Abstract
Introduction
Randomized trials have demonstrated significant improvements in progression-free survival (PFS) with consolidation paclitaxel (P) and bevacizumab (B) following cytoreduction and adjuvant carboplatin/paclitaxel (CP) for advanced epithelial ovarian cancer (EOC). We sought to evaluate the cost-effectiveness (C/E) of these consolidation strategies.
Methods
A decision model was developed based on Gynecologic Oncology Group (GOG) protocols #178 and #218. Arm 1 is 6 cycles of CP. Arm 2 is 6 cycles of CP followed by 12 cycles of P (CP+P). Arm 3 is 1 cycle of CP, 5 cycles of CPB, and 16 cycles of B (CPB+B). Parameters include PFS, overall survival (OS), cost, complications (neuropathy for P and bowel perforation for B), and quality-of-life utility values. Sensitivity analyses were performed.
Results
The incremental cost-effectiveness ratio (ICER) for CT+T is $13,402/quality adjusted life year (QALY) gained compared to CP. For CPB+B compared to CP, the ICER is $326,530/QALY. When compared simultaneously, CPB+B is dominated, i.e. is more costly and less effective than CP+P. Results were robust to parameter variation. At a willingness to pay threshold of $100,000/QALY, CP+P was the preferred option throughout most of the decision space. Sensitivity analyses suggest that CPB+B would become the preferred option if it were to improve OS by 6.1 years over CP+P.
Conclusions
In this model, B consolidation for advanced EOC was associated with a modest improvement in effectiveness that is less than that with P consolidation and mor e costly. A statistically significant improvement in survival may improve the value of B consolidation.
Keywords: ovarian cancer, bevacizumab, paclitaxel, maintenance therapy, cost
Introduction
Epithelial ovarian cancer (EOC) is the most lethal gynecologic cancer, with an estimated 21,880 new cases resulting in 13, 850 deaths in 2010. [1] The high mortality rate is in part due to late diagnosis, as the majority of patients will be Federation of Gynecology and Obstetrics (FIGO) Stage III or greater. [2] It can also be attributed to other prognostic indicators such as the extent of residual disease following initial surgical cytoreduction and the presence of chemotherapy resistance. [3]
Standard therapy for advanced EOC is surgical cytoreduction followed by 6 cycles of carboplatin and paclitaxel (CP). [4] However, this “standard” regimen has been modified as alternative techniques (intraperitoneal (IP) administration) and dosing schedules (consolidation therapy, dose-dense therapy) are developed. The Gynecologic Oncology Group (GOG) study protocol 178 showed that 12 cycles of P significantly improves progression-free survival (PFS) by a median of 8 months in patients with advanced EOC who had achieved a complete response to primary chemotherapy. [5,6] This regimen, however, showed no statistically significant benefit for overall survival (OS).
Novel biologic agents are also being developed, with the rationale that these therapies may overcome the intrinsic and acquired drug resistance seen in patients with advanced and/or recurrent disease. One such agent is bevacizumab (B), an anti-angiogenic compound. Multiple phase II clinical trials of B have shown good tolerability and impressive response rates, even in patients with platinum-resistant disease. [7,8] The preliminary results presented in abstract form at the ASCO Annual Meeting, 2010, of the phase III evaluation of consolidation B therapy also demonstrated an improvement in PFS by a median of 4 months. This strategy is under further investigation in the current phase III trials for advanced ovarian cancer (GOG protocols 252 and 262). [9]
Despite improvements in PFS, the use of consolidation chemotherapy following upfront treatment of advanced EOC remains to be decided. The lack of OS benefit in the setting of increased neurotoxicity with P consolidation has thwarted its acceptance. B is an expensive therapy, and this has hampered excitement over relatively minimal improvement in PFS. Therefore, we sought to evaluate the cost-effectiveness (C/E) of consolidation P versus B following primary treatment with surgical cytoreduction and CP for advanced EOC.
