Abstract
Purpose
Transdermal insulin delivery is an attractive needle-free alternative to subcutaneous injection conventionally used to treat diabetes. However, skin’s barrier properties prevent insulin permeation at useful levels.
Methods
We investigated whether microdermabrasion can selectively remove skin’s surface layers to increase skin permeability as a method to administer insulin to diabetic rats. We further assessed the relative roles of stratum corneum and viable epidermis as barriers to insulin delivery.
Results
Pretreatment of skin with microdermabrasion to selectively remove stratum corneum did not have a significant effect on insulin delivery or reduction in blood glucose level (BGL). Removal of full epidermis by microdermabrasion significantly reduced BGL, similar to the positive control involving subcutaneous injection of 0.1U insulin. Significant pharmacokinetic differences between microdermabrasion and subcutaneous injection were faster time to peak insulin concentration after injection and larger peak insulin concentration and area-under-the-curve after microdermabrasion.
Conclusions
Microdermabrasion can increase skin permeability to insulin at levels sufficient to reduce BGL. Viable epidermis is a barrier to insulin delivery such that removal of full epidermis enables significantly more insulin delivery than removal of stratum corneum alone.
Keywords: epidermis, insulin, microdermabrasion, stratum corneum, transdermal drug delivery
INTRODUCTION
An estimated 150 million people worldwide suffer from diabetes, and many require insulin administration multiple times per day (1). Typically, insulin is given subcutaneously by injection or with a pump. These needle/catheter-based methods require significant training and diligent care by patients, which often leads to poor compliance (2). Inhaled insulin was recently introduced as a needle-free alternative, but subsequently discontinued due to relatively poor sales associated with limitations of marketing, device design, and other factors (3).
Transdermal delivery of insulin is attractive as a noninvasive method that offers the convenience of a transdermal patch (4). However, the skin’s barrier properties prevent absorption of useful amounts of insulin. Various approaches have been studied to increase transdermal delivery of insulin, including the use of chemical enhancers and iontophoresis (5), liposomes (6), ultrasound (7), thermal ablation (8), and microneedles (9,10).
In general, methods to increase transdermal delivery of insulin or other molecules target the stratum corneum as the primary barrier to transport into the skin (4). Stratum corneum is 10–15 µm thick in humans and is made up of keratin-filled, metabolically inactive corneocytes surrounded by a lipid matrix organized largely in bilayers. Below stratum corneum is the viable epidermis, which measures 50–100 µm thick and is densely filled with keratinocytes and other living cells surrounded by an aqueous extracellular matrix. Stratum corneum and viable epidermis together form the full epidermis. Deeper still is the dermis, which is a 1–2 mm thick tissue containing fibers of collagen and elastin embedded in an aqueous extracellular matrix that anchors sweat ducts, hair follicles, nerves, and vasculature (11).
Typically, transdermal drug delivery involves transporting drugs across the stratum corneum barrier, after which they diffuse through viable epidermis and the upper portion of dermis to the capillary bed found just below the dermal-epidermal junction. This junction features a basal lamina, which is known to limit transport of macromolecules, especially those larger than 40 kDa in size (12). There is also evidence suggesting that tight junctions in the viable epidermis may also provide a transport barrier (13). Because the stratum corneum barrier has, until recently, been the rate-limiting step for delivery of most drugs, there has been relatively little attention given to the role of viable epidermis and dermis as barriers to drug delivery.
In this study, we investigate the use of microdermabrasion to increase skin permeability to insulin. Microdermabrasion is a superficial cosmetic resurfacing technique that removes the skin’s layers by bombarding them with high-velocity abrasive particles (14,15). Traditionally, microdermabrasion is used to lessen the appearance of fine lines, wrinkles, and scars (15,16). In recent years, microdermabrasion has been used as a method of disrupting the stratum corneum for transdermal drug delivery (17–20).
In this study, we used microdermabrasion to address two related topics. First, we wanted to determine if microdermabrasion could be used to increase skin permeability to insulin at levels sufficient to lower blood glucose level (BGL) in diabetic rats. Previous studies have used microdermabrasion to increase skin permeability to small molecules with limited attention to macromolecules like insulin. Second, we wanted to determine the relative roles of stratum corneum and viable epidermis as barriers to insulin delivery. By optimizing microdermabrasion conditions to selectively remove just stratum corneum or remove the full epidermis, we were able to compare insulin delivery in these two situations and thereby determine whether viable epidermis presents a significant barrier to insulin delivery.
MATERIALS AND METHODS
Diabetic Animal Model
Hairless rats (CD Strain, 6–8 weeks old, Charles River Laboratories, Wilmington, MA) were made diabetic by injecting 300 µL of filter-sterilized streptozocin (Sigma, St. Louis, MO) in citrate buffer (pH 4.5, Sigma, St. Louis, MO) at a dose of 80–100 mg/kg into the tail vein (21,22). After 24 h, animals were anesthetized with isoflurane and monitored for 1 h before initiating insulin delivery experiments described immediately below. To be included in the study, rats were required to have (a) BGL >300 mg/dL, (b) variation in BGL <20% among two measurements at 0 h and 1 h after initiating anesthesia and (c) <20% body weight loss after streptozocin administration. Two rats, one from the positive control group and one from the stratum corneum removal group, were excluded from this study based on these criteria. BGL from these initial measurements was between 313 and 450 mg/dL due to the combined effects of streptozocin-induced diabetes and anesthesia. Isoflurane has been shown to increase the blood glucose in diabetic humans and may have similarly increased blood glucose levels in this study (23). The animal protocol was approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee (IACUC).
