Abstract
Fast ripples are high-frequency, 250-600 Hz field potential oscillations which can be recorded from hippocampal or neocortical structures. In the neocortex, fast ripples occur during both sensory information processing and under pathological, epileptic conditions. In the hippocampus and entorhinal cortex, fast ripples are exclusively associated with epilepsy and perhaps even mark the epileptogenic focus. In contrast to ripples, which regularly also occur in normal tissue and which are thought to reflect population spike bursts at 100 to 200 Hz paced and synchronised by recurrent inhibition, the fast ripple frequency range exceeds the maximal firing frequency of most neurones. Hence, particularly in the hippocampus, fast ripples must emerge as a network phenomenon and cannot reflect the activity of single spiking neurones. In this review, current views on the mechanisms and processes underlying fast ripples are discussed.
Keywords: ripples, fast ripples, oscillations, hippocampus, neocortex, epilepsy, GABA
1. Ripples vs. Fast Ripples: Definition and significance
Ripples, i.e. fast oscillations at 100-200 Hz, have been described in a variety conditions, and in different brain areas. In the neocortex, specifically the barrel cortex, they appear during sensory vibrissal stimulation (Jones and Barth, 1999, 2002, Barth 2003). In the hippocampus and entorhinal/perirhinal cortex, they were found to be associated with bursting episodes during slow-wave sleep, anaesthesia or behavioural immobility (Buzsaki et al., 1992; Ylinen et al 1995; Chrobak and Buszaki, 1996, Collins et al 1999, Csicsvari et al 1998, 1999,). Ripples were interpreted by the cited authors to serve important physiological functions, such as (sensory) information processing and memory consolidation. In the hippocampus, ripples are thought to reflect sparsely firing pyramidal cells and perisomatic inhibitory activity in phase with the oscillation (Ylinen et al., 1995). In the neocortex, highly synchronised population spikes appear to generate ripples, possibly synchronised via gap junctions – however without the involvement of GABAergic inhibition (Jones and Barth 2002; Barth, 2003). While a link of ripples to epilepsy might be suggested since they also appear in recordings from epileptic foci (Allen et al., 1992, Fisher et al., 1992), others actually think they may prevent epileptiform discharges by reducing pyramidal cell recruitment into such an event (Ylinen et al., 1995).
What about fast ripples then? They are defined as oscillations at yet higher frequencies than ripples, in a range of 250-600 Hz, and from this definition comes the synonym “high frequency oscillations”. In the neocortical literature, some authors do not distinguish between ripples (<200 Hz) and fast ripples (>250 Hz) as strictly. These terminology differences may have arisen due to difference in the association of fast ripples and pathology in the hippocampus and neocortex. Whereas hippocampal fast ripple activity is uniquely associated with epileptic activity (Engel et al. 2009; Jiruska et al. 2010a), fast ripple activity in the neocortex can be associated with either epileptic tissue (Jacobs et al. 2009, 2010) or physiological sensory information processessing (Barth 2003). Hippocampal fast ripples may represent a marker for hippocampal sclerosis and cell loss (Staba et al., 2007; Ogren et al., 2009, Bragin et al., 2010), but hippocampal lesions are not a prerequisite for their emergence (Jiruska et al., 2010a).
How are fast ripples generated? As already is evident from the frequency spectrum, it is difficult to conceive fast ripples to emerge from tightly synchronised population bursting, since none of the cells in the hippocampus or neocortex can fire at frequencies beyond 250 (or 300 Hz at most). Hence, frequency components above this range must originate form something else. The possible mechanisms underlying such fast ripples will be discussed in the following sections, first for the hippocampus and then for neocortical tissue.
