Abstract
Purpose
To evaluate the efficacy of human bone marrow-derived mesenchymal stem cells (hBMSCs) as gene delivery vehicles to simultaneously express human hepatocyte growth factor (HGF) and interleukin 1 receptor antagonist (IL-1Ra) to improve the outcome of islet transplantation.
Methods
Morphology and islet-binding affinity of hBMSCs were checked by microscope. The expression of target genes and endogenous genes was determined by ELISA. Protection of islets by hBMSCs was evaluated in vitro by Calcein-AM/Propidium Iodide staining and in vivo by allogeneic islet transplantation study. Function and revascularization of islets was evaluated by immune fluorescence study.
Results
Non-donor-specific hBMSCs showed strong binding affinity to human islets and protected viability and function. Transduction of hBMSCs with adenovirus encoding human HGF and human IL-1Ra (Adv-hHGF-hIL-1Ra) prior to co-culturing with islets further protected from apoptotic cell death, helped maintain 3D structures and morphology, and enhanced insulin secretion. Transplantation of human islets reconstituted with Adv-hHGF-hIL-1Ra transduced hBMSCs under the kidney capsule of streptozotocin-induced diabetic non-obese diabetic/severe combined immunodeficient (NOD-SCID) mice reversed diabetes by reducing blood glucose levels to ≤200 mg/dL for up to 15 weeks and reduced the number of islets required to achieving normoglycemia. Blood glucose levels of mice transplanted with islets alone reversed to ≥500 mg/dL 4 weeks post-transplantation.
Conclusions
Results indentified hBMSCs as effective gene delivery vehicles to improve the outcome of islet transplantation.
Keywords: adenovirus, gene therapy, islet transplantation, mesenchymal stem cells
INTRODUCTION
Islet transplantation has the potential to treat type I diabetes mellitus if we could prevent the primary nonfunctioning of islet grafts. Among several factors contributing to the loss of islet viability, the two most important are the acute attacks from inflammatory cytokines at the transplantation site and the long-term graft rejection from the recipient's immune system (1).
Human bone marrow-derived mesenchymal stem cells (hBMSCs) are adult progenitor cells with differentiation potential and self-renewal capacity. Their proliferation capacity reduces following the differentiation induction phase and ceases to proliferate after transplantation. hBMSCs have great promise in repairing injured tissues, including pancreatic islets (2). hBMSCs also hold hypoimmunogenic property (3), since they are in lack of major histocompatibility complex II (MHC-II) and co-stimulatory molecules, such as CD14, CD86, CD40L and CD95L. MSCs suppress inflammation, either by receptor-mediated signal-transduction, probably through MMP2/MMP9-CD25 signal pathway (4), or the secretion of immunosuppressive factors such as transforming growth factor β1 (TGF-β1), interleukin 10 (IL-10) and prostaglandin E2 (PGE2). Therefore, MSCs are unlikely to cause inflammation at the transplant site. These characteristics make MSCs attractive vehicles for virus-based gene therapy.
However, MSCs alone, through systemic administration or local transplantation, cannot prevent apoptotic cell death from the sudden boost of inflammatory cytokines at the transplantation site, which accounts for more than 70% of islet loss in the first week after transplantation (5,6). This phenomenon probably explains the inadequate control of the blood glucose level in the early stage (<3 weeks) after transplantation in most islet/MSC co-transplantation studies (7,8).
Replication-deficient adenoviral vectors (Adv) can be used for silencing malevolent genes and expressing benevolent genes to prevent acute graft failure immediately after islet transplantation (9). Adv transduction of islets prior to transplantation has been proven to protect islets from inflammatory cytokines and promote revascularization of islet grafts (10). However, the transient gene expression or gene silencing may not prevent long-term islet graft rejection. Most genes delivered by Adv vectors remained active less than two weeks. Moreover, some reports suggested that Adv transduction led to immune reaction to the Adv-bearing organs and made these organs more susceptible to attack from the recipient's immune system(11,12).
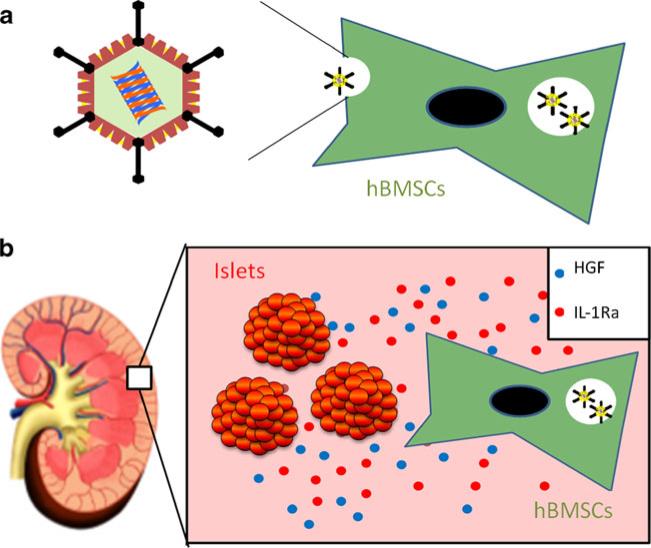
In this study, we evaluated the capability of primary hBMSCs as gene delivery vehicles to protect the graft viability of human islets in a streptozotocin-induced diabetic non-obese diabetic/severe combined immunodeficient (NOD-SCID) mice model (Fig. 1). Adv-hHGF-hIL-1Ra-transduced hBMSCs served as gene delivery vehicles to express hepatocyte growth factor (HGF) to promote islet revascularization, and interleukin 1 receptor antagonist (IL-1Ra) to reduce acute islet loss from inflammatory cytokines. We also determined the capability of hBMSCs as nursing cells to prevent long-term islet graft rejection.
Fig. 1.
A schematic demonstration of Adv-hHGF-hIL-1Ra-transduced hBMSCs co-transplantation with human islets. (a) hBMSCs were transduced with Adv-hHGF-hIL-1Ra prior to islet transplantation. (b) After co-transplantation with human islets under the kidney capsule of diabetic NODSCID mice, hBMSCs expressed HGF and IL-1Ra into the surrounding microenvironment to support islet viability and function.