Methods
Baseline Model
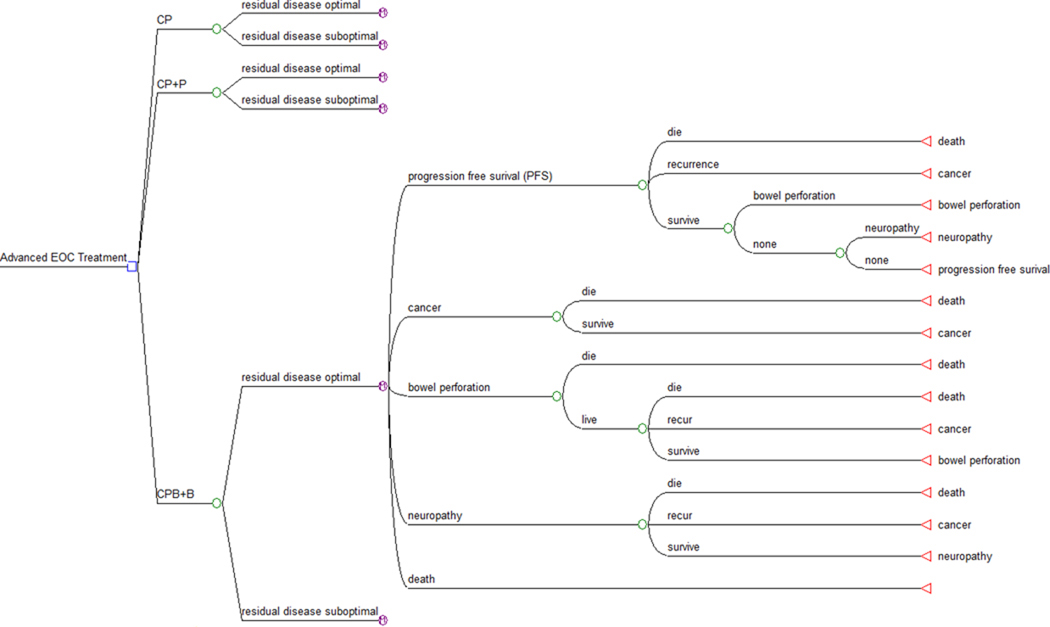
A Markov state transition model was constructed using commercially available software (TreeAge Inc., Williamstown, MA). The model compared the reference treatment arm with two consolidation strategies for a hypothetical cohort of patients with advanced stage EOC. (Figure 1) The patients are those with FIGO Stage III or greater disease who have undergone surgical cytoreduction, with residual disease being classified as either optimal or suboptimal. Treatment in the reference arm (Arm 1) consisted of six cycles of CP. In Arm 2, patients are treated with 6 cycles of CP followed by 12 cycles of T (CP+P). Arm 3 includes patients treated with one cycle of CP, 5 cycles of CP and B (CPB), followed by 16 cycles of B (CPB+B). These regimens are based on GOG protocols 158, 178 and 218, respectively. (Table 1)
Figure 1.
Decision tree structure for the clinical c ourse of patients with advanced epithelial ovarian cancer. Final Markov node expanded.
Table 1.
Treatment Arms
| Treatment Arms | Study Reference | Dosage and Treatment Times |
|---|---|---|
| Arm 1 (CP) | Ozols et al, 2003 [4] | 135 mg/m2 P over 3 hrs + C (AUC 7.5) 21-day cycle × 6 |
| Arm 2 (CP+P) | Markman et al, 2003 [5] | CP as above followed by *175mg/m2 IV P over 3 hrs 28-day cycles × 12 |
| Arm 3 (CPB+B) | Markman et al, 2003 [5] | 175 mg/m2 P + C (AUC 6) × 1 cycle 175 mg/m2 P + C (AUC 6) + B 15 mg/kg × 5 cycles B 15 mg/kg × 16 cycles |
Note. CP – carboplatin and paclitaxel; CP+P, carboplatin, paclitaxel and paclitaxel maintenance; CPB+B, carboplatin, paclitaxel, bevacizumab, and bevacizumab maintenance; AUC, area under curve
Markov states were PFS, recurrence, and death. During the consolidation phase of treatments, Markov states also included treatment complications (neuropathy and bowel perforation). A cohort of women entered the model at age 58. [5] All patients were assumed to survive surgical cytoreduction after which they entered the Markov model where the likelihood of transitioning to a cancer recurrence or death was determined via survival data translated into 28-day increments and followed for 10 years.
The base case model was developed from the perspective of the health care system. Costs included hospital and professional costs associated with chemotherapy and treatment of toxicities and complications. All costs estimates were calculated in 2009 U.S. dollars and discounted at a rate of 3% per year. Toxicity was accounted for within the model by its effects on the actual cost of treatment as well as effect on quality-adjusted life years (QALY). All QALY estimates were discounted at a rate of 3% per year.
Model Parameters
Parameters include PFS, OS, costs, and quality-of-life (QOL) utility values. These were obtained from review of the reference trials used to define the arms as well as general search of the literature through PubMed. The key clinical assumptions are illustrated in Table 2.
Table 2.
