Abstract
Background
Post-traumatic stress disorder (PTSD) is associated with increased risk for age-related diseases and early mortality. An accelerated rate of biological aging could contribute to this increased risk. To investigate, we assessed leukocyte telomere length (LTL), an emerging marker of biological age, in men and women with and without PTSD. We also examined childhood trauma, a risk factor for both PTSD and short LTL, as a potential contributor to short LTL in PTSD.
Methods
Participants included 43 adults with chronic PTSD (n=18 with multiple categories of childhood trauma) and 47 controls (none with multiple categories of childhood trauma) (M age = 30.55, SD = 7.44). Exclusion criteria included physical illness, medication use, obesity, alcohol or substance abuse, and pregnancy. Structured clinical interviews were conducted to assess PTSD and other psychiatric disorders and childhood trauma exposure. LTL was measured with quantitative polymerase chain reaction.
Results
As predicted, participants with PTSD had shorter age-adjusted LTL than controls. Exposure to childhood trauma was also associated with short LTL. In fact, childhood trauma appeared to account for the PTSD group difference in LTL; only participants with PTSD and exposure to multiple categories of childhood trauma had significantly shorter LTL than controls.
Conclusions
Childhood trauma is associated with short LTL in individuals with PTSD. Chronic exposure to the psychobiological sequelae of childhood trauma could increase risk for PTSD and short LTL. Thus, the lasting psychological impact of exposure to trauma in childhood may be accompanied by equally enduring changes at the molecular level.
Keywords: anxiety, biological aging, childhood trauma, post-traumatic stress disorder, telomere length
Post-traumatic stress disorder (PTSD) is an anxiety disorder that develops in a minority of individuals following exposure to traumatic stress (1). Accumulating evidence indicates that PTSD substantially increases risk for age-related diseases, such as cardiovascular, autoimmune, and neurodegenerative diseases, and early mortality (2–6). However, the biological mechanisms mediating these associations remain unclear. Accelerated biological aging is a potential mechanism of such increased risk, and PTSD is associated with dysregulation of bodily systems that have been linked with biological aging (7, 8). However, to our knowledge, there are no studies examining indices of biological aging in PTSD.
Leukocyte telomere length (LTL) is an emerging marker of biological age that predicts incidence of age-related diseases and mortality (9–12). Factors associated with shorter LTL include higher body mass index (BMI), cigarette smoking, and male gender (13–15). Exposure to chronic psychological stress and affective disorders has been also associated with short LTL (16–18). Repeated and prolonged activation of the biological stress response is a potential contributor to an accelerated rate of leukocyte telomere shortening in chronically stressed individuals. As evidence, shorter LTL has been observed in individuals who have higher nocturnal levels of the stress hormones cortisol and catecholamines (19, 20). Furthermore, anxiety and depression related elevations in inflammatory activity may promote telomere shortening by increasing cell turnover and promoting the release of reactive oxygen species that damage telomeric DNA via oxidative stress (7, 21, 22). Individuals with PTSD, who demonstrate dysregulation of the hypothalamic-pituitary adrenal (HPA) axis, increased sympathetic nervous system (SNS) activation, and elevated inflammatory activity (8, 23–25), may also be susceptible to accelerated shortening of LTL.
Childhood trauma is a significant risk factor for the development of PTSD later in life (26, 27), and is also associated with short LTL in some, but not all, studies of all adult samples (28–30). These consequences of childhood trauma may be due to early life experiences shaping later life biological stress responses (31, 32). Individuals who experience childhood trauma have increased neuroendocrine and immune system responses to stress, glucocorticoid resistance, and reduced hippocampal volume compared to those who do not experience childhood trauma (33, 34). Many of these biological factors confer increased risk for developing PTSD after exposure to trauma (35). Insofar as childhood trauma leads to exaggerated and prolonged biological stress responses, childhood trauma may also increase risk for short LTL in adulthood. Thus, it is possible that childhood trauma may play a role in any observed association between PTSD and LTL.
In the present study, we examined LTL in individuals with PTSD with and without exposure to multiple categories of childhood trauma and in control participants, hypothesizing that: 1) the PTSD group overall would have shorter LTL than controls; 2) across the sample, childhood trauma would be related to shorter LTL; and 3) participants with PTSD would have greater childhood trauma exposure, which could account for any observations of short LTL in PTSD.
