Abstract
Intrauterine growth restriction (IUGR) increases the risk of postnatal lung disease, with males more affected. In rat lungs, IUGR impairs alveolarization in conjunction with altered expression of peroxisome proliferator activated receptor gamma (PPARγ). In non-lung cells, PPARγ transcription is regulated in part by the epigenetic modifying enzyme, methyl CpG binding protein 2 (MeCP2). However, it is unknown if IUGR affects MeCP2 expression or its interaction with PPARγ in the rat lung during alveolarization. In this study, we hypothesized that the rat lung would be characterized by the presence of MeCP2 short and long mRNA transcripts, MeCP2 protein isoforms and the MeCP2 regulatory micro RNA, miR132. We further hypothesized that IUGR would, in a gender-specific manner, alter the levels of MeCP2 components in association with changes in PPARγ mRNA, MeCP2 occupancy at the PPARγ promoters, and PPARγ histone 3, lysine 9, trimethylation (H3K9Me3). To test these hypotheses, we used a well characterized rat model of uteroplacental insufficiency induced IUGR. We demonstrated the presence of MeCP2 mRNA, protein and miR132 in the rat lung throughout alveolarization. We also demonstrated that IUGR alters MeCP2 expression and its interaction with PPARγ in a gender-divergent manner. We conclude that IUGR induces gender-specific alterations in the epigenetic milieu in the rat lung. We speculate that in the IUGR rat lung, this altered epigenetic milieu may predispose to gender-specific alterations in alveolarization.
Keywords: MeCP2, IUGR, epigenetics, lung development
Introduction
Intrauterine growth restriction (IUGR) increases the risk of lung disease in the immediate neonatal period as well as later in life (Dezateux and others, 2004; Hoo and others, 2004; Kotecha and others, 2010; Laughon and others, 2010; Lawlor and others, 2005; Lucas and others, 2004; Tyson and others, 1995). Importantly, this is true of IUGR babies that are born at term as well as those born preterm (Hoo and others, 2004; Kotecha and others, 2010). In general, male IUGR infants tend to be more severely affected (Frusca and others, 1997; Melamed and others, 2009; Spinillo and others, 2009; Torrance and others, 2010; Zimmermann and others, 1997). In animal models, studies have demonstrated that IUGR produces a lung that is structurally and functionally different from the control lung (O’Brien and others, 2007), often with impaired alveolarization (Harding and others, 2000; Karadag and others, 2009; Maritz and others, 2001; Maritz and others, 2004; O’Brien and others, 2007).
Alveolarization, the last stage of lung development, is characterized by thinning of the lung mesenchyme, extension of secondary septa, and remodeling of the microvasculature (Roth-Kleiner and Post, 2003). This process is conserved between human and rat lungs, with the primary difference being timing relative to birth. In the human lung, the majority of alveolarization takes place prenatally, at approximately weeks 28–32 of gestation. In the rat lung, alveolarization takes place entirely in the postnatal period, postnatal days 4–14. In addition to the structural similarities between human and rat lung development, the molecular pathways involved are also similar (Bland and others, 2007; Groenman and others, 2005; Mariani and others, 1997; Roth-Kleiner and Post, 2003; Wendel and others, 2000). Appropriate activation of these pathways requires precisely timed regulation of gene expression.
The regulation and timing of gene expression is modulated by epigenetics. Epigenetics refers to modifications of DNA and histone proteins that form chromatin. These modifications affect the interactions and targeting of transcription factors, and the transcription machinery, to the DNA. Epigenetics can function to silence a gene entirely, as in developmental gene regulation and gene imprinting (Laprise, 2009; Weaver and others, 2009). Alternatively, epigenetics can provide a means of modulating the transcription level of actively transcribed genes (Liu and others, 2008). The latter effect is often observed in genes that are responding to a stressful stimulus, including IUGR (Fu and others, 2009; Joss-Moore and others, 2010a; Joss-Moore and others, 2010b; Ke and others, 2006).
Our group has previously demonstrated that IUGR alters gene expression and epigenetics in the lung (Joss-Moore and others, 2010a; O’Brien and others, 2007). One gene affected by IUGR is peroxisome proliferator activated receptor gamma (PPARγ) (Joss-Moore and others, 2010b; Joss-Moore and others, 2010c). PPARγ is a member of the nuclear receptor family of transcription factors and is crucial for appropriate lung development (Cerny and others, 2008; Simon and others, 2006). We have demonstrated that IUGR decreases PPARγ mRNA variants (transcribed from multiple promoters), protein abundance and downstream targets at birth in the rat lung (Joss-Moore and others, 2010b). However, while the downstream targets of PPARγ have received some attention (Joss-Moore and others, 2010b; Karadag and others, 2009; Rehan and others, 2006) little is known about the regulation of transcription of PPARγ itself.
PPARγ transcription is regulated in part by methyl CpG binding protein 2 (MeCP2) (Mann and others, 2010). In hepatic cells, MeCP2 associates with the PPARγ promoters reducing PPARγ transcription. In these cells, this “repressive” state is also marked by the presence of the MePC2 associated histone modification, histone 3 lysine 9 tri-methylation (H3K9Me3) on histones surrounding the PPARγ promoters (Fuks and others, 2003). Reduction of MeCP2 at the PPARγ promoters removes the repression and allows PPARγ transcription to increase (Mann and others, 2010).
MeCP2 is an alternatively spliced gene that gives rise to multiple mRNA variants and protein isoforms (Fuks and others, 2003; Kriaucionis and Bird, 2004; Mnatzakanian and others, 2004; Pelka and others, 2005). The MeCP2 gene produces mRNA transcripts with differing 3′ UTR lengths. A short 3′UTR (transcript 1.8kb) and a long 3′UTR (transcript 10.2kb) have been identified in the mouse lung both pre and postnatally (Pelka and others, 2005). The long 3′ UTR has a number of regions that are highly conserved between human and rodent. One of these highly conserved regions is a binding site for the micro RNA, miR132 (Klein and others, 2007). MeCP2 translation is inhibited by miR132 binding to the long 3′UTR (Klein and others, 2007). In addition to the 3′UTR splice variants, the MeCP2 gene also undergoes alternative splicing in the coding region, giving rise to two protein isoforms, MeCP2-e1 and MeCP2-e2 (Kriaucionis and Bird, 2004; Mnatzakanian and others, 2004). These isoforms of MeCP2 differ in their N-terminal region, with the –e1 isoform containing additional N-terminal amino acids. While all of the above MeCP2 variants have been identified in the brain, the combined use of coding variants and 3′UTR variants has not been described.
While the short and long 3′UTR MeCP2 mRNA transcripts have been identified in the lung, the presence and ontogeny of MeCP2 protein isoforms and miR132, during alveolar formation, is unknown. It is also unknown whether IUGR alters levels of MeCP2 mRNA, protein or the miR132. Finally, it is unknown whether MeCP2 associates with PPARγ promoter in the lung and, if so, whether IUGR affects this association.