Model Estimates - Clinical Assumptions
| Clinical Parameter | Estimate [refs] | Range |
|---|---|---|
| Probabilities | ||
| Surgical cytoreduction suboptimal | 0.33a | 0.00 – 1.00 |
| Neuropathy | CP | 0.07a | 0.03 – 0.14 |
| Neuropathy | CP+P | 0.20a | 0.10 – 0.40 |
| Neuropathy | CPB+B | 0.07a | 0.03 – 0.14 |
| Bowel Perforation | CPB+B | 0.03a | 0.015 – 0.10 |
| Death | Bowel Perforation | 0.50a | 0.00 – 0.75 |
| Progression Free Survival (months) | ||
| CP | ||
| Optimal | 24a | reference |
| Suboptimal | 14a | reference |
| CP+P | ||
| Optimal | 35a | 17.5 – 105 |
| Suboptimal | 20a | 10 – 60 |
| CPB+B | ||
| Optimal | 35a | 17.5 – 105 |
| Suboptimal | 20a | 10 – 60 |
| Overall Survival (months) | ||
| CP | ||
| Optimal | 48a | reference |
| Suboptimal | 35a | reference |
| CP+P | ||
| Optimal | 70a | 35 – 210 |
| Suboptimal | 42a | 21 – 126 |
| CPB+B | ||
| Optimal | 70a | 35 – 210 |
| Suboptimal | 42a | 21 – 126 |
| Quality of Life Utility Index | ||
| Chemotherapy | ||
| CP Cycles | 0.77a | 0.64 – 0.85 |
| CPB Cycles | 0.77a | 0.64 – 0.85 |
| P Maintenance Cycles | 0.80a | 0.66 – 0.88 |
| B Maintenance Cycles | 0.82a | 0.68 – 0.90 |
| Months 1–6 Recovery | 0.84a | 0.70 – 0.93 |
| PFS | 0.85 [34] | 0.75 – 1.00 |
| Cancer recurrence | 0.65 [34] | 0.50 – 0.85 |
| Neuropathy | 0.94 [35] | 0.80 – 1.00 |
| Bowel perforation | 0.85 [36] | 0.50 – 0.95 |
| Discount rate | 0.03 | 0.00 – 0.05 |
See text for rationale
Note. CP: Carboplatin and Paclitaxel; CP+P: Carboplatin and Paclitaxel followed by Paclitaxel consolidation; CPB+B: Carboplatin and Paclitaxel and Bevacizumab followed by Bevacizumab consolidation.
Clinical assumptions
Assumptions were made about the likelihood an optimally versus suboptimally debulked patient would present clinically. GOG 158 included only optimally debulked patients. In GOG 178, approximately two-thirds of the patients enrolled were optimal Stage III. GOG 218 patients were predominately suboptimal Stage III and IV (66%). Given this variation, the first key assumption is that one-third of patients entering the model will be suboptimal.
PFS and OS times were estimated from the published data. Regarding Arm 1, Winter et al reported OS rates for microscopic disease (71 months), macroscopic but <1cm disease (42 months), and >1cm (suboptimal) disease (35 months). [10] We estimated an OS for optimal patients within the microscopic and <1cm groups at 58 months and used 35 months for suboptimal patients. A similar compromise was made for PFS, estimating 24 months for optimal and 14 months for suboptimal patients. These estimates are also consistent with phase III studies not included in this review, GOG protocol 182. [11]
For the CP+P cohort, Abaid et al reported on cohort of patients who received P consolidation with specific survival estimates for optimal and suboptimal patients. [12,13] They reported OS rates for optimal patients receiving 12 cycles of P consolidation of 80 months. [13] The GOG 178 follow-up study reported an OS of 53 months for 12 cycles of P consolidation. [6] PFS numbers also varied between studies: 22 months for 12 cycles in GOG 178, 35 months for optimal and 20 months for suboptimal in the Abaid studies. The key clinical estimates take into account both. OS was estimated to be 70 months for optimal and 42 months for suboptimal patients. PFS was estimated at 35 months for optimal and 20 months for suboptimal patients.
The CPB+B cohort estimates were derived from GOG 218 data (available only in abstract form at the time of this manuscript). [9] The OS data is not yet mature. Early estimates for PFS are 14.1 months. Recurrent disease could be defined by a rising CA125, which has been argued to hasten the median PFS. [14,15] Given this potential limitation, equivalent survival estimates for B and P consolidation were used. This provides a liberal estimate of the potential survival improvement seen with B as it more than doubles the 14-month PFS estimate reported to 35 months for optimal and increases it to 20 months for suboptimal patients.
