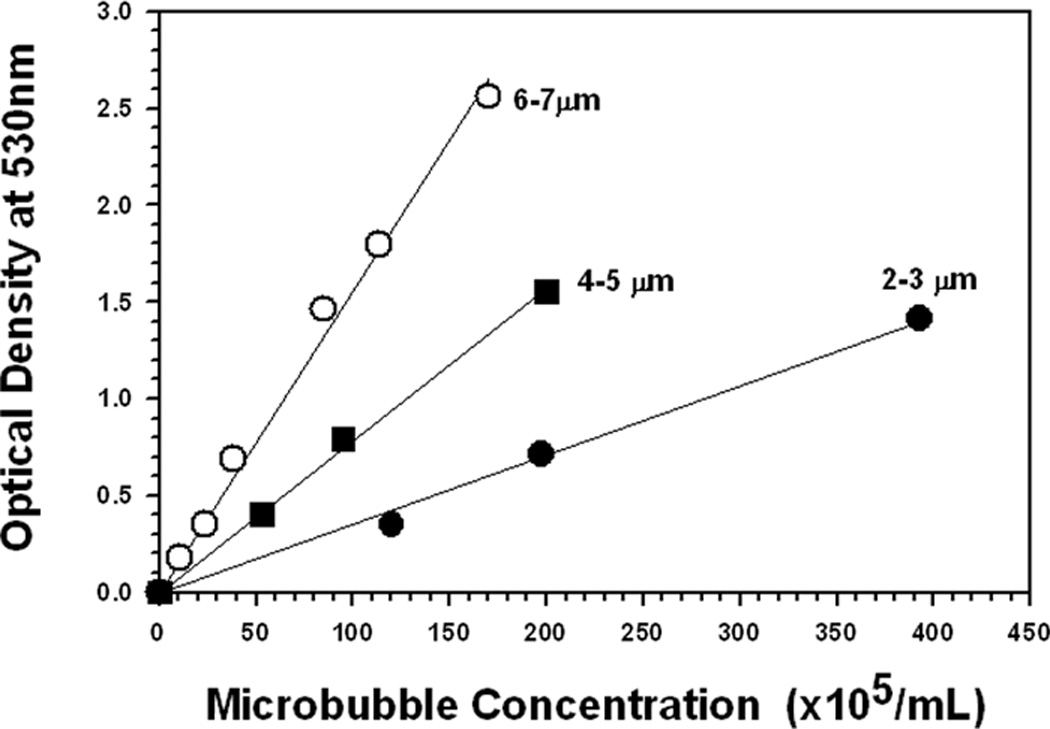
Acceptable concentration measurements were made using OD530 values in the range of 0 to 2.4, but OD530 values lower than 2.0 resulted in greater accuracy. Consequently, highly concentrated MB preparations were serially diluted until their OD530 reading was lower than 2.0 and the resultant concentration value was adjusted for the dilution.
3.6 ACOUSTIC DURABILITY AND DESTRUCTION THRESHOLD TEST RESULTS
The ultrasound durability and destruction threshold measurements for Table 1 MBs are presented in Table 3. Both of these measurements were very reliable indicators that new MB preparations had acoustic characteristics similar to those of earlier preparations made using the same protocol. These acoustic assays complemented the size stability assays to provide quality control benchmarks to ensure batch to batch consistency in physical and acoustical parameters for produced MBs. If test results for a new MB preparation deviate substantially from the values listed in Tables 2 and 3 that preparation should be discarded.
Unfortunately, the acoustic durability assay could not be used successfully with MBs with diameters of 5 µm or larger. These larger MBs rose rapidly to the top surface of the Mylar insonation chamber and formed tight clusters almost immediately after the ultrasound was turned on; possibly related to the ultrasound-induced MB coalescence described by Postema et al. [83]. This clustering led to inconsistent test results, possibly due to wide variations in MB shielding effects and other ultrasound induced MB-MB interactions.
The Mettler system could not be used for the destruction threshold assay, at 1 MHz, with several MB preparations because its inherent timing pulse was sufficient to destroy the MBs before they traveled 5 mm. The timing pulse could be used as a crude benchmark for certain MBs. For example, if the timing pulse cannot destroy the 0.3 µm protocol B MBs after traveling 5 mm then that preparation is more resistant to ultrasonic lysis than it should be.
3.7 MODIFYING MB SIZE DISTRIBUTION AND ACOUSTIC PERFORMANCE
Experience with manipulating production parameters to develop new MB protocols revealed general trends concerning how changing each parameter produced desired changes in MB size distribution, size stability, and acoustic performance. Understanding how adjusting each protocol parameter affects MB physical and acoustical properties is the most crucial aspect of producing MBs and developing new production protocols.
3.7.1 Modifying Serum Albumin and Dextrose Concentrations
Increasing SA concentration can: improve MB size-stability, prolong storage time, make MBs more resistant to ultrasonic lysis, or shift the size distribution to larger diameter MBs. Small increases in SA concentration (3–10%) improved size stability and storage time without affecting MB size distribution and acoustic performance. Greater increases in SA concentration shifted the MB size distribution to larger diameters and also made the larger diameter MBs more resistant to ultrasonic lysis. Converse changes resulted when SA concentration was reduced.