Insulin Delivery Protocol
Rats that met the inclusion criteria were divided into four groups: untreated negative control, subcutaneous injection positive control, stratum corneum abrasion, and full epidermis abrasion. The negative control group was not abraded and did not receive insulin. The positive control group received a 500 µL subcutaneous injection of 0.1 U insulin diluted in sterile phosphate buffered saline (Mediatech Inc., Manassas, VA) from U100 Humalog (Eli Lilly, Indianapolis, IN). The rats in the positive control group had an average weight of 0.29 ± 0.04 kg, and the insulin dose was therefore 0.35 ± 0.05 U/kg.
Microdermabrasion was carried out on the two abrasion groups as described in the next section. Then, a transdermal reservoir patch (Altea Therapeutics, Atlanta, GA) filled with 200 µL of a solution containing 80 vol.% Humalog (100 U/mL) and 20 vol.% FITC-labeled bovine insulin (2.5 mg/mL, Sigma-Aldrich, St. Louis, MO) was applied to the site of abrasion for 4 h. The FITC-labeled insulin was included to allow visualization of the insulin penetration depth into the skin during subsequent histological analysis.
For all four groups, BGL was measured every 30 min for the first 2 h and every 2 h for hours 2 to 8 using a NovaMax meter (Nova Biomedical, Bedford, MA) with blood obtained from tail vein laceration. At each time point, 200 µl of blood was also collected in blood serum tubes that contained gel and a clot activator (Capiject, Somerset, NJ) for insulin quantification by enzyme-linked immunoassay (ELISA). The insulin-filled patches were removed after 4 h, and the skin was wiped clean with tissue paper. The animals remained under anesthesia throughout the experiment and until they were euthanized by carbon dioxide asphyxiation at the conclusion of the 8 h experiment. Animals were euthanized during the experiment if their BGL exceeded 475 mg/dL or fell below 45 mg/dL, and their data were discarded.
Microdermabrasion Protocol
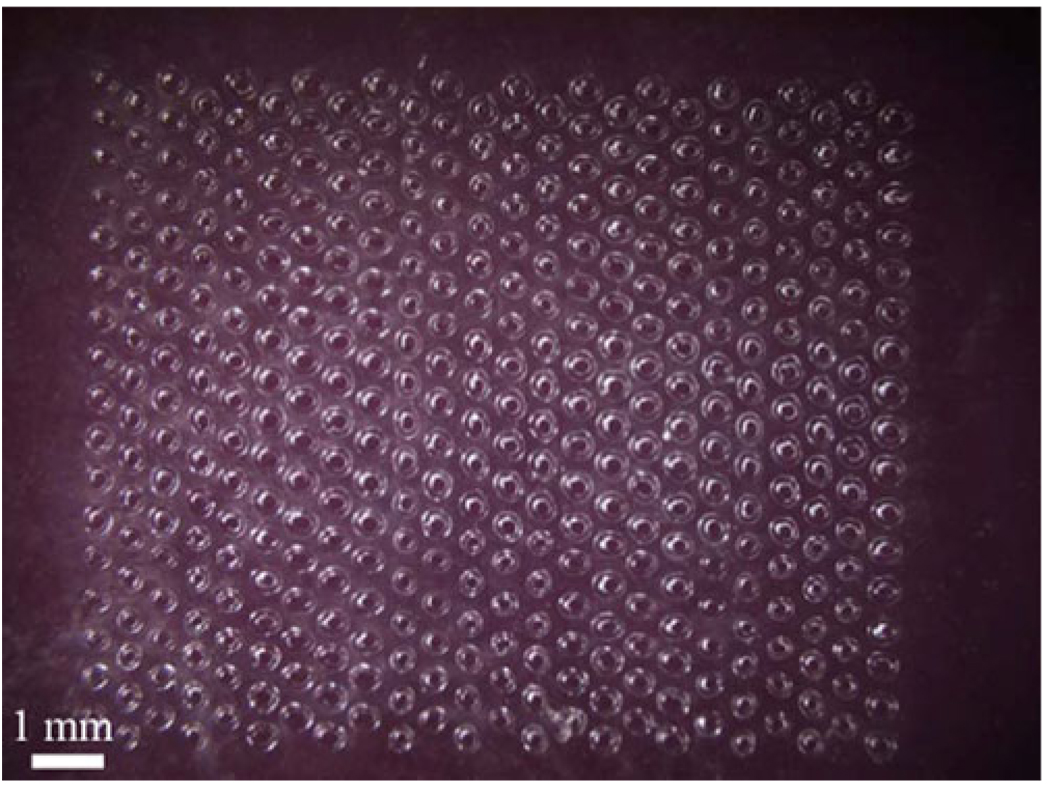
To carry out microdermabrasion, a few square centimeters of skin on the back of the rat was gently cleaned with alcohol swabs and allowed to air dry. A mask measuring 1 cm by 0.8 cm was then applied to the cleaned skin surface using adhesive tape. As shown in Fig. 1, the mask had an array of 408 circular holes (125 µm in diameter) with a center-to-center spacing of 500 µm. The masks were fabricated out of 70 µm-thick polyethylene terephthalate (McMaster Carr, Aurora, OH), and the holes were laser cut with a Hermes LS500XL CO2 laser (Gravograph, Duluth, GA). The mask was used to limit the area of skin tissue removal to an array of micron-size holes.
Fig. 1.
Mask used to selectively expose areas of skin to microdermabrasion. The 408 approximately circular holes of 125 µm diameter were drilled into the polymer mask using a CO2 laser. The mask was adhered to the skin with tape, and the microdermabrasion tip was moved across the surface of the mask to abrade the skin exposed through the holes.
The skin was microdermabraded using a Gold Series MegaPeel machine (DermaMed USA, Lenni, PA) with the gold handpiece assembly by manually moving the handpiece continuously across the mask. The skin was abraded at a suction pressure of −30 kPa for 30 s at a crystal flow rate of 0.19 g/s (6 turns of the crystal flow rate knob) to remove the stratum corneum. The full epidermis was removed using a suction pressure of −50 kPa for 60 s at a crystal flow of 0.95 g/s (0 turns of the crystal flow rate knob). After abrasion, a green dye (McCormick & Co, Hunt Valley, MD) was applied to the skin for 20 s and wiped cleaned with alcohol swabs to stain holes in the skin.