QOL adjustments for toxicities, recurrence, and PFS are listed in Table 2. As there are no published utility estimates for patients with EOC undergoing the regimens described in this model, a panel of three gynecological oncology experts (JL, TK, MM) provided consensus judgments for utility values. These were based on similar models and published literature regarding the QOL of ovarian cancer patients. [16,17] For the reference arm (CP), the utility index was estimated to be 0.77, which approaches values cited for patients generally on chemotherapy. [16] The addition of B in Arm 3, because it is very tolerable, was assumed to leave the estimate unchanged. [18] The utility values during the maintenance phase of CP+P, the maintenance phase of CPB+B, and the first six, 28-day cycles of recovery from each chemotherapy regimen were estimated to be 0.80, 0.82, and 0.84, respectively.
For the purposes of calculating medication costs, patients were assumed to enter the model with an average weight of 76.9 kg and average height of 1.62 meters. [5,19]
Toxicity
Since each arm has equal cycles of CP, common hematologic and gastrointestinal toxicities were assumed to occur at equivalent rates and omitted from analysis. Arm 3 does include B with 5 of the 6 initial CP cycles; however, phase II data does not indicate that B increases the rate of these toxicities. [8,9] Thus, the key toxicities included in this model include neuropathy and bowel perforations.
In GOG 178, the rate of Grade 3 neuropathy was reported as 5% for 12 cycles. [6] Being conservative, our key assumption for Grade 3 or 4 neuropathy was a basal rate of 7% in arms 1 and 3 during CP administration, and 20% in arm 2 during P consolidation.
There are several B-associated side effects to consider for inclusion in the model, including hypertension, thromboembolism, and gastrointestinal perforations. [18] B-induced hypertension is usually mild and medically managed with little increase in the total cost of the therapy. [18] It is unclear whether thromboembolic events are increased in patients receiving B over the baseline malignancy rate [20,21] As a result, these toxicities were excluded from the model. Gastrointestinal perforations were included as the main toxicity associated with the addition of B to cytotoxic therapy. The rates of perforation associated with B vary in the literature between ~1.5 and 10%. [7–9] Our key estimate was a 3% rate of bowel perforations with a 50% risk of death. [22,23]
Costs
The cost estimates incorporate the reimbursement costs of medication and administration, major complications, and surveillance. [Table 3] With the exception of B, all costs were estimated based on hospital costs, Medicare reimbursement rates as documented in the available literature, the Agency for Healthcare Research and Quality (AHRQ) database, the AHRQ Health Care Utilization Project (HCUP) database, the American Medical Association (AMA) database, the CMS Physician Payment database, or Red Book AWP medication costs. B was included at the cost to the author’s home institution, which is less than published AWP values. Estimates were adjusted via the consumer price index to 2009 U.S. dollars.
Table 3.
Model Estimates - Costs
| Clinical parameter | Estimate [refs] | Range |
|---|---|---|
| Administration of infusion | $551 [37] | $0 – $551 |
| Carboplatin (C), 150 mg, AWP | $268.75 [38] | $0 – $268.75 |
| Paclitaxel (P), 6 mg/ml, 50 ml, AWP | $155.16 [38] | $0 – $155.16 |
| Bevacizumab, 25 mg/ml, 16 ml, AWP | $2191.45a | $0 – $2191.45 |
| Surveillance | ||
| Office visit | $205a | $0 – $205 |
| Lab work | $125a | $0 – $125 |
| CA-125 | $98a | $0 – $98 |
| CT scan | $2841a | |
| Toxicities | ||
| Bowel perforation (ICD-9 569.83, CPT 44604) | $31,113b[39] | $15–60,000 |
| Neuropathy (per episode) | $844 [40] | $400–1600 |
Cost to University of Pittsburgh Medical Center
Agency for Healthcare Research and Quality (AHRQ) [41] hospital cost estimate and American Medical Association (AMA) provider charge estimate
Estimating surveillance costs required the following assumptions, which were extrapolated from clinical trial protocols for the purpose of the model. Following completion of the chemotherapy regimen, patients were assumed to return for an office visit, laboratory tests and CA-125 testing every 3 months for the first 2 years, and then every 6 months thereafter provided they remained progression-free. A computed tomography scan of the abdomen and pelvis with oral and IV contrast was assumed to occur yearly for five years.