Changing the dextrose concentration had similar effects on MB properties. However, dextrose concentration changes of 1.5 to 2.0 fold were required to produce any detectable changes in MB size, size stability, or acoustic performance.
3.7.2 Changing Power and Duration in Single Sonication Protocols
Increasing the power for single sonication protocols by 5–15% improved MB size stability and increased the storage time for larger diameter MBs. Larger increases in sonication power further enhanced MB size stability and also increased MB resistance to ultrasonic destruction. Increasing the sonication time also made MBs more size-stable but tended to reduce MB size. Thus, MBs can be made more size stable and more resistant to ultrasonic destruction with little or no change in diameter by increasing both the sonication power and duration. Conversely, more stable, larger diameter MBs can be produced by increasing the sonication intensity and shortening sonication time.
3.7.3 Modifying Sonication Parameters for Multiple Sonication Protocols
Using two sonication steps was the best way we found to produce size-stable MBs larger than 1 µm. The first sonication step produced some bubbles and MBs, which accounted for solution’s increase in opacity; but MBs larger than 1 µm were not size-stable and quickly started shrinking. However, the first sonication made it possible to produce greater numbers of larger diameter MBs during a second sonication. These larger MBs were size stable, could be separated into uniformly sized fractions and stored long term.
Increasing the power and/or duration of the first sonication yielded a greater number of larger diameter MBs during the second sonication and shifted the size distribution towards the larger diameters. Resultant MBs were also more resistant to ultrasonic lysis. However, there were thresholds beyond which increasing first sonication power and/or duration produced fewer, smaller, and less stable MBs. Such threshold levels had to be determined empirically for each SA-dextrose formulation.
Increasing the power of the second sonication step decreased average MB size while increasing the duration of the second sonication step increased MB size stability. Thus, the first sonication step alters the SA-Dextrose solution such that larger diameter, stable MBs can be produced while the second sonication determines MB size distribution. The magnitude of size stabilization achievable by increasing the second sonication duration was significantly less than the effects achieved by modifying first sonication parameters.
The most effective way to increase MB size stability without altering size distribution was to increase the power and/or duration of both the first and second sonication steps. The tendency to increase MB size with greater power and/or duration during the first sonication step was offset by the tendency to decrease size using more power for the second sonication. Extending the duration of both sonication steps contributed to better size stability, longer storability, and greater resistance to ultrasonic destruction. The converse adjustments are made to decrease MB size and/or stability.
3.7.4 Production Trends with 3 µm MBs
The 3 µm diameter MBs were the most difficult to produce, size separate, and make stable for protracted storage and resisting ultrasonic lysis. Hence, more details on protocol adjustments required to produce and modify 3 µm MBs will provide better insight into how similar procedural changes will affect MBs with other diameters.
Table 1 shows the parameter changes required to translate protocols for making 2 µm MBs to ones that produced more 3 µm diameter MBs. The 2 µm A protocol was modified by increasing the dextrose solution from 5% to 15% and increasing the first sonication power from 175 W to 250 W. These two changes produced more, larger diameter MBs that were size-stable and more resistant to ultrasonic lysis. The second sonication power increase from 350 W to 450 W and the concomitant decrease in duration from 40 sec to 20 sec shifted the MB size distribution peak from 2 µm to 3 µm to yield the 3 µm A protocol in Table 1.
The 2 µm B protocol was modified into the 3 µm B protocol by increasing the concentration of serum albumin (4.0 mL 15% albumin to 5.3 mL 15% albumin), lengthening the first sonication by 5 sec, and increasing the power of the second sonication from 350 W to 450 W. The serum albumin increase was likely the most significant change for making more stable, larger diameter MBs, and was supported by the slight increase in first sonication duration. Initial tests showed that modifications of the first sonication produced a high yield of 4 µm diameter MBs (data not shown), which is why the second sonication power was increased from 350 W to 450 W to produce more 3 µm diameter MBs.
Increasing the duration of both the first and second sonications to, respectively, 40 sec and 30 sec yielded the 3 µm C protocol from the 3 µm B protocol. As expected, MB size was unchanged while size stability, storability, and ultrasonic stability (Tables 2 and 3) increased.
3.8 CONCLUDING REMARKS
Table 1 protocols were used to reproducibly make preparations enriched with MBs of a selected diameter that were then size-separated into fractions with a narrow MB size distribution about the selected mean diameter. MB size distribution could be made more narrow using additional size fractionation steps, but yield was reduced. The quality control (QC) assays developed to characterize MB size stability and acoustic performance were used successfully to confirm that MB preparations achieved the benchmarks characteristic for each MB protocol, within acceptable tolerance ranges. Unacceptable MB preparations can thus be identified and excluded to ensure batch to batch consistency of MB performance. The acoustic performance assays were designed strictly as quality control indicators for MB production reproducibility and are not intended for assessing MB performance for specific applications such as ultrasonic image contrast agents, sontothrombolysis, sonoporation, etc.