MATERIALS AND METHODS
Materials
The replication-deficient (ΔE1/ΔE3) Adv-hHGF-hIL-1Ra containing a cytomegalovirus (CMV) promoter, hHGF cDNA, and rabbit β-globin poly A in the E-1 region, and a CMV promoter, hIL-1Ra cDNA, and rabbit β-globin poly A in the E-3 region was constructed and amplified, and the titer measured in our lab as previously described (13). The control virus encoding green fluorescent protein (Adv-GFP) was constructed and kept in our lab. Primary hBMSCs, HyClone Advanced Stem Cell Medium and Medium Supplement were purchased from Thermo Fisher Scientific (Waltham, MA), passaged, and frozen in our lab. Human islets were received from Integrated Islet Distribution Program (Duarte, CA). CMRL-1066 medium for islet culture and 6-diamidino-2-phenylindole (DAPI) were purchased from Sigma Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was purchased from MediaTech Cellgro (Herndon, VA). Phosphate-buffered solution (PBS) was purchased from GIBCO-BRL (Gaithersburg, MD). Human HGF ELISA kits, IL-1Ra ELISA kits, IL-10 ELISA kits, vascular endothelial growth factor (VEGF) ELISA kits, stem cell factor (SCF) ELISA kits and recombinant IL-1β were purchased from R&D Systems (Minneapolis, MN). Human insulin and c-peptide ELISA kits were purchased from Alpco Diagnostics (Windham, NH). The primary antibodies for hHGF, hIL-1Ra, insulin, von-willebrand factor (vWF), and the Dylight 488-conjugated secondary antibody were purchased from Abcam (Cambridge, MA). The Alexa Fluor 568-conjugated secondary antibody and 0.25% trypsin were purchased from Invitrogen (Carlsbad, CA). Eight-well Lab-Tek Chamber Slides were purchased from Nalge Nunc. (Rochester, NY). Ultrasensitive One Touch glucose test strips and One Touch Ultra glucometer were purchased from LifeScan (Milpitas, CA). Tissue-Tek O.C.T. compounds were purchased from Sakura Finetek (Torrance, CA).
Adv Transduction of hBMSCs
Primary hBMSCs were seeded at a density of 5×105 cells per well with 2 ml HyClone Advanced Stem Cell Medium in a six-well plate and allowed 24 h for adherence. Prior to transduction, morphology of hBMSCs was checked under a bright field microscope. To determine the optimal multiplicity of infection (MOI) for Adv transduction, primary hBMSCs were transduced with Adv-GFP at 60 MOI and 120 MOI for 3 h followed by washing three times. The GFP expression was determined under a fluorescent microscope at 2 days after transduction. Primary hBMSCs were then transduced with Adv-hHGF-hIL-1Ra at 120 MOI for 3 h followed by washing three times. The expressions of HGF, IL-1Ra, IL-10, VEGF, and SCF were measured by ELISA at 0, 2, 4, 6, 8, 10, 12, 14 days after transduction according to the manufacturer's instructions. The protein concentration was normalized using the number of cells in each well.
Islet Viability Study
After transduction with Adv-hHGF-hIL-1Ra at 120 MOI for 3 h, 5×105 primary hBMSCs were digested with 0.25% trypsin and co-culture with 500 IEs in a 10 cm dish of CMRL-1066 medium with 10% FBS for 2 days. Then, the islet/hBMSC co-culture was stimulated with 5 μg/ml IL-1β for 4 days and stained with 5 μg/ml calcein-AM and 2 μg/ml propidium iodide for 30 min. Cell viability was monitored under a fluorescent microscope.
Viability of human islets was also determined by staining with dithizone, a zinc chelating agent. Briefly, at 4 weeks after cultured alone or co-cultured with Adv-hHGF-hIL-1Ra-transduced primary hBMSCs, human islets were stained with dithizone as described previously (14).
The insulin production of human islets was quantified by the static insulin release method. Briefly, 500 IEs were cultured alone or with 5×105 untransduced primary hBMSCs or with 5×105 Adv-hHGF-IL-1Ra-transduced primary hBMSCs (120 MOI) in a 10-cm dish of CMRL-1066 medium with 10% FBS for 10 days. At day 10, 5 μg/ml IL-1β was added to the medium, and the islet/hBMSC co-culture was incubated with IL-1β for 4 days. Insulin release from islets was measured at day 0, 10 and 14 by sequentially stimulated islets with the media containing 2.5 mM (basal) and 22 mM glucose (stimulated) at 37°C for 1 h.
Islet Transplantation
Animal experiments were performed following NIH (http://grants1.nih.gov/grants/olaw/references/phspol.htm) and institutional animal care and use guidelines using an approved protocol. To induce diabetes, streptozotocin (STZ) (70 mg/kg) was administered to NOD-SCID mice by intraperitoneal injection for two consecutive days. Animals were considered to be diabetic after two consecutive measurements of blood glucose≥400 mg/dl using a glucometer. Primary hBMSCs were transduced with AdvhHGF-hIL-1Ra at 120 MOI and labeled with Qdot 565 prior to transplantation according to the manufacturer's instructions. Then islets were transplanted into the kidney capsule of diabetic NOD-SCID mice alone or with AdvhHGF-hIL-1Ra-transduced primary hBMSCs at constant ratio (islet: hBMSC=1:100). The non-fasted glucose levels and body weights of all the mice were measured from the snipped tail of each animal up to 15 weeks after transplantation. Then the mice were anesthetized to collect blood to measure serum insulin and c-peptide levels by ELISA. The graft-bearing kidneys were then removed from some animals to confirm the function of islet grafts by the return of blood glucose levels to ≥400 mg/dL for two consecutive days.
Intraperitoneal Glucose Tolerance Test
Two weeks after islet transplantation, glucose tolerance was analyzed in overnight fasted mice as described by Garcia-Ocana et al. (10). Briefly, the mice were subjected to intraperitoneal injection of glucose at 2 g/kg of body weight. Blood samples were obtained from the snipped tail at 15, 30, 60, 90, 120, and 180 min after injection and analyzed for glucose levels using a glucometer.
