Abstract
Background & Aims
Hand2 is a basic helix-loop-helix transcription factor required for terminal differentiation of enteric neurons. We studied Hand2 haploinsufficient mice, to determine whether reduced expression of Hand2 allows sufficient enteric neurogenesis for survival but not for development of a normal enteric nervous system (ENS).
Methods
Enteric transcripts that encode Hand2 and the neuron-specific embryonic lethal abnormal vision proteins HuB, HuC, and HuD were quantified. Immunocytochemistry was used to identify and quantify neurons. Apoptosis was analyzed with the TUNEL procedure. Intracellular microelectrodes were used to record inhibitory junction potentials. Gastrointestinal transit and colonic motility were measured in vivo.
Results
Levels of of enteric Hand2 transcripts were associated with genotypes of mice, in the following order: Hand2+/+ > Hand2LoxP/+ > Hand2+/− > Hand2LoxP/−. Parallel reductions were found in expression of HuD and, in regional and phenotypic manners, numbers of neurons; numbers of nNOS+ and calretinin+, but not substance P+ or vasoactive intestinal peptide+ neurons, decreased. No effects were observed in stomach or cecum. Apoptosis was not detected, consistent with the concept that Hand2 inhibits neuronal differentiation, rather than regulates survival. The amplitude of inhibitory junction potentials in colonic circular muscle was similar in Hand2 wild-type and haploinsufficient mice, although in haploinsufficient mice, the purinergic component was reduced and a nitrergic component appeared. The abnormal ENS of haploinsufficient mice slowed gastrointestinal motility but protected mice against colitis.
Conclusion
Reduced expression of factors required for development of the ENS can cause defects in the ENS that are subtle enough to escape detection yet cause significant abnormalities in bowel function.
Keywords: transcriptional regulation, nervous system development, ELAV, mouse model
INTRODUCTION
Hand1 and Hand2, which encode basic helix-loop-helix transcription factors 1, are expressed in developing gut 2, 3. Enteric expression of Hand2 in mice is developmentally regulated and restricted to crest-derived cells, while that of Hand1 occurs in muscle and interstitial cells of Cajal. Although deletion of Hand2 does not interfere with the colonization of the bowel by crest-derived cells, these cells are unable to form neurons in vitro 3. Transfection of enteric crest-derived cells (ENCDC) with siRNA to silence Hand2 in vitro also prevents neuronal differentiation. The Wnt1-Cre-mediated conditional inactivation of Hand2 in migrating crest-derived cells interferes with the terminal differentiation of HuD-expressing enteric neurons; nevertheless, enteric crest-derived precursors express early pan-neuronal markers, such as β3-tubulin. It has thus been suggested that Hand2 expression is required for terminal differentiation of enteric neurons, albeit not for ENCDC to colonize the bowel, commit to a neuronal lineage, or form glia.
Exon 1 of Hand2 was flanked with loxP sites to generate conditional knockout mice 3. Although Hand2LoxP/LoxP mice appear normal, a cleft palate develops in Hand2LoxP/− fetuses, suggesting that flanking intron 1 of the Hand2 gene with loxP sites generates a hypomorphic allele that impairs development, at least of the palate. Similarly, in vitro experiments with siRNA have suggested that there is a minimum essential threshold of Hand2 expression that must be exceeded for terminal differentiation of enteric neurons 3.
We now show that enteric neurogenesis is a quantitative function of Hand2 expression, which is reduced in the bowel of Hand2+/− and further reduced in Hand2LoxP/− mice. Parallel reductions occur in the enteric expression of the neuron-specific gene, HuD, and in the numbers of enteric neurons, especially those containing neuronal nitric oxide synthase (nNOS) and calretinin. The gene-dosage of Hand2 thus appears to be critical for the acquisition of a normal complement of enteric neurons. These molecular defects have functional consequences. Gastrointestinal (GI) motility is reduced in Hand2 haploinsufficient mice, which are also, paradoxically, resistant to intestinal inflammation.
MATERIALS AND METHODS
Animals and measures of motility
Hand2+/− mice were bred at Columbia University. The “floxed” Hand2 allele includes LoxP sites placed 5′ of the start of transcription and within the first intron 4. Mutant embryos were identified by PCR genotyping of extraembryonic membranes. A non-absorbed dye was used to measure total transit time, the time required to eject a glass bead placed into the rectum was employed to evaluate colonic motility, and conventional intracellular microelectrodes were employed to analyze inhibitory junction potentials in smooth muscle (see Supplemental Methods for details).
Real-time PCR
RNA extraction and cDNA preparation were as previously described 5. cDNA was amplified using sequence-specific primers (see supplemental data, Table 1). PCR products were sequenced and found to match the appropriate sequences in the GenBank. Real-time PCR was used to quantify transcripts extracted from mouse gut (SYBR Green I; LightCycler, Roche Molecular Biochemicals, Indianapolis, IN).
Immunocytochemistry and histochemistry
Tissues were fixed with 4% formaldehyde (from paraformaldehyde) in 0.2 M phosphate buffer at pH 7.4. Dissected bowel was fixed overnight at 4° C. Fixed preparations from E17 gut were then cryoprotected (30% sucrose; 4° C), embedded in Neg50™ (Richard Allan Scientist, Kalamazoo, MI), frozen (liquid N2), and sectioned with a cryostat-microtome. Fixed laminar preparations containing the submucosal or longitudinal muscle with attached myenteric plexus (LMMP) were prepared from 6-8 week old mice by dissection and examined as whole mounts. Procedures used for immunostaining have been described previously 6. Acetylcholinesterase activity was demonstrated histochemically 7. (see Supplemental Methods for details.)
TUNEL assay
Apoptosis was detected in tissue fixed as above, according to the manufacturer’s instructions, with the TMR red In Situ Cell Death Detection Kit (Roche, NJ), (supplemented with 0.1 % sodium citrate). Terminal transferase was omitted as a negative control. Tissue was exposed to DNase I prior to the assay (10 min; Roche, NJ) to provide a positive control.
TNBS-induced colitis
Acute colitis was induced with 2,4,6 trinitrobenzenesulfonic acid (TNBS; Fluka; 5mg/35 gram mouse) 8 in 6 age-, sex- and weight-matched Hand2+/− mice and 6 WT littermates. Mice were euthanized after seven days and tissue was taken for histological examination. The distal 2 cm of colon was embedded in paraffin and 5 μM transverse sections were stained with hematoxylin and eosin. An expert pathologist scored the histopathology without knowledge of the animals’ treatment. Scoring was based on inflammation, crypt damage and ulceration 9. (see Supplemental Methods)
Statistical analyses
One-way ANOVA or, when only 2 groups were studied, Student’s t test was used to compare means. Equality of variances was analyzed with an F test and Welch’s correction was employed when variances of populations was significantly different.