Producing MBs using Table 1 protocols requires ensuring that the ultrasonic power output of any 20 kHz sonicator used for MB production matches that of the Fisher 500 sonicator used to develop the Table 1 protocols. Running the sonicator power output test prior to beginning MB production will accomplish this by identifying the proper instrument settings for electrical power input to the sonic horn required to produce the required ultrasonic power output (measured colorimetrically) for each sonication step in MB production. This proved true for four different Fisher 500 20 kHz sonicators. This test should also work for other makes of 20 kHz sonicators using a 1.9 cm diameter acoustic horn because the measured temperature rise is indicative of ultrasonic power output from the sonic horn, which will be independent of the sonicator used. What is instrument dependent, and must be varied accordingly, is the electrical power that must be delivered to the sonic horn to produce the required ultrasonic power output.
The methods for testing MB physical and acoustic properties presented herein are rudimentary and are intended only as a means of ensuring batch to batch consistency for MB production. Additional and more sophisticated physical and acoustical measurements will most certainly be needed to explicate MB performance in MB-ultrasound experiments, model their behavior in response to ultrasound, and to utilize them effectively in experiments and other applications. Potential measurements include measuring the MBs’ Young modulus using atomic force microscopy [84], determining their acoustic collapse thresholds [46], and measuring MB shell thickness [9, 85].
The generalized schemes for modifying MB size and/or acoustic performance were compiled empirically while developing the Table 1 protocols. These schemes have been used consistently and effectively to develop new MB protocols using Table 1 protocols as templates, and to make the minor protocol changes required to compensate for lot to lot variations in materials (SA or decafluorobutane) and other factors to continue producing MBs with consistent physical and performance characteristics. Those attempting to produce MBs will need to employ these schemes immediately since they will be using different SA lots than those used to produce the Table 1 protocols. The recommendation is to begin using Table 1 protocols to produce MBs, test them to determine how their physical and acoustical characteristics differ from what is expected (Table 2 and Table 3), and then make the appropriate changes in protocol parameters to achieve expected results.
Once the Table 1 parameters have been adjusted successfully, the protocol adjustment schemes can be used to develop new MB protocols and to optimize MB performance for specific applications. Ostensibly, the preparation parameters (SA and dextrose concentrations, sonication intensities and duration, etc.) can be adjusted into innumerable combinations that go well beyond Table 1 protocols to produce a myriad of different MBs that encompass a wide range of physical and acoustic properties.
MBs larger than 2 µm in diameter could be produced using a single sonication step, but these were not very size stable and could not be stored for more than a few days without considerable changes in their physical and acoustic properties. Using two sonication steps clearly produced MBs that were more stable with respect to their performance characteristics and storage. The reasons for this are unknown. Significant heat was generated during the first sonication step and this might have denatured the SA such that MBs formed during the second sonication were stabilized. Differential scanning calorimetry [86] or some other protein denaturation assay will be required to make this determination. Another possibility is that SA denaturation and/or transient chemical species that formed during the first sonication, e.g. free radicals, produced SA-SA or SA-dextrose interactions that contributed to producing more stable MB shells during the second sonication.
Interestingly, different MB preparations with the same mean diameter exhibited significantly different size stabilities and acoustic performances. This demonstrated that other factors besides size, e.g. MBs shell strength and elasticity, affect MB stability and performance. Further investigation is required to identify, with certainty, which physical properties of the MBs determine their stability and acoustic performance and to quantify these. Such information is necessary to identify MB characteristics that are best suited for different applications, e.g. imaging, sonothrombolysis, sonophoresis, sonoporation etc., and then develop new MBs with these characteristics. Additionally, MB preparations with different average sizes and/or acoustic properties can be mixed in defined ratios to yield “customized”, multivariate preparations that might perform certain tasks better than those with more uniform size distributions and acoustic performance.
The observation that size-separated MBs shrink uniformly when maintained at an air-liquid interface suggests another means for adjusting MB size and acoustic performance. It is presumed that shrunken MBs will have thicker shells because the shell diameter will have changed while the shell mass remains constant. Because MBs shrink to assume a new steady state diameter, some gas must pass out from the MBs. Presumably, the lighter gasses, viz. those in air, will leak out before any decafluorobutane. Hence, shrunken MBs should be richer in the denser decafluorobutane. Both the thicker shell and greater decafluorobutane content should make the shrunken MBs respond differently to ultrasound, and they would likely be more resistant to ultrasonic lysis than other MBs with the same diameter. This possibility is being tested. One possibility for increasing MB diameter after they are produced is to sonicate them in a controlled manner to increase their diameter via rectified diffusion [87, 88]. While possible conceptually, the practicality of increasing MB diameter uniformly throughout the population and reproducibly needs to be tested.
MB concentration is an important parameter for any experiment or application. Consistently accurate MB counts were obtained with the hemocytometer. However, difficulty was experienced counting and obtaining size distributions for MBs smaller than 2 µm with Coulter Counter models Z1 or Z2, using either a 100 µm or 50 µm diameter counting orifice. The 50 µm counting orifice is the smallest made for these counter models, and the manufacturer states that the smallest sized particles that can be counted are 1–2 µm. Consequently, MBs with diameters of 2 µm and smaller are near the lower detection limit of these instruments, which is consistent with the difficulties in obtaining counts and size distributions with these smaller MBs. The spectrophotometric method for determining MB concentration using OD530 was much faster than the hemocytometer and just as accurate as the hemocytometer or cell counter. This method for determining MB concentration should be serviceable and effective since most laboratories have a spectrophotometer, or access to one.