Immunofluorescence Staining
For Adv-hHGF-hIL-1Ra-transduced hBMSCs, primary hBMSCs were seeded at the density of 5×104 each well into an eight-well chamber slide. After 24 h, these cells were transduced with Adv-hHGF-hIL-1Ra at 120 MOI for 3 h followed by washing three times and culturing an additional 48 h. The slides were then detached from the media chamber and fixed with ice-cold methanol. To detect HGF expression, the slides were stained with rabbit anti-HGF primary antibody (1:200) at 4°C overnight and Alexa Fluor 568-conjugated goat anti-rabbit secondary antibody (1:500) at room temperature for 1 h. To detect IL-1Ra expression, slides were stained with rabbit anti-IL-1Ra primary antibody (1:200) at 4°C overnight and Dylight 488-conjugated goat anti-rabbit secondary antibody (1:500) at room temperature for 1 h. Slides were counter-stained with DAPI.
For the kidney section, 1000 IEs were mixed with AdvhHGF-hIL-1Ra-transduced hBMSCs and co-transplanted under the kidney capsule of STZ-induced diabetic NOD-SCID mice. Mice receiving islets alone and islets co-transplanted with primary hBMSCs were sacrificed 4 weeks after islet transplantation. Mice receiving islets co-transplanted with Adv-hHGF-hIL-1Ra transduced hBMSCs were sacrificed at 4 weeks and 15 weeks after islet transplantation. The kidneys bearing islets were isolated, washed with PBS, fixed in 4% paraformaldehyde overnight, and embedded in optimal cutting temperature compound. Frozen sections of 5 μm thickness were cut. To detect insulin-positive human islets, the slides were stained with guinea pig anti-insulin primary antibody (1:200) at 4°C overnight and Alexa Fluor 568 conjugated goat anti-guinea-pig secondary antibody (1:500) at room temperature for 1 h. To detect revascularization, the slides were stained with rabbit anti-vWF primary antibody (1:500) at 4°C overnight and Dylight 488-conjugated goat anti-rabbit secondary antibody (1:500) at room temperature for 1 h. Slides were counter-stained with DAPI. Three independent images were analyzed using the Measure RGB function of ImageJ to quantify the relative intensity of protein expression.
Statistical Analysis
Statistical significance of the difference between the two groups was determined by unpaired t-test and between several groups by one-way ANOVA.
RESULTS
Characterization of Adv-hHGF-hIL-Ra-Transduced hBMSCs
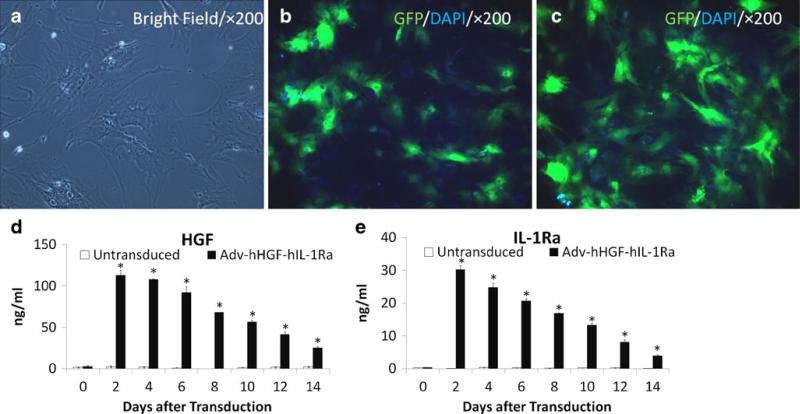
Primary hBMSCs exhibit a spindle-shaped fibroblastic morphology after ex vivo expansion (Fig. 2a). The optimal MOI was determined by transduction of hBMSCs with Adv-GFP at 60 MOI and 120 MOI. Results showed that the Adv transduction efficiency into primary hBMSCs is >30% at 60 MOI and >80% at 120 MOI (Figs. 2b, c). Then 120 MOI was selected for optimal transduction of Adv-hHGF-hIL-1Ra. Results showed that primary hBMSCs transduced with Adv-hHGF-hIL-Ra at 120 MOI produced significantly elevated levels of hHGF and hIL-1Ra since two days after transduction. The expressions of HGF and IL-1Ra by Adv-hHGF-hIL-1Ra transduced primary hBMSCs decreased with time but were still significantly higher than the expressions by untransduced hBMSCs at 14 days after transduction (Figs. 2d, e). The exogenous expressions of HGF and IL-1Ra by Adv-hHGF-hIL-1Ra transduced primary hBMSCs were validated by immunofluorescence staining (Fig. S1). Results also showed that primary hBMSCs produced IL-10 in picogram level and VEGF in nanogram level, and the native expressions of IL-10 and VEGF were not changed by Adv transduction (Fig. S2). As in previous reports (15,16), we did not detect expression of SCF from primary hBMSCs (data not shown). In summary, these results indicated that primary hBMSCs simultaneously express the target exogenous gene after transduction by Adv-hHGF-hIL-1Ra without losing their native characteristics.
Fig. 2.
Transduction efficiency of Adv-hHGF-hIL-1Ra into hBMSCs. (a) Primary hBMSCs exhibit a spindle-shaped fibroblastic morphology after ex vivo expansion. (b) GFP expression in primary hBMSCs after the transduction of Adv-GFP at 60 MOI. (c) GFP expression in primary hBMSCs after the transduction of Adv-GFP at 120 MOI. (c) Time profile of HGF gene expression in primary hBMSCs after the transduction of Adv-hHGF-hIL-1Ra at 120 MOI as determined by ELISA. (d) Time profile of IL-1Ra gene expression in primary hBMSCs after the transduction of Adv-hHGF-hIL-1Ra at 120 MOI as determined by ELISA. Data are represented as the mean ± SD, n=3. *P<0.05 under t test.