Methods
Design and Sample
The present study has a cross-sectional, 2 × 2 design (PTSD/ Control × Male/Female) with medically healthy medication-free subjects. Participants were recruited through ads and flyers distributed in the community, as well as through relevant local clinics for the PTSD sample. The final sample included 43 individuals with current chronic PTSD (47% female; M age = 30.60, SD = 6.63) and 47 controls without PTSD (56% female; M age = 30.68, SD = 8.19), ranging in age from 21 to 49 years. Chronic PTSD was defined by fulfillment of DSM-IV criteria or Clinician Administered PTSD Scale (CAPS, 36) score > 40 for at least three months. Controls were negative for lifetime PTSD and had current CAPS scores < 20. Women took part in the study during the follicular phase of the menstrual cycle. Exclusion criteria included the presence of neurologic disorders or systemic illness, use of psychiatric, anticonvulsant, antihypertensive, sympathomimetic, estrogen replacement therapy, steroidal, statin or other prescription medications, obesity (BMI > 30), alcohol abuse or dependence in the previous two years, substance abuse or dependence in the previous year, any psychiatric disorder with psychotic features, bipolar disorder, or obsessive-compulsive disorder, and pregnancy. Controls were excluded if they had a lifetime history of PTSD, major depressive disorder (MDD) or panic disorder. All participants provided written informed consent and the study protocol was approved by the Committee on Human Research at the University of California, San Francisco and at the San Francisco Veteran’s Affairs Medical Center.
Psychiatric Diagnoses
Lifetime and current PTSD were assessed with the Clinician-Administered PTSD Scale (CAPS), a structured interview measure that corresponds to DSM-IV criteria for PTSD (36). The CAPS is a 30-item scale that assesses the frequency and intensity of re-experiencing, avoidance and hyperarousal symptoms of PTSD. Diagnosis of PTSD was based on symptoms experienced in the previous month associated with the subject’s self-identified worst traumatic event. Other psychiatric disorders were assessed by administration of the Structured Clinical Interview for DSM-IV (37). All diagnoses were made by trained clinical interviewers who calibrated their assessments at weekly case consensus meetings, supervised by an experienced PhD-level clinical psychologist.
Childhood Trauma
Five items from the interview version of the Life Stressor Checklist (LSC) were modified to assess exposure to childhood trauma at or before age 14 (38). Participants were asked if they had been exposed to any of the following experiences to the extent that they felt that they could die or be physically harmed, physical neglect, family violence, physical abuse, forced sexual touch, or forced sexual intercourse at or before age 14. These items were administered during a structured interview conducted by trained clinical interviewers who calibrated their assessments at weekly case consensus meetings, supervised by an experienced PhD-level clinical psychologist. Childhood trauma data was missing for one participant with PTSD.
Leukocyte Telomere Length (LTL)
Samples were collected in 10-ml heparin tubes (Becton–Dickinson, Franklin Lakes, NJ). Leukocytes were isolated and frozen at −80°C. DNA was extracted from leukocytes by the University of California San Francisco DNA bank. Genomic DNA isolation was performed using a standardized and quality-controlled PureGene DNA isolation system (Gentra Systems, Minneapolis). The quantity and quality of the genomic DNA isolate was determined by 260/280 UV spectrophotometery. At regular intervals, the integrity of isolated DNA was evaluated by agarose gel electrophoresis performed on randomly selected isolates. DNA was analyzed for LTL using quantitative polymerase chain reaction (qPCR) as previously described (39) with modifications as described in (40). The quantities of telomeric product (T) and single copy gene (S) were determined relative to the reference DNA by the standard curve method (see Supplementary Materials). All PCRs were carried out on a Roche Lightcycler 480 real-time PCR machine with 384-tube capacity (Roche Diagnostics Corporation, Indianapolis, IN). To convert T/S values to basepairs, the T/S ratios for a set of genomic DNA samples from the human fibroblast primary cell line IMR90 at different population doubling (PD) as well as with the telomerase protein subunit gene hTERT in a lentiviral construct were determined. This set of DNA samples represents different T/S ratios from the same parental cell line. The mean telomeric restriction fragment (TRF) length from these DNA samples is determined using Southern blot analysis and the slope of the plot of mean TRF length versus T/S for these samples serves as the conversion factor for calculation of approximate telomere length, in base-pairs (bp), for each T/S ratio in this study. The conversion formula was determined to be: base pairs=3274+2413*(T/S).