In this study, we hypothesized that the rat lung would be characterized by the presence of MeCP2 short and long mRNA transcripts, MeCP2-e1 and –e2 protein isoforms and the micro RNA, miR132 throughout alveolarization. We further hypothesized that IUGR would alter the levels of MeCP2 mRNA and protein as well as miR132 and that this will be associated with changes in PPARγ mRNA levels, MeCP2 occupancy at the PPARγ promoters, and PPARγ H3K9Me3. To test these hypotheses, we used a well characterized rat model of uteroplacental insufficiency (UPI) induced IUGR (Baserga and others, 2005; Lane and others, 1996; Lane and others, 2002b; Lane and others, 2000; Ogata and others, 1986). We have previously demonstrated that lung development is impaired at birth in this model (O’Brien and others, 2007).
Methods
Animals
The rat uteroplacental insufficiency model of IUGR has been described in detail previously (Lane and others, 1998; Lane and others, 2002a; Unterman and others, 1993). All procedures were approved by the University of Utah Animal Care Committee and are in accordance with the American Physiological Society’s guiding principles (2002). The surgical procedures have been described previously (Kloesz and others, 2001; Pham and others, 2003). Briefly, on day 19 of gestation, pregnant Sprague-Dawley rats were anesthetized with intraperitonealxylazine (8 mg/kg) and ketamine (40 mg/kg), and both uterine arteries ligated giving rise to IUGR pups. Control dams underwent identical anestheticprocedures. Body weights of IUGR pups used in this study, were approximately 25% less than control pups at birth and 15% less than control pups at d21 (Joss-Moore and others, 2010c).
Day 0 (d0) pups were delivered by caesarian section at term, 2.5 days after bilateraluterine artery ligation. For other ages, dams were allowed to deliver spontaneously and litters randomly culled to 6 pups (3 male and 3 female). Pups remained with the dam for 7 (d7) or 21 (d21) days before sacrifice. Lungs were removed, flash-frozen in liquid nitrogen, and stored at −80°C. For each experiment, each group (control, IUGR) had 6 male pups and 6 female pups, unless otherwise noted. Pups within each group were derived from different litters.
Real-Time RT PCR
Real-time reverse transcriptase PCR was used to evaluate mRNA abundance of long or short MeCp2 mRNA, PPARγ variants (PPARγ1a, PAPRγ1b and PPARγ2) as well the micro RNA, miR132. Real-time RT-PCR was performed as previously described, with GAPDH as an internal control (Joss-Moore and others, 2010c). Primers were as follows: MeCP2 short 3′ UTR, 5′-ctctgccttgcagtcaggtt-3′ and 5′-cagcgaaagataccacccata-3′, MeCP2 long 3′ UTR, 5′-aaaaacaaaaggcaatttattaagga-3′ and 5′-aacaaaagacacaaacggaca-3′, GAPDH 5′-caagatggtgaaggtcggtgt-3′ and 5′-caagagaaggcagccctggt-3′. For PPARγ the following Assay-on-demand primer/probe sets were used: PPARγ1a- Rn01492275_m1, PPARγ1b -Rn01492273_m1, PPARγ2 - Rn00440940_m1, (Applied Biosystems).
Micro RNA’s were extracted using the mirVana miRNA Isolation Kit (Ambion). RT-PCR for mature microRNAs was performed using TaqMan microRNA assays (Applied Biosystems) according to the manufacturer’s protocol. miR-103 was used as a reference miRNA as it has levels have been shown to remain constant across different tissues and different disease states (Peltier and Latham, 2008). In addition, we have observed no change in levels between control and IUGR lung. Primers were miR-132 (assay# 000457) and control, miR-103 (assay# 000439) (Applied Biosystems).
Immunoblot
Immunoblotting was used to determine relative levels of lung MeCP2 protein abundance in IUGR and control rats. Whole lung protein was isolated as previously described (Joss-Moore and others, 2010b). Immunoblotting was performed as previously described (Joss-Moore and others, 2010c) with primary antibody against MeCP2 (Abcam, ab2828).
Chromatin immunoprecipitation (ChIP)
A revised ChIP protocol, based primarily on the methods of Farnham and Bomstztyk (Nelson and others, 2006; Wells and Farnham, 2002), was used to investigate 1) the relative MeCP2 occupancy of the PPARγ promoter 1 (P1) and promoter 2 (P2) and 2) the levels of histone modifications along the PPARγ gene at the following positions, 5′ of P1, at P1, P2, Exon 4 and at the 3′ end of the PPARγ gene. Briefly, chromatin isolation from male and female whole lung (190–280 mg) was performed as follows; tissue was fixed in 1% formaldehyde for 10 minutes at room temperature, after which the reaction was stopped by the addition of glycine to a final concentration of 125 mM. Samples were centrifuged, washed twice with PBS and resuspended in PBS with added protease inhibitor cocktail (PIC) at manufacture’s recommended concentration (Complete, Mini. Roche, Indianapolis, IN). After centrifugation, cell pellets were resuspended in lysis buffer (5 mM PIPES, pH 8.0, 85 mM KCl, 0.5% Igepal) with PIC and dounced on ice using a tight pestle. After centrifuging, pelleted nuclei were resuspended in nuclei lysis buffer (50 mM Tris-Cl, pH 8.1, 10 mM EDTA, 1% SDS) with PIC, incubated for 20 minutes on ice and split into aliquots of approximately 200 μl (in 1.5 ml tubes) for sonication. Due to the importance of the sonication step in producing relatively uniform chromatin fragments while maintaining protein integrity for subsequent immuoprecipitation (IP), significant optimization was performed. Sonication was performed on ice using a Fisher Scientific Model 100 sonicator with microtip attachment (Fisher Scientific, Pittsburgh, PA). Each sample was pulsed 10 times with the micotip placed near the base of the tube and returned to ice, this was repeated a total of 10 times. After centrifuging, chromatin containing supernatants for each of the 4 samples were frozen at −80°C.
Immunoprecipitation (IP) were performed as previously described (Joss-Moore and others, 2010b) using anti-MeCP2 (Abcam, ab2828) and anti-H3K9Me3 antibody (Abcam, ab8898). For MeCP2 IP percent of input was used as a control and for H3K9Me3 IP a non-transcribing intergenic region was used as a control (Fu and others, 2009). Primers sequences for RT-PCR of IP DNA: 5′ of P1 5′ tcgacggctttctgaatgtg and 5′ cttgccctctttcagctctttc; P1 5′ aaaaacaaacttctgcgtgacagt and 5′ ggtcccacgttcctcagaca; P2 5′ ccaagtcttgccaaagaagca and 5′ gattgagagccagctgtgacaa; exon 4 5′ ccatcaggtttgggcgaat and 5′ gatctccgccaacagcttct; 3′ end 5′ cgccaaggtgctccagaa and 5′ ctgcacgtgctctgtgacaa.
Statistics
Ontogeny data are presented as mean ± SD. Statistical significance was assessed using ANOVA and Fisher’s Projected Least Significant Difference test between d0, d7 and d21. IUGR data are presented as IUGR as percent of gender and age matched controls ± SD. Statistical significance was determined by Student’s unpaired t-test with comparison between IUGR and gender-matched control. In both cases, the Statview software package (SAS Institute, Cary, NC) was used to determine statistical significance. p ≤ 0.05 was considered significant.