RESULTS
Hand2 regulation of ENS development is gene dosage dependent
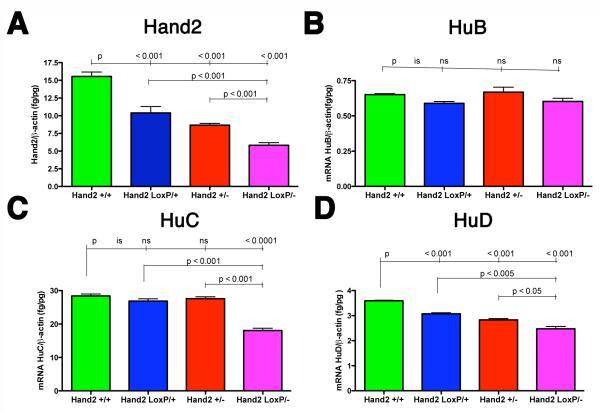
We tested the hypothesis that, like the palate, the ENS is more sensitive than other targets to reduced levels of Hand2 during development. We therefore analyzed the Hand2+/− and Hand2LoxP/− gut to determine whether hypomorphic effects could be detected in the ENS. The abundance of transcripts encoding Hand2 in the E13 gut, analyzed by real-time PCR, reflected the animals’ genotype and decreased in the rank order Hand2+/+ > Hand2LoxP/+ > Hand2+/− > Hand2LoxP/−. Differences between groups were significant. To determine whether Hand2 expression dose-dependently affects neuronal development, transcripts encoding the neuron-specific proteins, HuB, HuC, and HuD were quantified in E13 gut (Fig. 1B-D). The abundance of transcripts encoding HuB, which was relatively low, did not differ significantly in Hand2+/+, Hand2LoxP/+, Hand2+/−, and Hand2LoxP/− animals (Fig. 1B). Although the enteric abundance of transcripts encoding HuC in Hand2LoxP/+ and Hand2+/− mice was not different from that in WT animals, it was significantly lower in the bowel of Hand2LoxP/− mice (Fig. 1C). In contrast, the abundance of enteric transcripts encoding HuD paralleled the abundance of transcripts encoding Hand2; transcripts encoding HuD decreased in the order Hand2+/+ > Hand2LoxP/+ > Hand2+/− > Hand2LoxP/− (Fig. 1D). Differences between groups were significant.
Figure 1. Hand2+/− and Hand2LoxP/− mice are hypomorphic.
(A-D) Real-time PCR was used to quantify transcripts encoding Hand2, HuB, HuC, and HuD in the gut of Hand2+/+, Hand2LoxP/+, Hand2+/−, and Hand2LoxP/− mice at E13. (A) The abundance of transcripts encoding Hand2 in the gut of Hand2+/+ mice > Hand2LoxP/+ mice > Hand2+/− mice > Hand2LoxP/− mice. (B). No significant differences are found in abundance of enteric transcripts encoding HuB in Hand2+/+, Hand2LoxP/+, Hand2+/−, and Hand2LoxP/− mice. (C) The abundance of enteric transcripts encoding HuC is significantly decreased in Hand2LoxP/− mice. (D) The abundance of enteric transcripts encoding HuD in Hand2+/+ > Hand2LoxP/+ > Hand2+/− > Hand2LoxP/−. Each parameter (A-D) was analyzed in 3 mice of each genotype
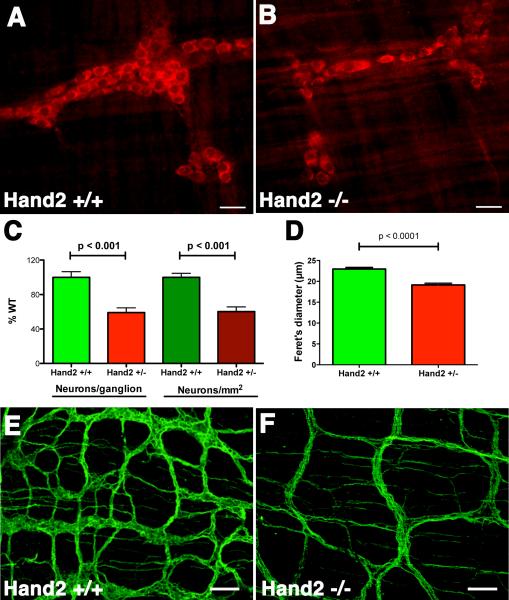
The effects of Hand2 haploinsufficiency on the generation and/or survival of neurons in the mature ENS was evaluated by counting the number and determining the size of neurons in the bowel of 6-8 week old Hand2+/+ and Hand2+/− mice. Antibodies to HuD were used to identify neurons immunocytochemically (Fig. 2A, B). Because of the cleft palates that develop in Hand2loxP/− mice, these animals do not survive and thus their postnatal bowel could not be investigated. The numbers of neurons, per ganglion (Fig. 2C) or per mm2 (Fig. 2D) were significantly lower in Hand2+/− mice at 6-8 wks than in their Hand2+/+ littermates. Similar changes in neuronal numbers were observed at 3 wks, although ganglia were less well organized; the numbers in Hand 2+/− mice were 73.5 ± 6.4 % that of Hand2+/+; p < 0.001). Neurons were smaller in Hand2+/− than in Hand2+/+ mice at 6 but not 3-wks of age. The relative abundance of neurites and connectives, evaluated in LMMP preparations in which β3-tubulin immunoreactivity (Fig. 2E, F) or AChE (Supplemental Fig. 1) activity was demonstrated, were also found to be lower (and space between connectives greater) in the myenteric plexus of Hand2+/− than WT mice. As with neurons visualized with antibodies to HuD, fewer neurons could be detected with antibodies to β3-tubulin in myenteric ganglia of Hand2+/− than in Hand2+/+ mice (Supplemental Fig. 2A, B, E). Neurons were smaller and their cytoplasm not as well-filled with β3-tubulin immunoreactivity in the Hand2+/− neurons as in their Hand2+/+ counterparts (compare Supplemental Figs. 2A and B). In contrast to neurons, glia were not decreased in myenteric ganglia of Hand2+/− animals (Supplemental Fig. 2 C, D, E, and F). The glia to neuron ratio was thus greater in Hand2+/− than in Hand2+/+ mice (Supplemental Fig. 2G). These data suggest that both the number and eventual sizes of neurons in the postnatal ENS parallels the abundance of transcripts encoding HuD, which in turn depends upon the abundance of transcripts encoding Hand2.