At the conclusion of the experiment, green dye was applied to the skin to visualize the holes. The skin was excised, embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA), and frozen using dry ice. The skin was sectioned at a thickness of 10 µm using a Leica 3050S cryostat (Leica Microsystems, Wetzlar, Germany). The skin was imaged with a Nikon E600 microscope (Nikon, Tokyo, Japan) with a QCapture camera and software (Q Imaging, Pleasanton, CA) to visualize the fluorescence. The sections were also stained using routine hematoxylin and eosin and photographed.
Serum Insulin Determination
Collected blood samples were allowed to clot at room temperature for 2 h after collection and then centrifuged (Eppendorf, Hamburg, Germany) at 1,200 g for 10 min. After centrifugation, the serum was removed and stored at −70°C. The concentration of insulin in the serum was quantified using a rat insulin ELISA kit (Alpco Diagnostics, Salem, NH) that detected both Humalog and rat insulin. The rat insulin kit was used because ELISA insulin kits that detect Humalog also cross-react with endogenous rat insulin. Human insulin kits were not used because they cannot detect Humalog, since the amino acid structure of native insulin and synthetic insulin differ. The bovine insulin did not cross-react with the kit. The assay was carried out according to the manufacturer’s protocol. The provided insulin control solutions ranging from 0 to 5.5 ng/mL were run for calibration with every experiment.
Data Analysis
The area under the curve for the BGL and serum insulin data was determined with NCSS software (NCSS, Kaysville, UT). The statistical analysis was performed using Minitab software (Minitab, State College, PA). One-way and two-way analysis of variance (ANOVA), general linear model (unbalanced ANOVA), and the Student’s t-test were conducted on the data, and a p-value less than 0.05 was considered significant.
RESULTS
Skin Histology
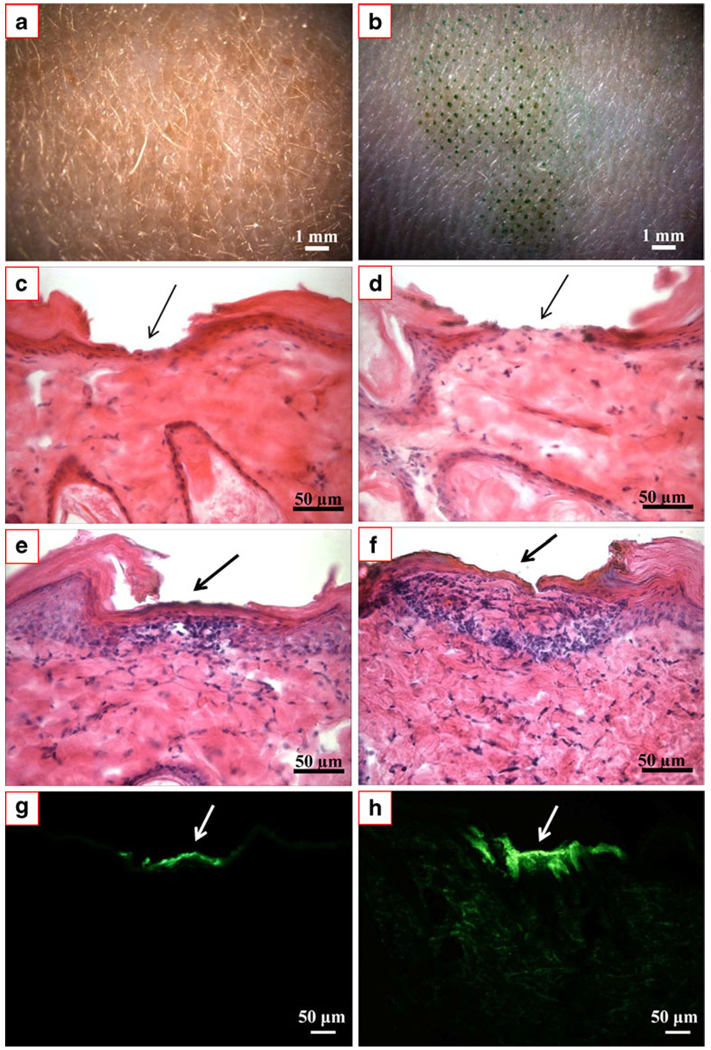
We wanted to determine if microdermabrasion can increase skin permeability to deliver a therapeutic dose of insulin and if the depth of abrasion across the stratum corneum versus the full epidermis had an effect on the extent and rate of insulin delivery. We therefore used microscopic imaging and histological staining to qualitatively determine the pattern and depth of tissue removal and the ability to deliver insulin into the skin, as shown in Fig. 2.
Fig. 2.
Representative microscopic images of hairless rat skin exposed to microdermabrasion. (a) En face view of untreated, negative control skin. (b) En face view of skin after microdermabrasion. Staining with green dye identifies sites of skin abrasion, which follow the pattern of the mask shown in Fig. 1. Histological sections of hairless rat skin with H&E staining biopsied and frozen immediately after microdermabrasion to remove (c) stratum corneum and (d) full epidermis. Histological sections of hairless rat skin with H&E staining biopsied and frozen 8 h after microdermabrasion to remove (e) stratum corneum and (f) full epidermis. Comparison of (c) vs. (e) and (d) vs. (f) shows partial skin repair after 8 h. The same histological sections shown in (e) and (f) are shown before H&E staining under fluorescence optics to reveal green-fluorescent insulin penetration into skin microdermabraded to remove (g) stratum corneum and (h) full epidermis.