Statistical Analysis
Data screening procedures showed that both PTSD and control groups included one statistical outlier on LTL, defined as LTL greater than twice the interquartile range. Data from these two participants were excluded for the purpose of analysis. When excluding these outliers, LTL was normally distributed (Shapiro-Wilks = .98, df = 88, p = .34) with acceptable skewness (skewness = .24, SE = .26) and kurtosis (kurtosis = −.31, SE = .51). Our primary hypothesis was tested using an analysis of covariance (ANCOVA) model, comparing differences in LTL in participants with and without PTSD. Partial correlation was used to explore the association between PTSD severity (assessed by CAPS score) and LTL. To examine if MDD contributed to our findings, we examined if participants with PTSD and a history of MDD had significantly shorter LTL than participants with PTSD who were without a history of MDD. For our childhood trauma analyses, we used a partial correlation to examine the association between number of categories of childhood trauma exposure and LTL. For categorical analyses, given that children exposed to more than one category of childhood trauma have been found to be more at risk for PTSD and other adverse outcomes compared with those exposed to one category or none (26, 27, 41), we categorized participants into two groups: those having none or one category of exposure and two or more categories of exposure and examined differences in LTL between these two groups using ANCOVA. Age was included as a covariate in all primary LTL analyses. Given the directionality of our hypotheses regarding LTL, one-tailed p values were used to assess statistical significance in analyses with LTL as outcome. For the purpose of graphically representing our findings, age-adjusted LTL for each participant was calculated as the observed LTL plus the difference between the mean and actual age multiplied by the unstandardized beta obtained by regressing LTL on age. We also reran our correlational analyses using this age-adjusted LTL variable, and found that Spearman’s correlations on this age-adjusted LTL variable yielded the same pattern of results as observed using Pearson’s partial correlations. Data analysis was conducted with PASW Statistics 18 (SPSS Inc., 2009).
Results
Descriptive data for the sample are presented in Table 1. There were no significant age differences between PTSD (age range: 21–49) and control participants (age range: 20–50), and there were no gender distribution or education differences between groups (p = ns). However, participants with PTSD did have significantly higher BMI than controls (t = 2.62, p = .01). There were no differences between male and female participants in LTL (p = ns). Shortening LTL was associated with increasing age (r = −.29, p = .007), but BMI, smoking and years of education were not associated with LTL and there were no gender differences in LTL (p = ns). Participants with PTSD had high mean CAPS scores, indicating moderately severe symptoms of PTSD (M = 54.7, SD = 15.8). Eighteen participants with PTSD and no controls reported a history of exposure to multiple categories of childhood trauma.
Table 1.