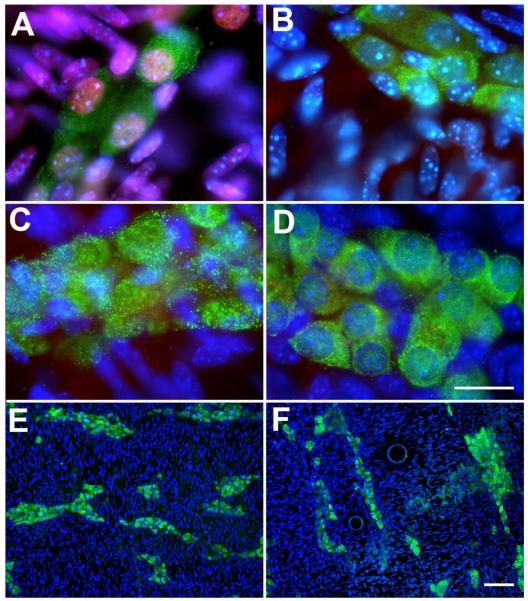
Figure 2. Neuronal numbers are reduced in the ENS of Hand2+/− mice.
HuD immunoreactivity in the myenteric plexus of the colon of (A) a Hand2+/+ mouse and (B) a Hand2+/− littermate. C, D, E. Numbers of HuD-immunoreactive neurons were quantified and compared in Hand2+/+ and Hand2+/− bowel as a function of ganglia (C) and area (D). Significantly more neurons are found in the myenteric plexus of the Hand2+/+ than in the Hand2+/− gut, both counted as neurons per ganglia and neurons/mm2 E. Neurons are significantly smaller in Hand2+/− than in the Hand2+/+ colon.
Enteric nNOS-expressing neurons are reduced in Hand2 haploinsufficient mice
We investigated the possibility that differentiation of subsets of enteric neurons might be more sensitive than others to the reduction of Hand2 expression that occurs in the Hand2+/− or Hand2LoxP/− mice and fail to develop in the ENS of these animals. The nature of the defect in the ENS associated with Hand2 haploinsufficiency, therefore, might be reflected in a change in the “chemical coding” of the ENS 10. Enteric neurons of different phenotypes are born at different ages 11; moreover, enteric neuronal phenotypes reflect the number of proliferative division precursors undergo prior to exiting the cell cycle 12. Particularly because terminal differentiation is Hand2-dependent, we used Hand2 haploinsufficient mice to test the hypothesis that the development of early- and late-born types of enteric neuron, which have, respectively, undergone fewer or greater numbers of proliferative divisions, differentially depend on Hand2 regulation.
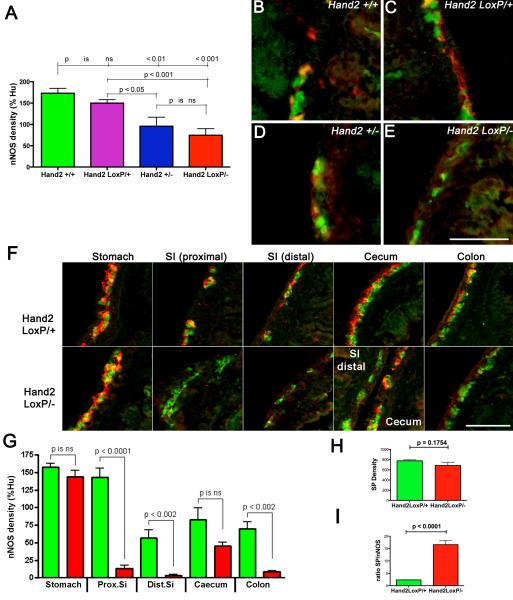
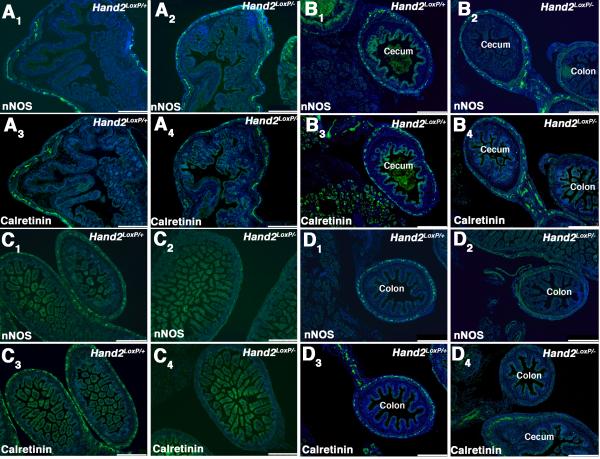
Cholinergic neurons, which coexpress calretinin, are born early while many neurons that contain nNOS are born late 12. Vasoactive intestinal peptide (VIP), which is coexpressed with nNOS 10, is found in late-born neurons 11, although nitrergic neurons can also be detected as early as E11.5 13-15. The effects of Hand2 haploinsufficiency on the development of calretinin- and nNOS-immunoreactive (+) neurons were investigated in the gut of Hand2+/+, Hand2LoxP/+, Hand2+/−, and Hand2LoxP/− mice at E17. Hu immunoreactivity was used as a neuronal marker. The densities of nNOS+ neurons, normalized to that of Hu+ cells, were significantly lower in the intestines of Hand2+/− and Hand2LoxP/− mice than in those of Hand2+/+ or Hand2LoxP/+ animals (Fig. 3A). The reduction of the density of nNOS+ neurons in the Hand2LoxP/− and Hand2+/− gut, furthermore, was due to a decrease in the numbers of nNOS+ neurons, rather than to a reduction in the intensity of nNOS immunoreactivity in individual cells (Fig. 3B-E). The numbers (as % Hand2LoxP/+) of Phox2b+ ENCDC (106 ± 7; n = 6), BrdU-labeled cells 2 hrs after administration of BrdU to pregnant dams (104 ± 9; n = 6), and the ratio of BrdU-labeled to Phox2b+ cells (98 ± 4; n = 6) in the foregut at E12 of Hand2LoxP/− mice did not differ significantly (p = 0.67) from those in Hand2LoxP/+ animals. It thus seems unlikely that Hand2 haploinsufficiency impairs the colonization of the bowel by ENCDC or their subsequent proliferation. Surprisingly, the decrease in relative density of nNOS+ neurons in Hand2LoxP/− gut relative to Hand2LoxP/+ controls was regionally specific in that it was seen in the small intestine, proximal, and distal colon, but not in the stomach or cecum (Fig. 3F, G). Some loss of cecal nNOS+ neurons might be demonstrable if more animals were to be examined. In contrast to nNOS+ neurons, the density of substance P (the best known tachykinin)-immunoreactive neurons (examined as an example of a late appearing neuronal phenotype 16,17) in Hand2LoxP/− mice was not significantly different from that of Hand2LoxP/+ mice (Fig. 3H; n = 9 mice). Because of the smaller number of nNOS+ neurons, the nNOS+/substance P+ ratio was significantly greater in Hand2LoxP/− than in Hand2LoxP/+ mice (Fig. 3I). The effect of Hand2 haploinsufficiency on the development of nNOS+ and calretinin+ neurons, studied in the gut of Hand2LoxP/+ and Hand2LoxP/− mice at E18, was found to be remarkably similar. For both types of neuron, there was a preservation of development in the stomach (Fig. 4A) and cecum (Fig. 4B); however, development of nNOS and calretinin-containing neurons was markedly deficient in the small intestine (Fig. 4C) and the colon (Fig. 4D). Effects of the reduction of Hand2 expression are thus selective. These observations are consistent with the idea that phenotypic expression in the ENS is Hand2-dependent and that the level of Hand2 expression necessary to support the acquisition of a full neurotransmitter-defined neuronal phenotype varies as a function of neuronal subtype and bowel region.