Fig. 2a shows an en face view of untreated hairless rat skin. For comparison, Fig. 2b shows hairless rat skin after microdermabrasion was carried out in vivo. The sites of tissue removal are highlighted by selective staining with a green dye in the pattern of the mask shown in Fig. 1.
Microdermabrasion was carried out under two different sets of operating conditions: one to remove just stratum corneum and another to remove the full epidermis. As shown in Fig. 2c, microdermabrasion at the milder conditions removed stratum corneum, which is the pink-stained tissue at the top of the skin, but left the viable epidermis intact, as shown by the tissue with a dense population of blue-stained nuclei. Microdermabrasion at the stronger conditions is shown in Fig. 2d, where the stratum corneum and viable epidermis are both removed.
The final four images in Fig. 2 show representative images of skin 8 h after microdermabrasion and exposure to a topical insulin solution for the first 4 h. Fig. 2e and f show skin with stratum corneum removal and full epidermis removal, respectively. It is evident in these images, stained with hematoxylin and eosin (H&E) to highlight anatomical features, that there has already been significant skin repair during the 8 h after microdermabrasion. In Fig. 2e, there appears to be pink-stained tissue forming above the viable epidermis. In Fig. 2f, the epidermis is partially reformed as shown by the presence of cells with blue-stained nuclei and pink-stained tissue above. These rapid kinetics of apparent skin recovery can be explained by the observation that rodents are known to have remarkably fast wound repair (24). The speed of repair here was probably enhanced by the highly localized, micron-scale damage caused to the skin by microdermabrasion under the conditions used in this study in combination with a mask.
Figure 2g and h show the same histological sections shown in Fig. 2e and f, but with different staining and imaging conditions. These final images show fluorescence of FITC-labeled insulin administered to microdermabraded skin. In the skin with only stratum corneum removal (Fig. 2g), insulin has penetrated into the upper part of the epidermis, but not significantly deeper, which suggests it may not have reached the capillary bed in the upper dermis. In the skin with full epidermis removal (Fig. 2h), the insulin can be seen not only at the site of tissue removal, but also deep into the dermis as well.
BGL Pharmacodynamics
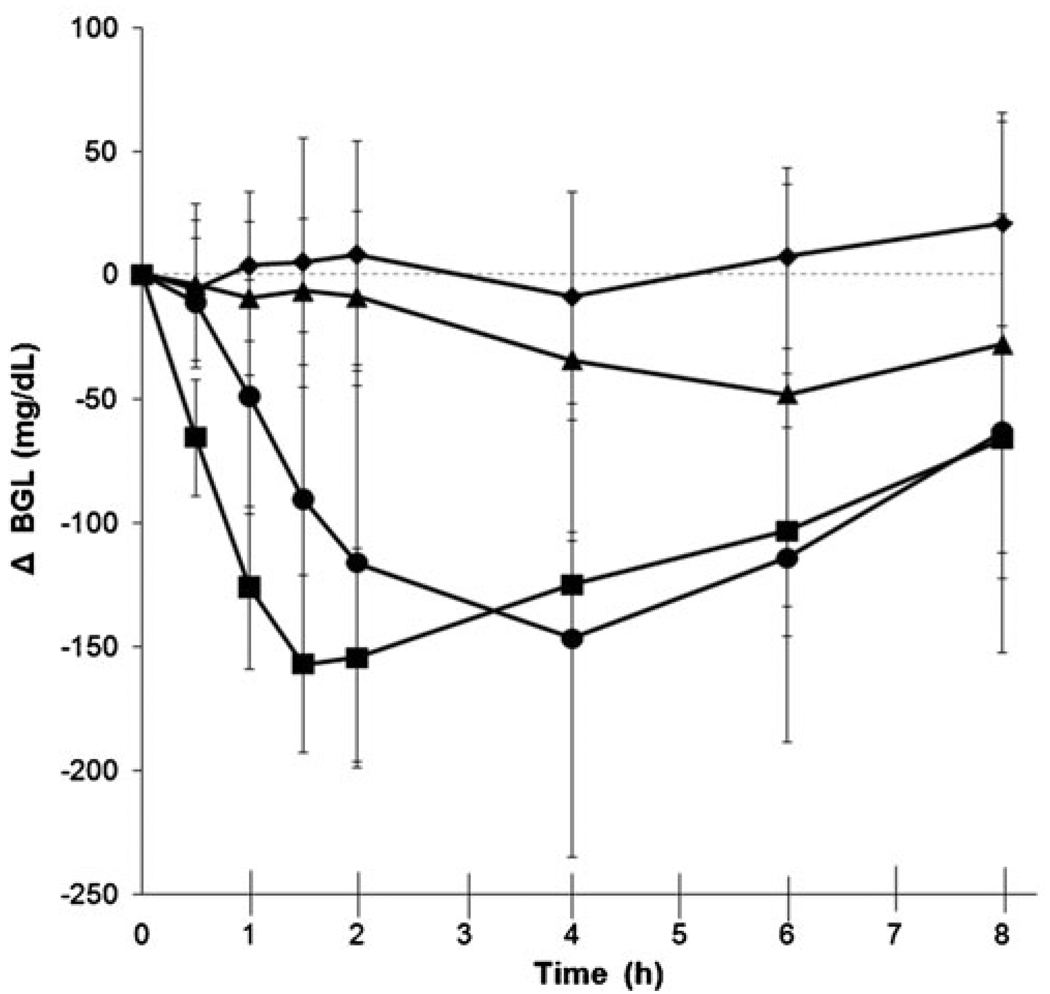
We next assessed changes in BGL among four experimental groups, as shown in Fig. 3 and Table I. These groups included (a) untreated negative control, (b) subcutaneous injection positive control, (c) microdermabrasion to remove stratum corneum and (d) microdermabrasion to remove full epidermis. The microdermabraded groups had a topical insulin patch applied to the skin for 4 h. All groups had blood collected periodically to measure glucose and insulin concentrations for 8 h. The untreated negative control group maintained high blood glucose values throughout the experiment, consistent with our use of a diabetic animal model.
