Abstract
Low pH treatment of influenza virus hemagglutinin (HA) exposes its relatively conserved stalk domain, suggesting a potential immunogen with capability to induce broader immune responses. Here, we describe characterization, immunogenicity, antigenicity, and protective immunity induced by low pH treated inactivated whole viral vaccine in comparison with the untreated vaccine. The acidic pH treated viral vaccine showed high susceptibility to proteolytic cleavage and low hemagglutination activity indicating conformational changes. Immunization of mice with low pH treated viral vaccine induced lower levels of homologous or heterologous virus-specific binding and neutralizing antibodies compared to the untreated vaccine. Also, low pH treated influenza viral antigen showed lower antigenicity compared to the untreated influenza viral antigen. Lower efficacy of cross-protection against heterosubtypic virus was observed in the low-pH treated vaccine group. The results provide evidence that there is a correlation between protective efficacy and the stability of vaccines.
Keywords: Low pH, conformational change, immunogenicity, vaccine, influenza
Introduction
Influenza virus hemagglutinin (HA) is the major target for inducing virus-neutralizing antibodies after vaccination. Cleavage of the HA precursor molecule HAo is a required step to activate virus infectivity (Klenk et al., 1975; Lazarowitz and Choppin, 1975). The distribution of activating proteases in the host is one of the determinants of tropism and pathogenicity (Steinhauer, 1999). HA is a homotrimeric molecule, each monomer consisting of two disulfide-linked glycoproteins, a globular head of HA1 and a stem domain composed of part of HA1 and all of HA2 (Wilson, Skehel, and Wiley, 1981). The globular head contains the receptor-binding pocket surrounded by variable antigenic sites that have been identified by amino acid sequence changes in escape mutants selected by monoclonal antibodies and in natural variants (Laver et al., 1980; Laver et al., 1979). The locations of four antigenic sites, designated A, B, C, and D, agree well with antigenic mapping studies of the HA molecule using monoclonal antibodies (Gerhard et al., 1981; Wiley and Skehel, 1987). The recurrence of influenza virus infection in humans is primarily due to mutations occurring in the antigenic sites of the HA1 globular head domains.
Influenza viruses are known to fuse with liposomes at the acidic pH found in lysosomes but not at neutral pH (Maeda, Kawasaki, and Ohnishi, 1981; Yoshimura et al., 1982). Structural analysis of the HA molecule shows that the hydrophobic fusion peptide is located in the stem region of the HA spike proximal to the viral envelope (Wiley, Wilson, and Skehel, 1981; Wilson, Skehel, and Wiley, 1981). The stem is mainly composed of two antiparallel α-helices of unequal length linked by an extended polypeptide chain. At the pH of membrane fusion, HA increases its hydrophobic properties due to exposure of the fusion peptide, and undergoes additional conformational changes that allow for proteolytic cleavage at newly-susceptible residues in the HA1 subunit (Skehel et al., 1982). This acid-induced change of HA is largely irreversible, even when the molecule is returned to neutral pH, and includes the loss of the trimeric structure of the globular head domains (Bizebard et al., 1995; Ruigrok et al., 1988; Skehel et al., 1982). Meanwhile, the stem structure is known to remain in a trimeric structure despite its extensive refolding and reorganization (Bullough et al., 1994; Ruigrok et al., 1988).
Previous studies demonstrated that certain monoclonal antibodies were reactive to the low-pH induced but not to the native conformation of HA, especially on the stem region of the HA molecule, where HA2 is relatively well conserved among influenza A viruses (Ekiert et al., 2009; Kostolansky et al., 1988; Vanlandschoot et al., 1998; Webster, Brown, and Jackson, 1983). Some of these monoclonal antibodies have been reported to show broadly neutralizing activity, probably by preventing the fusion step of virus entry (Prabhu et al., 2009; Sui et al., 2009; Wang et al., 2010b). Also, vaccines designed to induce antibodies against the stalk of HA were recently shown to provide cross protection against lethal challenge (Bommakanti et al., 2010; Hashem et al., 2010; Stanekova et al., 2011; Steel et al., 2010; Wang et al., 2010a). However, the efficacy of cross protection was relatively low, not being able to prevent weight loss.
In the present study, we have addressed the question whether the low-pH induced structural and conformational changes in the context of whole influenza virus might provide an immunogen that can induce broad cross protection. We compared immunogenicity, antigenicity, and cross protection of the inactivated H1 influenza virus as a vaccine before and after treatment at pH 5.0.
MATERIALS AND METHODS
Virus and cells
Influenza viruses A/PR8/34 (H1N1), A/WSN/33 (H1N1) and A/Philippines/2/82 (H3N2) were grown in 10-day-old embryonated hen's eggs and purified from allantoic fluids by using a discontinuous sucrose gradient (15%, 30%, and 60%). Inactivation of the purified virus was performed by mixing the virus with formalin at a final concentration of 1:4,000 (vol/vol) as described previously (Quan et al., 2008). Inactivation of the virus was confirmed by plaque assay on confluent monolayers of Madin-Darby canine kidney (MDCK) cells and by inoculation of the virus into 10-day-old embryonated hen's eggs. For challenge experiments, mouse-adapted A/Philippines/2/82 (H3N2) virus was prepared as lung homogenates from intranasally infected mice and used for challenge.