Figure 3. nNOS+ neurons are Hand2-dependent.
(A-G) The density (pixels/mm gut length, normalized to the density of Hu+ neurons) of nNOS+ neurons was analyzed in the intestine of Hand2+/+, Hand2LoxP/+, Hand2+/−, and Hand2LoxP/− mice at E17. (A) Although the density of nNOS+ neurons in Hand2LoxP/+ was similar to that of Hand2+/+ controls, nNOS+ neuronal density was significantly reduced in Hand2+/−, and Hand2LoxP/− fetuses. (B-E) The myenteric plexus, doubly immunostained to demonstrate nNOS (red) and Hu immunoreactivities (green). B. Hand2+/+. C. Hand2LoxP/+. D. Hand2+/−. E. Hand2LoxP/−. The fluorescence of nitrergic neurons is yellow because antibodies to Hu and nNOS doubly immunostain these cells. The proportion of nNOS+ neurons to Hu+ neurons is about the same in Hand2+/+ and Hand2LoxP/+ gut but is reduced in Hand2+/− and especially in Hand2LoxP/− bowel. (F, G) The density of nNOS immunoreactivity in Hand2LoxP/+ and Hand2LoxP/− mice, normalized to that of Hu, was compared in the stomach, proximal small intestine (SI prox), distal small intestine (SI dist), cecum, and colon. F. The myenteric plexus, doubly immunostained to demonstrate nNOS (red) and Hu (green) is illustrated for each of the regions analyzed in G. The regional specificity of effect of genotype on nNOS+ neuronal density is particularly evident in the panel illustrating the distal small intestine and cecum of the Hand2LoxP/− mouse where a loop from each of these regions from the same animal appear in a single section; nNOS+ neurons are virtually absent in the distal small intestine but abundant in the cecum. G. Quantitation of the ratios of the regional density of nNOS to Hu immunoreactivities in the bowel of Hand2LoxP/+ and Hand2LoxP/− mice. The nNOS+ neuronal density in the stomach and cecum was similar in Hand2LoxP/+ and Hand2LoxP/− fetuses; however, in SI prox, SI dist, and colon the nNOS+ neuronal density in Hand2LoxP/+ was significantly greater than that in Hand2LoxP/−. (H) The density of SP immunoreactivity/mm length of bowel in Hand2LoxP/+ proximal small intestine does not differ significantly from that in the gut of E17 Hand2LoxP/− mice. I. The ratios of the densities of SP to nNOS immunoreactivity were measured in proximal small intestine. Because of the decrease in nNOS without a corresponding decrease in SP, the SP/nNOS ratio is significantly greater in Hand2LoxP− than Hand2LoxP+ animals. Each parameter (A-G) was analyzed in 3 mice of each genotype. Five additional Hand2LoxP+ and 4 Hand2LoxP/− animals were analyzed in H-I. Scale bars: 50mm.
Figure 4. Regional effects of Hand2 haploinsufficiency on enteric calretinin+ neurons and nNOS+ are similar.
Representative sections are shown at low magnification to facilitate comparisons between nNOS+ and calretinin+ neurons at various levels of the E18 bowel of Hand2LoxP/+ (A1, A3, B1, B3, C1, C3, D1, D3) and Hand2LoxP/− (A2, A4, B2, B4, C2, C4, D2, D4) mice. Two mice of each genotype are illustrated. A. Stomach. Numbers of nNOS+ and calretinin+ neurons are similar in Hand2LoxP/+ (A1, A3) and Hand2LoxP/− (A2, A4) mice. B. Cecum. Numbers of nNOS+ and calretinin+ neurons are similar in Hand2LoxP/+ (B1, B3) and Hand2LoxP/− (B2, B4) mice. The section in C4 contains a segment of colon, which can be compared to the segment of cecum in the same field of view. There is little calretinin immunoreactivity in the colon, but calretinin immunoreactivity is prominent in the cecum of the same Hand2LoxP/− animal. C. Small intestine. The immunoreactivities of nNOS and calretinin are prominent in the developing myenteric plexus in Hand2LoxP/+(C1, C3) mice but deficient in that of Hand2LoxP/− (C2, C4) littermates. D. Colon. nNOS and calretinin immunoreactivities are prominent in the developing myenteric plexus of Hand2LoxP/+(D1, D3) mice but lacking in that of Hand2LoxP/− (D2, D4) mice. D4 contains cecum, which can be compared to colon in the same field. Calretinin immunoreactivity is evident in the cecum, but lacking in the colon of the same Hand2LoxP/− animal. The bars = 50 μm.
The reduction of nNOS and calretinin-expressing neurons in Hand2 haploinsufficient mice was not confined to fetal gut, but persisted through postnatal weeks 6-8. Significantly fewer nNOS and calretinin-expressing neurons were found in the ENS of Hand2+/− than in Hand2+/+ intestine at 6 weeks of age (Supplemental Fig. 2); moreover, the deficit in each type of neuron was of equal magnitude in both the submucosal (nNOS = 50.4 ± 4.6 %WT; p < 0.001; calretinin = 31.9 ± 2.2 %WT; p < 0.001) and myenteric plexus (nNOS = 55.6 ± 4.6 %WT; p < 0.001; calretinin = 32.9 ± 2.9 %WT; p < 0.001) (Supplemental Fig. 2). In situ hybridization and immunocytochemistry were used to investigate transcripts encoding VIP to determine whether the nNOS deficiency in Hand2+/− gut is due to an absence of a type of neuron or a failure of an existing neuron to acquire its full terminally differentiated phenotype (Supplemental Fig. 4). VIP transcripts, but not immunoreactivity , were detected at E14 (not illustrated)16; however, both were found at E18 in Hand2LoxP/+ and Hand2LoxP/− mice. At that age, nNOS and VIP immunoreactivities were mostly coincident in Hand2 LoxP/+ mice but not in the Hand2 LoxP/− ENS. Because VIP immunoreactivity in neuronal perikarya is inconsistent in the absence of colchicine treatment, in situ hybridization was used to quantify VIP-expressing and nNOS+ neurons in the proximal small intestines of the same Hand2LoxP/+ and Hand2LoxP/− animals (Supplemental Fig. 4C; n = 3). Despite the significantly smaller number of nNOS+ neurons in the Hand2LoxP/− gut, the number of VIP-expressing neurons did not differ in Hand2LoxP/+ and Hand2LoxP/− mice. As a result, there was a significant increase in the ratio of VIP-expressing to nNOS+ neurons in the Hand2LoxP/− bowel (Supplemental Fig. 4D).