Results
MeCp2 ontogeny throughout alveolarization
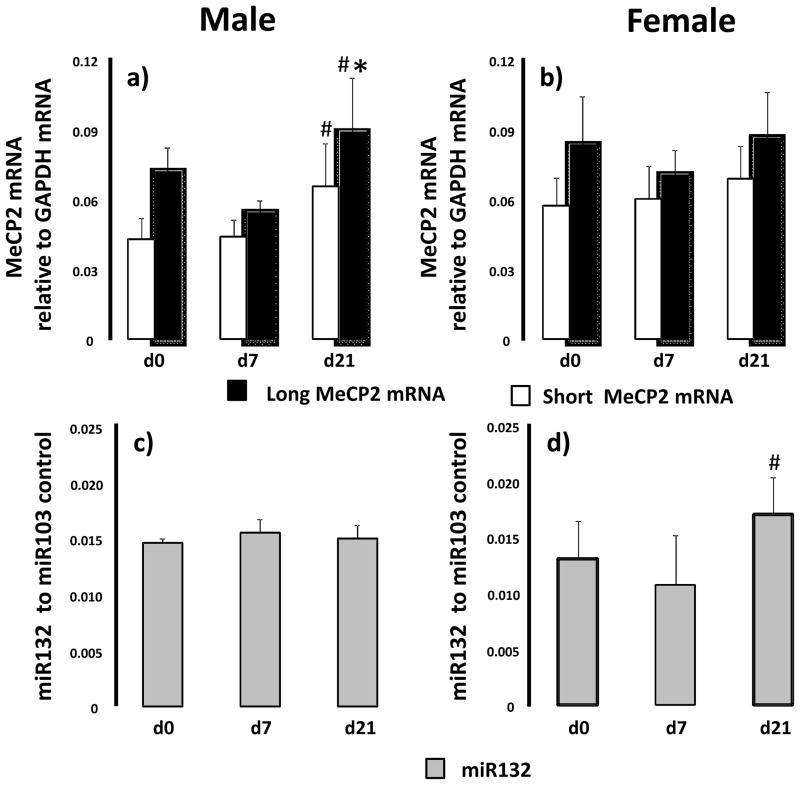
MeCP2 mRNA transcript levels, MeCP2 protein abundance and miR132 levels were quantified in control rat lung at d0 (pre-alveolarization), d7 (mid-alveolarization) and d21 (post-alveolarization). MeCp2 mRNA transcripts with a long (~10kb) 3′UTR and short (~2kb) 3′UTR were detectable in both male and female lung throughout alveolarization. In males, long MeCP2 mRNA transcript levels increased significantly between d7 and d21 (p=0.01) (Figure 1a). Short MeCP2 mRNA transcript levels increased between d7 and d21 (p=0.009) in male rat lung. In contrast, MeCP2 long and short mRNA transcript levels did not change during development in the female lungs (Figure 1b).
Figure 1.
Ontogeny of MeCP2 mRNA and miR132 in rat lung at birth (d0), postnatal day 7 (d7) and day 21 (d21). Male (panel a and c) and female (panel b and d) lungs were examined. Differences in MeCP2 mRNA and miR132 between d0, d7 and d21, for each gender, were assessed with ANOVA with Fisher’s PLSD. Significant difference to d0 indicated by *, significant difference to d7 indicated by #. n=6.
The long 3′ UTR MeCP2 transcript contains a binding site for the micro RNA, miR132. Because the presence of miR132 has been shown to inhibit translation of the long MeCP2 transcript (Klein and others, 2007), we also examined levels of miR132 during alveolarization in control rat lung. Both male and female lungs had detectible levels of miR132 from birth to d21. In male lung, levels of miR132 did not change during alveolarization (Figure 1c). In female lungs, levels of miR132 increased between d7 and d21 (p=0.03) (Figure 1d).
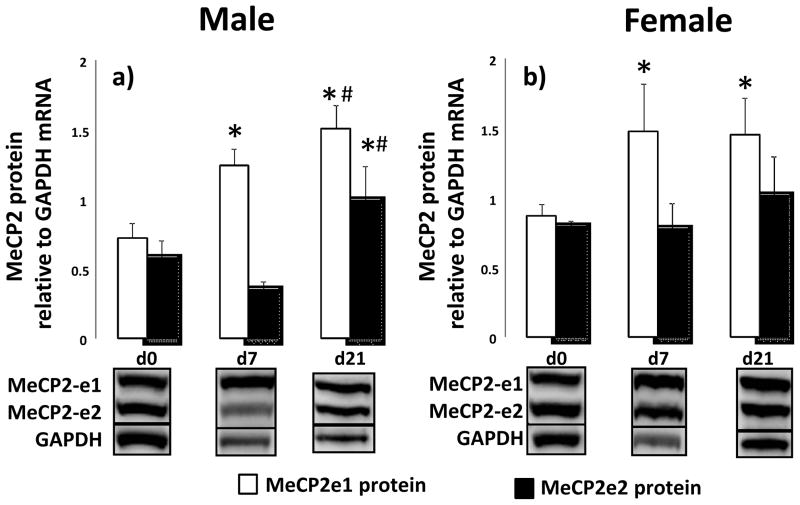
MeCP2 exists as two isoforms that differ in the N-terminus, MeCp2-e1 and -e2 (Giacometti and others, 2007; Mnatzakanian and others, 2004; Quenard and others, 2006). We detected a doublet band for MeCP2 with immunoblot in control rat lung tissue (inserts Figure 2). In male rat lungs, MeCP2-e1 (upper band) abundance increases between d0 and d7 (p=0.001) as well as between d7 and d21 (p=0.017). MeCP2-e2 (lower band) abundance increases between d7 and d21 (p=0.001) in male rat lung (Figure 2a). In female lungs, MeCp2-e1 protein abundance increases between d0 and d7 (p=0.001) with no other changes in MeCp2-e1 or MeCP2-e2 at any time point (Figure 2b).
Figure 2.
Ontogeny of MeCP2 protein isoforms in rat lung at birth (d0), postnatal day 7 (d7) and day 21 (d21). Male (panel a) and female (panel b) lungs were examined. Inserts are representative western blots. Differences in MeCP2 protein between d0, d7 and d21, for each gender, were assessed with ANOVA with Fisher’s PLSD. Significant difference to d0 indicated by *, significant difference to d7 indicated by #. n=6.