Low pH treatment of inactivated virus and trypsin cleavage
Inactivated egg-grown A/PR8/34 virus (A/PR8) in 150 μl of phosphate-buffered saline (PBS) at a protein concentration of 3 mg/ml was mixed with 24.4 μl of low pH buffer (30 mM H3PO4, 150mM NaCl, 2.7mM KCl) to reduce pH to 5.0, followed by incubation at 37°C for 10 min. 174 μl of neutralizing buffer (86mM Na2HPO4, 2.7mM KCl, and 150mM NaCl) were added to the mixture to neutralize the pH. To examine the low pH conformational change, the PBS buffer (mock) treated control and low-pH treated inactivated A/PR8 viruses (1.29 mg/ml after treatment) were digested in the absence or presence of 20 μg/ml of TPCK trypsin at 37°C for 10 min. The trypsin-mediated cleavage products were confirmed by Coomassie blue staining and Western blot using mouse anti-A/PR8/34 virus serum. .
Hemagglutination activity assay
Low-pH treated and untreated inactivated A/PR8 virus vaccines were serially diluted and used to determine their hemagglutination activity titers. Serially diluted A/PR8 virus vaccines (50 μl) were incubated with 50 μl of 0.5% chicken erythrocytes in each well. The highest dilution of low-pH treated and untreated inactivated A/PR8 viruses capable of hemagglutination was scored as the HA titer. Presented data are the means with standard deviation from three independent replicate experiments.
Immunization and challenge
Female BALB/c mice aged 6 to 8 weeks were purchased from Charles River Laboratories and used for immunization studies. Mice were intranasally immunized with 50 μl phosphate-buffered saline (PBS) containing 25 μg of inactivated A/PR8 virus treated with low-pH at days 0 and 30. The same amount of inactivated A/PR8 virus was used as an untreated control for comparison. For challenge infections, isoflurane-anesthetized mice were challenged with A/Philippines/82 (2 × LD50) at week 4 after boost. Mice were observed daily to monitor changes in body weight and to record survival rates (25% loss in body weight as the Institutional Animal Care and Use Committee (IACUC) endpoint). All animal experiments and husbandry involved in the studies presented in this manuscript were conducted under the guidelines of the Emory University IACUC. Emory IACUC operates under the federal Animal Welfare Law (administered by the USDA) and regulations of the Department of Health and Human Services.
Enzyme-linked immunosorbent assay (ELISA)
Blood samples were collected by retro-orbital plexus puncture before immunization and 3 weeks after boost. Samples were then spun in a microcentrifuge for 10 min and supernatants were collected. Influenza virus-specific immunoglobulin IgG, IgG1, IgG2a, and IgG2b antibodies (isotypes) were determined in sera by enzyme-linked immunosorbent assay (ELISA). As ELISA coating antigens, purified egg-grown inactivated influenza A/PR8, A/WSN, or A/Philippines/2/82 virus (4 μg/ml) was coated onto 96-well microtiter plates using 100 μl in coating buffer (0.1 M sodium carbonate, pH 9.5) at 4°C overnight. The serum samples were serially diluted and added onto plates after blocking with 3% bovine serum albumin. The plates were then incubated with horseradish peroxidase-labeled goat anti-mouse IgG, IgG1, IgG2a and IgG2b antibodies at 37°C for 1.5 hrs. The substrate O-phenylenediamine in citrate-phosphate buffer (pH 5.0) containing 0.03% H2O2 was used to develop color. The optical density at 450 nm was read using an ELISA reader.
Neutralizing activities
Mouse sera were inactivated at 56°C for 30 min and then serially diluted in DMEM using 96-well assay plates and virus neutralizing activities were determined as described (Quan et al., 2007). Live influenza virus (A/PR8, A/WSN, or A/Philippines/2/82) was diluted in DMEM media and incubated with serially diluted mouse sera at 37°C for 1 hr and then added to prewashed, confluent monolayers of MDCK cells. Plates were incubated for 2 days, the cells were fixed with 0.25% glutaraldehyde and stained with 1% crystal violet to visualize plaques. The mean percent plaque reduction by sera from vaccinated mice compared to sera from naïve and medium control were determined. The highest serum dilution showing 50% plaque reduction in comparison to the negative control was taken as the neutralizing-antibody titer.
Statistics
All parameters were recorded for individuals within all groups. Statistical comparisons of data were carried out using the analysis of variance and Npar one-way Kruskal-Wallis tests of the PC-SAS system. P values of < 0.05 were considered significant.
Results
Exposure of inactivated virus to acidic pH lowers hemagglutination activity
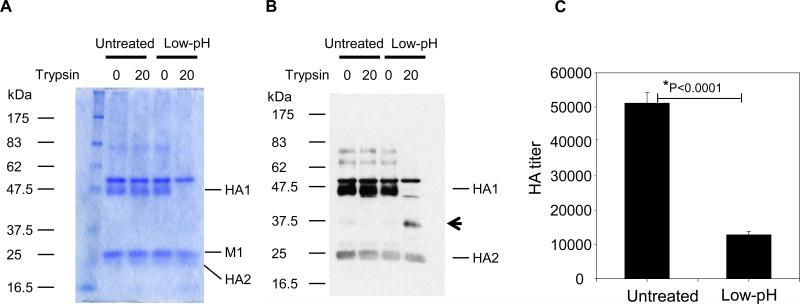
In order to expose conserved domains of HA2, inactivated influenza virus (A/PR8) was exposed to the acidic pH of 5.0. It is known that low pH induced conformational changes in HA result in susceptibility to proteolytic cleavage (Skehel et al., 1982). Untreated influenza virus did not show differences in the pattern of viral proteins separated on the SDS-PAGE before and after proteolytic digestion with a low concentration of trypsin as shown by coomassie blue staining (Fig. 1A) and western blot (Fig. 1B). In contrast, treatment of low pH treated inactivated A/PR8 virus with the same concentration of typsin resulted in cleavage of the HA proteins as shown by a decrease in the full length HA1 protein (Fig. 1A and 1B), and the appearance of lower molecular weight digestion products (Fig. 1B, column 4). These observations confirm that exposure of inactivated virus to low pH resulted in the characteristic HA structural rearrangements, and are consistent with a previous study on bromelain-released ectodomains of HA (Skehel et al., 1982). The hemagglutination activity of inactivated virus after exposure to low pH was found to be significantly reduced by 4 to 5 fold compared to the untreated inactivated influenza virus (Fig. 1C). These results indicate that low pH induced conformational changes in inactivated vaccines result in structural changes that confer increased susceptibility to proteolytic cleavage as well as a decrease in receptor binding activity.