Apoptosis is not detectable in enteric neurons of newborn or postnatal mice
Because the myenteric plexus of mature Hand2+/− mice contains fewer neurons than that of their Hand2+/+ littermates, it is possible that decreased Hand2 expression adversely affects enteric neuronal survival. The density of Hu-expressing neurons in Hand2+/+ and +/− mice was found to be the same at E17, but to decline in Hand2+/− mice at 3 and 6 weeks of age; therefore, apoptosis was compared in the myenteric plexuses of 3 week-old Hand2+/+ and Hand2+/− mice. The TUNEL assay was used for this purpose in LMMP preparations (Fig. 5). The immunoreactivity of HuD was used as a neuronal marker and DNA was stained with bisbenzimide. The TUNEL assay failed to detect apoptosis in myenteric neurons either in Hand2+/+ mice (Fig. 5A-D, K) or in their Hand2+/− littermates (Fig. 5E-H, L). In contrast, in a positive control treated with DNAase before the TUNEL assay, all of the nuclei in both smooth muscle and ganglia showed the red fluorescence indicative of DNA fragmentation (Fig. 5I). No non-specific TUNEL staining was detected when terminal transferase was omitted (Fig. 5J). These observations suggest that if apoptosis had been present in myenteric neurons, the TUNEL assay would have revealed it. Similar results were obtained with the TUNEL assay in frozen sections cut through the gut of newborn Hand2+/+ or Hand2+/− mice (data not illustrated). Again, no evidence of apoptosis was detected in myenteric ganglia of either type of animal; therefore, Hand2 haploinsufficiency does not seem to lead to apoptotic death of neurons through the first 3 weeks of life.
Figure 5. Little or no apoptosis of myenteric neurons is seen in either Hand2+/+ or Hand2+/− mice.
Laminar whole mounts of longitudinal muscle with adherent myenteric plexus were prepared from the bowel of 3 week-old Hand2+/+ and Hand2+/− mice. The TUNEL procedure (red) was carried out to detect apoptosis in neurons identified as Hu+ cells (green). DNA was stained with bisbenzimide (blue). A-D. A field from a Hand2+/+ mouse, imaged to show the TUNEL reaction (A), DNA (B), Hu immunoreactivity (C) and merged images (D). No structures show TUNEL staining (A); therefore, no red fluorescence appears in the merged image (D). The blue fluorescence of smooth muscle nuclei (B) forms a bed to which the myenteric ganglia adhere (D). E-F. A field from a Hand2+/− mouse, imaged to show the TUNEL reaction (E), DNA (F), Hu immunoreactivity (G) and the merged images (H). Again, no structures show TUNEL staining (E) and no red fluorescence appears in the merged image (H). More neurons are evident in the ganglia of Hand2+/+ mice (C, D) than in those of Hand2+/− animals (G, H). I. The preparation was treated with DNAase to provide a positive control for the TUNEL reaction. A high power merged image is shown in which the DNA fragmentation is made evident by the superimposed merged red fluorescence of the TUNEL reaction and the blue fluorescence of DNA. Hu+ neurons are marked by their green fluorescence. J. Terminal transferase was omitted to provide a negative control for the TUNEL reaction. A merged image is shown at the same magnification as that of the positive control (I). There is no red fluorescence; therefore, nuclei display unmodified blue fluorescence in the merged image. Again, Hu+ neurons are marked by their green fluorescence. K. A high power merged image of a myenteric ganglion from a Hand2+/+ mouse subjected to the TUNEL procedure. No red fluorescence indicative of DNA fragmentation has been produced by the TUNEL assay. L. A high power merged image of myenteric ganglion from a Hand2+/− mouse subjected to the TUNEL procedure. Again, no red fluorescence indicative of apoptosis has been produced by the TUNEL assay. The markers A-H = 100 μm; I-L = 20 μm.
Enteric motility is abnormal in Hand2+/− mice
Because Hand2+/− mice are fertile and survive with a typical lifespan, they have been considered to be “normal”. There are, nevertheless, significant reductions in the enteric expression of Hand2 (Fig. 1A) and HuD (Fig. 1D), while numbers of total, nNOS+ and calretinin+ enteric neurons are also decreased (Fig. 3A-G, 4). ENS development, therefore, is abnormal in Hand2+/− mice even though the severity of the defects does not lead to pseudoobstruction or to the death of the animals. To determine whether these defects have functional consequences, we measured total GI transit time, colonic motility, and IJPs in Hand2+/− mice. Hand2LoxP/− mice die at birth; therefore, these animals cannot be used to study postnatal intestinal motility.
Total gastrointestinal transit time 18 was significantly greater in Hand2+/− than in Hand2+/+ mice (Fig. 6A). Because gastric emptying is included in total gastrointestinal transit time, and nNOS+ neurons were not affected in the stomachs of Hand2+/− mice, colonic motility was separately evaluated. Colonic motility was significantly slower in Hand2+/− than in Hand2+/+ mice (Fig. 6B). These data confirm that Hand2 haploinsufficiency not only alters numbers and phenotypes of enteric neurons, but also causes gastrointestinal motility to become abnormal.
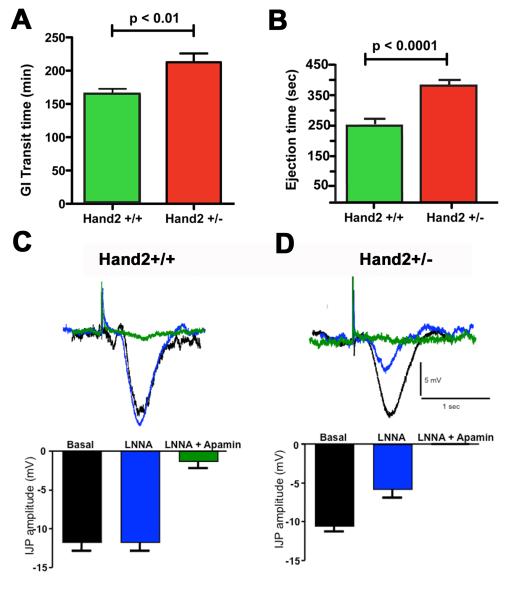
Figure 6. Hand2 haploinsufficiency slows total gastrointestinal transit and colonic motility.