2.1 Generation of Ripples and Fast Ripples in the epileptic Hippocampus
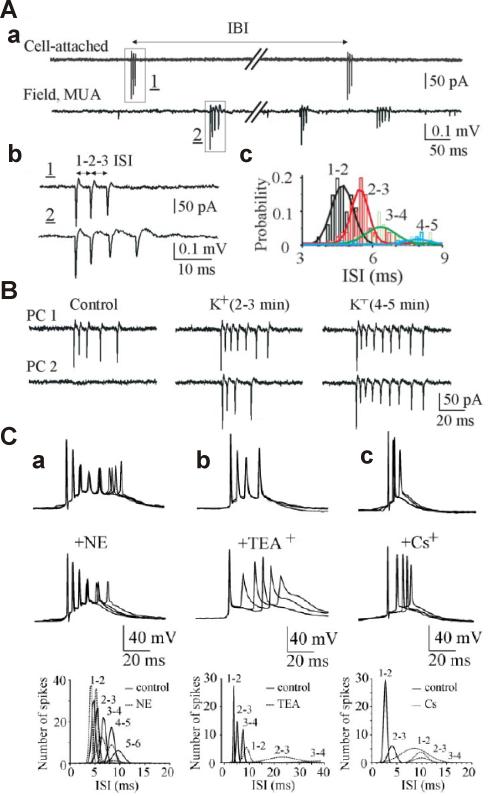
What is the neuronal basis of fast ripples? To address this question, one should again take a closer look to cellular mechanisms of ripple generation first. From the work of group of Buszaki, we know much about the in vivo situation, where it appears that ripples are being generated by sparse pyramidal firing (sinks in the cellular layers) and more importantly, in-phase perisomatic strong inhibition generating currents large enough to generate sources in the extracellular field, which together with the sinks make up the oscillation (Ylinen et al., 1995). While in vivo, pyramidal neurones fire sparsely and not on every cycle, the inhibitory circuitry that subserves sparse firing is disrupted by brain slice preparation. Thus, in vitro, pyramidal cells fire more consistently with each cycle (Dzhala and Staley, 2004): Under typical in vitro recording conditions, pyramidal cells rarely fire action potential bursts, so when these bursts occur they are generally asynchronous both in CA1 and in CA3 (Fig. 1Aa). Such sparse firing would normally not be seen in field potential recordings (Fig. 2A). However, under epileptogenic conditions, for example when the extracellular K+ concentration is raised beyond 8 mM, the difference between resting membrane potential and action potential threshold is sufficiently reduced (Staley et al. 1998) that the recurrent excitatory connections that survive slicing are sufficient to subserve synchronous population bursts (Figs. 1B, 2B). These busts thus all possess very similar temporal structures (in other words, the inter-burst interspike intervals of individual pyramidal cells are nearly identical) due to similar spike timing mechanisms in each pyramidal cell: namely the afterhyperpolarisation current (IAHP), the delayed rectifier potassium (IKd) and fast potassium currents (IA). Thus population synchrony under relatively disinhibited conditions in vitro arise from the similar clocking mechanisms in sparsely connected pyramidal cells. The relative importance of the clocking mechanism is by the substantial desynchronizatoin of the population response after blockade of the potassium conductances (Fig. 1C). Taken together, intrinsic firing properties of neurones generate ~200 Hz firing patterns, which, when afferent synaptic activity is strong enough, will be initiated more or less synchronously in a large number of neurones due to extensive afferent and recurrent innervation within the hippocampus.
Figure 1.