Fig 1. Low pH treated inactivated virus is susceptible to proteolytic cleavage and shows lower hemagglutination activity.
(A) Coomassie blue staining of SDS-PAGE. (B) Western blot of low pH treated and mock treated A/PR8 virus. (A-B) Inactivated A/PR8 virus was exposed to pH 5.0 at 37°C for 10 min, and then returned to neutral pH. Proteolytic digestion was performed at neutral pH with TPCK-treated trypsin. Western blot was probed with mouse anti-A/PR8/34 virus serum to detect protein bands on SDS-PAGE. M1 is the matrix protein. Arrowhead indicates an HA1 cleavage product after trypsin treatment. Untreated: virus at neutral PBS buffer. 0 and 20 indicate the concentration of trypsin (μg/ml) treated. Low pH: treatment at pH 5.0. Concentrations of trypsin used are indicated 0 and 20 (μg/ml). (C) Hemagglutination activity of low pH treated and untreated A/PR8 virus. The same amount of untreated or low-pH treated A/PR8 virus (1.29 mg/ml, 50μl) was used to determine hemagglutination activity. HA titers were determined as the highest dilution of highly purified influenza A/PR8 virus using chicken red blood cells. Titers shown in this figure represent approximately 50 fold concentrated virus after sucrose gradient ultracentrifugation. Untreated: A/PR8 virus suspended in neutral pH PBS buffer, Low-pH: low-pH treated A/PR8 virus.
Antibody responses to low pH exposed influenza vaccines
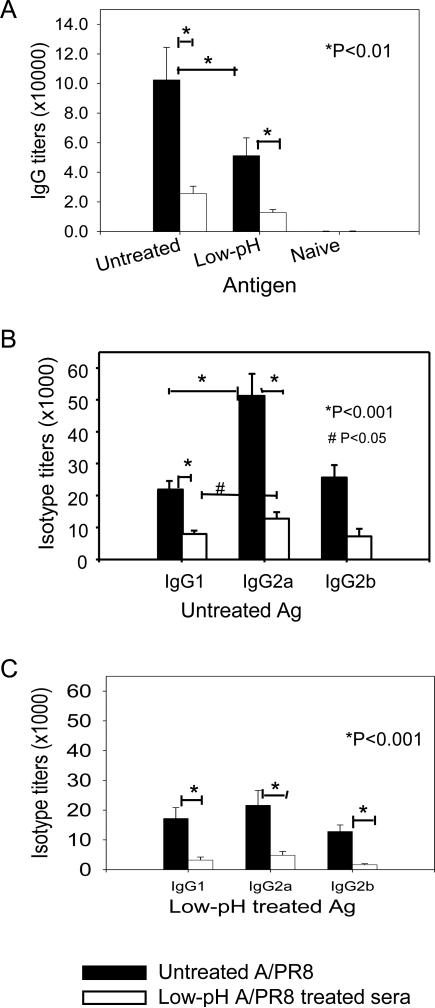
We hypothesized that changes in the conformation and receptor binding sites in HA would affect the immunogenicity and protective efficacy of the influenza virus vaccine. To test this hypothesis, groups of mice were intranasally immunized with 25 μg of untreated or low-pH treated A/PR8 vaccines at weeks 0 and 4. Serum samples were collected at 3 weeks after boost vaccination and used for determination of antibody titers. When antibody responses were compared by using untreated A/PR8 virus as an ELISA coating antigen (Fig. 2A), total IgG antibody titers were higher in the untreated A/PR8 group compared to the group that was immunized with low pH treated A/PR8 virus vaccine. The untreated A/PR8 group showed higher levels of antibodies even to the low-pH treated A/PR8 antigen compared to the low-pH treated A/PR8 group.
Fig 2. Low pH treated inactivated virus induces lower antibody responses and shows less reactivity with immune sera.
(A) Total IgG antibody responses reactive to untreated or low-pH treated A/PR8 viral antigens. Untreated A/PR8 sera: Immune sera from mice immunized with untreated inactivated A/PR8 virus kept at neutral pH, Low-pH A/PR8 sera: Immune sera from mice immunized with low-pH treated inactivated A/PR8 virus. X-axis; untreated or untreated Ag: antibody titers reactive to untreated A/PR8 virus used as an ELISA antigen, Low-pH: antibody titers reactive to low-pH treated A/PR8 virus used as an ELISA antigen, Significances were found between low-pH and untreated groups either using low-pH treated or untreated A/PR8 viral antigen (P < 0.01). (B) Isotype antibodies reactive to untreated A/PR8 viral antigen. IgG1, IgG2a and IgG2b antibodies were determined by ELISA using untreated A/PR8 virus as an antigen. Significance was found between IgG2a and IgG1 from the untreated control (P < 0.001) and between untreated A/PR8 and low-pH treated A/PR8 groups (P < 0.001). (C) Isotype antibodies reactive to low-pH treated A/PR8 viral antigen. IgG1, IgG2a and IgG2b antibodies were determined by ELISA using low-pH treated A/PR8 virus as an antigen. A significant difference was found between untreated A/PR8 and low-pH treated A/PR8 groups (P < 0.001).