Measuring MB size distribution using image analysis software was applicable to all MBs, even those significantly smaller than 1 µm in diameter (Fig. 4A). Since most laboratories have a digital photographic microscope or ready access to one, this methodology for determining MB size distributions is recommended.
Surprisingly, MB QC analyses could be performed consistently using either the inexpensive Mettler therapeutic ultrasound unit or the more expensive system comprised of a random signal generator and a 50 dB RF amplifier. Although the values of the measured benchmarks obtained with the two ultrasound systems were slightly different, both systems were consistent internally. Thus, either system will serve for measuring MB ultrasonic performance for QC purposes. The Mettler system may not be suitable for all MB-ultrasound experiments, but this will have to be determined empirically for each experiment and experimental set up.
The protocols and procedures presented in this report will provide sufficient information to begin producing and QC testing SA-dextrose MBs. Obtaining expertise in producing MBs and modifying MB protocols requires practice, persistence, and observational acuity. Experience has shown that novitiates to MB production and development start out slowly because they make more frequent observations and measurements in order to become more familiar with the sounds (pitch and intensity of the cavitation sounds during sonication), feel (vibration of the tubes and temperature increases), and sights (foam formation and turbulence) associated with the process. Experience quickly leads to faster MB production, more precise and accurate size separation and QC assessment, and improved success with adjusting protocol production parameters to modify MB size and acoustic properties.
It is hoped that this manuscript instills interest in MB research and facilitates execution of new and ongoing studies. More detailed protocols for producing MBs and details for building the exposure chambers will be supplied upon request after contacting the authors.
ACKNOWLEDGEMENTS
This research was supported by NIH Grants R01CA99178 (MJB), R37EB002641 (WDO, MLO), and R01HL82481 (WCC).
ABBREVIATIONS
- BSA
bovine serum albumin
- CW
continuous wave
- HAS
human serum albumin
- MBs
microbubbles
- PBS
phosphate buffered saline
- QC
quality control
- SA
serum albumin
- STT
size transition time point
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
REFERENCES
- 1.Keller MW, Segal SS, Kaul S, Duling B. The behavior of sonicated albumin Microbubbles within the microcirculation: a basis for their use during myocardial contrast echocardiography. Circ. Res. 1989;65:458–467. doi: 10.1161/01.res.65.2.458. [DOI] [PubMed] [Google Scholar]
- 2.Grinstaff MW, Suslick KS. Air-filled proteinaceous microbubbles: Synthesis of an echo-contrast agent. Proc. Nat. Acad. Sci. USA. 1991;88:7708–7710. doi: 10.1073/pnas.88.17.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter TR, Xie F, Kricsfeld A, Chiou A, Dabestani A. Improved endocardial border resolution during dobutamine stress echocardiography with intravenous sonicated dextrose albumin. J. Am. Coll. Cardiol. 1994;23:1440–1443. doi: 10.1016/0735-1097(94)90389-1. [DOI] [PubMed] [Google Scholar]
- 4.Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, Grayburn PA. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000;101:2554–2556. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 5.Simon RH, Ho SY, D'Arrigo J, Wakefield A, Hamilton SG. Lipid-coated ultrastable microbubbles as a contrast agent in neurosonography. Invest. Radiol. 1990;25:1300–1304. doi: 10.1097/00004424-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Borden MA, Kruse DE, Caskey CF, Zhao S, Dayton PA, Ferrara KW. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2005;52:1992–2002. doi: 10.1109/tuffc.2005.1561668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmann C, Klibanov AI, Olson TS, Sonnenschein JR, Rivera–Nieves J, Cominelli F, Ley KF, Lindner JR, Pizarro TT. Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology. 2006;130:8–16. doi: 10.1053/j.gastro.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Wheatley MA, Schrope B, Shen P. Contrast agents for diagnostic ultrasound: development and evaluation of polymer-coated microbubbles. Biomaterials. 1990;119:713–717. doi: 10.1016/0142-9612(90)90033-m. [DOI] [PubMed] [Google Scholar]