Protection of Human Islets by Adv-hHGF-hIL-Ra-Transduced hBMSCs
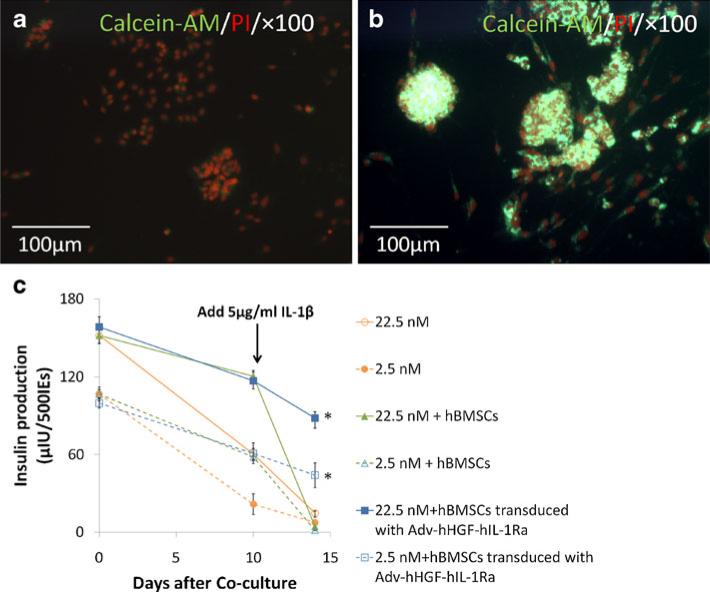
Primary hBMSCs participated extensively in organ repair (17). HGF and IL-1Ra were widely reported to hold anti-apoptotic ability (18,19). We tested the protection of human islets against inflammatory cytokine from Adv-hHGF-hIL-1Ra transduced primary hBMSCs in an ex vivo co-culturing study. Results showed that after stimulating with 5 ng/ml recombinant human IL-1β for 4 days, the cluster-like structures of islets were destroyed and viability of islets decreased (Fig. 3a). However, the viability and morphological integrity were better preserved in the islets co-cultured with Adv-hHGF-hIL-1Ra-transduced primary hBMSCs (Fig. 3b). The protection on cultured human islets from Adv-hHGF-hIL-1Ra-transduced primary hBMSCs even lasted to 4 weeks (Fig. S3). To determine whether this protection effect was a result of the Adv-hHGF-hIL-1Ra transduction on hBMSCs or the organ repairing function of primary hBMSCs, the insulin release profiles over time from islets cultured alone, islets cultured with untransduced primary hBMSCs and islets cultured with Adv-hHGF-hIL-1Ra-transduced primary hBMSCs were monitored (Fig. 3c). Results showed that the insulin releases from islets cultured with primary hBMSCs were significantly higher than the islets cultured alone at day 10 of culture, indicating that hBMSCs did help to protect islets viability. However, untransduced hBMSCs did not provide sufficient protection against the apoptotic islet death caused by IL-1β. Results showed that insulin releases from islets cultured with untransduced primary hBMSCs decreased dramatically (75.1±9.7%) in 4 days after stimulating with 5 ng/ml recombinant human IL-1β. However, insulin releases from islets cultured with Adv-hHGF-hIL-1Ra-transduced hBMSCs decreased only slightly (23.4±5.6%).
Fig. 3.
Adv-hHGF-hIL-1Ra-transduced hBMSCs helped to preserve islet viability against IL-1β stimulation. (a) Viability of naked islets after IL-1β stimulation for 4 days, as determined by calcein-AM/PI double staining. (b) Viability of islets co-cultured with AdvhHGF-hIL-1Ra-transduced hBMSCs after IL-1β stimulation for 4 days, as determined by calcein-AM/PI double staining. (c) Glucose-stimulated insulin release from islets alone, islets co-cultured with primary hBMSCs and islets IEs co-cultured with Adv-hHGF-hIL-1Ra-transduced hBMSCs at day 0, day 10, and day 14 after co-culturing, as determined by ELISA. 5 μg/ml IL-1β was added at day 10 (arrow) and incubated with islets for 4 days. Data are presented as the mean±SD, n=3. *P<0.05 under one-way ANOVA test.
In summary, these results demonstrated that primary hBMSCs supported islet viability and function, but primary hBMSCs alone failed provide islets with sufficient protection against inflammatory cytokines in the physiology condition. Adv-hHGF-hIL-1Ra-transduced primary hBMSCs effectively prevented the apoptotic islet death caused by inflammatory cytokines.