IUGR alters MeCP2 mRNA, protein and miRNA in a gender and age specific manner
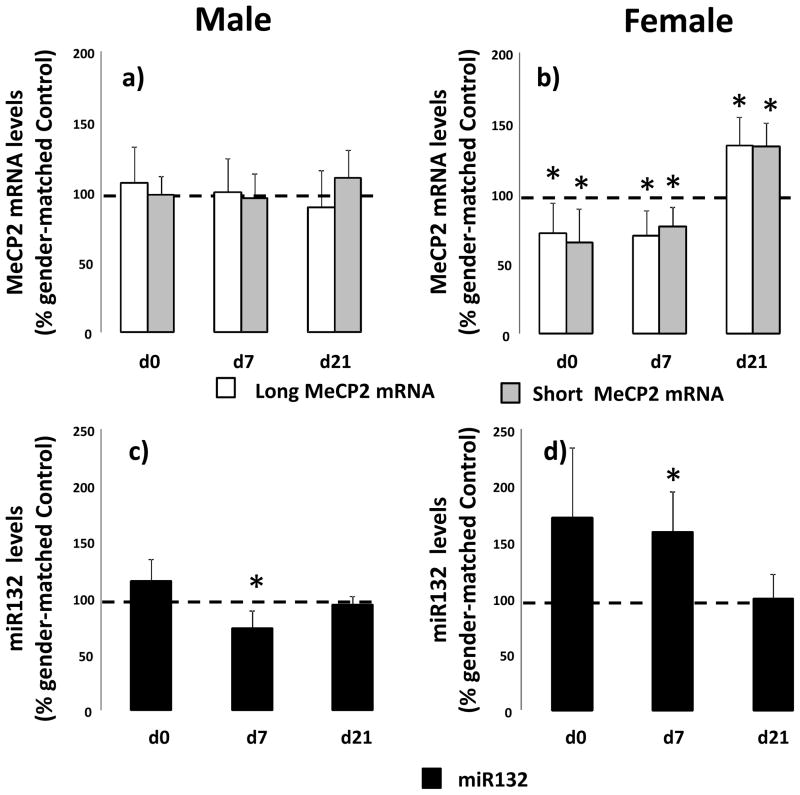
We examined the effect of IUGR on long and short MeCP2 mRNA, MeCP2 protein and miR132 before, during and after alveolarization in male and female rats. In males, IUGR does not alter long or short MeCP2 mRNA transcript levels at any stage examined (Figure 3a). In female lung, IUGR decreases long (p=0.05) and short (p=0.05) MeCP2 mRNA transcript levels at d0 as well as long (p=0.05) and short (p=0.03) MeCP2 mRNA transcript levels at d7 compared to age-matched female control. Conversely at d21 IUGR increases long (p=0.02) and short (p=0.01) MeCP2 mRNA transcript levels in female lung compared to d21 female control (Figure 3b).
Figure 3.
IUGR alters MeCP2 mRNA and miR132 levels in a gender-specific manner in rat lung. Male (panel a and c) and female (panel b and d) control and IUGR lungs were examined at birth (d0), postnatal day 7 (d7) and day 21 (d21). Data are IUGR as a % of age and gender matched control, error bars are standard deviation. Asterisks denote significant differences between IUGR and gender-matched control determined by unpaired t-test. n=6.
We also examined the effect of IUGR on miR132 in male and female lungs. In male lung, IUGR decreases miR132 at d7 (p=0.05) compared to d7 control male (Figure 3c). In female lung, IUGR decreases miR132 d0 (0.08) and at d7 (p=0.05) compared to age-matched female controls (Figure 3d).
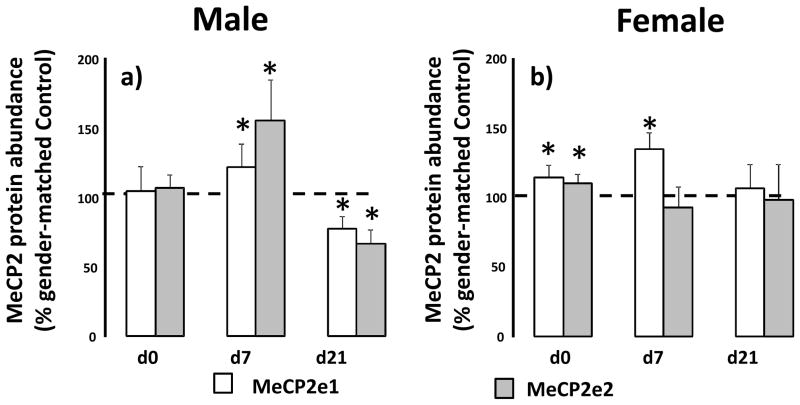
IUGR does not alter levels of male lung MeCP2-e1 or–e2 protein abundance at d0 relative to male d0 control. At d7, IUGR increases MeCp2-e1 abundance (p=0.03) and MeCP2-e2 abundance (p=0.004) relative to male d7 control. However at d21, IUGR decreases MeCP2-e1 (p=0.002) and MeCP2-e2 (p=0.01) abundance relative to male d21 control (Figure 4a). In females lung, IUGR increases both MeCP2-e1 (p=0.01) and MeCP2-e2 (p=0.01) relative to d0 female control. At d7, IUGR only increases MeCP2-e1 abundance (p=0.009) in female lung, with no change at d21 (Figure 4b).
Figure 4.
IUGR alters MeCP2 protein isoform levels in a gender-specific manner in rat lung. Male (panel a) and female (panel b) control and IUGR lungs were examined at birth (d0), postnatal day 7 (d7) and day 21 (d21). Data are IUGR as a % of age and gender matched control, error bars are standard deviation. Asterisks denote significant differences between IUGR and gender-matched control determined by unpaired t-test. n=6.
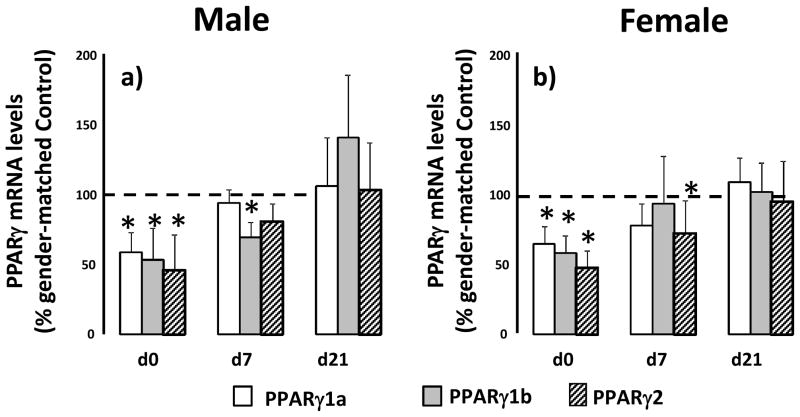
IUGR decreases PPARγ mRNA variants before and during alveolarization
Because MeCP2 has been demonstrated to regulate PPARγ transcription, we measured mRNA transcript levels of PPARγ variants PPARγ1a, PPARγ1b and PPARγ2 before, during and after alveolarization. In male lung, IUGR decreases PPARγ1a (p=0.001), PPARγ1b (p=0.001) and PPARγ2 (p=0.003) mRNA transcript levels at d0 compared to d0 male control. At d7, IUGR decreases PPARγ1b (p=0.004) and PPARγ2 (p=0.07) mRNA transcripts only. There was no change in PPARγ variant mRNA at d21 (Figure 5a). In female lungs at d0, IUGR also decreases PPARγ1a (p=0.007), PPARγ1b (0.001) and PPARγ2 (p=0.02) mRNA transcript levels compared to female d0 controls. At d7, IUGR decreases PPPARγ2 mRNA transcript (p=0.04) relative to d7 female control. There were no changes in PPARγ variants in d21 female lung (Figure 5b).
Figure 5.
IUGR decreases PPARγ variant mRNA levels at birth at d7 in rat lung. Male (panel a) and female (panel b) control and IUGR lungs were examined at birth (d0), postnatal day 7 (d7) and day 21 (d21). Data are IUGR as a % of age and gender matched control, error bars are standard deviation. Asterisks denote significant differences between IUGR and gender-matched control determined by unpaired t-test. n=6.