(A) Total gastrointestinal transit time in Hand2+/− mice is significantly greater than that of their Hand2+/+ littermates. (B) Colonic motility in Hand2+/− mice is significantly slower than in their Hand2+/+ littermates. N=4 (Hand2+/+), N=4 (Hand2+/−). (C, D) The neurochemistry of inhibitory junction potentials from circular muscle in Hand2+/+ mice is different from that in Hand2+/− animals. C. Intracellular recordings (above) and graph (below) demonstrating that in Hand2+/+ mice, the NOS inhibitor LNNA did not affect the IJP; nevertheless, the IJP was almost completely eliminated by apamin, which inhibits purinergic neuromuscular signals. B. Intracellular recordings (above) and graph (below) demonstrating that LNNA significantly reduced the amplitude of the IJP in Hand2+/− mice and the IJP was eliminated in the presence of LNNA plus apamin. Under basal conditions, the amplitude of the IJP was approximately the same in Hand2+/+ and +/− mice. * indicates p<0.01 vs basal; ** indicates p<0.0001 vs basal
We investigated IJPs to determine whether the defect in colonic motility in Hand2+/− mice could be related to a defect in nitrergic inhibition of smooth muscle. IJPs are thought to be mediated in part by NO 19, although prior studies in mouse colon have suggested that inhibitory neurotransmission is purinergic, rather than nitrergic 20,21. IJPs were recorded in the circular muscle of the colons of Hand2+/+ (Fig. 6C) and Hand2+/− mice (Fig. 6D). The NOS inhibitor, Nω-nitro-L-arginine (LNNA) did not affect IJPs in Hand2+/+ mice (Fig. 6C). The IJP, however, was almost completely eliminated by apamin, which blocks the small conductance Ca2+-activated K+ channels upon which purinergic hyperpolarization of smooth muscle depends. In contrast, LNNA significantly reduced the amplitude of IJPs in Hand2+/− mice and IJPs were eliminated in the presence of LNNA plus apamin (Fig. 6D). In the absence of inhibitors, IJP amplitude was approximately the same in Hand2+/+ and Hand2+/− mice. These findings suggest that IJPs are predominantly purinergic in Hand2+/+ mice, but in Hand2+/− animals, IJPs have a nitrergic as well as a purinergic component.
Hand2 haploinsufficient mice are protected from inflammation
Motility of the bowel is thought to protect the intestine from infection and inflammation 22. Because motility is abnormal in Hand2+/− mice, we studied the susceptibility of the colon of Hand2+/− mice to tissue damage from experimental inflammation. TNBS was used induce colitis. Clinical symptoms, including weight loss, diarrhea, and blood in the stool, were followed. Animals were permitted to survive for 7 days after the administration of TNBS after which the colons were fixed to permit the severity of the inflammation-induced tissue damage to be quantified histologically. Scores were assigned, on a scale of zero to three, taking account of ulceration, crypt damage and leukocyte infiltration 23 (Fig. 7A, B). Despite the slower intestinal transit and colonic motility, the extent of tissue damage following the induction of colitis with TNBS was significantly less in Hand2+/− mice than in their Hand2+/+ littermates (Fig. 7A-C).
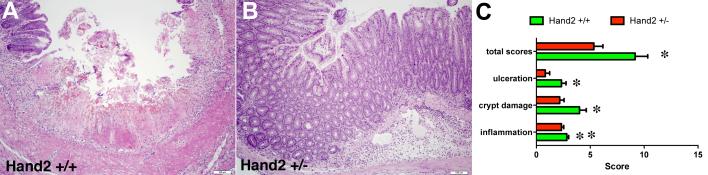
Figure 7. Histological signs of inflammation are greater in Hand2+/+ than Hand2+/− mice.
TNBS was used to induce colitis in Hand2+/+ and Hand2+/− mice. The colons were fixed 7 days later for histological evaluation. A. Hand2+/+ colon. Note the presence of mucosal ulceration and infiltration of the underlying tissue with leukocytes. B. Hand2+/− colon. Ulceration is absent and only moderate edema and leukocyte infiltration is evident. C. The severity of inflammation-induced tissue damage was quantified by assignment of histological scores on a scale of zero to three. The total score and the component scores for ulceration, crypt damage and leukocyte infiltration are illustrated. Total scores and each of the component scores were significantly higher in Hand2+/+ (green) than in Hand2+/− mice (red). * p < 0.01; ** p < 0.05.
DISCUSSION
Prior studies with Hand2+/− mice have assumed that the bowel of these animals is not abnormal because the mice survive and breed. On the contrary, we now find that substantial ENS abnormalities occur in Hand2+/− mice, which while not lethal, affect bowel function. Hand2 expression is reduced by about half in Hand2+/− animals and more so in Hand2LoxP/− mice; these reductions are associated with a decrease in HuD expression. Hu proteins affect neuronal differentiation 24-26. HuD is an RNA binding protein that is related to the ELAV (Embryonic Lethal Abnormal Vision) proteins of Drosophila, which are required for nervous system development and maintenance 27. ELAV proteins extend the longevity of mRNAs by binding to AU-rich elements in the 3′ untranslated region, thereby enhancing their stability 28. Within neurons, HuD competes with other RNA binding proteins that primarily destabilize transcripts 27. Because of this balance, reductions of HuD expression, secondary to decreased expression of Hand2 in Hand2+/− mice, might have the effect of destabilizing transcripts, which would, when sufficiently severe, interfere with neuronal differentiation and/or survival. In adult Hand2+/− animals, these changes in gene expression result in an ENS that has fewer and smaller neurons than in wild-type mice; the defects in the ENS could be due to a direct effect of Hand2 on neuronal differentiation/survival or they may be indirect and secondary to the associated decrease in expression of HuD.