Cost-Effectiveness and Sensitivity Analysis
Incremental cost-effectiveness ratios (ICER) were calculated as the ratio of the additional economic impact of a strategy relative to the improvement in quality-adjusted survival time of the more costly and effective strategy. A series of one-way and two-way sensitivity analyses were performed to account for uncertainty in clinical assumptions. Model probabilities, QOL indices, and toxicity costs were varied individually in one-way sensitivity analyses (see tables 2 and 3 for ranges). PFS and OS ranges were yoked across the suboptimal and optimal patient groups to maintain the survival benefit of an optimal cytoreduction, and then two-way sensitivity analyses were used to vary PFS in the CP+P versus CPB+B cohort and OS across the CP+P versus CPB+B cohort (see table 2 for ranges). The influence of costs on model conclusions was examined in a one-way sensitivity analysis that varied all CPB+B costs simultaneously. A willingness-to-pay threshold of $100,000/QALY was used to define strategies that are cost-effective from the standpoint of resource utilization in the U.S. healthcare system. [24,25]
Results
Cost-effectiveness analysis
The results of the C/E analysis are shown in Table 4. This model revealed that consolidation P had a cost of $23,886 per patient with an effectiveness of 3.36 QALY’s. Consolidation B cost $122,899 per patient with a similar effectiveness of 3.31 QALY’s. When compared to the reference arm, the ICER for CP+P is $13,402/QALY and is $326,530/QALY for CPB+B. When all three strategies are compared simultaneously, CPB+B is dominated by CP+P. In other words, CPB+B is more costly and less effective than CP+P.
Table 4.
Cost-effectiveness comparison
| Arm/Strategy | Cost | Incremental Cost |
Effectiveness (QALYs) |
Incremental Effectiveness (QALYs) |
Incremental C/E |
|---|---|---|---|---|---|
| CP | $18,877 | 2.99 | |||
| CP+P | $23,886 | $4,909 | 3.36 | 0.37 | $13,402 |
| CPB+B | $122,899 | $99,012 | 3.31 | −0.05 | Dominated |
Note. QALY, quality-adjusted life years; C/E, cost-effectiveness; CP – carboplatin and paclitaxel; CP+P, carboplatin, paclitaxel and paclitaxel maintenance; CPB+B, carboplatin, paclitaxel, bevacizumab, and bevucizumab maintenance
Sensitivity analyses
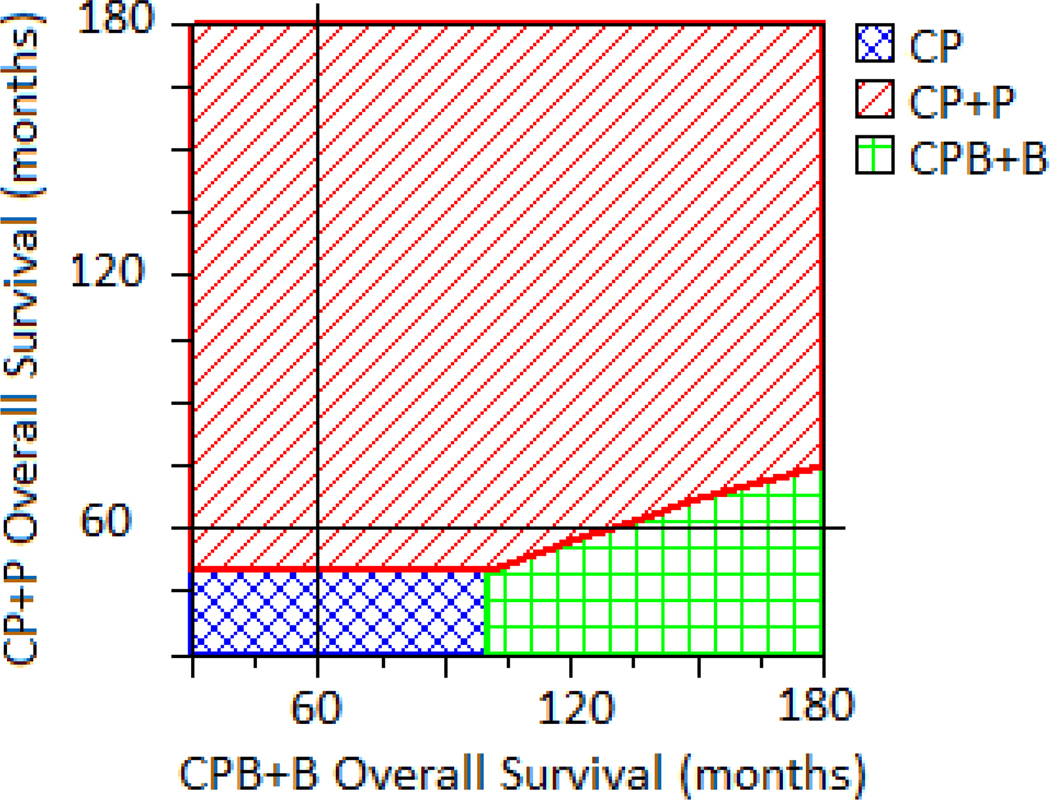
Sensitivity analyses demonstrated that model conclusions were robust to variation across parameters at a willingness-to-pay threshold of $100,000/QALY. CP+P was the preferred option throughout most of the decision space for all analyses. Two-way sensitivity analysis was used to vary PFS times for the CP+P and CPB+B cohorts simultaneously. Across a range of values, CPB+B would never become the cost-effective option even if it were to improve PFS by 10 years or more over CP+P. For OS, sensitivity analyses suggest that CPB+B would become a preferred option (i.e., C/E at a $100,000/QALY threshold) if it were to improve OS by 6.1 years beyond baseline CP+P improvement. (Figure 2) A one-way sensitivity analysis varied the total cost of CPB+B treatment from zero to the base case value. If the total cost of CPB+B were to drop below 37% of the total current cost, the ICER would drop below $100,000 as compared to the CP arm, but the strategy would still be dominated by CP+P. The total cost of CPB+B would have to drop to less than 12% of the current cost before it would become a cost-effective alternative to CP+P.