Characteristics of the sample
| Female | Male | Group | Gender | |||
|---|---|---|---|---|---|---|
| PTSD+ (n=20) | Control (n=25) | PTSD+ (n=22) | Control (n=21) | p | p | |
| Age | 29.4 (6.1) | 30.6 (8.1) | 30.9 (6.4) | 30.7 (8.7) | .75 | .61 |
|
| ||||||
| Education | 15.2 (2.1) | 15.4 (2.0) | 14.4 (2.3) | 15.5 (2.1) | .16 | .45 |
|
| ||||||
| Body mass index | 23.9 (4.3) | 25.2 (4.2) | 29.5 (4.3) | 23.6 (3.1) | .005* | .01* |
|
| ||||||
| Current Smoker n (%) | 6 (30) | 5 (20) | 4 (18) | 3 (14) | .46 | .34 |
|
| ||||||
| Marital Status n (%) | ||||||
| Single | 16 (80) | 20 (80) | 16 (73) | 19 (90) | ||
| Married | 1 (5) | 4 (16) | 1 (4) | 2 (10) | ||
| Divorced/Separated | 3 (15) | 1 (4) | 5 (23) | 0 (0) | .04* | .85 |
|
| ||||||
| Ethnicity n (%) | ||||||
| African American | 2 (10) | 0 (0) | 3 (14) | 1 (5) | ||
| Asian American | 2 (10) | 3 (12) | 1 (4) | 3 (14) | ||
| White | 12 (60) | 18 (72) | 10 (45) | 17 (81) | ||
| Hispanic | 0 (0) | 1 (4) | 0 (0) | 0 (0) | ||
| Hawaiian | 0 (0) | 0 (0) | 2 (9) | 0 (0) | ||
| Pacific Islander | 1 (5) | 0 (0) | 3 (14) | 0 (0) | ||
| Multi-ethnic | 3 (15) | 3 (12) | 3 (14) | 0 (0) | .07 | .40 |
|
| ||||||
| Alcohol Use n (%) | ||||||
| Past Abuse | 4 (20) | 0 (0) | 4 (18) | 1 (5) | ||
| Past Dependence | 4 (20) | 0 (0) | 3 (14) | 0 (0) | .002* | .85 |
|
| ||||||
| Substance Use n (%) | ||||||
| Past Abuse | 1 (5) | 1 (4) | 0 (0) | 0 (0) | ||
| Past Dependence | 2 (10) | 0 (0) | 4 (18) | 0 (0) | .03* | .26 |
|
| ||||||
| MDD History n (%) | ||||||
| Current | 3 (15) | 0 (0) | 5 (23) | 0 (0) | .002* | .42 |
| Past | 14 (70) | 0 (0) | 9 (41) | 0 (0) | ||
| Lifetime | <.001* | .61 | ||||
|
| ||||||
| Childhood Trauma n (%) | 11 (55) | 2 (8) | 9 (43) | 2 (10) | ||
| Neglect | 3 (15) | 0 (0) | 3 (14) | 0 (0) | ||
| Family Violence | 9 (45) | 2 (8) | 7 (33) | 1 (5) | ||
| Physical Abuse | 2 (10) | 0 (0) | 7 (33) | 0 (0) | ||
| Forced Touch | 4 (20) | 0 (0) | 6 (28) | 2 (10) | ||
| Forced Sex | 5 (25) | 0 (0) | 4 (19) | 1 (5) | <.001* | .53 |
Notes. Numbers refer to mean and standard deviation for age, education and body mass index, and to number of participants and percentage of cell for all other variables. Childhood trauma information was missing for one male participant with PTSD; thus, percentages are based on n = 21 for that cell. Some participants experienced multiple categories of childhood trauma. P values are based on Student’s t-tests for continuous data and on Mann Whitney U tests for categorical data.
denotes statistical significance at p < .05.
PTSD and LTL
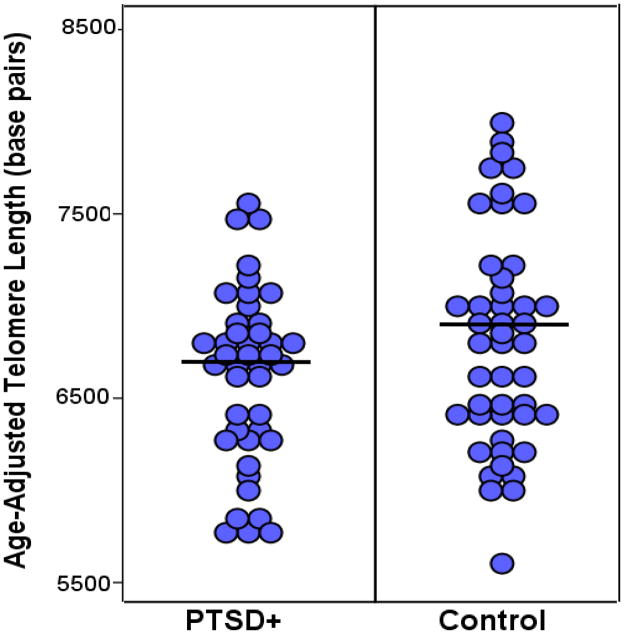
Participants with PTSD had significantly shorter age-adjusted LTL than control participants, F(1,85) = 3.29, p = .03, partial η2 = .04, (Figure 1). On average, participants with PTSD had age-adjusted LTL 204 base pairs shorter (M = 6594.10, SE = 81.50) than control participants (M = 6798.61, SE = 77.88). Next, we examined if PTSD symptom severity as assessed by CAPS was associated with LTL within participants with PTSD, but found no significant associations between CAPS scores and LTL (rpartial = −.12, p = ns). Finally, we examined if the 31 participants in our sample with PTSD and a history of MDD had significantly shorter LTL than those participants with PTSD who did not have a history of MDD. First, we confirmed that there was no significant difference in the gender composition of groups with and without a history of MDD, χ2 = 2.47, p = ns. Then, using ANCOVA, we found no difference in LTL between the 31 participants with PTSD and a history of MDD and the participants with PTSD who did not have a history of MDD, F(1,39) = 2.15, p = ns.