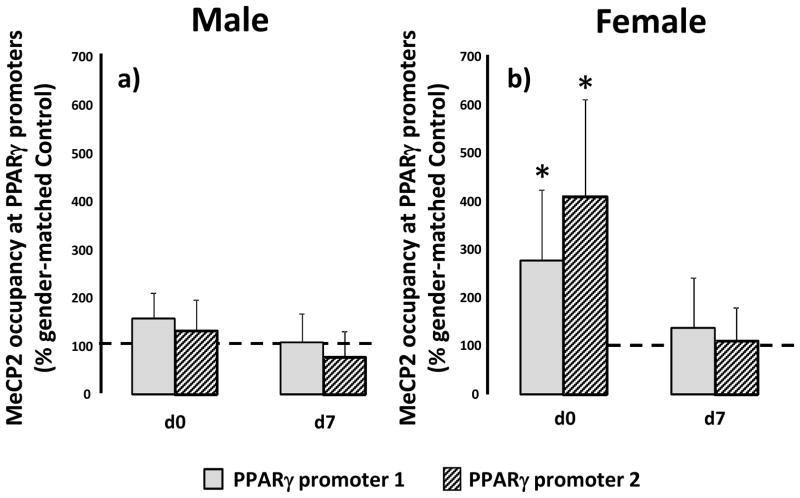
IUGR increases MeCp2 occupancy at the PPARγ promoters in female d0 lung
Because MeCP2 presence at the PPARγ promoters was demonstrated to alter PPARγ expression in hepatocytes, we used ChIP to determine whether MeCP2 also associates with the PPARγ promoters in rat lung. MeCP2 was detectable at both promoter 1 (P1) and promoter 2 (P2) of the PPARγ gene at birth and d7 in male and female rat lungs. In male lungs, IUGR did not alter levels of MeCP2 associated with either PPARγ P1 or P2 relative to age-matched male controls (Figure 6a). In female lungs however, IUGR increased levels of MeCP2 associated with PPARγ P1 (p=0.04) and P2 (p=0.03) at d0 relative to d0 female control, with no change observed at d7 (Figure 6b). At d21, MeCP2 was undetectable at the PPARγ promoters in control and IUGR, male and female rat lung.
Figure 6.
IUGR increases MeCP2 occupancy at PPARγ promoter 1 (P1) and promoter 2 (P2) in d0 rat lung. Male (panel a) and female (panel b) control and IUGR lungs were examined at birth (d0), postnatal day 7 (d7) and day 21 (d21). MeCP2 occupancy at the PPARγ promoters was not detectable at d21 in male or female lung. Data are IUGR as a % of age and gender matched control, error bars are standard deviation. Asterisks denote significant differences between IUGR and gender-matched control determined by unpaired t-test. n=6.
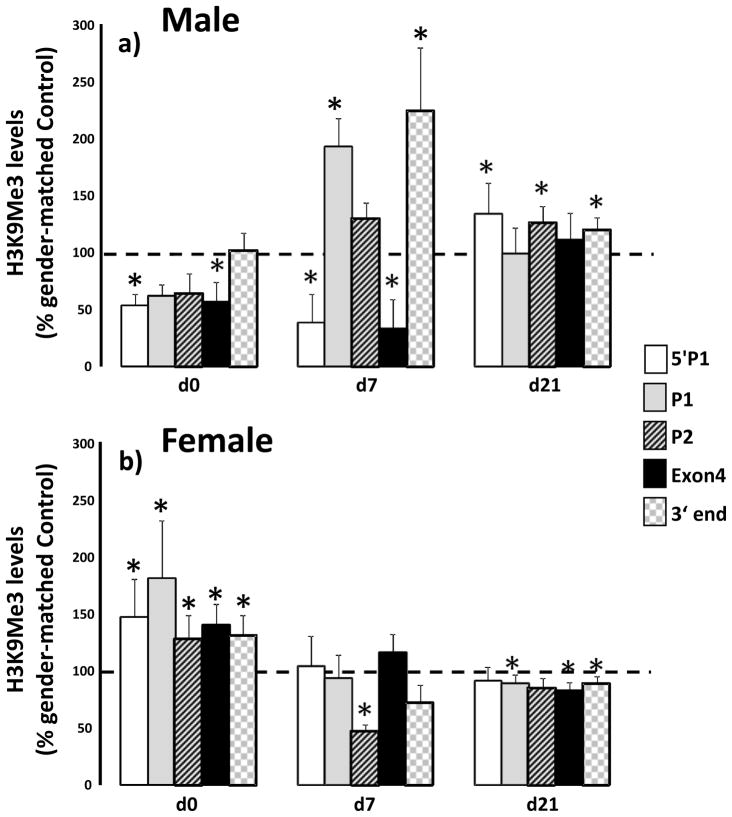
IUGR induces gender-specific changes to H3K9Me3 along the PPARγ gene
We also examined the effect of IUGR on levels of the MeCP2 dependent histone modification, H3K9Me3, along the PPARγ gene. IUGR altered H3K9Me3 along the PPARγ gene differentially in male and female lung. In male lung, levels of H3K9Me3 5′P1 were decreased at d0 (p=0.005) and d7 (p=0.02) compared to age and gender matched control. At d21, IUGR increased H3K9Me3 (p=0.02) at 5′P1. At P1, IUGR increased H3K9Me3 at d7 (p=0.016) while at P2 H3K9Me3 increased at d21 (p=0.01) relative to age matched male control. At Exon 4, IUGR decreased H3K9Me3 at d0 (p=0.05) and d7 (p=0.03) with no change at d21 relative to age matched male control. At the 3′ end of PPARγ IUGR increased H3K9Me3 at d7 (p=0.03) and d21 (p=0.003) relative to age matched male control (Figure 5a).
In female lungs, IUGR increased H3K9Me3 at all positions examined at d0 (5′P1-p=0.04, p1 p=0.01, P2 p=0.04, Exon 4 p=0.07, 3′ p=0.04) relative to female d0 control. At d7 IUGR decreased H3K9Me3 at P2 only (p=0.03). At d21, IUGR decreased H3K9Me3 at P1 (p=0.02), Exon 4 (p=0.008), and 3′end (p= 0.02) relative to d21 female control.
Discussion
The most significant finding of this study is that IUGR alters lung levels of MeCP2 mRNA transcript, MeCP2 protein abundance, and miR132 throughout alveolarization in the rat. Further, these changes in MeCP2 occur in conjunction with altered MeCP2 occupancy of the PPARγ promoters and altered levels of H3K9Me3 on the PPARγ gene. In addition, IUGR alters MeCP2 related findings in a gender-divergent manner. These findings are novel and imply that IUGR induces gender-divergent alterations in lung MeCP2 expression. These IUGR induced changes in MeCP2 likely alter the expression of MeCP2 target genes and may impact alveolarization in the rat.
The effect of IUGR on MeCP2 during alveolarization has not been previously characterized. MeCP2 mRNA lung expression has been reported (Pelka and others, 2005), however little else is known about MeCP2 in the lung. Our findings of the presence of long and short 3′UTR MeCP2 transcripts throughout alveolarization are consistent with this previous report. In the brain, where it has been extensively studied, MeCp2 is important for normal neuronal development and a number of MeCP2 target genes undergo stimuli-dependent activation (Gonzales and LaSalle, 2010). MeCP2 is often associated with actively transcribed genes and is found at active promoters (Chahrour and others, 2008; Yasui and others, 2007). These findings lead to the notion that MeCP2 may play a role in the dynamic regulation of specific gene activity, or modulate transcription in response to a stimulus (Thambirajah and Ausio, 2009).