- 9.Cavalieri F, El Hamassi A, Chiessi E, Paradossi G. Stable polymeric microballoons as multifunctional device for biomedical use: Synthesis and characterization. Langmuir. 2005;21:8758–8764. doi: 10.1021/la050287j. [DOI] [PubMed] [Google Scholar]
- 10.Porter TR, Xie F, Li S. Differences in myocardial contrast produced with transient response imaging when using intravenous microbubbles containing gases of different molecular weight. Echocardiography. 1997;14:441–446. doi: 10.1111/j.1540-8175.1997.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 11.Bommannan D, Okuyama H, Stauffer P, Guy RH. Sonophoresis. I. The use of high-frequency ultrasound to enhance transdermal drug delivery. Pharm. Res. 1992;9:559–564. doi: 10.1023/a:1015808917491. [DOI] [PubMed] [Google Scholar]
- 12.Mitragotri S, Farrell J, Tang H, Terahara T, Kost J, Langer R. Determination of threshold energy dose for ultrasound-induced transdermal drug transport. J. Control. Release. 2000;63:41–52. doi: 10.1016/s0168-3659(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 13.Lavon I, Grossman N, Kost J, Kimmel E, Enden G. Bubble growth within the skin by rectified diffusion might play a significant role in sonophoresis. J. Control. Release. 2007;117:246–255. doi: 10.1016/j.jconrel.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita M, Hynynen K. Intracellular Delivery of Bak BH3 peptide by microbubble-enhanced ultrasound. Pharm. Res. 2005;22:716–720. doi: 10.1007/s11095-005-2586-7. [DOI] [PubMed] [Google Scholar]
- 15.Lai CY, Wu CH, Chen CC, Li PC. Quantitative relations of acoustic inertial cavitation with sonoporation and cell viability. Ultrasound Med. Biol. 2006;32:1931–1941. doi: 10.1016/j.ultrasmedbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Ohta S, Suzuki K, Ogino Y, Miyagawa S, Murashima A, Matsumaru D, Yamada G. Gene transduction by sonoporation. Develop. Growth Differ. 2008;50:517–520. doi: 10.1111/j.1440-169X.2008.01026.x. [DOI] [PubMed] [Google Scholar]
- 17.Forbes MM, Steinberg RL, O’Brien WD., Jr Examination of Inertial Cavitation of Optison™ in Producing Sonoporation of Chinese Hamster Ovary Cells. Ultrasound Med. Biol. 2008;34:2009–2018. doi: 10.1016/j.ultrasmedbio.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter TR, LeVeen RF, Fox R, Kricsfeld A, Xie F. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. Am. Heart J. 1996;132:964–968. doi: 10.1016/s0002-8703(96)90006-x. [DOI] [PubMed] [Google Scholar]
- 19.Culp WC, Porter TR, Xie F, Goertzen TC, McCowan TC, Vonk BN, Baxter BT. Microbubble potentiated ultrasound as a method of declotting thrombosed dialysis grafts: experimental study in dogs. Vasc. Interv. Radiol. 2001;11:351–358. doi: 10.1007/s00270-001-0052-4. [DOI] [PubMed] [Google Scholar]
- 20.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N. Engl. J. Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 21.Eggers J, Seidel G, Koch B, König R. Sonothrombolysis in acute ischemic stroke for patients ineligible for rt-PA. Neurology. 2005;64:1052–1054. doi: 10.1212/01.WNL.0000154599.45969.D6. [DOI] [PubMed] [Google Scholar]
- 22.Holland CK, Vaidya SS, Datta S, Coussios CC, Shaw GJ. Ultrasound-enhanced tissue plasminogen activator thrombolysis in an in vitro porcine clot model. Thromb, Res. 2008;121:663–673. doi: 10.1016/j.thromres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Non-invasive opening of BBB by focused ultrasound. Acta. Neurochir. Suppl. 2003;86:555–558. doi: 10.1007/978-3-7091-0651-8_113. [DOI] [PubMed] [Google Scholar]
- 24.Choi JJ, Pernot M, Brown TR, Small SA, Konofagou EE. Spatio-temporal analysis of molecular delivery through the blood-brain barrier using focused ultrasound. Phys. Med. Biol. 2007;52:5509–5530. doi: 10.1088/0031-9155/52/18/004. [DOI] [PubMed] [Google Scholar]
- 25.Hynynen K. Macromolecular delivery across the blood-brain barrier. Meth. Mol. Biol. 2009;480:175–185. doi: 10.1007/978-1-59745-429-2_13. [DOI] [PubMed] [Google Scholar]
- 26.Tran BC, Seo J, Hall TL, Fowlkes JB, Cain CA. Effects of contrast agent infusion rates on thresholds for tissue damage produced by single exposures of high-intensity ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2005;52:1121–1130. doi: 10.1109/tuffc.2005.1503998. [DOI] [PubMed] [Google Scholar]
- 27.Hanajiri K, Maruyama T, Kaneko Y, Mitsui H, Watanabe S, Sata M, Nagai R, Kashima T, et al. Microbubble-induced increase in ablation of liver tumors by high-intensity focused ultrasound. Hepatol. Res. 2006;36:308–314. doi: 10.1016/j.hepres.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Yu T, Fan X, Xiong S, Hu K, Wang Z. Microbubbles assist goat liver ablation by high intensity focused ultrasound. Eur. Radiol. 2006;16:1557–1563. doi: 10.1007/s00330-006-0176-7. [DOI] [PubMed] [Google Scholar]