Figure 1.
Dot plot illustrating age-adjusted LTL in participants with PTSD and controls. Participants with PTSD had significantly shorter age-adjusted LTL than control participants, F(1,85) = 3.29, η2 = .04, p = .03.
Childhood Trauma
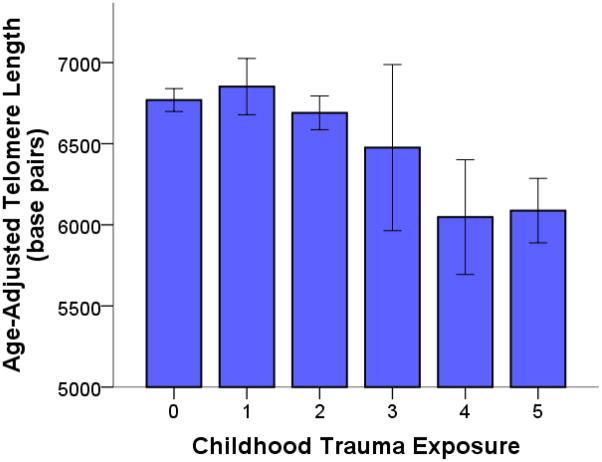
Exposure to more categories of childhood trauma, or cumulative childhood trauma exposure, was linearly associated with shortening LTL in the combined sample of participants with PTSD and controls (rpartial = −.27, p = .005; Figure 2). However, childhood trauma was significantly more prevalent in participants with PTSD and no controls had experienced more than one category of childhood trauma (Table 1). Because there was insufficient variance in childhood trauma to examine relations between childhood trauma and LTL within the control group, and because PTSD and childhood trauma were confounded, we focused subsequent analyses on the effect of childhood trauma on LTL within participants with PTSD.
Figure 2.
Cumulative exposure to traumatic events in childhood is associated with short leukocyte telomere length (LTL). Age-adjusted LTL tended to decrease across participants exposed to more categories of childhood trauma, controlling for gender (rpartial = −.27, p = .005). Notably, however, all of the participants exposed to two or more categories of childhood trauma had a diagnosis of PTSD.
Cumulative childhood trauma exposure was even more strongly associated with shortening LTL in the subsample including only participants with PTSD (r = −.42, p = .003). Participants with PTSD who endorsed experiences of multiple childhood traumas (n = 18) versus one or no categories of childhood trauma (n = 23) were not significantly different in gender, BMI, years of education, or PTSD severity. However, results indicated that participants with PTSD who had experienced multiple childhood traumas (M TL = 6480.45, SD = 107.80) had significantly shorter LTL than those who had experienced one category of childhood trauma or none (M TL = 6727.88, SD = 94.94), exhibiting LTL on average 247 base pairs shorter, F(1,38) = 2.86, p = .04, partial η2 = .07.
We then examined the relative contribution of participants with PTSD and childhood trauma to the observed association between PTSD and LTL. Results indicated that only participants with PTSD and more than one type of childhood trauma had significantly shorter LTL than controls. Specifically, participants with PTSD and more than one category of childhood trauma had LTL 316 base pairs shorter than controls, F(1,61) = 4.07, p = .02, partial η2 = .06, whereas participants with PTSD and one or no categories of childhood trauma were not significantly different from controls on LTL, F(1,66) = .41, p = ns. Furthermore, the group difference in LTL between participants with PTSD and controls was no longer significant when controlling for childhood trauma. Overall, these results indicated that only participants with the combination of PTSD and childhood trauma were significantly different than control participants in LTL, and that these participants accounted for the observed group difference in LTL between participants with PTSD and controls.