Spontaneous action potential bursts do not occur synchronously in hippocampal slices, synchronise under epileptogenic conditions (high K+) but have similar inter-spike-intervals as determined by intrinsic firing properties. A. Burst-type action-potential activity of CA3 pyramidal cells. Aa, Simultaneous cell-attached recording from the CA3 pyramidal cell (top trace) and 200-μm-apart extracellular field potential recording (bottom trace) of multiple unit activity in the CA3 pyramidal cell layer in a rat hippocampal slice in vitro. Spontaneous bursts of action potentials are present in both records. Ab, Spontaneous bursts from cell-attached and extracellular recordings of two different cells were superimposed and shown on an expanded time scale. The superposition demonstrates that both neurones have nearly identical inter-spike-intervals, suggesting that intrinsic firing properties of neurones lead to similar burst rhythms. Ac, Distributions of ISIs (bin size, 0.2 msec) from extracellular recording of multiple unit activity in CA3 pyramidal cell. ISIs are range between 4.7±0.05 msec (1–2 ISI) and 5.6±0.08 msec (2–3 ISI). A Gaussian equation was used to fit a curve to the active data plots. B. Effects of establishing epileptogenic conditions by raising extracellular K+-concentration [K+]o to 8.5 mM on spontaneous burst-type discharges. Dual cell-attached recordings from neighbouring CA3 pyramidal cells (PC1 and PC2) in a hippocampal slice. Bath application of 8.5mM [K+]o increased neuronal firing rate and decreased time delay between burst onset in individual pyramidal cells, resulting in their synchronisation. C. Contribution of intrinsic cellular properties to the intraburst ISIs. a-c, CA3 pyramidal cells were recorded in whole cell current-clamp mode at the resting membrane potential. Superimposed traces represent spontaneous burst-type action potential activity in control (top traces) and after application of norepinephrine (NE; 20 μM), TEA+ (2 mM), and Cs− (3 mM) (bottom traces). Plots below represent distribution of the intraburst ISIs in control and after drug applications (bin lag, 0.5 msec). ISIs were decreased after suppression of IAHP and increased after IKd and Ih suppressions. A Gaussian probability distribution function was used to fit a curve to the active data plot. (from Dzhala & Staley, The Journal of Neuroscience 24, 8896-8906, 2004, with permission)
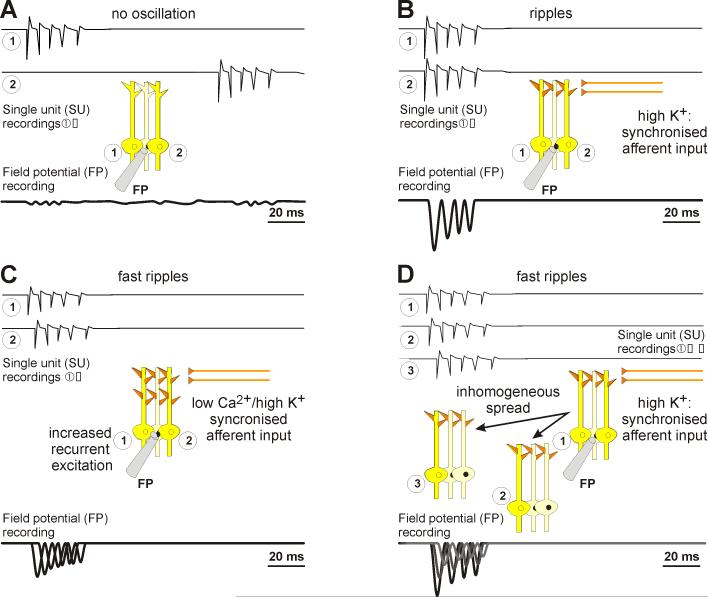
Figure 2.
Schematic illustrations of ripple and fast ripple generation. A. Under non-epileptogenic conditions, neurones fire bursts, but too rarely to coincide temporally. Hence, bursts of similar frequency and inter-spike intervals can be observed in single unit (SU, numbered circles), but not in field potential (FP) recordings. B. Elevating extracellular K+ concentration raises the bursting propensity in neurones. These will hence start to bust synchronously. Since their intrinsic firing properties are similar, inter-spike intervals are similar (~ 5 ms) and thus, enough SUs discharge to cause ripples (~200 Hz) to emerge in FP recordings. C. If neurons fire out of phase, the summed activity can be at a higher frequency than the discharge of any one neuron. However, if the neurons are too close physically, current sources and sinks may overlap, reducing the amplitude of extracellularly-recorded field potentials. D. Phase differences between groups of neurons, where neurons fire synchronously within each group, can also result in frequency multiplication at the level of the field potential. The number of groups and the group firing frequency appears to vary substantially.
What does this mean for fast ripples? It was suggested that fast ripples emerge as hypersynchronous firing of highly interconnected neuronal clusters, in which feedback inhibition had lost effectiveness (Bragin et al., 2002). Indeed, small and defined regions of high-frequency oscillations can be identified and recorded from in human epileptic brain (Bragin et al., 1999, 2010), as well as in chronic animal models (Bragin et al., 2002), the latter of which expand to become larger areas once inhibition is blocked (Bragin et al., 2002). Hence, very focal fast ripple activity has been shown to be under inhibitory control regarding its spatial extent, but independent of inhibition regarding its generation. This is well in line with intrinsically generated bursts. However, as we will recall, fast ripples would require intrinsic busting of neurones to exceed firing frequencies of 250 Hz (up to 600 Hz), which for a single cell with at least some refractoriness and a certain spike duration (usually around 1.5 to 2 ms) is impossible.