Fig. 3.
Change in blood glucose level (BGL) relative to pre-treatment values. The negative control group (◆) was not abraded or given insulin. The positive control group (■) was injected subcutaneously at time = 0 with 0.1 U of Humalog. The stratum corneum (▲) and full epidermis (●) abrasion groups were administered a topical insulin patch at the abrasion site for 4 h starting at time = 0. All data points represent the average ± standard deviation of measurements in 6–8 different rats.
Table I.
Pharmacodynamic Parameters Associated with BGL Reduction After Insulin Administration by Microdermabrasion
| Group | tmax, BGL (h)a | ΔBGLmax (mg/dL)b | AUCBGL (mg h/dL)c |
|---|---|---|---|
| Stratum corneum removal | n.s.d | n.s.d | n.s.d |
| Full epidermis removal | 3.0 ± 2.0 | −144 ± 69 | −805 ± 524 |
| Subcutaneous injection (0.1 U) | 3.9 ± 3.0 | −165 ± 29 | −846 ± 129 |
In this table, the pharmacodynamic parameters were determined for each individual animal and then averaged within each group. For this reason, these pharmacodynamic parameter values differ from those that would be obtained by directly reading off the graph in Fig. 3, which contains averaged data from all animals in each group.
tmax, BGL is the time until the maximum reduction in BGL was achieved.
ΔBGLmax is the maximum reduction in BGL achieved.
AUCBGL is the integral of the ΔBGL vs time curve.
n.s. = not significant (unbalanced ANOVA analysis showed that there were no significant changes in BGL in the stratum corneum removal group compared to the untreated control group (P = 0.08)).
Animals microdermabraded to remove only the stratum corneum appeared to have a reduction in BGL, but it was not significantly different from the untreated control group (unbalanced ANOVA, P= 0.08; Fig. 3). In contrast, microdermabrasion that removed the full epidermis had a much more dramatic and significant reduction in BGL compared to the untreated control group (unbalanced ANOVA, P= 0.01; Fig. 3) and compared to the stratum corneum removal group (unbalanced ANOVA, P= 0.001; Fig. 3). The full epidermis removal group had a maximum BGL reduction of 144 mg/dL that was achieved after 3.0 h, on average (Table I).
In comparison, the positive control animals received a bolus subcutaneous injection of 0.1 U insulin and exhibited a significant reduction in BGL compared to untreated controls (unbalanced ANOVA, P< 0.001; Fig. 3) that had a maximum BGL reduction of 165 mg/dL after 3.9 h, on average (Table I). Comparison of the three pharmacodynamic parameters shown in Table I showed no significant differences between insulin delivery across skin with full epidermis removed and subcutaneous injection (Student’s t-test, P> 0.05). Altogether, these data show that although stratum corneum removal did not have a significant effect, insulin delivery after full epidermis removal significantly reduced BGL at levels and with kinetics similar to subcutaneous injection.
Insulin Pharmacokinetics
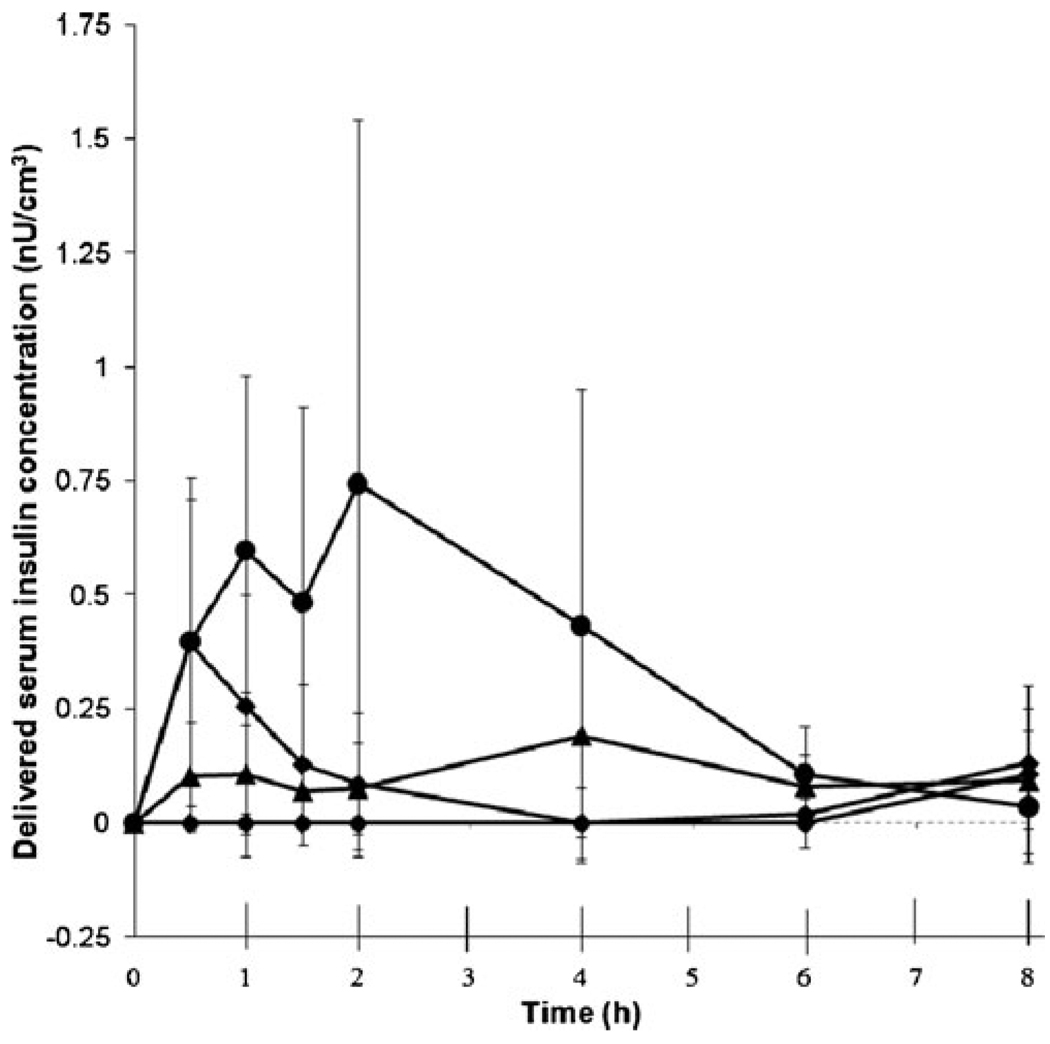
As a companion to the BGL measurements, we also determined the total serum insulin concentration by ELISA, as shown in Fig. 4. Consistent with the BGL measurements, serum insulin concentration increased after insulin administration, and the extent and kinetics depended on the method of administration.