This concept is consistent with MeCP2 being involved in the modulation of PPARγ transcription during a dynamic process such as alveolar formation. Inhibition of PPARγ transcription by MeCP2 in hepatic fibroblasts is associated with transdifferentiation of hepatic fibroblasts to the fibrotic myofibroblast phenotype (Mann and others, 2010). In these cells, when MeCP2 is bound to the PPARγ promoter, transcription is inhibited and histones in the promoter region are characterized by increased H3K9Me3, a MeCP2 dependent repressive mark (Fuks and others, 2003). We have previously reported that PPARγ is reduced at birth in IUGR rat lungs (Joss-Moore and others, 2010b). In this study, we have shown that effects of IUGR on PPARγ levels persist at d7 and normalize by d21. Interestingly, while MeCP2 does associate with both male and female PPARγ promoters at birth, only female lungs are characterized by the expected IUGR induced increase in MeCP2 occupancy and H3K9Me3 at the PPARγ promoters. These finding are consistent with the observed decrease in PPARγ mRNA in females at birth. In contrast, male IUGR lungs display a reduction in H3K9Me3 on PPARγ promoters in the context of decreased PPARγ mRNA at birth. This suggests that other histone modifications or modifying enzymes contribute to PPARγ mRNA transcription in male rat lungs. It will be important to indentify other epigenetic modifiers that regulate PPARγ transcription in male rat lung. Interestingly, it has also been shown that rat lungs undergo a PPARγ dependent transdifferentiation to a fibrotic phenotype in response to hyperoxia (Rehan and others, 2007; Rehan and others, 2006). The differences in MeCP2/PPARγ interactions observed in this study may predispose IUGR lungs to a gender-divergent PPARγ response in the face of a secondary insult, such as hyperoxia exposure.
The gender-divergent effects observed in this study are interesting and currently unexplained. Gender-divergence in molecular regulation during lung development is consistent with previous observations of subtle differences in the timing of lung maturation between males and females (Torday and others, 1981). Gender-specific epigenetics may also be important in priming the immature lung for future changes. For example, after sexual maturation, female lungs have greater mass-specific surface area in order to accommodate increased oxygen needs of pregnancy (Massaro and others, 1995). It is possible that the epigenetic regulatory mechanisms governing MeCP2 expression and function may exhibit subtle differences in male and female lungs under basil conditions. However, in the face of a stressor such as IUGR, these differences become more pronounced resulting in a gender-divergence in MeCP2 response. This response may predispose to gender-specific differences in the severity and timing or lung morbidities.
MeCP2 binds to unmethylated DNA (Thambirajah and Ausio, 2009; Yasui and others, 2007) potentially directed by histone modifications in, and proximal to, the promoter. However, in vitro, MeCP2 preferentially binds to methylated CpG’s. A limitation of this study is that we did not assess the DNA methylation status of the PPARγ promoters. It is possible that the association of MeCP2 with the PPARγ promoters may be influenced by the methylation status of the promoters. Interestingly, the PPARγ1 promoter is CpG rich while the PPARγ2 promoter is CpG sparse. This may influence MeCP2 binding and needs to be explored in future studies. A further limitation is the use of a rat model. While rat lung development follows a similar course to human lung development, it is important to remain cognoscente of the fundamental differences. For example, the rat lung is adequately functional when in the sacular stage of development, as opposed to the human lung at the same stage of development. However, an advantage of using a rat model to study lung development and the effects IUGR on lung development, however, is that the immature lung can be interrogated, without the systemic complications of prematurity.
In conclusion, this is the first study to examine the effects of IUGR on MeCP2 expression, miR132 and the interaction of MeCP2 with PPARγ in the developing rat lung. We demonstrated that IUGR alters levels of lung MeCP2 biology in a gender-divergent manner. While the results of this study are primarily correlative, this data sets the stage for future studies that explore mechanisms by which alterations in MeCP2 related epigenetics impact lung development. We speculate that in the IUGR rat lung, an altered epigenetic milieu during alveolar formation may predispose to gender-specific differences in subsequent susceptibility to lung injury.
Figure 7.
IUGR alters levels of H3K9Me3 along the PPARγ gene in a gender-specific manner. Male (panel a) and female (panel b) control and IUGR lungs were examined at birth (d0), postnatal day 7 (d7) and day 21 (d21). Positions examined were: 5′P1 (5′ of promoter 1), at P1, at promoter 2 (P2), in Exon 4 and at the 3′ end of the PPARγ gene. Data are IUGR as a % of age and gender matched control, error bars are standard deviation. Asterisks denote significant differences between IUGR and gender-matched control determined by unpaired t-test. n=6.
Table 1.
Primer/probe sets for real-time RT-PCR
| Transcript position relative to PPARγ gene | Sequence |
|---|---|
| 5′ of P1 | For: 5′ TCGACGGCTTTCTGAATGTG |
| Rev: 5′ CTTGCCCTCTTTCAGCTCTTTC | |
| Probe: 5′ ATCTTTAGGACAGATCATG | |
|
| |
| P1 | For: 5′ AAAAACAAACTTCTGCGTGACAGT |
| Rev: 5′ GGTCCCACGTTCCTCAGACA | |
| Probe: 5′ AGGGCACCAGCCGG | |
|
| |
| P2 | For: 5′ CCAAGTCTTGCCAAAGAAGCA |
| Rev: 5′ GATTGAGAGCCAGCTGTGACAA | |
| Probe: 5′ ACAGCATTATGACACACCAT | |
|
| |
| Exon 4 | For: 5′ CCATCAGGTTTGGGCGAAT |
| Rev: 5′ GATCTCCGCCAACAGCTTCT | |
| Probe: 5′ CCACAGGCCGAGAAG | |
|
| |
| 3′ End | For: 5′ CGCCAAGGTGCTCCAGAA |
| Rev: 5′ CTGCACGTGCTCTGTGACAA | |
| Probe: 5′ ATGACAGACCTCAGGCAG | |
GAPDH primer and probe sequences; Forward: CAAGATGGTGAAGGTCGGTGT; Reverse: CAAGAGAAGGCAGCCCTGGT; Probe: GCGTCCGATACGGCCAAATCCG.
Add MeCP2 primers
Acknowledgments
We wish to acknowledge the support of J. Ross Milley and Ronald S. Bloom, Division of Neonatology. This work was supported by an NIH K01-DK084036-01A1 (LJM), and the Primary Children’s Medical Center Foundation (LJM).