Figure 2.
Two-way sensitivity analysis varying OS for CP+P and CPB+B. Each solid black line is the base case model OS assumption (60 months for each arm). The intersection of these lines falls in a demarcated region where a given treatment strategy is the preferred one given a willingness to pay threshold of $100,000/QALY. In the current model, the intersection falls in the CP+P region, suggesting it is the preferred strategy, but if OS for CPB+B were 160 months and OS for CP+P remained the same, the intersection would move to the CPB+B region, suggesting it is the cost-effective strategy.
Discussion
In this model, treatment of advanced EOC with CP followed by B consolidation (GOG 218) was associated with a modest improvement in effectiveness that is less than that with P consolidation and more costly. With an ICER of $13,402/QALY for 12 cycles of P consolidation, CP+P is more cost-effective than the reference strategy of CP and dominates B consolidation.
The optimal treatment strategy for advanced EOC following surgical debulking is still a matter of debate. Multiple studies have demonstrated improvements in survival with the use of IP chemotherapy compared to IV therapy; however, concerns about toxicity, administration, and QOL continue to prevail. [2] P consolidation has not been routinely adopted secondary to lack of demonstration of an OS benefit as well as concerns about toxicity. [5,6] Biological agents hold promise for the treatment of EOC, with B being one of the most studied. [7,8] Despite its tolerability, critics of B cite modest improvements in survival as well as significant costs associated with the drug. [8] The actual cost of B at our institution starts at more than $2000/cycle, which is nearly ten times the cost of P. Granted, this increased cost would likely be readily adopted if survival benefits were clear, but the lack of data demonstrating an OS benefit may be one of the key factors impairing its widespread adoption and use. Moreover, extending the use of B to progression needs to be assessed in the setting of objective efficacy and cost data.
We recognize that there are several limitations to this model. First, the basis of the creation of the model is to compare three separate and different clinical trials; thus, assumptions are made to define the parameters of the model to allow such comparisons. Although the estimates are based on published literature and available data sets, the final value is based on judgment. For instance, survival estimates are based on published trials, but given the variation of the study populations and definitions of recurrence, it is difficult to define a single estimate that would be globally applicable. There is also significant variation in the patients enrolled. In GOG 178, approximately 66% were optimal stage III patients, while the majority (66%) of patients enrolled on GOG 218 were either suboptimal stage III or stage IV. [5,9] The model accounted for this variation and forced all arms to treat the same proportion of suboptimal patients. Another important distinction is the patients who received maintenance chemotherapy. In GOG 178, patients had to attain a clinically defined complete response in order to be enrolled; whereas, GOG 218 included patients with or without measurable disease, so that the primary endpoint of PFS may have been based on patients with stable disease. [5,9] This difference may have impacted the magnitude of PFS, which would ultimately be reflected in the model parameters.
Another limitation is the QOL utility value estimates. There are no utility values that have been validated for patients specifically with EOC, nor for the therapies included in this model. The cost estimates are based on institutional costs of the drug and infusion, but these may be regionally dependent. Furthermore, the cost estimates do not account for outpatient issues, hospitalizations, delays in therapy requiring additional laboratory testing and imaging, etc. We attempted to account for discrepancies in estimates with the sensitivity analyses, and our results remained consistent.
Finally, this model uses CP as the reference treatment. There are, however, other options for upfront therapy that some may argue is more appropriate for advanced EOC, namely IP chemotherapy or dose-dense P. [30–31] The authors recognize the optimal analysis would be to include a C/E evaluation in a prospective trial comparing P to B consolidation.