Thus neurons cannot fire on every cycle of a fast ripple. What has been proposed instead is that smaller groups of neurons fire at lower frequencies (Foffani et al. 2007; Jiruska et al. 2010b; Ibarz et al. 2010). Ordinarily, this would engender very little power in extracellular recordings, because the synchronously firing groups of neurons would discharge at random phase intervals (i.e. fractions of a cycle) with respect to other groups, and this random phasing would result in destructive interference; current sinks generated by one group would coincide with current sources from another, and no extracellular potentials would be generated (Staley 2007). However, under epileptic conditions these synchronously firing groups of neurons begin to fire with fixed phase relations to each other, at least during fast ripples. Here it gets a bit tricky. The neurons in any one group are firing in phase with each other, i.e. during peaks of the local ripple oscillation. But during fast ripples, the oscillating groups are out of phase with each other. This results in frequency multiplication: one group is firing when another is silent, so a distant, extracellular electrode will record twice as many peaks per unit time. A minor complication of this hypothesis is that there are some anatomical constraints imposed by this hypothetical mechanism: as discussed earlier, the out-of-phase groups of oscillating neurons can’t be too close, otherwise the current sources and sinks will generate destructive interference, abolishing the fast ripple.
The group-phase-difference hypothesis was first proposed by Foffani et al. 2007. They found high frequency oscillations in slices from epileptic animals, but not in control slices. Although the initial proposed mechanism for phase differences between groups was jitter in neuronal spike timing, this hypothesis has evolved in light of the evidence that such jitter reduces in-group synchrony (Dzhala and Staley 2003; Ibarz et al. 2010). The mechanism for the phase differences between groups remains unclear, but new studies have provided additional experimental evidence supporting the basic hypothesis of group phase differences. Jiruska et al., (2010b) show, in acute epilepsy models, both with synaptic transmission intact (high-K+ model) and abolished (low-Ca2+ model), that hippocampal CA1 neurones fire nearly synchronously with the fast ripple oscillation, but at much lower frequencies (single action potentials at 4 Hz), with coincident firing of ~4% of neurons per cycle of the fast ripple. These oscillations were attributed to pyramidal cell firing, as firing probability only slightly increased during oscillations for interneurones. Since fast ripples emerged also in the absence of synaptic transmission, and in the presence of gap junction blockers, the authors conclude ephaptic interactions to be responsible to synchronise co-firing of neurones during these fast ripples, at least in the densely packed CA1 pyramidal layer (Jiruska et al., 2010b). The primary differences between the chronically epileptic tissue (Foffani et al. 2007; Ibarz et al. 2010) and acutely epileptic preparations (Jiruska et al. 2010) has been the size of the groups of synchronously firing neurons: many small, slowly firing groups in acute preparations whereas in chronically epileptic tissue, only two groups of neurons were firing, but each group was oscillating at high (ripple) frequencies (Fig. 2C).
This leaves open the key question as to what engenders the phase differences between groups of synchronously firing neurons. One possibility is irregular spread of neuronal activity (Fig. 2D). In hippocampal slices, very local discharges occur which do not manage to spread throughout the neuronal network (de la Prida et al. 2006). Because such local discharges may create locally refractory areas of the network (Staley et al. 1998), the subsequent spread of epileptic activity may be spatially inhomogeneous, resulting in groups of neurones firing synchronous bursts that are out of phase with other groups due to differences in conduction delays as activity spreads around the refractory areas. This possibility is could be evaluated using activity-dependent calcium imaging (Staley 2007).