Co-Transplantation of Islets with Adv-hHGF-hIL-1Ra-Transduced hBMSCs Improved Outcome of Islet Transplantation
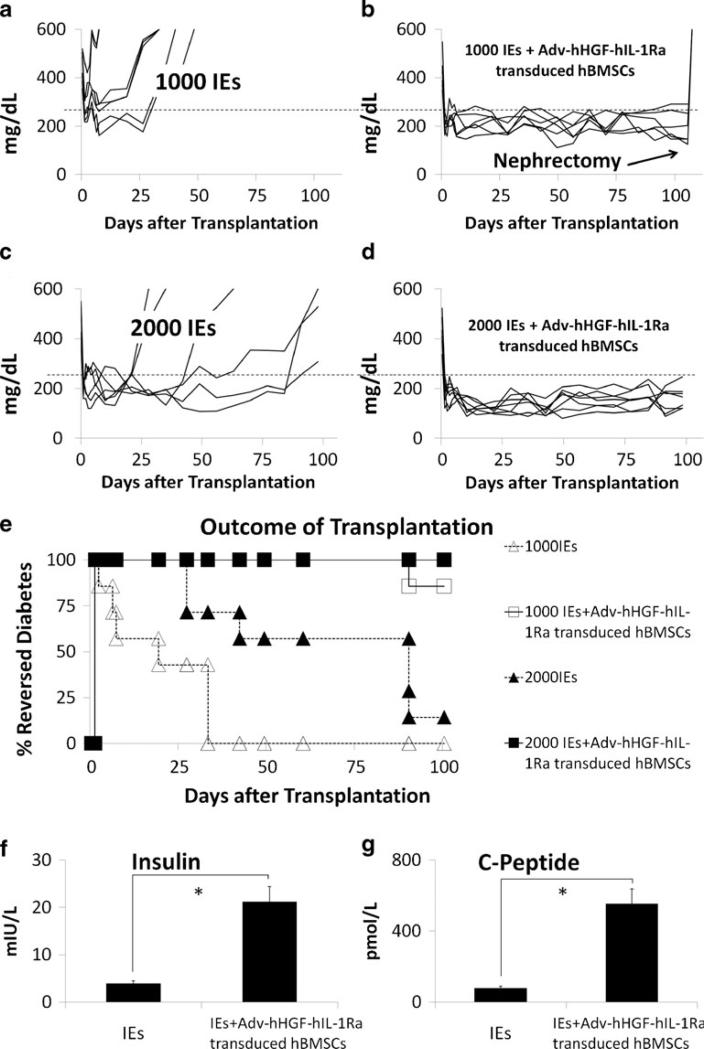
Transplantation of islets under the kidney capsule of diabetic NOD-SCID mice reversed diabetes in every subject. The mice transplanted with 1000 Islet Equivalents (IEs) all reverted to diabetes after only 4 weeks (Fig. 4a). However, the diabetic mice receiving 1000 IEs and AdvhHGF-hIL-Ra-transduced hBMSCs maintained the blood glucose level ≤250 mg/dL for 15 weeks, suggesting prolonged protection of islets by Adv-hHGF-hIL-Ra-transduced primary hBMSCs (Fig. 4b). Results also showed that increasing islet mass from 1000 IEs to 2000 IEs effectively improved the outcome of islet transplantation (Fig. 4c, d), although rapid increase in blood glucose level was still observed when no additional hBMSCs were co-transplanted (Fig. 4c). We considered mice with blood glucose ≤250 mg/dL as the reversed-diabetes mice to determine the cumulative outcome of islet transplantation. Co-transplantation of islets with Adv-hHGF-hIL-1Ra-transduced hBMSCs significantly increased the reversed-diabetes ratio, suggesting the positive role of Adv-hHGF-hIL-Ra-transduced primary hBMSCs after islet transplantation (Fig. 4e). To determine the islet function at 15 weeks after islet transplantation, the mice were anesthetized to collect blood to measure serum insulin and c-peptide level by ELISA. Results showed that the levels of insulin and c-peptide of the mice transplanted with 1000 IEs co-transplanted with Adv-hHGF-hIL-1Ra-transduced primary hBMSCs were significantly higher than those of the mice transplanted with islets alone (Fig. 4f, g). The body weight was also measured to validate the blood glucose measurement. The increases in body weight were significantly slower in mice receiving islets alone, since their blood glucose reverted to diabetes sooner after transplantation (Fig. S4). However, this phenomenon was not observed in mice receiving islets and Adv-hHGF-hIL-1Ra-transduced primary hBMSCs.
Fig. 4.
Outcome of islet transplantation after being cotransplanted with Adv-hHGF-hIL-1Ra-transduced hBMSCs. (a–d) The blood glucose level of every single mouse after receiving 1000 IEs (a), 1000 IEs co-transplanted with Adv-hHGF-hIL-1Ra-transduced hBMSCs (b), 2000 IEs (c) and 2000 IEs co-transplanted with Adv-hHGF-hIL-1Ra-transduced hBMSCs (d). Blood glucose≤250 mg/dL was identified as reversed-diabetes (dashed line)(e). Reversed-diabetes ratio of the NOD-SCID mice after islet transplantation. White triangles = mice receiving 1000 IEs, white squares = mice receiving 1000 IEs co-transplanted with Adv-hHGF-hIL-1Ra-transduced hBMSCs, black triangles = mice receiving 2000 IEs, black squares = mice receiving 2000 IEs co-transplanted with AdvhHGF-hIL-1Ra-transduced hBMSCs. (f) Average serum insulin level of the mice receiving 1000 IEs and 1000 IEs co-transplanted with Adv-hHGF-hIL-1Ra-transduced hBMSCs 15 weeks after islet transplantation, as determined by ELISA. (g) Average serum c-peptide level of the mice receiving 1000 IEs and 1000 IEs co-transplanted with Adv-hHGF-hIL-1Ra-transduced hBMSCs 15 weeks after islet transplantation, as determined by ELISA. Data are presented as the mean ± SD, n=7. *P<0.05 under t test.
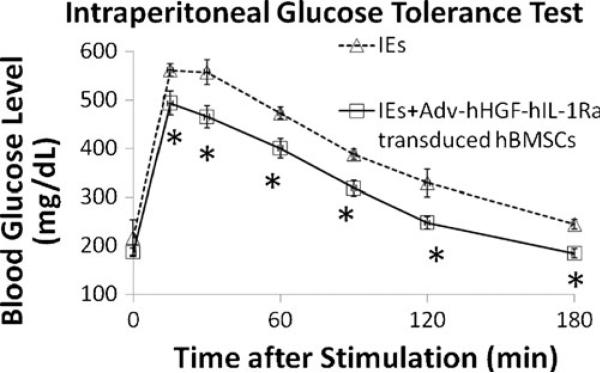
Intraperitoneal glucose tolerance test was used to determine islet engraftment and function 2 weeks after transplantation. Results showed faster and better response to the glucose boost in mice receiving 1000 IEs co-transplanted with Adv-hHGF-hIL-1Ra-transduced primary hBMSCs compared with mice receiving islets alone (Fig. 5). These results demonstrated the improvement in islet engraftment and function after cotransplantation of islets with Adv-hHGF-hIL-1Ra-transduced primary hBMSCs and agreed with recently published results (4).
Fig. 5.
Intraperitoneal glucose tolerance test (IPGTT) of the mice receiving 1000 IEs and 1000 IEs co-transplanted with Adv-hHGF-hIL-1Ra-transduced hBMSCs at 2 weeks after islet transplantation. Data are presented as the mean ± SD, n=7. *P<0.05 under t test.