Since we observed some differences in total IgG antibody responses, isotypes of IgG antibodies were determined. High levels of IgG2a and similar levels of IgG1 and IgG2b isotype antibodies reactive to untreated A/PR8 virus antigen were observed in the untreated A/PR8 group (Fig. 2B). In the low pH treated A/PR8 group, both antibody isotypes reactive to untreated A/PR8 virus antigen were detected at lower levels compared to the untreated A/PR8 group (Fig. 2B). The untreated A/PR8 group showed higher levels of isotype antibodies even when isotype antibodies were determined using low-pH treated A/PR8 virus as an ELISA antigen. Particularly, immunization with low-pH treated A/PR8 antigen was not as effective in inducing IgG isotype antibodies reactive even to the same low-pH treated vaccine antigen. These results suggest that the low pH exposed A/PR8 virus is less effective in eliciting antibodies reactive to itself or A/PR8 antigen.
Low pH treated influenza vaccines do not induce increased cross-reactive antibody responses
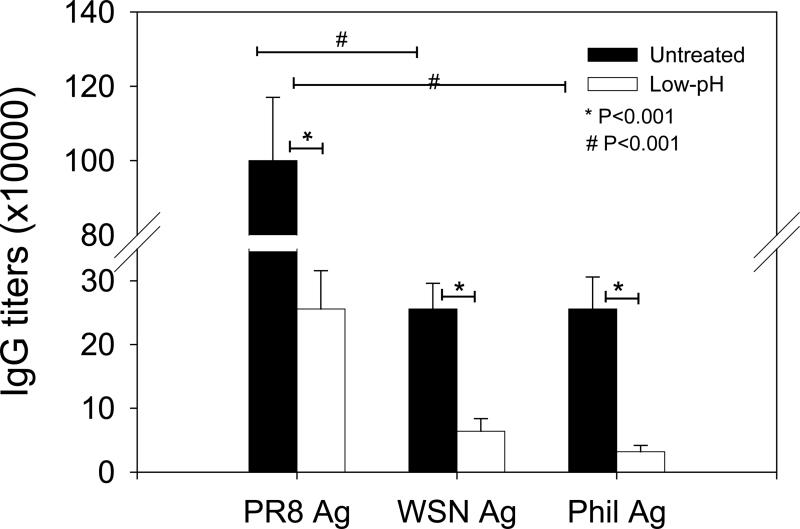
We determined whether the low-pH treated inactivated virus might induce enhanced cross reactivity to different viruses. The group of mice immunized with low-pH exposed A/PR8 vaccines showed approximately 4 fold lower titers of antibodies reactive to the homologous A/PR8 virus compared to the untreated A/PR8 vaccine group (Fig. 3). When the H3N2 heterosubtypic viral antigen (A/Philippines/82) was used as an ELISA antigen, 4 fold lower antibody titers were also observed, and they were proportionally reduced in both untreated and low-pH treated A/PR8 groups (Fig. 3). The low-pH treated influenza A/PR8 virus vaccine induced approximately 4 fold lower antibody titers cross-reactive to the heterologous H1N1 A/WSN/33 or heterosubtypic H3N2 A/Philippines/82 virus antigens compared to untreated A/PR8 viral vaccine. Therefore, the antibody reactivity of immune sera from low-pH treated virus is rather specific although the levels were low. These results indicate that low-pH treated whole inactivated vaccine is not more effective in inducing cross reactive antibody responses compared to the untreated viral vaccine.
Fig 3. Antibody responses cross-reactive to heterologous and heterosubtypic influenza viruses.
IgG antibody responses were determined and compared using homologous (A/PR8 H1N1), heterologous (A/WSN/33 H1N1), or heterosubtypic (A/Philippines/82 H3N2) virus as an ELISA antigen. Untreated A/PR8 sera: Immune sera from mice immunized with neutral pH inactivated A/PR8 virus kept at neutral pH, Low-pH A/PR8 sera: Immune sera from mice immunized with low-pH treated inactivated A/PR8 virus. Significance was found between low-pH and untreated mouse groups (P < 0.001) in antibody titers reactive to different influenza viral antigens tested. IgG antibody titers reactive to the homologous virus were higher than those binding to heterologous influenza antigens (P < 0.001).