In view of the important role of IPSP synchronised in phase to the oscillation in case of ripples, as suggested by Ylinen et al (1995), one important question which remains is whether also fast ripples might be dependent on inhibitory activity. This, however, does not seem to be the case, independent of whether neurones are firing sparsely at low frequencies (but in phase to the oscillation), or whether they fire intrinsically at high frequencies, but with temporal jitter. Thus, in the first case, inhibitory activity is not necessary, since oscillations arose in conditions of suppressed synaptic transmission (Jiruska et al., 2010b). In the other case, blockade of GABAA-ergic transmission does not suppress fast ripples (Foffani et al., 2007), and neither is this the case in a model of dentate-gyrus fast ripples induced by picrotoxin-application, hence in a model relying on GABA-A suppression (D’Antuono et al., 2005). Save for the non-synaptic model, AMPA-receptor-mediated, glutamatergic transmission, however, is essential, presumably to generate enough recurrent excitation (Dzhala and Staley, 2004, D’Antuono et al., 2005, Foffani et al., 2007). Equally, gap junctional coupling may have some synchronising role (D’Antuono et al., 2005), although not, again, in the non-synaptic 0-Ca2+-model with enhanced ephaptic effects (Jiruska et al., 2010b).
2.2 Generation of Ripples and Fast Ripples in the epileptic Neocortex
Do the same mechanisms as in the hippocampus hold true for the generation of fast ripples in the neocortex? Data correlating field potential oscillations to intracellular behaviour, which would reveal the cellular nature of neocortical fast ripples, are much scarcer than from hippocampus. Nevertheless, differences might be present, as fast ripples actually also occur in the neocortex not only under pathological conditions, but also with sensory information processing (Jones and Barth, 1999, 2002; Jones et al., 2000, Barth 2003). Importantly, in the neocortex some cells, namely fast-spiking interneurones, actually discharge at fast ripple frequency (~ 500 Hz), and in phase of the oscillation, suggesting that they contribute to its emergence (Jones et al., 2000). In this study, besides interneurones, also regular spiking neurones show phase-coupled membrane potential oscillations, which however do not always result in spikes, and usually only yield doublet action potentials, even though the oscillation obviously consists of more oscillatory cycles. It is hence likely that at least some high frequency oscillations actually faithfully mirror interneuron spiking in the neocortex. Interestingly, the rhythm of this oscillation is not disturbed by GABAA-blockade (Jones and Barth, 2002), but Kynurenate suppresses them (Ikeda et al., 2002), so that while the precise rhythm may be generated intrinsically in fast spiking interneurons, excitatory drive is still required for summation to extracellularly recorded fast ripple activity. Both fast ripples (Barth, 2003) and ripples (Grenier et al., 2001) synchronise in the submillisecond range; hence if chemical synaptic transmission is a synchonising factor, many active synaptic inputs would be required per neuron to generate sufficiently precise spike timing between neurons. Gap junctions could also provide this synchronization, and ripples are reduced or abolished with halothane anaesthesia, which might indicate gap junctional coupling to be necessary for them (Grenier et al., 2001, Timofeev and Steriade, 2004). However, these anesthetics have many effects, including substantial prolongation of GABAA receptor-mediated conductances (Banks and Pierce 1999), so further testing of the role of gap junctions awaits sufficiently selective pharmacological antagonists and knockout animals. The data cited regarding the neocortex mainly pertain to normal cortex, and sleep or sensory information processing states, and hence, further investigations are necessary to clarify whether the epileptic neocortex generates fast ripples in the same way, or by different mechanisms. Yet, at least ripple-frequency oscillations seem to be associated with epilepsy (Timofeev and Steriade, 2004), and also in these conditions, it is fast spiking neurones, albeit fast rhythmic bursting neurones and less fast spiking ones are thought to sustain them.