Despite these limitations, the main findings of this C/E analysis suggest that P consolidation is more C/E than B consolidation. With an ICER of $13,402/QALY, P consolidation appears to be the agent of choice for consolidation chemotherapy in this model. B is both more costly and less effective. Furthermore, variations within the model that would make B the treatment of choice would require significant increases in survival (6.1 years OS) which are not likely to be realized based on current information. Also, from a purely cost standpoint, the price of B would have to significantly drop (less than 12% of its current cost) for it to be a C/E alternative to CP. This is an important issue when choosing which patient to offer consolidation chemotherapy and with what agent.
According to the 2009 World Health Organization statistics report, the U.S. spends nearly 16% of its gross domestic product on healthcare, yet indicators of health, such as mortality rates and life expectancy, are not higher than comparable Western countries. [32,33] The reasons for this paradox are complex and multi-factorial. Pharmaceutical costs and provider variation are only part of the equation. While biological agents are an attractive treatment strategy for advanced EOC, objective survival benefits need to be assessed in the setting of costs of treatment and toxicities. This model suggests that consolidation B is not a C/E strategy, both when compared to consolidation P as well as treatment with CP. The decrease in actuarial costs and increases in survival necessary to promote this strategy are not likely.
Research Highlights.
Paclitaxel is a more cost-effective consolidation option in advanced ovarian cancer
Consolidation Bevacizumab is not cost-effective for advanced ovarian cancer
Acknowledgments
This research was supported by the NIH/NCRR/CTSA Grant UL1 RR024153.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This abstract will be presented as a poster at the Society for Gynecologic Oncology Annual Meeting, Orlando FL, March 2011.
Conflict of Interest Statement
Dr. Markman is a consultant for Genetech. There are no other conflicts of interest to declare.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Pecorelli S, Creaseman WT, Petterson F, Benedet JL, Shepard JH. FIGO annual report on the results of treatment in gynaecological cancer. J Epidemiol Biostat. 1998;3:75–102. [Google Scholar]
- 3.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R Gynecologic Oncology Group. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, Jiang C, Alberts D. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003;21(13):2460–2465. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Markman M, Liu PY, Moon J, Monk BJ, Copeland L, Wilczynski S, Alberts D. Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered o patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gyn Oncol. 2009;114:195–198. doi: 10.1016/j.ygyno.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer; a Gynecologic Oncology Group study. J Clin Oncol. 2007;25(33):5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 8.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, Douglas J, Burger RA, Armstrong D, Wenham R, McGuire W. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25(33):5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 9.Burger RA, Brady MF, Bookman MA, Walker JL, Homesley HD, Fowler J, Monk BJ, Greer BE, Boente M, Liang SX. Phase III trial of bevacizumab in the primary treatment of advanced epithelial ovarian, primary peritoneal, or fallopian tube cancer: a Gynecologic Oncology Group study. ASCO Annual Meeting; Chicago, Il. 2010. [Google Scholar]
- 10.Winter WE, et al. Prognostic factors for stage III epithelial ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2007;25(24):3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 11.Bookman M, Brady M, McGuire W, Harper P, Alberts D, Friedlander M, Colombo N, Fowler J, Argenta J, DeGeest K, Mutch D, Burger R, Swart AM, Trimble E, Accario-Winslow C, Roth L. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27(9):1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abaid L, et al. Improved overall survival with 12 cycles of single-agent paclitaxel maintenance therapy following a complete response to induction chemotherapy in advanced ovarian carcinoma. Oncology. 2010;78:389–393. doi: 10.1159/000320579. [DOI] [PubMed] [Google Scholar]
- 13.Abaid LN, et al. The prognostic significance of optimal debulking in the setting of a complete clinical response for advanced ovarian carcinoma patients receiving maintenance chemotherapy. Arch Gynecol Oncol. 2010 June 26; doi: 10.1007/s00404-010-1571-5. [DOI] [PubMed] [Google Scholar]
- 14.Guppy AE, Rustin GJ. CA125 response: can it replace the traditional response criteria in ovarian cancer? Oncologist. 2002;7(5):437–443. doi: 10.1634/theoncologist.7-5-437. [DOI] [PubMed] [Google Scholar]