Low-pH treated influenza vaccines are less effective in inducing cross-reactive neutralizing antibody responses
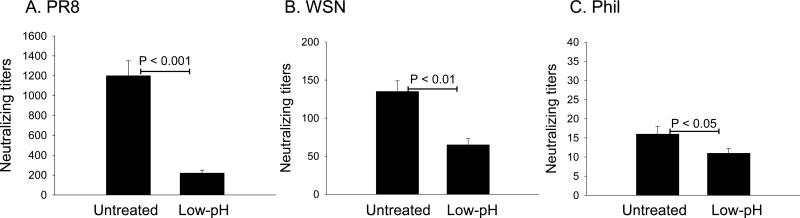
The A/PR8 virus vaccine was effective in inducing high titers of neutralizing antibody responses against the homologous A/PR8 virus, which were 6 fold higher than responses induced by the low-pH treated vaccine (Fig. 4). Significant levels of cross-neutralizing antibodies against the closely related A/WSN/33 virus were observed in the sera of the untreated A/PR8 immunized group although these titers were approximately 9 fold lower than those against the homologous virus. The low-pH treated group also showed significant cross neutralizing antibody titers only 2 fold lower than the untreated group. Cross- neutralizing antibodies against the antigenically unrelated heterosubtypic H3N2 A/Philippines/82 were observed with 80 fold lower levels in the sera of the untreated A/PR8 vaccine immunized group compared to those against the homologous virus. The low-pH treated A/PR8 vaccine group did not increase levels of antibodies neutralizing heterosubtypic A/Philippines/82 virus compared to the untreated group (Fig. 4). Therefore, these results suggest that low-pH induced conformational changes in HA do not increase cross-neutralizing antibody responses compared to the untreated influenza vaccine.
Fig 4. Cross-reactive neutralizing activity.
Neutralizing activities against (A) homologous H1N1 A/PR8 virus, (B) heterologous H1N1 A/WSN/33 virus, and (C) heterosubtypic H3N2 A/Philippines/82 virus were determined using immune sera from mice immunized with untreated or low-pH treated A/PR8 virus vaccine. The highest serum dilution showing 50% plaque reduction was taken as the neutralizing antibody titer. Significant differences in titers were found between low-pH treated group and untreated control group against PR8 (P < 0.001), WSN (P < 0.01) and Phil (P < 0.05).
Low-pH treated influenza vaccine confers reduced cross protection
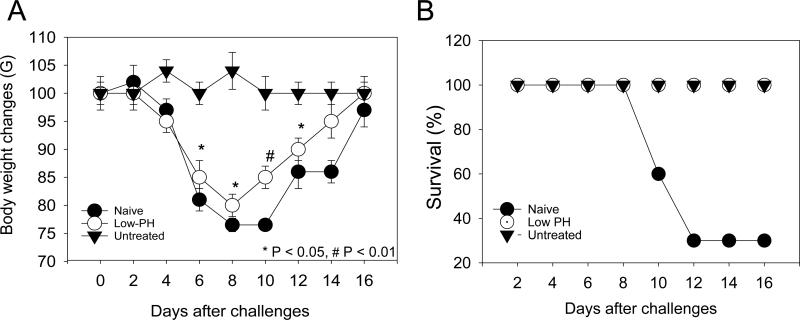
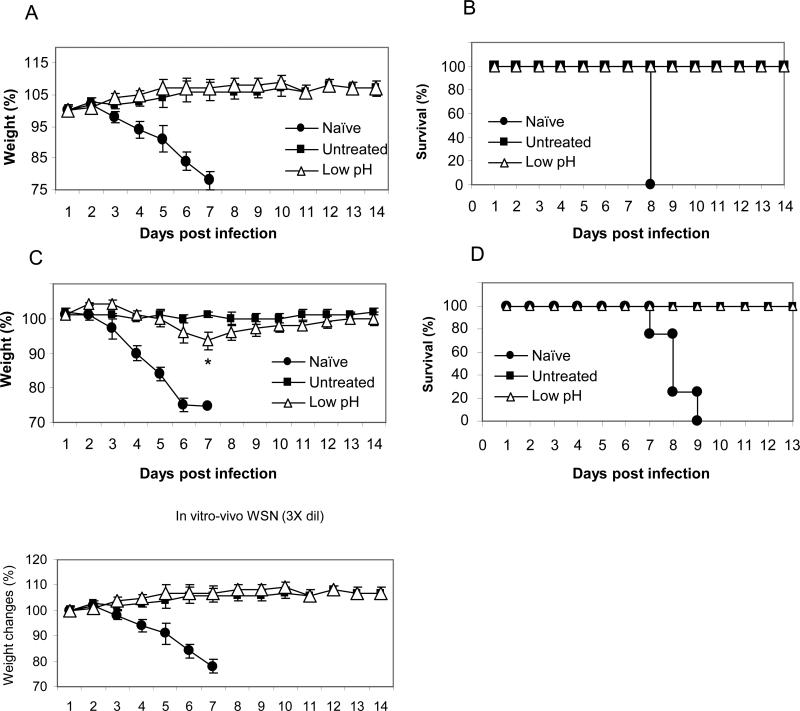
To determine heterosubtypic protection against challenge with H3N2 A/Philippines/82 virus, groups of mice were challenged with 2 LD50 dose of A/Philippines/82 virus, and their body weight changes and survival rates were monitored for 16 days. Unimmunized naïve mice displayed a rapid weight loss until day 8 post infection and the surviving mice showed a slow recovery (Fig. 5). Mice that were immunized with the untreated A/PR8 virus did not show any changes in body weights and all survived the challenge. In contrast, a substantial loss in body weight was observed in mice immunized with the low-pH treated virus (Fig. 5A). Despite the loss in body weight, the low-pH treated vaccine provided 100% survival against the heterosubtypic virus challenge compared to the 30% survival in naïve mice (Fig. 5B). Consistent with the results of antibody responses, the heterosubtypic challenge experiment suggests that the low-pH treated vaccine is less effective in inducing cross-protective immunity than the untreated influenza vaccine.
Fig 5. Low-pH treated vaccine is less effective in inducing heterosubtypic protection.
Mice that were immunized with low-pH treated or untreated A/PR8 viral vaccine were challenged with heterosubtypic A/Philippines/82 virus. Body weight loss (A) and survival rates of mice (B) were monitored for 16 days. Significant difference was found on body weight loss during days 6 – 12 post challenge between low-pH treated and untreated control (P < 0.01).