Prior in vitro experiments using siRNA to disrupt Hand2 function suggested that there is a threshold for Hand2 expression, below which neurogenesis fails 3. Current observations support this suggestion, however, they also indicate that such a threshold cannot be the same for all classes of enteric neuron. Hand2 haploinsufficiency interferes with the development of nNOS+ and calretinin+ neurons but not with that of substance P+ neurons; moreover, subsets of nNOS+ and calretinin+ neurons in the stomach and cecum evidently can develop and survive even when Hand2 is 30-50% of normal (in hypomorphic and haploinsufficient animals), while most of those in the remainder of the intestine cannot. It appears, therefore, that the nNOS+ and calretinin+ neuronal sets are heterogeneous with regard to the levels of Hand2, and probably also HuD, expression required to support their development/survival.
Apoptosis was not detected in the postnatal bowel of either Hand2+/+ or Hand2+/− mice. The TUNEL assay, which was employed for this investigation, was able to detect DNA fragmentation when it was induced as a positive control prior to the procedure; moreover, the use of LMMP preparations enable large regions and many neurons of the myenteric plexus to be examined. It is likely, therefore, that the failure of the TUNEL assay to detect apoptosis in 3 week-old animals of either genotype is due to the relative absence of apoptosis during normal ENS development 29, which is not altered by Hand2 haploinsufficiency. The reduction of neurons that occurs in the Hand2+/− ENS, in the absence of evidence for increased apoptosis, is compatible with the idea that the haploinsufficiency of Hand2 interferes with terminal differentiation, rather than survival, of neurons. That likelihood is supported by the observation in Hand2 LoxP/−, but not Hand2 LoxP/+ mice, that VIP expression occurs in neurons that lack nNOS (with a consequent increase in the VIP-expressing/nNOS+ ratio in hypomorphic animals). The nNOS component of the phenotype of neurons that normally contain both VIP and nNOS is not acquired but the incompletely developed VIP-expressing neurons do not die. Prior observations have also suggested that the deletion or silencing of Hand2 interferes with the terminal differentiation of enteric neurons 3. It remains formally possible, however, that non-apoptotic death of neurons 30 or early apoptotic death of a subset of precursors contributes to the deficient numbers of neurons found in the Hand2+/− ENS.
The reduction of the total number of enteric neurons and alteration of the distribution of neurons of different phenotypes that occurs in adult Hand2 haploinsufficient mice, moreover, was associated with abnormal gastrointestinal motility; total gastrointestinal transit time increased and colonic motility slowed. These motor malfunctions suggest that the changes in phenotypic expression within the ENS of Hand2+/− mice disturb the intrinsic reflex circuits upon which motility depends. The observed deficiencies in numbers of nNOS+ and calretinin+ neurons in the small intestine and colon might contribute to the slowing of motility in Hand2+/− mice; however, the maintenance of IJP amplitude and the surprising acquisition of a nitrergic component to IJPs in these animals suggest that the defect is not a simple loss of nitrergic inhibition of smooth muscle. It is thus likely that additional defects exist in the Hand2+/− ENS, perhaps involving a purinergic deficiency and/or nitrergic and Ach/calretinin-expressing interneurons, which also contribute to the abnormal motility of the Hand2+/− bowel.
The motility of the gut is thought to be important in protecting it from microbial invasion 22. One might anticipate, therefore, that the decrease in motility associated with Hand2 haploinsufficiency would predispose the bowel to inflammation, perhaps due to increased invasion by commensal organisms, which is thought to be a factor in the etiology of human inflammatory bowel disease 31, 32. Instead, the Hand2+/− phenotype was found to confer a resistance to the inflammation induced in the colon by instillation of TNBS. TNBS acts as a haptene, which binds to tissue and evokes a cellular immune response. Neuropeptides and neurotransmitters have been found to affect immune responses and inflammation 33. The change in neuronal numbers and the altered distribution of chemically-defined types of neuron that occur in Hand2+/− mice could therefore confer resistance to inflammation by affecting the balance of pro- and anti-inflammatory neuropeptides and/or neurotransmitters that enteric neurons release. A net decrease in the drive to inflammation provided by the ENS may contribute to the resistance of Hand2+/− mice to TNBS-induced colitis.
The observations that reductions in Hand2 expression, which are relatively modest in magnitude, disturb the development of the ENS and the behavior of the mature bowel is of considerable interest. The defect in the Hand2+/− gut is not an aganglionosis and the animals are clearly not a model for Hirschsprung’s disease. In fact, a similar defect arising in the human gut would almost certainly not be noticed by a pathologist examining the bowel. Such diagnoses tend to be binary; ganglia are rated as present or absent 34. Only an investigator looking for it, as some have 35, 36, would recognize a subtle decrease in numbers of neurons or in subsets expressing particular chemically-defined phenotypes, such as those defined by nNOS or calretinin immunoreactivities. In contrast, the pathophysiology of abnormally slow transit in most patients probably would not be diagnosed and, in fact, is common in chronic constipation or constipation-predominant irritable bowel syndrome. Constipation in the absence of an aganglionosis or myopathy is usually considered a functional gastrointestinal disease (FGID) 34. It is thus possible that FGID or subsets of it arise from subtle defects in the ENS resulting from genetic factors that reduce expression of genes such as Hand2 or HuD that are required in ENS development. Similarly, subtle inherited alterations in the neuronal composition of the ENS might contribute individual variations in sensitivity to perturbations of the bowel that give rise to intestinal inflammation.
Supplementary Material
Acknowledgments
Grant Support: National Association for Pediatric Gastroenterology, Hepatology and Nutrition (KGM) NIH NS15547 and NIH NS12969 (MDG)
Footnotes
Disclosures: None of the authors of this manuscript have any financial, professional, or personal disclosures to reveal.