Discussion
The present data demonstrate that young to middle-aged adults with PTSD have shorter LTL than do non-psychiatric control persons, even in the absence of chronic physical illness. This finding extends a small but growing body of literature showing short LTL in individuals experiencing chronic psychological stress (17, 18) or psychiatric illness (16, 42). However, our data additionally indicated that only participants with PTSD who had been exposed to multiple types of childhood trauma had significantly shorter LTL than controls, indicating that childhood trauma may have accounted for the observed finding of short LTL in PTSD. Moreover, our data indicated that exposure to multiple categories of childhood trauma may have a cumulative effect on LTL, as number of categories of trauma exposure was linearly associated with shortening LTL across the entire sample and within the group of participants with PTSD. These findings of short LTL in association with childhood trauma add to a growing literature specifically showing short LTL in individuals exposed to traumatic events in childhood (29, 30). Our results are particularly compelling in light of the relatively young age and good physical health of our sample.
Our primary finding was that individuals with PTSD and multiple categories of childhood trauma had significantly shorter LTL than both controls and participants with PTSD who had none or one category of childhood trauma. Childhood trauma and PTSD could exert separate and combined effects on the rate of telomere shortening through biological and behavioral pathways. First, both childhood trauma and PTSD are associated with exaggerated reactivity to negative stimuli and events, which may lead to repeated and prolonged activation of the biological stress response (43–45). Products of the biological stress response, in turn, have been causally linked with leukocyte telomere shortening. High levels of stress hormones including cortisol and catecholamines indexing activation of the HPA axis and SNS respectively have been associated with short LTL (8, 19, 20, 23–25). Stress-related elevations in inflammatory activity may also promote leukocyte telomere shortening in individuals with PTSD and childhood trauma (8, 21, 46, 47). Second, individuals with PTSD or childhood trauma exposure may be less likely to engage in the kinds of behaviors, such as maintaining a healthy weight, exercising regularly and not smoking, that could protect against the negative effects of psychological stress on TL (48–52). While smoking and obesity, two major risk factors for short LTL, did not appear to contribute to our findings, other behavioral factors such as exercise and diet remain plausible as mediators of the relationship between PTSD and childhood trauma and LTL.
A particularly striking finding from our study is that childhood trauma was linearly associated with shorter LTL across the full sample and in the subsample with PTSD, indicating an additive effect of childhood trauma on LTL. Previous research has shown that cumulative childhood trauma is associated with increased risk for PTSD and other adverse outcomes (26, 27, 41). In our sample, groups with and without cumulative childhood trauma were not significantly different on severity of overall or domain-specific symptoms of PTSD. However, exposure to multiple traumatic events in childhood has been associated with greater complexity of trauma-related symptoms in adulthood (53, 54). Thus, participants in our study with more categories of childhood trauma may have greater complexity of trauma-related symptoms, which could extend to greater physiological dysregulation and more accelerated telomere shortening. Further research will be necessary to test this hypothesis.
Our finding of short LTL in participants with PTSD and multiple categories of childhood trauma contributes to a recent debate concerning observed associations between childhood trauma and short LTL. In particular, one published study indicated that self-reported childhood maltreatment, including neglect, was associated with short LTL in a sample of 31 women and men without current Axis I disorders (29). In a subsequent study, 20 participants who reported experiences of physical abuse in childhood did not have significantly different LTL than 520 controls, and 34 participants who reported experiences of sexual abuse in childhood did not have significantly different LTL than 516 controls (28). The authors of the first paper argue that difficulties with accurate measurement of childhood trauma with only 2 questionnaire items in the latter study, as well as disparate controls for concomitant psychiatric and medical illnesses and medications may have contributed to the inconsistent findings between the two studies (55). Our study has the potential to contribute to this debate. First, our study was focused on PTSD and trauma exposure and as such, childhood trauma was carefully assessed and quantified by expert clinical interviewers using methods validated by our group in previous research (45, 56). Second, we had strict inclusion criteria for both participants with PTSD and controls, selecting participants who were physically healthy and free of potential confounding medications. Finally, we tested for the contribution of age, sex, smoking, BMI and education to our findings. Thus, we believe that our finding reinforces the conclusion that childhood trauma impacts LTL.