It is also important to determine the protective efficacy against influenza A/WSN/33 virus since cross-reactive antibodies induced by low-pH treated vaccines were lower for this virus (Figs. 3 and 4). As shown in Fig. 6, we determined the capability of immune sera to provide protection against A/WSN/33 virus in naïve mice as described previously (Song et al., 2011). All mice that received naïve sera showed severe body weight loss and were not protected (Fig. 6). Immune sera from both untreated and low-pH vaccination conferred complete protection against A/WSN virus at the dilution of 3 fold (Fig. 6A, 6B). With 6 fold-diluted immune sera, mice that received immune sera from the low-pH treated vaccine group displayed a transient loss of body weight which was not seen with serum from mice immunized with untreated vaccine (Fig. 6C, 6D). These results provide further evidence that low-pH treated vaccine does not induce more effective cross protective immunity compared to the untreated vaccine.
Fig. 6. Protective efficacy against influenza A/WSN/33 virus.
Immune sera collected from untreated or low pH treated A/PR8 virus vaccinated mice at 4 weeks after boost vaccination were incubated with a lethal dose of A/WSN/33 (H1N1) influenza virus at room temperature for 30 min. Groups of mice (n=4) were intranasally challenged with a lethal infectious dose (5 LD50) mixed with immune sera. (A-B) 3 fold diluted immune sera were used for incubating with A/WSN/33 virus. Body weight (A) and survival rate (B) were monitored for 14 days. (C-D) 6 fold diluted immune sera were used for incubating with A/WSN/33 virus. Body weight (C) and survival rate (D) were monitored for 14 days. *: p<0.05 at day 7 post infection.
Discussion
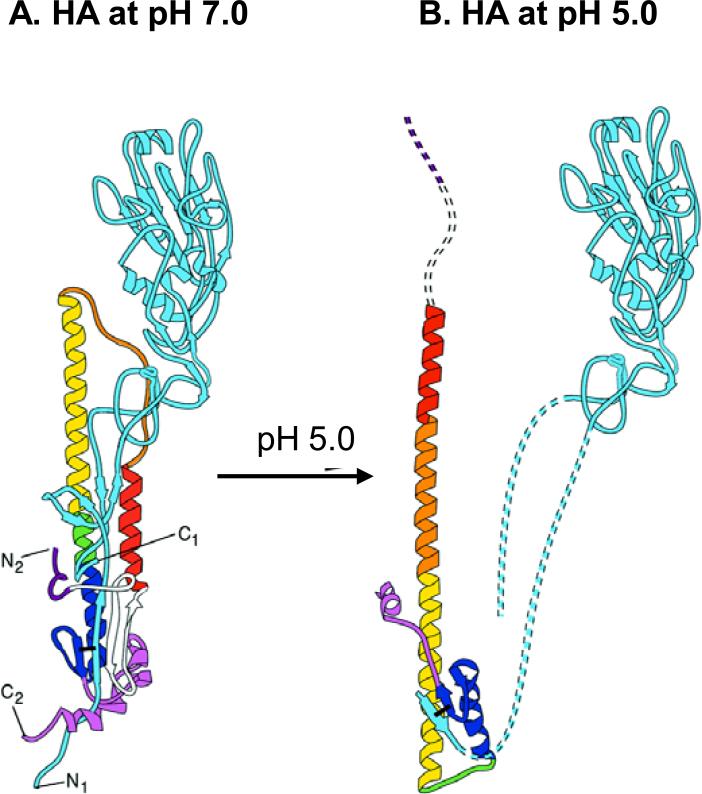
Exposing the untreated virus to an acid environment was shown to induce a significant conformational change in HA such that the HA2 domain is relocated to a favorable position to trigger membrane fusion of the virus and the target cell (Maeda, Kawasaki, and Ohnishi, 1981; Ruigrok et al., 1988; Skehel et al., 1982; White, Kartenbeck, and Helenius, 1982). In the present study, we observed two obvious changes after exposing inactivated influenza virus to pH 5.0. The HA protein in the low-pH treated inactivated virus became highly susceptible to proteolytic cleavage by trypsin, which is consistent with previous observations of increased HA hydrophobic properties and proteolytic cleavage after acidification (Skehel et al., 1982). Fig. 7 shows a structural depiction of the HA conformational changes that are induced upon exposure to low pH. In this model, hydrophobic fusion domains are likely to be exposed. Also, an approximately 4 fold decrease in hemagglutination activity was observed in the low-pH treated inactivated virus compared to the untreated virus vaccine, indicating potential changes in the receptor binding globular regions. It is possible that low-pH induced conformational changes might have occurred in the HA2 hydrophobic stalk region domains proximal to the viral membrane as well as in the globular head regions of receptor binding sites. These changes are consistent with an electron microscopic observation that stalks in the HA2 domain change to thinner and longer spikes on virus particles in the fusion pH conformation (Wharton et al., 1995).
Fig. 7. A diagram of low-pH induced conformational changes in HA.
(A) HA monomer at neutral pH. (B) HA monomer at low pH. The HA molecule undergoes several major conformational changes at pH 5.0. Step 1: HA1 domain in blue is detrimerized. Step 2: The fusion peptide of HA2 is extruded (red). Step 3: The loop region between short α-helix and long α-helix become α-helix (orange). Step 4: HA2 residues (106-112) shown in green that are located in the long α-helix are converted to loop with both N-terminal and C-terminal of HA2 on the same end. This diagram of low-pH conformational changes in HA is modeled as previously described (Skehel et al., 1995).