Adv-hHGF-hIL-Ra-Transduced hBMSCs Promoted Islet Engraftment and Revascularization
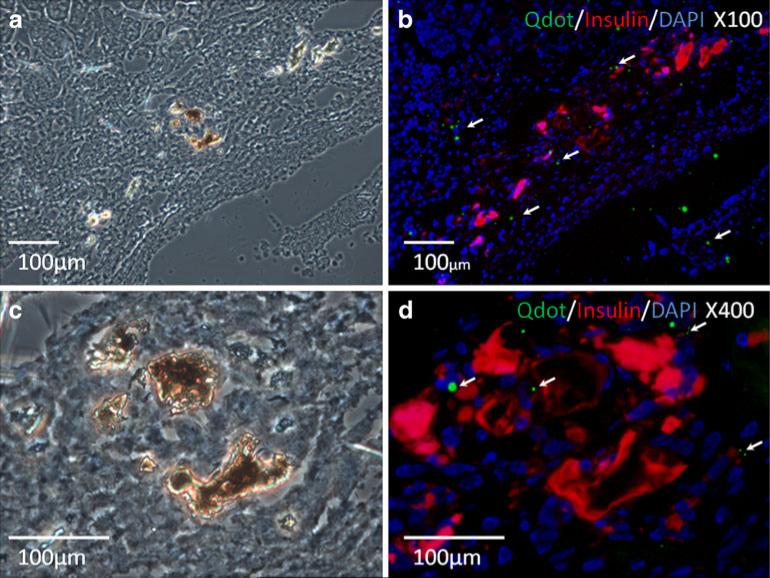
Immunofluorescence staining showed the engraftments of insulin-positive islets and Qdot-565-labeled hBMSCs in the proximity of engrafted islets under the kidney capsule (Fig. 6). These results suggested the supporting role of primary hBMSCs to islet grafts after co-transplantation. The staining of vWF was undetectable in the kidney sections bearing islets alone (Fig. 7a) and sparse in the kidney sections bearing islets and untransduced primary hBMSCs (Fig. 7b). However, evident vWF staining in the kidney sections bearing islets co-transplanted with AdvhHGF-hIL-1Ra-transduced primary hBMSCs was detected at both 4 weeks and 15 weeks after islet transplantation (Fig. 7c, 7d). Analysis of the relative vWF intensity from three independent regions using ImageJ suggested increased revascularization by Adv-hHGF-hIL-1Ra-transduced primary hBMSCs (Fig. 7e).
Fig. 6.
Immunofluorescence staining of the kidney section bearing islets and Adv-hHGF-hIL-1Ra-transduced hBMSCs. Images from bright field microscope (a, c) and fluorescent microscope (b, d) were contrasted for morphological evaluation of transplanted islets. (c) and (d) are magnified images of (a) and (b), respectively. Insulin was stained in red to indicate the functional human islets. Qdot 565 labeled hBMSCs were green “blinking” dots in close proximity of transplanted islets (white arrows).
Fig. 7.
Adv-hHGF-hIL-1Ra-transduced hBMSCs promoted revascularization of the transplanted islets. (a-d) Representative staining of vWF factor (green) in the kidney bearing islets alone at 4 weeks after transplantation (a), the kidney bearing islets co-transplanted with untransduced primary hBMSCs at 4 weeks after transplantation (b), the kidney bearing islets co-transplanted with AdvhHGF-hIL-1Ra-transduced primary hBMSCs at 4 weeks after transplantation (c) and the kidney bearing islets co-transplanted with Adv-hHGF-hIL-1Ra-transduced primary hBMSCs at 15 weeks after transplantation (d). (e) vWF fluorescent intensity was quantified using ImageJ from three independent regions. Data are presented as the mean ± SD, n=3, *P< 0.05 under t test.
DISCUSSION
Islet transplantation has been demonstrated in a limited number of patients to treat type I diabetes. Despite the tremendous progress in the isolation, transportation, and preservation of islets, the clinical application of this treatment is still limited by the huge loss of islet mass after transplantation (5,6). Therefore, islets from more than two donors are required to achieve an optimal outcome (20). Post-transplantation challenges causing islet loss include 1) long-term hyperglycemia challenge, 2) acute inflammation at the transplantation site, 3) hypoxic environment and failure to revascularize, and 4) innate immune rejection from the recipient (1).
The purpose of this study was to combine mesenchymal stem cell-based cell therapy with adenovirus-based gene therapy to improve the outcome of islet transplantation. We have been working on Adv-based gene therapy for years. Replication-deficient Adv vectors are extensively reported to be effective in blocking the inflammatory cytokine pathway and increasing the islet resistance to post-transplantation challenges (21). HGF is known to induce angiogenesis, promote β-cell proliferation and protect islets from apoptosis and eventually helps to improve the transplantation outcome in streptozotocin-induced diabetic mice (10,22). IL-1Ra binds to the cell surface interleukin-1 receptor and prevents IL-1 from sending a signal to that cell. Ex vivo transduction of human islets with Adv-hIL-1Ra has been reported to prevent IL-1β cell-induced apoptotic cell death in islets (23). We previously reported the improved islet transplantation using Adv-hHGF-hIL-1Ra-transduced human islets (13). However, most Adv vectors failed to support the long-term survival of islet grafts, probably due to the short expression profile of Adv. For example, the expression of HGF and IL-1Ra drastically decreased at 14 days after Adv transduction (Fig. 1d, e). Lentivrus could be an alternative solution for long-term gene therapy. But since lentivirus integrates into the host genome and has the potential for generation of replication competent lentivirus and oncogenesis (24), great caution should be used before starting in this area.