Primary limitations of the present study include the cross-sectional design, the relatively small sample size and the absence of a control sample who experienced multiple categories of childhood trauma. Combined, these limitations reduce the extent to which any statements can be made about causality in relationships among PTSD, childhood trauma and short LTL. In particular, it is not clear whether more chronic experiences of PTSD or childhood trauma per se contributed to the observed difference in LTL between groups. However, given that cumulative childhood trauma is such a strong risk factor for the development of later PTSD, individuals with PTSD and childhood trauma form a large proportion of the PTSD population (27). Thus, the present findings may be applicable to a large number of the approximately 8% of people who experience PTSD in their lifetime (57).
A further limitation is the use of a mixed sample of leukocytes including both granulocytes and agranulocytes in our measurement of telomere length. Future research focused on elucidating the relationship between psychosocial factors and LTL might productively measure telomere length in specific leukocyte subpopulations. A history of serious illness may lead to T-cell clonal expansion and thus contribute to short leukocyte telomere length in later life. We excluded participants with current chronic illness, but did not systematically assess experiences of chronic illness throughout the life course. We did collect data on lifetime history of hospitalization and found that participants who had experienced either childhood or lifetime hospitalization for illness (n = 10 and n = 20 respectively) or any cause (n = 12 and n = 33 respectively) were not significantly different from other participants in leukocyte telomere length (all p’s > .44). Our data indicates that the relationship of PTSD and childhood trauma with short LTL is independent of several major potential confounds and mediators including age, BMI, gender and years of education. However, behavioral factors including supplement use and physical activity and indicators of socioeconomic status beyond years of education were not assessed in our study and cannot be ruled out as potential confounds or mediators of the observed relationships.
Conclusions
This study represents the first demonstration of short LTL in PTSD, and additionally indicates that only patients with PTSD and a substantial history of childhood trauma have short LTL. Our findings that even physically healthy young to middle-aged adults with PTSD and childhood trauma bear markers of cellular aging suggest the need for further research to understand the biologic affects of trauma and to prevent future adverse health outcomes in this population.
Supplementary Material
Acknowledgments
This research was supported in part by a Society in Science: Branco Weiss Fellowship (AOD), and grants from the National Institute for Mental Health (TCN: 5R01MH073978-04, 5R34MH077667-03), the Bernard and Barbro Foundation (EB), the O’Shaugnessy Foundation (OW), the Mental Illness Research and Education Clinical Center (MIRECC) of the US Veterans Health Administration, and the Clinical Research Center of the Clinical & Translational Science Institute at the University of California, San Francisco (CTSI: UL1 RR024131), as well as by a VA Health Services Research and Development Career Development Award (to SM) and a NIH/NHLBI grant (to BC: K23 HL 094765-01). This material is the result of work supported with resources and the use of facilities at the Veterans Administration Medical Center, San Francisco, California.