We found that immunization with low-pH treated influenza vaccines induced 3 to 4 fold lower levels of antibodies reactive to either untreated or low-pH treated influenza antigens compared to standard untreated influenza vaccines. These results have important implications. Protective immune responses by current influenza vaccination are largely targeted to the major antigenic regions in the HA1 globular head domain where receptor binding pockets are located. Low pH treatment causes changes in the receptor binding sites and in the globular head domain, which might alter the effective antigenic and immunogenic target sites. These changes in tertiary structures lead to specific changes in the antigenic sites B and D as shown by reactivity of monoclonal antibodies (Daniels et al., 1983; Yewdell, Gerhard, and Bachi, 1983). A study using monoclonal antibodies revealed that two antigenic regions that are located at the tip and interface of the HA molecule at neutral pH were lost or modified (Webster, Brown, and Jackson, 1983). It is also speculated that maintenance of HA receptor binding activity might be needed for effective interaction with antigen presenting cells. In previous studies, the stability of influenza vaccines as measured by levels of hemagglutination activity was shown to correlate with ability to induce protective immune responses (Kim et al., 2010; Quan et al., 2010; Quan et al., 2009). Another possibility is that the head domains of these immunogens might have short half-lives probably due to proteolytic degradation. Thus, these results suggest that low immunogenicity and antigenicity of acidic pH treated virus might be related to changes in globular head regions.
Regions of HA that are cross-reactive antibody targets have not been well defined yet. Cross reactive antibody responses elicited by immunization with low-pH treated influenza vaccines were several fold lower levels compared to the untreated vaccine. Similarly, low-pH treated influenza vaccines were not as effective in inducing neutralizing antibody responses against homologous or closely related strains. Both the untreated and low-pH treated vaccines showed low levels of heterosubtypic cross neutralizing antibody titers. In contrast, there was over 4 fold difference in levels of cross reactive binding antibodies between the untreated and low-pH treated vaccines. Therefore, it is speculated that the globular head domain of HA could be a target for inducing more cross-reactive antibodies than the exposed stalk domain in the low-pH treated vaccine. Consistent with our observations, previous studies demonstrated that monoclonal antibodies specific for the low pH treated influenza viruses were not capable of blocking viral growth whereas those specific for the untreated virus neutralized virus to high titers (Webster, Brown, and Jackson, 1983).
It is interesting to note that the low-pH vaccine conferred 100% protection against the A/Philippines H3N2 virus. Low levels of cross-reactive antibody responses might play a role in conferring protection against lethal infection with a low dose of heterosubtypic virus. There is also a possibility that cellular and/or humoral immunity to internal proteins might have contributed to protection against the heterosubtypic virus. In previous studies, approaches presenting headless HA containing the conserved stalk domain or HA2-based synthetic peptide coupled to the carrier protein keyhole limpet hemocyanin were recently demonstrated to confer potential broad cross-protection against lethal infection (Steel et al., 2010; Wang et al., 2010a). Also, recent studies demonstrated that antibodies to the conserved fusion peptides contribute to weak heterosubtypic cross protection against lethal infection (Hashem et al., 2010; Prabhu et al., 2009; Stanekova et al., 2011).
The current study indicates important implications on immunogenicity and cross protective efficacy of influenza vaccines containing HA with conformational alterations by a low pH environment. Also, the present study provides relevant information regarding vaccine stability, formulation, and protective efficacy. Storage and/or vaccine inactivation conditions that might alter HA functional activity might be parameters affecting the efficacy of vaccines. Nevertheless, future studies may enable development of influenza vaccines without alterations in the receptor binding sites but with conserved epitopes exposed.
Acknowledgments
This work was supported in part by NIH/NIAID grant AI0680003 (R.W.C.), NIH/NIAID contract HHSN2662007000006C (D.A.S.), the Georgia Research Alliance (S.M.K), and NIH/NIAID grants AI081385 (S.M.K.) and AI093772 (S.M.K.). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bizebard T, Gigant B, Rigolet P, Rasmussen B, Diat O, Bosecke P, Wharton SA, Skehel JJ, Knossow M. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature. 1995;376(6535):92–4. doi: 10.1038/376092a0. [DOI] [PubMed] [Google Scholar]
- Bommakanti G, Citron MP, Hepler RW, Callahan C, Heidecker GJ, Najar TA, Lu X, Joyce JG, Shiver JW, Casimiro DR, ter Meulen J, Liang X, Varadarajan R. Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc Natl Acad Sci U S A. 2010;107(31):13701–6. doi: 10.1073/pnas.1007465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- Daniels RS, Douglas AR, Skehel JJ, Wiley DC. Analyses of the antigenicity of influenza haemagglutinin at the pH optimum for virus-mediated membrane fusion. J Gen Virol. 1983;64(Pt 8):1657–62. doi: 10.1099/0022-1317-64-8-1657. [DOI] [PubMed] [Google Scholar]
- Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–51. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard W, Yewdell J, Frankel ME, Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981;290(5808):713–7. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Hashem AM, Van Domselaar G, Li C, Wang J, She YM, Cyr TD, Sui J, He R, Marasco WA, Li X. Universal antibodies against the highly conserved influenza fusion peptide cross-neutralize several subtypes of influenza A virus. Biochem Biophys Res Commun. 2010;403(2):247–51. doi: 10.1016/j.bbrc.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142(2):187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk HD, Rott R, Orlich M, Blodorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975;68(2):426–39. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Kostolansky F, Russ G, Mucha V, Styk B. Changes in the influenza virus haemagglutinin at acid pH detected by monoclonal antibodies to glycopolypeptides HA1 and HA2. Arch Virol. 1988;101(1-2):13–24. doi: 10.1007/BF01314648. [DOI] [PubMed] [Google Scholar]