- 29.Borrelli MJ, Frizzell LA, Dunn F. Ultrasonically induced morphological changes in the mammalian neonatal spinal cord. Ultrasound Med. Biol. 1986;12:285–295. doi: 10.1016/0301-5629(86)90338-8. [DOI] [PubMed] [Google Scholar]
- 30.Miller DL, Li P, Dou C, Gordon D, Edwards CA, Armstrong WF. Influence of contrast agent dose and ultrasound exposure on cardiomyocyte injury induced by myocardial contrast echocardiography in rats. Radiology. 2005;237:137–143. doi: 10.1148/radiol.2371041467. [DOI] [PubMed] [Google Scholar]
- 31.Zachary JF, Blue JP, Miller RJ, O’Brien WD., Jr Vascular lesions and S-thrombomodulin concentrations from auricular arteries of rabbits infused with MB contrast agent and exposed to pulsed ultrasound. Ultrasound Med. Biol. 2006;32:1781–1791. doi: 10.1016/j.ultrasmedbio.2005.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiménez C, de Gracia R, Aguilera A, Alonso S, Cirugeda A, Benito J, Regojo RM, Aguilar R, et al. In situ kidney insonation with MB contrast agents does not cause renal tissue damage in a porcine model. J. Ultrasound Med. 12008;27:607–1615. doi: 10.7863/jum.2008.27.11.1607. [DOI] [PubMed] [Google Scholar]
- 33.Nyborg WL. Ultrasonic microstreaming and related phenomena. Br. J. Cancer Suppl. 1982;5:156–160. [PMC free article] [PubMed] [Google Scholar]
- 34.Holland CK, Apfel RE. An improved theory for the prediction of microcavitation thresholds. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1989;36:204–208. doi: 10.1109/58.19152. [DOI] [PubMed] [Google Scholar]
- 35.de Jong N, Hoff L, Skotland T, Bom N. Absorption and scatter of encapsulated gas filled microspheres: Theoretical consideration and some measurements. Ultrasonics. 1992;30:95–103. doi: 10.1016/0041-624x(92)90041-j. [DOI] [PubMed] [Google Scholar]
- 36.Church CC. The effects of an elastic solid surface layer on the radial pulsations of gas bubbles. J. Acoust. Soc. Am. 1995;97:1510–1521. [Google Scholar]
- 37.Khismatullin DB. Resonance frequency of microbubbles: effect of viscosity. J Acoust. Soc. Am. 2004;116:1463–1473. doi: 10.1121/1.1778835. [DOI] [PubMed] [Google Scholar]
- 38.Fong SW, Klaseboer E, Khoo BC. Interaction of microbubbles with high intensity pulsed ultrasound. J. Acoust. Soc. Am. 2008;123:1784–1793. doi: 10.1121/1.2836746. [DOI] [PubMed] [Google Scholar]
- 39.Doinikov AA, Haac JF, Dayton PA. Modeling of nonlinear viscous stress in encapsulating shells of lipid-coated contrast agent microbubbles. Ultrasonics. 2009;49:269–275. doi: 10.1016/j.ultras.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atchley AA, Frizzell LA, Apfel RE, Holland CK, Madanshetty S, Roy RA. Thresholds for cavitation produced in water by pulsed ultrasound. Ultrasonics. 1988;26:280–285. doi: 10.1016/0041-624x(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 41.Porter TR, Xie F, Li S, D'Sa A, Rafter P. Increased ultrasound contrast and decreased microbubble destruction rates with triggered ultrasound imaging. J. Am. Soc. Echocardiogr. 1996;9:599–605. doi: 10.1016/s0894-7317(96)90054-1. [DOI] [PubMed] [Google Scholar]
- 42.Klibanov AL, Ferrara FW, Hughes MS, Wible JH, Jr, Wojdyla JK, Dayton PA, Morgan KE, Brandenburger GH. Direct video-microscopic observation of the dynamic effects of medical ultrasound on ultrasound contrast microspheres. Invest. Radiol. 1998;33:863–870. doi: 10.1097/00004424-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Chang PP, Chen WS, Mourad PD, Poliachik SL, Crum LA. Thresholds for inertial cavitation in albunex suspensions under pulsed ultrasound conditions. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2001;48:161–170. doi: 10.1109/58.895927. [DOI] [PubMed] [Google Scholar]
- 44.Sboros V, MacDonald CA, Pye SD, Moran CM, Gomatam J, McDicken WN. The dependence of ultrasound contrast agents backscatter on acoustic pressure: theory versus experiment. Ultrasonics. 2002;40:579–583. doi: 10.1016/s0041-624x(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar K, Shi WT, Chatterjee D, Forsberg F. Characterization of ultrasound contrast microbubbles using in vitro experiments and viscous and viscoelastic interface models for encapsulation. J. Acoust. Soc. Am. 2005;118:539–550. doi: 10.1121/1.1923367. [DOI] [PubMed] [Google Scholar]