References
- American Physiologic Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002:R281–283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- Baserga M, Hale MA, McKnight RA, Yu X, Callaway CW, Lane RH. Uteroplacental insufficiency alters hepatic expression, phosphorylation, and activity of the glucocorticoid receptor in fetal IUGR rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1348–1353. doi: 10.1152/ajpregu.00211.2005. [DOI] [PubMed] [Google Scholar]
- Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, Kumar VH, Ryan RM, Swartz DD, Csiszar K, Fong KS. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1370–1384. doi: 10.1152/ajplung.00367.2006. [DOI] [PubMed] [Google Scholar]
- Cerny L, Torday JS, Rehan VK. Prevention and treatment of bronchopulmonary dysplasia: contemporary status and future outlook. Lung. 2008;186(2):75–89. doi: 10.1007/s00408-007-9069-z. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320(5880):1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezateux C, Lum S, Hoo AF, Hawdon J, Costeloe K, Stocks J. Low birth weight for gestation and airway function in infancy: exploring the fetal origins hypothesis. Thorax. 2004;59(1):60–66. [PMC free article] [PubMed] [Google Scholar]
- Frusca T, Soregaroli M, Valcamonico A, Guandalini F, Danti L. Doppler velocimetry of the uterine arteries in nulliparous women. Early Hum Dev. 1997;48(1–2):177–185. doi: 10.1016/s0378-3782(96)01854-3. [DOI] [PubMed] [Google Scholar]
- Fu Q, Yu X, Callaway CW, Lane RH, McKnight RA. Epigenetics: intrauterine growth retardation (IUGR) modifies the histone code along the rat hepatic IGF-1 gene. FASEB J. 2009;23(8):2438–2449. doi: 10.1096/fj.08-124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem. 2003;278(6):4035–4040. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci U S A. 2007;104(6):1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales ML, LaSalle JM. The role of MeCP2 in brain development and neurodevelopmental disorders. Curr Psychiatry Rep. 2010;12(2):127–134. doi: 10.1007/s11920-010-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenman F, Unger S, Post M. The molecular basis for abnormal human lung development. Biol Neonate. 2005;87(3):164–177. doi: 10.1159/000082595. [DOI] [PubMed] [Google Scholar]
- Harding R, Cock ML, Louey S, Joyce BJ, Davey MG, Albuquerque CA, Hooper SB, Maritz GS. The compromised intra-uterine environment: implications for future lung health. Clin Exp Pharmacol Physiol. 2000;27(12):965–974. doi: 10.1046/j.1440-1681.2000.03379.x. [DOI] [PubMed] [Google Scholar]
- Hoo AF, Stocks J, Lum S, Wade AM, Castle RA, Costeloe KL, Dezateux C. Development of lung function in early life: influence of birth weight in infants of nonsmokers. Am J Respir Crit Care Med. 2004;170(5):527–533. doi: 10.1164/rccm.200311-1552OC. [DOI] [PubMed] [Google Scholar]
- Joss-Moore LA, Metcalfe DB, Albertine KH, McKnight RA, Lane RH. Epigenetics and fetal adaptation to perinatal events: diversity through fidelity. J Anim Sci. 2010a;88(13 Suppl):E216–222. doi: 10.2527/jas.2009-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss-Moore LA, Wang Y, Baack ML, Yao J, Norris AW, Yu X, Callaway CW, McKnight RA, Albertine KH, Lane RH. IUGR decreases PPARgamma and SETD8 Expression in neonatal rat lung and these effects are ameliorated by maternal DHA supplementation. Early Hum Dev. 2010b doi: 10.1016/j.earlhumdev.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss-Moore LA, Wang Y, Campbell MS, Moore B, Yu X, Callaway CW, McKnight RA, Desai M, Moyer-Mileur LJ, Lane RH. Uteroplacental insufficiency increases visceral adiposity and visceral adipose PPARgamma2 expression in male rat offspring prior to the onset of obesity. Early Hum Dev. 2010c;86(3):179–185. doi: 10.1016/j.earlhumdev.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadag A, Sakurai R, Wang Y, Guo P, Desai M, Ross MG, Torday JS, Rehan VK. Effect of maternal food restriction on fetal rat lung lipid differentiation program. Pediatr Pulmonol. 2009;44(7):635–644. doi: 10.1002/ppul.21030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, Yu X, Wang L, Callaway CW, Gill G, Chan GM, Albertine KH, McKnight RA, Lane RH. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics. 2006;25(1):16–28. doi: 10.1152/physiolgenomics.00093.2005. [DOI] [PubMed] [Google Scholar]
- Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. 2007;10(12):1513–1514. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- Kloesz JL, Serdikoff CM, Maclennan NK, Adibi SA, Lane RH. Uteroplacental insufficiency alters liver and skeletal muscle branched-chain amino acid metabolism in intrauterine growth-restricted fetal rats. Pediatr Res. 2001;50(5):604–610. doi: 10.1203/00006450-200111000-00012. [DOI] [PubMed] [Google Scholar]
- Kotecha SJ, Watkins WJ, Heron J, Henderson J, Dunstan FD, Kotecha S. Spirometric lung function in school-age children: effect of intrauterine growth retardation and catch-up growth. Am J Respir Crit Care Med. 2010;181(9):969–974. doi: 10.1164/rccm.200906-0897OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriaucionis S, Bird A. The major form of MeCP2 has a novel N-terminus generated by alternative splicing. Nucleic Acids Res. 2004;32(5):1818–1823. doi: 10.1093/nar/gkh349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RH, Chandorkar AK, Flozak AS, Simmons RA. Intrauterine growth retardation alters mitochondrial gene expression and function in fetal and juvenile rat skeletal muscle. Pediatr Res. 1998;43(5):563–570. doi: 10.1203/00006450-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Lane RH, Dvorak B, MacLennan NK, Dvorakova K, Halpern MD, Pham TD, Philipps AF. IGF alters jejunal glucose transporter expression and serum glucose levels in immature rats. Am J Physiol Regul Integr Comp Physiol. 2002a;283(6):R1450–1460. doi: 10.1152/ajpregu.00172.2002. [DOI] [PubMed] [Google Scholar]
- Lane RH, Flozak AS, Ogata ES, Bell GI, Simmons RA. Altered hepatic gene expression of enzymes involved in energy metabolism in the growth-retarded fetal rat. Pediatr Res. 1996;39(3):390–394. doi: 10.1203/00006450-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD. Increased hepatic peroxisome proliferator-activated receptor-gamma coactivator-1 gene expression in a rat model of intrauterine growth retardation and subsequent insulin resistance. Endocrinology. 2002b;143(7):2486–2490. doi: 10.1210/endo.143.7.8898. [DOI] [PubMed] [Google Scholar]
- Lane RH, Tsirka AE, Gruetzmacher EM. Uteroplacental insufficiency alters cerebral mitochondrial gene expression and DNA in fetal and juvenile rats. Pediatr Res. 2000;47(6):792–797. doi: 10.1203/00006450-200006000-00019. [DOI] [PubMed] [Google Scholar]
- Laprise SL. Implications of epigenetics and genomic imprinting in assisted reproductive technologies. Mol Reprod Dev. 2009 doi: 10.1002/mrd.21058. [DOI] [PubMed] [Google Scholar]