Transcript Profiling: N/A
Writing Assistance: N/A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Firulli AB. A HANDful of questions: the molecular biology of the heart and neural crest derivatives (HAND)-subclass of basic helix-loop-helix transcription factors. Gene. 2003;312:27–40. doi: 10.1016/s0378-1119(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 2.Hendershot TJ, Liu H, Sarkar AA, Giovannucci DR, Clouthier DE, Abe M, Howard MJ. Expression of Hand2 is sufficient for neurogenesis and cell type-specific gene expression in the enteric nervous system. Dev Dyn. 2007;236:93–105. doi: 10.1002/dvdy.20989. [DOI] [PubMed] [Google Scholar]
- 3.D’Autreaux F, Morikawa Y, Cserjesi P, Gershon MD. Hand2 is necessary for terminal differentiation of enteric neurons from crest-derived precursors but not for their migration into the gut or for formation of glia. Development. 2007;134:2237–49. doi: 10.1242/dev.003814. [DOI] [PubMed] [Google Scholar]
- 4.Morikawa Y, D’Autreaux F, Gershon MD, Cserjesi P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol. 2007;307:114–26. doi: 10.1016/j.ydbio.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalazonitis A, D’Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA, Gershon MD. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J. Neurosci. 2004;24:4266–4282. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comoletti D, Flynn RE, Boucard AA, Demeler B, Schirf V, Shi J, Jennings LL, Newlin HR, Sudhof TC, Taylor P. Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for beta-neurexins. Biochemistry. 2006;45:12816–27. doi: 10.1021/bi0614131. [DOI] [PubMed] [Google Scholar]
- 7.Karnovsky MJ, Roots L. A “direct-coloring” method thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- 8.Castagliuolo I, Morteau O, Keates AC, Valenick L, Wang CC, Zacks J, Lu B, Gerard NP, Pothoulakis C. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. Br J Pharmacol. 2002;136:271–9. doi: 10.1038/sj.bjp.0704697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 10.Sang Q, Young H. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tiss. Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- 11.Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system. J. Comp. Neurol. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- 12.Chalazonitis A, Pham TD, Li Z, Roman D, Guha U, Gomes W, Kan L, Kessler JA, Gershon MD. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J Comp Neurol. 2008;509:474–92. doi: 10.1002/cne.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao MM, Moore RE, Roberts RR, Nguyen T, Furness JB, Anderson RB, Young HM. The role of neural activity in the migration and differentiation of enteric neuron precursors. Neurogastroenterol Motil. 2010;22:127–137. doi: 10.1111/j.1365-2982.2009.01462.x. [DOI] [PubMed] [Google Scholar]
- 14.Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J. Comp. Neurol. 1989;285:262–273. doi: 10.1002/cne.902850208. [DOI] [PubMed] [Google Scholar]
- 15.Young HM, Jones BR, McKeown SJ. The projections of early enteric neurons are influenced by the direction of neural crest cell migration. J Neurosci. 2002;22:6005–18. doi: 10.1523/JNEUROSCI.22-14-06005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman TP, Nilaver G, Gershon MD. Colonization of the developing murine enteric nervous system and subsequent phenotypic expression by the precursors of peptidergic neurons. J. Comp. Neurol. 1984;225:13–23. doi: 10.1002/cne.902250103. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine-Pérus JC, Chanconie M, Le Douarin NM. Differentiation of peptidergic neurons in quail-chick chimeric embryos. Cell Differ. 1982;11:183–193. doi: 10.1016/0045-6039(82)90065-3. [DOI] [PubMed] [Google Scholar]
- 18.Nagakura Y, Naitoh Y, Kamato T, Yamano M, Miyata K. Compounds possessing 5-HT3 receptor antagonistic activity inhibit intestinal propulsion in mice. Eur J Pharmacol. 1996;311:67–72. doi: 10.1016/0014-2999(96)00403-7. [DOI] [PubMed] [Google Scholar]
- 19.Hwang SJ, O’Kane N, Singer C, Ward SM, Sanders KM, Koh SD. Block of inhibitory junction potentials and TREK-1 channels in murine colon by Ca2+ store-active drugs. J Physiol. 2008;586:1169–84. doi: 10.1113/jphysiol.2007.148718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer NJ, Bywater RA, Holman ME, Taylor GS. Spontaneous and evoked inhibitory junction potentials in the circular muscle layer of mouse colon. J Auton Nerv Syst. 1998;69:115–21. doi: 10.1016/s0165-1838(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 21.Serio R, Alessandro M, Zizzo MG, Tamburello MP, Mule F. Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur J Pharmacol. 2003;460:183–90. doi: 10.1016/s0014-2999(02)02923-0. [DOI] [PubMed] [Google Scholar]
- 22.Powell DW. Neuroimmunophysiology of the gastrointestinal mucosa: implications for inflammatory diseases. Trans Am Clin Climatol Assoc. 1995;106:124–38. discussion 138-40. [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrance IC, Wu F, Leite AZ, Willis J, West GA, Fiocchi C, Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–61. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Akamatsu W, Fujihara H, Mitsuhashi T, Yano M, Shibata S, Hayakawa Y, Okano HJ, Sakakibara S, Takano H, Takano T, Takahashi T, Noda T, Okano H. The RNA-binding protein HuD regulates neuronal cell identity and maturation. Proc Natl Acad Sci U S A. 2005;102:4625–30. doi: 10.1073/pnas.0407523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakakibara S, Okano H. Expression of neural RNA-binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development. J Neurosci. 1997;17:8300–12. doi: 10.1523/JNEUROSCI.17-21-08300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratti A, Fallini C, Cova L, Fantozzi R, Calzarossa C, Zennaro E, Pascale A, Quattrone A, Silani V. A role for the ELAV RNA-binding proteins in neural stem cells: stabilization of Msi1 mRNA. J Cell Sci. 2006;119:1442–52. doi: 10.1242/jcs.02852. [DOI] [PubMed] [Google Scholar]
- 27.Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner JB, Furneaux HM. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell. 1991;67:325–33. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- 28.Bakheet T, Williams BR, Khabar KS. ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res. 2006;34:D111–4. doi: 10.1093/nar/gkj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130:2187–98. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- 30.Uesaka T, Jain S, Yonemura S, Uchiyama Y, Milbrandt J, Enomoto H. Conditional ablation of GFRalpha1 in postmigratory enteric neurons triggers unconventional neuronal death in the colon and causes a Hirschsprung’s disease phenotype. Development. 2007;134:2171–81. doi: 10.1242/dev.001388. [DOI] [PubMed] [Google Scholar]
- 31.McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–13. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 32.Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:503–11. doi: 10.1097/MOG.0b013e328331b69e. [DOI] [PubMed] [Google Scholar]
- 34.Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, Gershon MD, Hutson J, Lindberg G, Martin JE, Meier-Ruge WA, Milla PJ, Smith VV, Vandervinden JM, Veress B, Wedel T. Gastrointestinal neuromuscular pathology: guidelines for histological techniques and reporting on behalf of the Gastro 2009 International Working Group. Acta Neuropathol. 2009;118:271–301. doi: 10.1007/s00401-009-0527-y. [DOI] [PubMed] [Google Scholar]
- 35.Faussone-Pellegrini MS, Infantino A, Matini P, Masin A, Mayer B, Lise M. Neuronal anomalies and normal muscle morphology at the hypomotile ileocecocolonic region of patients affected by idiopathic chronic constipation. Histol Histopathol. 1999;14:1119–34. doi: 10.14670/HH-14.1119. [DOI] [PubMed] [Google Scholar]
- 36.Wattchow D, Brookes S, Murphy E, Carbone S, de Fontgalland D, Costa M. Regional variation in the neurochemical coding of the myenteric plexus of the human colon and changes in patients with slow transit constipation. Neurogastroenterol Motil. 2008;20:1298–305. doi: 10.1111/j.1365-2982.2008.01165.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.