Fig. 4.
Serum insulin levels. The four experimental groups are the same as in Fig. 3. All data points represent the average ± standard deviation of measurements in 6–8 different rats. Because the ELISA assay could not distinguish between endogenous rat insulin and exogenous Humalog insulin, the concentration of endogenous rat insulin was subtracted out, based on the level measured at time = 0 for each rat and making the imperfect assumption that this endogenous insulin level did not change over time (see Fig. S1 in Supplementary Material).
Insulin levels in the negative control animal that received no exogenous insulin were taken to be background levels and subtracted from insulin levels reported in the other animals. This background correction was necessary because the ELISA kit used for analysis could not distinguish between rat endogenous insulin and delivered Humalog. Thus, insulin levels in the negative control group were zero at all time points (Fig. 4).
Insulin delivery across skin with stratum corneum removed by microdermabrasion did not cause a significant change in serum insulin levels compared to the negative control group (unbalanced ANOVA, P= 0.78). Insulin delivery across skin with full epidermis removed resulted in elevated serum insulin levels that were significantly greater than the negative control (unbalanced ANOVA, P= 0.001), the stratum corneum removal group (unbalanced ANOVA, P< 0.001) and the subcutaneous injection group (unbalanced ANOVA, P= 0.001).
The main pharmacokinetic parameters are presented in Table II. Serum insulin concentration rose faster after subcutaneous injection compared to transdermal delivery (tmax, insulin in Table II, Student’s t-test, P< 0.05), whereas peak insulin concentration and area under the curve were higher for the full epidermis removal group (Cmax, insulin and AUCinsulin in Table II, Student’s t-test, P< 0.05). This contrasts with the pharmacodynamic data, in which reduction in BGL in the subcutaneous injection and full epidermis removal groups were similar.
Table II.
Pharmacokinetic Parameters Associated with Plasma Insulin Concentration After Insulin Administration by Microdermabrasion
| Group | tmax, insulin (h)a | Cmax, insulin (ng/mL)b | AUCinsulin (ng h/mL)c |
|---|---|---|---|
| Stratum corneum removal | n.s.d | n.s.d | n.s.d |
| Full epidermis removal | 2.6 ± 2.0 | 0.98 ± 0.73 | 2.58 ± 1.98 |
| Subcutaneous injection (0.1 U) | 1.0 ± 0.7 | 0.44 ± 0.30 | 0.77 ± 0.57 |
In this table, the pharmacokinetic parameters were determined for each individual animal and then averaged within each group. For this reason, these pharmacokinetic parameter values differ from those that would be obtained by directly reading off the graph in Fig. 4, which contains averaged data from all animals in each group.
tmax, insulin is the time until the maximum plasma insulin concentration was achieved.
Cmax, insulin is the maximum plasma insulin concentration achieved.
AUCinsulin is the integral of the plasma insulin concentration vs time curve. All calculations are based on data presented in Fig. 4.
n.s. = not significant (unbalanced ANOVA analysis showed that there were no significant changes in plasma insulin concentration in the stratum corneum removal group compared to the untreated control group (P = 0.78)).
DISCUSSION
The first objective of this study was to determine if microdermabrasion could be used to deliver a therapeutic dose of insulin to significantly decrease BGL in diabetic rats. We found that removing the stratum corneum did not significantly increase skin permeability to insulin or significantly affect BGL. In contrast, microdermabrasion that removed the full epidermis significantly increased insulin delivery across the skin, resulting in a 144 mg/dL drop in BGL at 3 h after patch application. The range of the plasma insulin in the rats treated by microdermabrasion was 0.04–0.75 ng/mL, which was similar to insulin delivered in previous studies using microneedles (0.2–1.2 ng/mL) (21,25). The plasma insulin levels were higher than those delivered previously using sonophoresis (7). Overall, we conclude that microdermabrasion is able to increase skin permeability to insulin at levels sufficient to lower BGL in the diabetic rat.
Our second objective was to compare the relative roles of stratum corneum and viable epidermis as barriers to transdermal delivery of insulin. Previous transdermal experiments have shown that insulin penetrates intact skin poorly, which results in difficulty to lower BGL in diabetic rats (7,10,25). In this study, removing only the stratum corneum was ineffective to administer insulin and lower BGL. However, removing the full epidermis was highly effective, exhibiting pharmcodynamic BGL responses similar to subcutaneous injection and pharmacokinetic serum insulin levels greater than subcutaneous injection. We conclude that viable epidermis was a significant barrier to transdermal insulin delivery.