- 46.Ammi AY, Cleveland RO, Mamou J, Wang GI, Bridal SL, O’Brien WD., Jr Ultrasonic contrast agent shell rupture detected by inertial cavitation and rebound signals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2006;53:126–136. doi: 10.1109/tuffc.2006.1588398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glynos E, Koutsos V, McDicken WN, Moran CM, Pye SD, Ross JA, Sboros V. Nanomechanics of biocompatible hollow thin-shell polymer microspheres. Langmuir. 2009;25:7514–7522. doi: 10.1021/la900317d. [DOI] [PubMed] [Google Scholar]
- 48.King DA, Haak A, Malloy MJ, Roberts AC, Yoder CC, O’Brien WD., Jr Determination of postexcitation thresholds for single ultrasound contrast agent microbubbles using double passive cavitation detection. J. Acoust. Soc. Am. doi: 10.1121/1.3373405. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Postema M, Schmitz G. Bubble dynamics involved in ultrasonic imaging. Expert. Rev. Mol. Diagn. 2006;6:493–502. doi: 10.1586/14737159.6.3.493. [DOI] [PubMed] [Google Scholar]
- 50.Sboros V. Response of contrast agents to ultrasound. Adv. Drug Deliv. Rev. 2008;60:1117–1136. doi: 10.1016/j.addr.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Wu J, Nyborg WL. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug Deliv. Rev. 2008;60:1103–1116. doi: 10.1016/j.addr.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys. Med. Biol. 2009;54:R27–R57. doi: 10.1088/0031-9155/54/6/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stride E. Physical principles of microbubbles for ultrasound imaging and therapy. Cerebrovasc. Dis. 2009;27:1–13. doi: 10.1159/000203122. [DOI] [PubMed] [Google Scholar]
- 54.Mayer CR, Bekeredjia R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv. Drug Deliv. Rev. 2008;60:177–1192. doi: 10.1016/j.addr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Laing ST, McPherson DD. Cardiovascular therapeutic uses of targeted ultrasound contrast agents. Cardiovasc. Res. 2009;83:626–635. doi: 10.1093/cvr/cvp192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porter TR. The utilization of ultrasound and microbubbles for therapy in acute coronary syndromes. Cardiovasc. Res. 2009;83:636–642. doi: 10.1093/cvr/cvp206. [DOI] [PubMed] [Google Scholar]
- 57.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv. Drug Deliv. Rev. 2004;56:1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery-A general review. Expert. Opin. Drug. Deliv. 2004;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Ann. Rev. Biomed. Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 60.Meairs S, Alonso A. Ultrasound, microbubbles and the blood-brain barrier. Prog. Biophys. Mol. Biol. 2007;93:354–362. doi: 10.1016/j.pbiomolbio.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 61.Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and Gene delivery. Adv. Drug Deliv. Rev. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tinkov S, Bekeredjian R, Winter G, Coester C. Microbubbles as ultrasound triggered drug carriers. J. Pharm. Sci. 2009;98:1935–1961. doi: 10.1002/jps.21571. [DOI] [PubMed] [Google Scholar]
- 63.Luo W, Zhou X, Tian X, Ren X, Zheng M, Gu K, He G. Enhancement of ultrasound contrast agent in high-intensity focused ultrasound ablation. Adv. Ther. 2006;23:861–888. doi: 10.1007/BF02850207. [DOI] [PubMed] [Google Scholar]
- 64.O'Neill BE, Li KC. Augmentation of targeted delivery with pulsed high intensity focused ultrasound. Int. J. Hyperthermia. 2008;24:506–520. doi: 10.1080/02656730802093661. [DOI] [PubMed] [Google Scholar]
- 65.Jolesz FA. MRI-guided focused ultrasound surgery. Ann. Rev Med. 2009;60:417–430. doi: 10.1146/annurev.med.60.041707.170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dayton PA, Rychak JJ. Molecular ultrasound imaging using microbubble contrast agents. Front. Biosci. 2007;12:5124–5142. doi: 10.2741/2553. [DOI] [PubMed] [Google Scholar]
- 67.Klibanov AL. Ultrasound molecular imaging with targeted microbubble contrast agents. J. Nucl. Cardiol. 2007;14:876–884. doi: 10.1016/j.nuclcard.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 68.Schmitz G. Ultrasonic imaging of molecular targets. Basic. Res. Cardiol. 2008;103:174–181. doi: 10.1007/s00395-008-0709-0. [DOI] [PubMed] [Google Scholar]
- 69.Kiessling F, Huppert J, Palmowski M. Functional and molecular ultrasound imaging: Concepts and contrast agents. Curr. Med. Chem. 2009;16:627–642. doi: 10.2174/092986709787458470. [DOI] [PubMed] [Google Scholar]
- 70.Miller DL, Averkiou MA, Brayman AA, Everbach EC, Holland CK, Wible JH, Wu J. Bioeffects considerations for diagnostic ultrasound contrast agents. J Ultrasound Med. 2008;27:611–632. doi: 10.7863/jum.2008.27.4.611. [DOI] [PubMed] [Google Scholar]