Footnotes
Financial Disclosures
Drs. Blackburn, Epel, and Lin, are co-founders in a company measuring telomere diagnostics, Telome Health, Inc. Dr. Neylan has served on an advisory board for Pfizer, and has received research support from Actelion and Glaxo Smith Kline. Dr. Wolkowitz has given lectures for Merck and Sunovion. All other authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among vietnam veterans: implications for surveillance and prevention. Psychosomatic Medicine. 2008;70:668–676. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boscarino JA, Forsberg CW, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med. 2010;72:481–486. doi: 10.1097/PSY.0b013e3181d9a80c. [DOI] [PubMed] [Google Scholar]
- 4.Cohen BE, Marmar CR, Neylan TC, Schiller NB, Ali S, Whooley MA. Posttraumatic stress disorder and health-related quality of life in patients with coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2009;66:1214–1220. doi: 10.1001/archgenpsychiatry.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qureshi SU, Pyne JM, Magruder KM, Schulz PE, Kunik ME. The link between post-traumatic stress disorder and physical comorbidities: a systematic review. Psychiatr Q. 2009;80:87–97. doi: 10.1007/s11126-009-9096-4. [DOI] [PubMed] [Google Scholar]
- 7.O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, et al. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 10.Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging. 2009;1:81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 12.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 13.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertens. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Parks CG, DeRoo LA, Chen H, Taylor JA, Cawthon RM, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18:816–820. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 16.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Nat Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s Disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 21.O’Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn E, et al. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23:446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- 23.Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: An epidemiologic community study. Arch Gen Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- 24.Yehuda R, Golier JA, Yang RK, Tischler L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biol Psychiatry. 2004;55:1110–1116. doi: 10.1016/j.biopsych.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, et al. PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology. 2005;30:373–381. doi: 10.1016/j.psyneuen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: results from the Detroit Area Survey of Trauma. Am J Psychiatry. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- 28.Glass D, Parts L, Knowles D, Aviv A, Spector TD. No Correlation Between Childhood Maltreatment and Telomere Length. Biol Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010a;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kananen L, Surakka I, Pirkola S, Suvisaari J, Lannqvist J, Peltonen L, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PloS ONE. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 32.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 33.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 35.Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology. 2010 doi: 10.1007/s00213-010-1969-6. In Press. [DOI] [PubMed] [Google Scholar]
- 36.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 37.First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) 4. New York: New York State Psychiatric Institute, Biometrics Research; 1996. [Google Scholar]
- 38.Wolfe J, Kimerling R, Brown P, Chresman K, Levin K. Psychometric review of the life stressor checklist-revised. In: Stamm B, editor. Instrumentation in stress, trauma, and adaptation. Lutherville, MD: Sidran Press; 1996. pp. 144–151. [Google Scholar]
- 39.Cawthon RM. Telomere measurement by quantitative PCR. Nucl Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kao HT, Cawthon RM, DeLisi LE, Bertisch HC, Ji F, Gordon D, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13:118–119. doi: 10.1038/sj.mp.4002105. [DOI] [PubMed] [Google Scholar]
- 43.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 44.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psych Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 45.Pole N, Neylan TC, Otte C, Metzler TJ, Best SR, Henn-Haase C, et al. Associations between childhood trauma and emotion-modulated psychophysiological responses to startling sounds: a study of police cadets. J Abnorm Psychol. 2007;116:352–361. doi: 10.1037/0021-843X.116.2.352. [DOI] [PubMed] [Google Scholar]
- 46.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 47.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Nat Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PloS ONE. 2010;5:e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spratt EG, Back SE, Yeatts SD, Simpson AN, McRae-Clark A, Moran-Santa Maria MM, et al. Relationship between child abuse and adult smoking. Int J Psychiatry Med. 2009;39:417–426. doi: 10.2190/PM.39.4.f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Assis MA, de Mello MF, Scorza FA, Cadrobbi MP, Schooedl AF, da Silva SG, et al. Evaluation of physical activity habits in patients with posttraumatic stress disorder. Clinics (Sao Paulo, Brazil) 2008;63:473–478. doi: 10.1590/S1807-59322008000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckley TC, Mozley SL, Bedard MA, Dewulf AC, Greif J. Preventive health behaviors, health-risk behaviors, physical morbidity, and health-related role functioning impairment in veterans with post-traumatic stress disorder. Mil Med. 2004;169:536–540. doi: 10.7205/milmed.169.7.536. [DOI] [PubMed] [Google Scholar]
- 52.Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 53.Cloitre M, Stolbach BC, Herman JL, Kolk BV, Pynoos R, Wang J, et al. A developmental approach to complex PTSD: Childhood and adult cumulative trauma as predictors of symptom complexity. J Trauma Stress. 2009 doi: 10.1002/jts.20444. [DOI] [PubMed] [Google Scholar]
- 54.Briere J, Kaltman S, Green BL. Accumulated childhood trauma and symptom complexity. J Trauma Stress. 2008;21:223–226. doi: 10.1002/jts.20317. [DOI] [PubMed] [Google Scholar]
- 55.Tyrka AR, Price LH, Kao HT, Porton B, Carpenter LL. In Response to “No Correlation Between Childhood Maltreatment and Telomere Length”. Biol Psychiatry. 2010b;68:e23–e24. [Google Scholar]
- 56.Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, et al. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Psychiatry. 2005;57:27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.