Another problem with Adv based gene therapy is the viral genome, more or less, co-expressed with target genes may trigger an increasing immune response to the transplanted organs. Some reports suggested that Adv transduction led to immune reaction to the Adv-bearing organs and made them more susceptible to attack from the recipient's immune system with potentially dangerous consequences (11,12). Therefore, how to find the perfect vehicles to provide prolonged protection to islet grafts with less immunogenicity is a critical question to be answered by traditional gene therapy.
hBMSCs seem to be one of these perfect vehicles for gene delivery. hBMSCs are pluripotent adult stem cells with the property of self-renewal and the potential to differentiate into many types of cells (25). They are open to genetic manipulation and capable of secreting endogenous growth factors to promote engraftment of human islets (26,27). They are also hypoimmunogenic, probably by releasing anti-inflammatory cytokines and inhibiting T-cell proliferation and activation (28,29). All these characteristics make hBMSCs good nursing cells to advance current clinical studies of islet transplantation by combining gene therapy with cell therapy.
However, probably because of the lack of standard operating protocols for isolating, preserving, and culturing hBMSCs, the supporting role of hBMSCs is still under discussion. The secretion of growth factors such as HGF, VEGF, and stem cell factor by hBMSCs is controversial. We did not detect SCF expression by primary hBMSCs, and the basal expression of HGF and VEGF from primary hBMSCs was too low to support an optimal revascularization for islet graft survival (Figs. 2d, 7b, and S2b), as noted in previous reports (30). We demonstrated that exogenous expression of HGF by Adv-hHGF-hIL-1Ra-transduced primary hBMSCs could be maintained for at least 2 weeks and may eventually lead to the notable increasing revascularization of islet grafts after transplantation (Figs. 2d, and 7a).
Despite many reports on hypoimmunogenic nature of hBMSCs, its mechanism is unclear. There are two hypotheses: 1) through the secretion of immunosuppressive cytokines, such as IL-10 and TGFβ (31) and 2) through cell surface molecules of hBMSCs to inhibit T-cell activity and proliferation (32). Previous reports showed that the cotransplantation of islets with primary hBMSCs prevented the allogeneic rejection and helped engraftment of islets, but one common feature in all these studies was that the hBMSCs were incompetent to reverse the blood glucose levels of recipients in the acute stage (<3 weeks) after transplantation, suggesting the slow engraftment of transplanted islets (7,8,33). To make the situation even worse, the hyperglycemia caused by slow engraftment in turn accelerated the apoptotic islet cell death (5). We hypothesized that this phenomenon was probably due to the incapability of primary hBMSCs to protect islets against the inflammatory-cytokine-induced apoptotic cell death. Consistent with previous report (34), we demonstrated that the native secretion of IL-10 by hBMSCs was insufficient to support islet survival against the inflammatory cytokine (Figs. 3,and S2a). We also demonstrated that Adv-hHGF-hIL-1Ra-transduced primary hBMSCs have clear advantages in preventing inflammatory-cytokine-induced islet death compared with untransduced primary hBMSCs (Fig. 3c).
hBMSCs are hypoimmunogenic cells in lack of the major histocompatibility complex class II (MHC II) (35), making them capable of escaping the recognition by Natural Killer cells and allreactive T-cells. This is an important feature for the gene therapy to improve islet transplantation because 1) adding primary hBMSCs into islets will not significantly raise allogeneic rejection, and 2) hBMSCs could serve as gene delivery vehicles to avoid the notable immunogenicity of Adv vectors. Primary hBMSCs were also capable of repairing injured tissues in long term (36). We demonstrated that when islets were co-transplanted with Adv-hHGF-hIL-Ra-transduced primary hBMSCs, the blood glucose levels of diabetic NOD-SCID mice were maintained ≤250 mg/dL for more than 15 weeks (Fig. 4), significantly longer than most reported islet transplantations using genetic modified islets (21,37,38). These results indicated the advantage of primary hBMSCs as gene delivery vehicles to improve the outcome of islet transplantation. Although an immunocompetent animal model should be used to further validate the benefit of hypoimmunogenic primary hBMSCs as gene delivery vehicles, our work provided useful information for further research.
The differentiation capability of hBMSCs is another interesting feature worth mentioning. The capacity of hBMSCs to differentiate into a variety of cells, including osteoblasts, adipocytes and chondrocytes and so on, is one of the most important mechanisms underlying its capability of tissue repair (36). Moreover, viral transduced hBMSCs still retained their differentiation capacity (39,40). This tissue repairing capacity of primary hBMSCs, as supported by a majority of reports, may eventually lead to the long-term protection on human islets and was further supported by the locations of hBMSCs which were in close proximity to the islet grafts (Figs. 4, and 6). Other reports showed that hBMSCs have the potential to differentiate into vascular endothelial cells (41). This is also possible because revascularization was observed mostly around islet grafts where most hBMSCs settled (Fig. 7). However, whether the neoforming blood vessels in the kidney-bearing islet grafts are from hBMSCs alone or other cell types remains to be clarified.
In agreement with other reports on islets/hBMSCs co-transplantation (42,43), our results indicated that the co-transplantation of islets with Adv-hHGF-hIL-1Ra-transduced primary hBMSCs into diabetic NOD-SCID mice promoted local revascularization, improved islet viability and reversed diabetes in long term. We are the first group to report the combination strategy of Adv-based gene therapy and hBMSC-based cell therapy to improve the outcome of islet transplantation. Our data indicated that hBMSCs were efficient gene delivery vehicles to improve the outcome of islet transplantation, as they did to treat other diseases (44,45). Adv-hHGF-hIL-1Ra-transduced primary hBMSCs secreted elevated levels of HGF and IL-1Ra and retained their protective capability for the long-term survival of islet grafts. Since current gene therapy approaches still focus on the expression of endogenous growth factors in human islets, our report provides a novel direction for future exploration of other factors and cells for co-transplantation. Our studies in rodents also provide useful information on the future studies on the immuno-competent animal model. However, several issues still need clarification before this approach can be clinically applicable, including the immunogenicity and in vivo fate of Advtransduced hBMSCs, mechanisms underlying the long-term protection of hBMSCs, and the optimal ratio between islet and hBMSC numbers.