Assessing the role of viable epidermis as a barrier is important as transdermal delivery of macromolecules becomes a reality (4). Most methods to increase skin permeability have focused on the stratum corneum barrier, largely by disrupting stratum corneum lipid structures using chemical enhancers, iontophoresis, and other methods. These methods have been able to increase skin permeability, but generally not enough to enable significant penetration of macromolecules. More recently, methods such as microdermabrasion, microneedles, and thermal ablation have been used to make micron-scale defects in the skin, thereby enabling transport of macromolecules at useful levels. However, it is unclear in the literature whether micron-scale pathways across stratum corneum are enough or if those pathways need to penetrate deeper to optimize delivery.
This study suggests that disrupting stratum corneum is not enough and that additional pathways across the viable epidermis can significantly increase transdermal delivery of macromolecules such as insulin into systemic circulation. Although the generality of this conclusion needs further validation with other molecules and in human skin, this finding could provide valuable insight into optimal design of skin permeability enhancement methods that have traditionally focused only on stratum corneum.
Further studies will be needed to determine which anatomical features give the viable epidermis its barrier function. Of particular interest is the basal lamina, which is located between the epidermis and dermis and serves as a semi-permeable membrane between the two skin layers (12). The basal lamina is mainly composed of type IV collagen and proteoglycans that provide an anchoring point for epidermal basal cells and control movement of substances across the membrane (26). It is thought that the basal lamina restricts movement of molecules that are larger than ~40 kDa (12). The insulin molecule has a molecular mass around 6 kDa (i.e., the size of the monomeric Humalog insulin used in this study), but the fluorescently tagged bovine insulin also used in this study probably existed in its hexameric form, which has a molecular mass of ~35 kDa, which is close to the expected molecular mass cut off of the basal lamina.
The possible role of tight junctions in the viable epidermis is also of interest. Although much less is known about the nature of this barrier, epidermal tight junctions are thought to restrict diffusion of molecules just a few kiloDaltons in size, which could include monomeric insulin (13). For this reason, epidermal tight junctions may represent an important barrier to transdermal delivery of macromolecules.
Diabetic patients often require fast-acting bolus insulin at meal times and slow release of basal insulin throughout the day. The slow, diffusion-based delivery of insulin across microdermabraded skinmay not be suitable for bolus delivery needs, butmay enable continuous basal delivery. The number and size of holes in the mask controls the area of skin abraded and, although not shown in this study, should likewise control the rate of insulin absorption into the skin. In this way, different basal delivery rates might be adjusted for a patient’s dosage by simply using different mask designs. In this initial study, there was large inter-animal variability in the pharmacodynamic and pharmacokinetic responses. This may be in part due to the wide range of pre-treatment blood glucose levels in the rats after diabetes induction, which is a limitation of this animal model. Additional device design and optimization will also be needed to better control insulin delivery after microdermabrasion.
Conventional cosmetic use of microdermabrasion partially removes small areas of stratum corneum, which is painless and requires no down time after treatment for most patients (14,19). In this study, we removed either full stratum corneum or epidermis, which is more aggressive than convention microdermabrasion, but similar in the level of skin trauma caused by thermal ablation, which has been well tolerated by patients in a number of clinical trials (27,28), and microneedle puncture, which has also been well tolerated in clinical trials (29–31) and is widely self-administered by patients for cosmetic purposes without pain or infection (32).
In the future, skin abrasion could be carried out using a small, hand-held, application-specific device instead of the large, multi-patient instrument currently used in medical clinics and spas. In this way, patients could pre-treat their skin with microdermabrasion and then apply a transdermal patch to administer basal insulin for the rest of the day. Although skin resealing is not expected to be as fast in people as seen for rats in this study, the patch could contain excipients that delay skin repair if needed (33). In cosmetic uses, microdermabrasion has been shown to be noninvasive, painless, and not result in scars. Using this technology to administer insulin could result in higher patient compliance, because it does not require the use of needles and does not cause pain. It would also decrease the amount of biohazardous sharps waste and its associated cost.
CONCLUSION
Most previous work on microdermabrasion for transdermal drug delivery has focused on transport of small molecules across the skin. In this study, we showed for the first time that microdermabrasion can increase skin permeability to deliver insulin and thereby lower BGL in diabetic rats. Because the lag time to peak serum insulin concentration and BGL reduction was a few hours, this method of delivery may be best suited to administer continuous, low-dose basal insulin rather than mealtime bolus doses. Use of microdermabrasion to remove stratum corneum was not significantly effective at delivering insulin, whereas removal of full epidermis was highly effective, which indicates an important barrier function in viable epidermis. This suggests that the conventional approach of focusing only on increasing stratum corneum permeability may be insufficient for optimal delivery of macromolecules into the skin, and methods that also permeabilize the viable epidermis may be more successful. Overall, we conclude that microdermabrasion that removes the full epidermis is an effective method to increase skin permeability for transdermal insulin delivery.
ACKNOWLEDGMENTS
We thank Dr. Laura O’Farrell for her assistance with the diabetes induction protocol, Dr. Tanicia Daley for assisting with the experiment, and Ms. Donna Bondy for administrative support. This work was carried out in the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at Georgia Tech with financial support in part from the National Institutes of Health.
Footnotes
Electronic Supplementary Material The online version of this article (doi:10.1007/s11095-011-0435-4) contains supplementary material, which is available to authorized users.
Contributor Information
Samantha Andrews, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, USA.
Jeong Woo Lee, School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Drive, Atlanta, Georgia 30332-0100, USA.
Seong-O Choi, School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Drive, Atlanta, Georgia 30332-0100, USA.