- 15.Rustin GJ, Timmers P, Nelstrop A, Shreeves G, Bentzen SM, Baron B, Piccart MJ, Bertelsen K, Stuart G, Cassidy J, Eisenhauer E. Comparison of CA-125 and standard definitions of progression of ovarian cancer in the intergroup trial of cisplatin and paclitaxel versus cisplatin and cyclophosphamide. J Clin Oncol. 2006;24(1):45–51. doi: 10.1200/JCO.2005.01.2757. [DOI] [PubMed] [Google Scholar]
- 16.Greving JP, Vernooij F, Heintz APM, van der Graaf Y, Buskens E. Is centralization of ovarian cancer care warranted? A cost-effective analysis. Gynecol Oncol. 2009;113:68–74. doi: 10.1016/j.ygyno.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Arriba LN, Fader AN, Frasure HE, von Gruenigen VE. A review of issues surrounding quality of life among women with ovarian cancer. Gynecol Oncol. 2010;119:390–396. doi: 10.1016/j.ygyno.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol. 2010;117:497–504. doi: 10.1016/j.ygyno.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States, 1960–2002. Advance Data from Vital and Health Statistics. 2004;347:1–17. [PubMed] [Google Scholar]
- 20.Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar R, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99(16):1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 21.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients. JAMA. 2008;300(19):2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 22.Diaz JP, et al. Incidence and management of bevacizumab-associated gastrointestinal perforation in patients with recurrent ovarian carcinoma. Gynecol Oncol. 2009;112(2) suppl1:S137. doi: 10.1016/j.ygyno.2009.11.017. (abstract 269] [DOI] [PubMed] [Google Scholar]
- 23.Richardson DL, et al. Which factors predict bowel complications in patients with recurrent epithelial ovarian cancer treated with bevacizumab? Gynecol Oncol. 2009;112(2) suppl1:S23. doi: 10.1016/j.ygyno.2010.01.011. [abstract 4] [DOI] [PubMed] [Google Scholar]
- 24.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 25.Garber AM, Phelps CE. Economic foundations of cost-effectiveness analysis. J Health Econ. 1997;16:1–31. doi: 10.1016/s0167-6296(96)00506-1. [DOI] [PubMed] [Google Scholar]
- 26.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 27.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 29.Markman M, Walker JL. Intraperitoneal che motherapy of ovarian cancer; a review, with a focus on practical aspects of treatment. J Clin Oncol. 2006;24:988–993. doi: 10.1200/JCO.2005.05.2456. [DOI] [PubMed] [Google Scholar]
- 30.Trimble EL, Christian MC. National Cancer Institute-United States strategy regarding intraperitoneal chemotherapy for ovarian cancer. Int J Gynecol Cancer. 2008;1:26–28. doi: 10.1111/j.1525-1438.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 31.Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura E, Ochiai K, Noda K Japanese Gynecologic Oncology Group. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomized controlled trial. Lancet. 2009;374(9698):1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 32.WHO. World Health Organization; 2009. May, World Health Statistics 2009”. [Google Scholar]
- 33.Spithoven AHGM. Why U.S. health care expenditure and ranking on health care indicators are so different from Canada’s. Int J Health Care Finance Econ. 2009;9:1–24. doi: 10.1007/s10754-008-9044-0. [DOI] [PubMed] [Google Scholar]
- 34.Greving JP, Vernooig F, Heintz APM, van der Graaf Y, Buskens E. Is centralization of ovarian cancer care warranted? A cost-effectiveness analysis. Gyn Oncol. 2009;113:68–74. doi: 10.1016/j.ygyno.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Schackman BR, Scott CA, Walensky RP, Losina E, Freedberg KA, Sax PE. The cost-effectiveness of HLA-B*5701 genetic screening to guide initial antiretroviral therapy. AIDS. 2008;22(15):2025–2033. doi: 10.1097/QAD.0b013e3283103ce6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyd NF, Sutherland HJ, Heasman KZ, Tritchler DL, Cummings BJ. Whose utilities for decision analysis? Med Dec Making. 1990;10:58–67. doi: 10.1177/0272989X9001000109. [DOI] [PubMed] [Google Scholar]
- 37.Havrilesky LJ, et al. Cost effectiveness of intraperitoneal compared with intravenous chemotherapy for women with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2008;26(25):4144–4150. doi: 10.1200/JCO.2007.13.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Red Book 2010. Montvale, NJ: PDR Network, LLC; 2010. [Google Scholar]
- 39.2010 ICD-9-CM Diagnosis Codes
- 40.Calhoun EA, et al. Evaluating the total costs of chemotherapy-induced toxicity: results from a pilot study with ovarian cancer patients. The Oncologist. 2001;6:441–445. doi: 10.1634/theoncologist.6-5-441. [DOI] [PubMed] [Google Scholar]
- 41.Agency for Healthcare Research and Quality Database. doi: 10.1080/15360280802537332. www.ahrq.gov. [DOI] [PubMed]