- Laughon M, Bose C, Allred EN, O’Shea TM, Ehrenkranz RA, Van Marter LJ, Leviton A. Antecedents of chronic lung disease following three patterns of early respiratory disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2010 doi: 10.1136/adc.2010.182865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Ebrahim S, Davey Smith G. Association of birth weight with adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax. 2005;60(10):851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li Y, Tollefsbol TO. Gene-environment interactions and epigenetic basis of human diseases. Curr Issues Mol Biol. 2008;10(1–2):25–36. [PMC free article] [PubMed] [Google Scholar]
- Lucas JS, Inskip HM, Godfrey KM, Foreman CT, Warner JO, Gregson RK, Clough JB. Small size at birth and greater postnatal weight gain: relationships to diminished infant lung function. Am J Respir Crit Care Med. 2004;170(5):534–540. doi: 10.1164/rccm.200311-1583OC. [DOI] [PubMed] [Google Scholar]
- Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, Mann DA. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138(2):705–714. 714, e701–704. doi: 10.1053/j.gastro.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani TJ, Sandefur S, Pierce RA. Elastin in lung development. Exp Lung Res. 1997;23(2):131–145. doi: 10.3109/01902149709074026. [DOI] [PubMed] [Google Scholar]
- Maritz GS, Cock ML, Louey S, Joyce BJ, Albuquerque CA, Harding R. Effects of fetal growth restriction on lung development before and after birth: a morphometric analysis. Pediatr Pulmonol. 2001;32(3):201–210. doi: 10.1002/ppul.1109. [DOI] [PubMed] [Google Scholar]
- Maritz GS, Cock ML, Louey S, Suzuki K, Harding R. Fetal growth restriction has long-term effects on postnatal lung structure in sheep. Pediatr Res. 2004;55(2):287–295. doi: 10.1203/01.PDR.0000106314.99930.65. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Mortola JP, Massaro D. Sexual dimorphism in the architecture of the lung’s gas-exchange region. Proc Natl Acad Sci U S A. 1995;92(4):1105–1107. doi: 10.1073/pnas.92.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed N, Yogev Y, Glezerman M. Effect of fetal sex on pregnancy outcome in twin pregnancies. Obstet Gynecol. 2009;114(5):1085–1092. doi: 10.1097/AOG.0b013e3181bd8874. [DOI] [PubMed] [Google Scholar]
- Mnatzakanian GN, Lohi H, Munteanu I, Alfred SE, Yamada T, MacLeod PJ, Jones JR, Scherer SW, Schanen NC, Friez MJ, Vincent JB, Minassian BA. A previously unidentified MECP2 open reading frame defines a new protein isoform relevant to Rett syndrome. Nat Genet. 2004;36(4):339–341. doi: 10.1038/ng1327. [DOI] [PubMed] [Google Scholar]
- Nelson JD, Denisenko O, Sova P, Bomsztyk K. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 2006;34(1):e2. doi: 10.1093/nar/gnj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien EA, Barnes V, Zhao L, McKnight RA, Yu X, Callaway CW, Wang L, Sun JC, Dahl MJ, Wint A, Wang Z, McIntyre TM, Albertine KH, Lane RH. Uteroplacental insufficiency decreases p53 serine-15 phosphorylation in term IUGR rat lungs. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R314–322. doi: 10.1152/ajpregu.00265.2005. [DOI] [PubMed] [Google Scholar]
- Ogata ES, Bussey ME, Finley S. Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism. 1986;35(10):970–977. doi: 10.1016/0026-0495(86)90064-8. [DOI] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Christodoulou J, Tam PP. Distinct expression profiles of Mecp2 transcripts with different lengths of 3′UTR in the brain and visceral organs during mouse development. Genomics. 2005;85(4):441–452. doi: 10.1016/j.ygeno.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14(5):844–852. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R962–970. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- Quenard A, Yilmaz S, Fontaine H, Bienvenu T, Moncla A, des Portes V, Rivier F, Mathieu M, Raux G, Jonveaux P, Philippe C. Deleterious mutations in exon 1 of MECP2 in Rett syndrome. Eur J Med Genet. 2006;49(4):313–322. doi: 10.1016/j.ejmg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Rehan VK, Sakurai R, Wang Y, Santos J, Huynh K, Torday JS. Reversal of nicotine-induced alveolar lipofibroblast-to-myofibroblast transdifferentiation by stimulants of parathyroid hormone-related protein signaling. Lung. 2007;185(3):151–159. doi: 10.1007/s00408-007-9007-0. [DOI] [PubMed] [Google Scholar]
- Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr Pulmonol. 2006;41(6):558–569. doi: 10.1002/ppul.20407. [DOI] [PubMed] [Google Scholar]
- Roth-Kleiner M, Post M. Genetic control of lung development. Biol Neonate. 2003;84(1):83–88. doi: 10.1159/000071009. [DOI] [PubMed] [Google Scholar]
- Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab. 2010;30(9):1564–1576. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, Gonzalez F, Shapiro SD, Mariani TJ. Epithelial cell PPAR[gamma] contributes to normal lung maturation. Faseb J. 2006;20(9):1507–1509. doi: 10.1096/fj.05-5410fje. [DOI] [PubMed] [Google Scholar]
- Spinillo A, Montanari L, Gardella B, Roccio M, Stronati M, Fazzi E. Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Dev Med Child Neurol. 2009;51(7):518–525. doi: 10.1111/j.1469-8749.2009.03273.x. [DOI] [PubMed] [Google Scholar]
- Thambirajah AA, Ausio J. A moment’s pause: putative nucleosome-based influences on MeCP2 regulation. Biochem Cell Biol. 2009;87(5):791–798. doi: 10.1139/O09-054. [DOI] [PubMed] [Google Scholar]
- Torday JS, Nielsen HC, Fencl Mde M, Avery ME. Sex differences in fetal lung maturation. Am Rev Respir Dis. 1981;123(2):205–208. doi: 10.1164/arrd.1981.123.2.205. [DOI] [PubMed] [Google Scholar]
- Torrance HL, Bloemen MC, Mulder EJ, Nikkels PG, Derks JB, de Vries LS, Visser GH. Predictors for outcome at two years of age after early intrauterine growth restriction. Ultrasound Obstet Gynecol. 2010 doi: 10.1002/uog.7627. [DOI] [PubMed] [Google Scholar]
- Tyson JE, Kennedy K, Broyles S, Rosenfeld CR. The small for gestational age infant: accelerated or delayed pulmonary maturation? Increased or decreased survival? Pediatrics. 1995;95(4):534–538. [PubMed] [Google Scholar]
- Unterman TG, Simmons RA, Glick RP, Ogata ES. Circulating levels of insulin, insulin-like growth factor-I (IGF-I), IGF-II, and IGF-binding proteins in the small for gestational age fetal rat. Endocrinology. 1993;132(1):327–336. doi: 10.1210/endo.132.1.7678218. [DOI] [PubMed] [Google Scholar]
- Weaver JR, Susiarjo M, Bartolomei MS. Imprinting and epigenetic changes in the early embryo. Mamm Genome. 2009;20(9–10):532–543. doi: 10.1007/s00335-009-9225-2. [DOI] [PubMed] [Google Scholar]
- Wells J, Farnham PJ. Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods. 2002;26(1):48–56. doi: 10.1016/S1046-2023(02)00007-5. [DOI] [PubMed] [Google Scholar]
- Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23(3):320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- Yasui DH, Peddada S, Bieda MC, Vallero RO, Hogart A, Nagarajan RP, Thatcher KN, Farnham PJ, Lasalle JM. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci U S A. 2007;104(49):19416–19421. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Eirio V, Koskinen J, Kujansuu E, Ranta T. Doppler assessment of the uterine and uteroplacental circulation in the second trimester in pregnancies at high risk for pre-eclampsia and/or intrauterine growth retardation: comparison and correlation between different Doppler parameters. Ultrasound Obstet Gynecol. 1997;9(5):330–338. doi: 10.1046/j.1469-0705.1997.09050330.x. [DOI] [PubMed] [Google Scholar]