Mark R. Prausnitz, Email: prausnitz@gatech.edu, Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, USA; School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Drive, Atlanta, Georgia 30332-0100, USA.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. 2008th ed. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 2.Asche CV, Shane-McWhorter L, Raparla S. Health economics and compliance of vials/syringes versus pen devices: a review of the evidence. Diabetes Technol Ther. 2010;12:S101–S108. doi: 10.1089/dia.2009.0180. [DOI] [PubMed] [Google Scholar]
- 3.Odegard FS, Capoccia KL. Inhaled insulin: exubera. Ann Pharmacother. 2005;39(5):843–853. doi: 10.1345/aph.1E522. [DOI] [PubMed] [Google Scholar]
- 4.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastogi R, Anand S, Dinda AK, Koul V. Investigation on the synergistic effect of a combination of chemical enhancers and modulated iontophoresis for transdermal delivery of insulin. Drug Dev Ind Pharm. 2010;36(8):993–1004. doi: 10.3109/03639041003682012. [DOI] [PubMed] [Google Scholar]
- 6.Cevc G. Transdermal drug delivery of insulin with ultradeformable carriers. Clin Pharmacokinet. 2003;42(5):461–474. doi: 10.2165/00003088-200342050-00004. [DOI] [PubMed] [Google Scholar]
- 7.Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269(5225):850–853. doi: 10.1126/science.7638603. [DOI] [PubMed] [Google Scholar]
- 8.Levin G. Advances in radio-frequency transdermal drug delivery. Pharm Technol. 2008;32(4):s12–s19. [Google Scholar]
- 9.Gupta J, Felner EI, Prausnitz MR. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol Ther. 2009;11(6):329–337. doi: 10.1089/dia.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K, Lee HC, Lee DS, Jung H. Drawing lithography: three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv Mater. 2010;22(4):483–486. doi: 10.1002/adma.200902418. [DOI] [PubMed] [Google Scholar]
- 11.Jean B, Joseph J, Ronald R. Dermatology. 2nd ed. St. Louis: Mosby; 2007. [Google Scholar]
- 12.Kydonieus AF, Berner B. Transdermal delivery of drugs. Boca Raton: CRC; 1987. [Google Scholar]
- 13.Kirschner N, Houdek P, Fromm M, Moll I, Brandner JM. Tight junctions form a barrier in human epidermis. Eur J Cell Biol. 2010;89(11):839–842. doi: 10.1016/j.ejcb.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Bhalla M, Thami GP. Microdermabrasion: reappraisal and brief review of literature. Dermatol Surg. 2006;32(6):809–814. doi: 10.1111/j.1524-4725.2006.32165.x. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto T, Shirakami K, Tojo K. Effect of microdermabrasion on barrier capacity of stratum corneum. Chem Pharm Bull. 2005;53(8):1014–1016. doi: 10.1248/cpb.53.1014. [DOI] [PubMed] [Google Scholar]
- 16.Freedman BM, Rueda-Pedraza E, Waddell SP. The epidermal and dermal changes associated with microdermabrasion. Dermatol Surg. 2001;27(12):1031–1034. doi: 10.1046/j.1524-4725.2001.01031.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee WR, Shen SC, Wang KH, Hu CH, Fang JY. Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J Invest Dermatol. 2003;121(5):1118–1125. doi: 10.1046/j.1523-1747.2003.12537.x. [DOI] [PubMed] [Google Scholar]
- 18.Fang JY, Lee WR, Shen SC, Fang YP, Hu CH. Enhancement of topical 5-aminolaevulinic acid delivery by erbium: YAG laser and microdermabrasion: a comparison with iontophoresis and electroporation. Br J Dermatol. 2004;151(1):132–140. doi: 10.1111/j.1365-2133.2004.06051.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee WR, Tsai RY, Fang CL, Liu CJ, Hu CH, Fang JY. Microdermabrasion as a novel tool to enhance drug delivery via the skin: an animal study. Dermatol Surg. 2006;32(8):1013–1022. doi: 10.1111/j.1524-4725.2006.32224.x. [DOI] [PubMed] [Google Scholar]
- 20.Gill HS, Andrews SN, Sakthivel SK, Fedanov A, Williams IR, Garber DA, et al. Selective removal of stratum corneum by microdermabrasion to increase skin permeability. Eur J Pharm Sci. 2009;38(2):95–103. doi: 10.1016/j.ejps.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52(5):909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- 22.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 23.Farrokhnia F, Lebaschi AH, Andalib N. A randomized clinical trial for the effects of halothane and isoflurane anesthesia on blood glucose levels in the diabetic patients. DARU. 2009;17(1):29–32. [Google Scholar]
- 24.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol. 2003;121(2):219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- 25.Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 26.Freinkel RK, Woodley DT. The biology of the skin. New York: Parthenon; 2000. [Google Scholar]
- 27.Böhler C. Large molecules cross the transdermal barrier. Drug Discovery Dev. 2009;12(10):36. [Google Scholar]
- 28.Kenan Y, Kochba E, Shahar M, Gadasi H, Levin G, Foldes AJ, et al. Comparison of transdermal and subcutaneous teriparatide effects on markers of bone turnover and safety in postmenopausal women. Bone. 2010;47(S1):S49. [Google Scholar]
- 29.Cosman F, Lane NE, Bolognese MA, Zanchetta JR, Garcia-Hernandez PA, Sees K, et al. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2010;95(1):151–158. doi: 10.1210/jc.2009-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198(5):650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 31.Wermeling DP, Banks SL, Huclson DA, Gill HS, Gupta J, Prausnitz MR, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci USA. 2008;105(6):2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doddaballapur S. Microneedling with dermaroller. J Cutan Aesthet Surg. 2009;2(2):110–111. doi: 10.4103/0974-2077.58529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milewski M, Brogden NK, Stinchcomb AL. Current aspects of formulation efforts and pore lifetime related to microneedle treatment of skin. Expert Opin Drug Deliv. 2010;7(5):617–629. doi: 10.1517/17425241003663228. [DOI] [PMC free article] [PubMed] [Google Scholar]