- Laver WG, Air GM, Dopheide TA, Ward CW. Amino acid sequence changes in the haemagglutinin of A/Hong Kong (H3N2) influenza virus during the period 1968--77. Nature. 1980;283(5746):454–7. doi: 10.1038/283454a0. [DOI] [PubMed] [Google Scholar]
- Laver WG, Gerhard W, Webster RG, Frankel ME, Air GM. Antigenic drift in type A influenza virus: peptide mapping and antigenic analysis of A/PR/8/34 (HON1) variants selected with monoclonal antibodies. Proc Natl Acad Sci U S A. 1979;76(3):1425–9. doi: 10.1073/pnas.76.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz SG, Choppin PW. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975;68(2):440–54. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kawasaki K, Ohnishi S. Interaction of influenza virus hemagglutinin with target membrane lipids is a key step in virus-induced hemolysis and fusion at pH 5.2. Proc Natl Acad Sci U S A. 1981;78(7):4133–7. doi: 10.1073/pnas.78.7.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu N, Prabakaran M, Ho HT, Velumani S, Qiang J, Goutama M, Kwang J. Monoclonal antibodies against the fusion peptide of hemagglutinin protect mice from lethal influenza A virus H5N1 infection. J Virol. 2009;83(6):2553–62. doi: 10.1128/JVI.02165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82(3):1350–9. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81(7):3514–24. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, Compans RW, Kang SM. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84(15):7760–9. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4(9):e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok RW, Aitken A, Calder LJ, Martin SR, Skehel JJ, Wharton SA, Weis W, Wiley DC. Studies on the structure of the influenza virus haemagglutinin at the pH of membrane fusion. J Gen Virol. 1988;69(Pt 11):2785–95. doi: 10.1099/0022-1317-69-11-2785. [DOI] [PubMed] [Google Scholar]
- Skehel JJ, Bayley PM, Brown EB, Martin SR, Waterfield MD, White JM, Wilson IA, Wiley DC. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982;79(4):968–72. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Bizebard T, Bullough PA, Hughson FM, Knossow M, Steinhauer DA, Wharton SA, Wiley DC. Cold Spring Harbor Symposia on Quantitative Biology. LX. Cold Spring Harbor Laboratory Press; 1995. Membrane fusion by influenza hemagglutinin. pp. 573–580. [DOI] [PubMed] [Google Scholar]
- Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, Jin HT, Pekosz A, Compans RW, Kang SM. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011;6(1):e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanekova Z, Kiraly J, Stropkovska A, Mikuskova T, Mucha V, Kostolansky F, Vareckova E. Heterosubtypic protective immunity against influenza A virus induced by fusion peptide of the hemagglutinin in comparison to ectodomain of M2 protein. Acta Virol. 2011;55(1):61–7. doi: 10.4149/av_2011_01_61. [DOI] [PubMed] [Google Scholar]
- Steel J, Lowen AC, Wang T, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1(1) doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer DA. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology. 1999;258(1):1–20. doi: 10.1006/viro.1999.9716. [DOI] [PubMed] [Google Scholar]
- Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16(3):265–73. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandschoot P, Beirnaert E, Barrere B, Calder L, Millar B, Wharton S, Jou WM, Fiers W. An antibody which binds to the membrane-proximal end of influenza virus haemagglutinin (H3 subtype) inhibits the low-pH-induced conformational change and cell-cell fusion but does not neutralize virus. J Gen Virol. 1998;79(Pt 7):1781–91. doi: 10.1099/0022-1317-79-7-1781. [DOI] [PubMed] [Google Scholar]
- Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, Garcia- Sastre A, Moran TM, Palese P. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010a;107(44):18979–84. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese P. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 2010;6(2):e1000796. doi: 10.1371/journal.ppat.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Brown LE, Jackson DC. Changes in the antigenicity of the hemagglutinin molecule of H3 influenza virus at acidic pH. Virology. 1983;126(2):587–99. doi: 10.1016/s0042-6822(83)80015-4. [DOI] [PubMed] [Google Scholar]
- Wharton SA, Calder LJ, Ruigrok RW, Skehel JJ, Steinhauer DA, Wiley DC. Electron microscopy of antibody complexes of influenza virus haemagglutinin in the fusion pH conformation. EMBO J. 1995;14(2):240–6. doi: 10.1002/j.1460-2075.1995.tb06997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Kartenbeck J, Helenius A. Membrane fusion activity of influenza virus. Embo J. 1982;1(2):217–22. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley DC, Skehel JJ. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–94. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wiley DC, Wilson IA, Skehel JJ. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981;289(5796):373–8. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289(5796):366–73. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Gerhard W, Bachi T. Monoclonal anti-hemagglutinin antibodies detect irreversible antigenic alterations that coincide with the acid activation of influenza virus A/PR/834-mediated hemolysis. J Virol. 1983;48(1):239–48. doi: 10.1128/jvi.48.1.239-248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Kuroda K, Kawasaki K, Yamashina S, Maeda T, Ohnishi S. Infectious cell entry mechanism of influenza virus. J Virol. 1982;43(1):284–93. doi: 10.1128/jvi.43.1.284-293.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]