- 71.Aggeli C, Giannopoulos G, Lampropoulos K, Pitsavos C, Stefanadis C. Adverse bioeffects of ultrasound contrast agents used in echocardiography: true safety issue or "much ado about nothing"? Curr. Vasc. Pharmacol. 2009;7:338–346. doi: 10.2174/157016109788340695. [DOI] [PubMed] [Google Scholar]
- 72.Juffermans LJ, van Dijk A, Jongenelen CA, Drukarch B, Reijerkerk A, de Vries HE, Kamp O, Musters RJ. Ultrasound and microbubble-induced intra- and intercellular bioeffects in primary endothelial cells. Ultrasound Med. Biol. 2009;35:1917–1927. doi: 10.1016/j.ultrasmedbio.2009.06.1091. [DOI] [PubMed] [Google Scholar]
- 73.Daffertshofer M, Hennerici M. Sonothrombolysis: Experimental evidence. Front. Neurol. Neurosci. 2006;21:140–149. doi: 10.1159/000092396. [DOI] [PubMed] [Google Scholar]
- 74.Tsivgoulis G, Culp WC, Alexandrov AV. Ultrasound enhanced thrombolysis in acute arterial ischemia. Ultrasonics. 2008;48:303–311. doi: 10.1016/j.ultras.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Meairs S, Culp WC. Microbubbles for thrombolysis of acute ischemic stroke. Cerebrovasc. Dis. 2009;27:55–65. doi: 10.1159/000203127. [DOI] [PubMed] [Google Scholar]
- 76.Medel R, Crowley RW, Dumont AS, Kassell NF. Sonothrombolysis: An emerging modality for the management of stroke. Neurosurgery. 2009;65:979–993. doi: 10.1227/01.NEU.0000350226.30382.98. [DOI] [PubMed] [Google Scholar]
- 77.Chen S, Wang Z, Zhou YT, Grayburn PA. Optimization of the size distribution and myocardial contrast effect of perfluorocarbon-filled albumin microbubbles by lyophilization under continuous negative pressure. J. Am. Soc. Echocardiogr. 2000;13:748–753. doi: 10.1067/mje.2000.104644. [DOI] [PubMed] [Google Scholar]
- 78.Weller GER, Villanueva FS, Klibanov AL, Wagner WR. Modulating targeted adhesion of an ultrasound control agent to dysfunctional endothelium. Ann. Biomed. Eng. 2002;30:1012–1019. doi: 10.1114/1.1513565. [DOI] [PubMed] [Google Scholar]
- 79.Feshitan JA, Chen CC, Kwan JJ, Borden MA. Mictobubble size isolation by differential centrifugation. J. Colloid. Interface. Sci. 2009;329:316–324. doi: 10.1016/j.jcis.2008.09.066. [DOI] [PubMed] [Google Scholar]
- 80.Talu E, Hettiarachchi K, Zhao S, Powell RL, Lee AP, Longo ML, Dayton PL. Tailoring the size distribution of ultrasound contrast agents: Possible method for improving sensitivity in molecular imaging. Mol. Imaging. 2007;6:384–392. [PMC free article] [PubMed] [Google Scholar]
- 81.Porter TR, Kricsfeld D, Lof J, Everbach EC, Xie F. Effectiveness of transcranial and transthoracic ultrasound and microbubbles in dissolving intravascular thrombi. J Ultrasound Med. 2001;20:1313–1325. doi: 10.7863/jum.2001.20.12.1313. [DOI] [PubMed] [Google Scholar]
- 82.Culp WC, Porter TR, Lowery J, Xie F, Roberson PK, Marky L. Intracranial clot lysis with intravenous microbubbles and transcranial ultrasound in swine. Stroke. 2004;35:2407–2411. doi: 10.1161/01.STR.0000140890.86779.79. [DOI] [PubMed] [Google Scholar]
- 83.Postema M, Marmottant P, Lancee CT, Hilgenfeldt S, De Jong N. Ultrasound-Induced microbubble coalescence. Ultrasound Med. Biol. 2004;30:1337–1344. doi: 10.1016/j.ultrasmedbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Sboros V, Glynos E, Pye SD, Moran CM, Butler M, Ross J, Short R, McDicken WN, Koutsos V. Nanointerrogation of ultrasonic contrast agent microbubbles using atomic force microscopy. Ultrasound Med. Biol. 2006;32:579–585. doi: 10.1016/j.ultrasmedbio.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 85.T, Tomita N, Horie S, Sax N, Iwasaki H, Suzuki R, Maruyama K, Mori S, Manabu F. Morphological study of acoustic liposomes using transmission electron microscopy. J. Electron. Micros. (Tokyo) 2010;59:187–196. doi: 10.1093/jmicro/dfp056. [DOI] [PubMed] [Google Scholar]
- 86.Lepock JR, Ritchie KP, Kolios MC, Rodahl AM, Heinz KA, Kruuv J. Influence of transition rates and scan rate on kinetic simulations of differential scanning calorimetry profiles of reversible and irreversible protein denaturation. Biochemistry. 1992;31:12706–12712. doi: 10.1021/bi00165a023. [DOI] [PubMed] [Google Scholar]
- 87.Church CC. Prediction of rectified diffusion during nonlinear bubble pulsations at biomedical frequencies. J. Acoust. Soc. Am. 1988;83:2210–2217. doi: 10.1121/1.396349. [DOI] [PubMed] [Google Scholar]
- 88.Church CC. A method to account for acoustic microstreaming when predicting bubble growth rates produced by rectified diffusion. J. Acoust. Soc. Am. 1988;84:1758–1764. doi: 10.1121/1.397192. [DOI] [PubMed] [Google Scholar]