3. Conclusion
Summarising the literature findings, it seems rather likely that fast ripples mirror groups of synchronously firing neurons whose oscillations are out of phase with other groups. Recent findings suggest that both the size of the groups and the frequency of the group oscillations vary widely depending on experimental conditions. The fact that fast ripples persist after GABAA-receptor blockade suggests that although interneurones can participate, their contribution is not necessary. The roles of gap junctional coupling or ephaptic interactions are controversial, but in most models, either of the two may play a role in synchronising larger networks (Traub, 2003). As a general technical note, care should be taken when recording fast activity, since filtering itself can generate fast components which actually do not occur in the raw data (Staley 2007; Bénar et al., 2010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen PJ, Fish DR, Smith SJ. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1992;82:155–159. doi: 10.1016/0013-4694(92)90160-j. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Dual actions of volatile anesthetics on GABA(A) IPSCs: dissociation of blocking and prolonging effects. Anesthesiology. 1999;90:120–134. doi: 10.1097/00000542-199901000-00018. [DOI] [PubMed] [Google Scholar]
- Barth DS. Submillisecond synchronization of fast electrical oscillations in neocortex. J Neurosci. 2003;23:2502–2510. doi: 10.1523/JNEUROSCI.23-06-02502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr., Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23:151–156. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr. Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr., Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci. 1999;19:RC20. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsáki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- Collins DR, Lang EJ, Paré D. Spontaneous activity of the perirhinal cortex in behaving cats. Neuroscience. 1999;89:1025–1039. doi: 10.1016/s0306-4522(98)00396-0. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, de Guzman P, Kano T, Avoli M. Ripple activity in the dentate gyrus of dishinibited hippocampus-entorhinal cortex slices. J Neurosci Res. 2005;80:92–103. doi: 10.1002/jnr.20440. [DOI] [PubMed] [Google Scholar]
- de la Prida LM, Huberfeld G, Cohen I, Miles R. Threshold behavior in the initiation of hippocampal population bursts. Neuron. 2006;49:131–142. doi: 10.1016/j.neuron.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci. 2004;24:8896–8906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Jr., Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, de la Prida L. Menendez. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:930–941. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80-200Hz) in neocortex and their neuronal correlates. J Neurophysiol. 2001;86:1884–1898. doi: 10.1152/jn.2001.86.4.1884. [DOI] [PubMed] [Google Scholar]
- Ibarz JM, Foffani G, Cid E, Inostroza M, de la Prida L. Menendez. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci. 2010;30:16249–61621. doi: 10.1523/JNEUROSCI.3357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Leyba L, Bartolo A, Wang Y, Okada YC. Synchronized spikes of thalamocortical axonal terminals and cortical neurons are detectable outside the pig brain with MEG. J Neurophysiol. 2002;87:626–630. doi: 10.1152/jn.00332.2001. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Châtillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Finnerty GT, Powell AD, Lofti N, Cmejla R, Jefferys JG. Epileptic high-frequency network activity in a model of non-lesional temporal lobe epilepsy. Brain. 2010;133:1380–1390. doi: 10.1093/brain/awq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, Csicsvari J, Powell AD, Fox JE, Chang WC, Vreugdenhil M, Li X, Palus M, Bujan AF, Dearden RW, Jefferys JG. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010;30:5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Effects of bicuculline methiodide on fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2002;88:1016–1025. doi: 10.1152/jn.2002.88.2.1016. [DOI] [PubMed] [Google Scholar]
- Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2000;84:1505–1518. doi: 10.1152/jn.2000.84.3.1505. [DOI] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Spatiotemporal organization of fast (>200 Hz) electrical oscillations in rat Vibrissa/Barrel cortex. J Neurophysiol. 1999;82:1599–1609. doi: 10.1152/jn.1999.82.3.1599. [DOI] [PubMed] [Google Scholar]
- Ogren JA, Wilson CL, Bragin A, Lin JJ, Salamon N, Dutton RA, Luders E, Fields TA, Fried I, Toga AW, Thompson PM, Engel J, Jr, Staba RJ. Three-dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66:783–791. doi: 10.1002/ana.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, Ogren J, Fried I, Wilson CL, Engel J., Jr. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Longacher M, Bains JS, Yee A. Presynaptic modulation of CA3 network activity. Nat Neurosci. 1998;1:201–209. doi: 10.1038/651. [DOI] [PubMed] [Google Scholar]; Nat Neurosci. 1998 Aug;1(4):331. Erratum in: [Google Scholar]
- Staley KJ. Neurons skip a beat during fast ripples. Neuron. 2007;55:828–830. doi: 10.1016/j.neuron.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Traub R. Fast oscillations and epilepsy. Epil. Curr. 2003;3:77–79. doi: 10.1046/j.1535-7597.2003.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]