In our previous and present studies (13,46), most transplanted human islets survived only up to 4~6 weeks in diabetic NOD-SCID mice without any treatment. We can also find out similar length of islet survival in other reports (47,48). We are aware of several reports of islet survival beyond 60 days post-transplantation. This discrepancy is due to the fact that islet survival and function post-transplantation depends on multiple factors, including donor condition, islet purity, immunocytes, hyperglycemia, hypoxia and inflammation after transplantation. The degree of diabetes and variability in mice used for transplantation also greatly affect the length of islet survival and function. The other reason may be the pre-transplantation treatment after islet isolation. When hand-picked islets were used for transplantation, the length of islet survival was significantly improved, possibly due to discarding of lymph nodes in the hand-picking process, as the existence of lymph nodes is detrimental to the survival of islets after transplantation. However, we are not personally in charge of the isolation process, and we do not prefer to use hand-picked islets for transplantation.
In summary, co-transplantation of islets and hBMSCs promoted revascularization, protected islet viability, and significantly reduced the islet mass required for reverse diabetes. The production of HGF and IL-1Ra by AdvhHGF-hIL-1Ra-transduced primary hBMSCs and the protective capability of hBMSCs synergistically helped to improve the islet survival in the early stage and graft function in the long run after transplantation.
ACKNOWLEDGMENTS
We thank the National Institutes of Health (NIH) for financial support (RO1 DK69968). We also thank Dr. David Armbruster from the University of Tennessee Health Science Center for kindly editing this manuscript.
ABBREVIATIONS
- Adv
adenoviral vector
- DAPI
6-diamidino-2-phenylindole
- GFP
green fluorescent protein
- hBMSCs
human bone marrow derived mesenchymal stem cells
- HGF
hepatocyte growth factor
- IL-1β
interleukin-1 beta
- IL-1Ra
interleukin 1 receptor antagonist
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinases
- MOI
multiplicity of infection
- NOD-SCID
non-obese diabetic/severe combined immunodeficient
- PBS
phosphate-buffered solution
- PGE
prostaglandin E
- SCF
stem cell factor
- STZ
streptozotocin
- TGF-β1
transforming growth factor-beta 1
- VEGF
vascular endothelial growth factor
- vWF
von-willebrand factor
Footnotes
Electronic Supplementary Material The online version of this article (doi:10.1007/s11095-011-0434-5) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Narangand AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev. 2006;58:194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 2.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–43. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm Lond. 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and −9. Diabetes. 2009;58:1797–806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 7.Figliuzzi M, Cornolti R, Perico N, Rota C, Morigi M, Remuzzi G, et al. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 2009;41:1797–800. doi: 10.1016/j.transproceed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, et al. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438–45. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 9.Mahato RI. Gene expression and silencing for improved islet transplantation. J Control Release. 2009;140:262–7. doi: 10.1016/j.jconrel.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Ocana A, Takane KK, Reddy VT, Lopez-Talavera JC, Vasavada RC, Stewart AF. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces beta cell death. J Biol Chem. 2003;278:343–51. doi: 10.1074/jbc.M207848200. [DOI] [PubMed] [Google Scholar]
- 11.Beardsley T. Gene therapy setback. Sci Am. 2000;282:36–7. doi: 10.1038/scientificamerican0200-36. [DOI] [PubMed] [Google Scholar]
- 12.Stolberg SG. Trials are halted on gene therapy: child in experiment falls ill–new setback for research. NY Times (Print); 2002. p. A1.p. A25. [PubMed] [Google Scholar]
- 13.Panakantiand R, Mahato RI. Bipartite adenoviral vector encoding hHGF and hIL-1Ra for improved human islet transplantation. Pharm Res. 2009;26:587–96. doi: 10.1007/s11095-008-9777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narang AS, Cheng K, Henry J, Zhang C, Sabek O, Fraga D, et al. Vascular endothelial growth factor gene delivery for revascularization in transplanted human islets. Pharm Res. 2004;21:15–25. doi: 10.1023/b:pham.0000012147.52900.b8. [DOI] [PubMed] [Google Scholar]
- 15.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–92. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Silva Jr WA, Covas DT, Panepucci RA, Proto-Siqueira R, Siufi JL, Zanette DL, et al. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells. 2003;21:661–9. doi: 10.1634/stemcells.21-6-661. [DOI] [PubMed] [Google Scholar]
- 17.Prockop DJ. Marrow stromal cells as stem cells for continual renewal of nonhematopoietic tissues and as potential vectors for gene therapy. Journal of Cellular Biochemistry. 1998:284–285. doi: 10.1002/(SICI)1097-4644(1998)72:30/31+<284::AID-JCB34>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, et al. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 1996;15:6205–12. [PMC free article] [PubMed] [Google Scholar]
- 19.Friedlander RM, Gagliardini V, Rotello RJ, Yuan J. Functional role of interleukin 1 beta (IL-1 beta) in IL-1 beta-converting enzyme-mediated apoptosis. J Exp Med. 1996;184:717–24. doi: 10.1084/jem.184.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 21.Panakantiand R, Mahato RI. Bipartite vector encoding hVEGF and hIL-1Ra for ex vivo transduction into human islets. Mol Pharm. 2009;6:274–84. doi: 10.1021/mp800183b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano M, Yasunami Y, Maki T, Kodama S, Ikehara Y, Nakamura T, et al. Hepatocyte growth factor is essential for amelioration of hyperglycemia in streptozotocin-induced diabetic mice receiving a marginal mass of intrahepatic islet grafts. Transplantation. 2000;69:214–21. doi: 10.1097/00007890-200001270-00004. [DOI] [PubMed] [Google Scholar]
- 23.Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, et al. Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1beta-induced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes. 1999;48:1730–6. doi: 10.2337/diabetes.48.9.1730. [DOI] [PubMed] [Google Scholar]
- 24.Themis M, Waddington SN, Schmidt M, von Kalle C, Wang Y, Al-Allaf F, et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol Ther. 2005;12:763–71. doi: 10.1016/j.ymthe.2005.07.358. [DOI] [PubMed] [Google Scholar]
- 25.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 26.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 28.Liu N, Chen R, Du H, Wang J, Zhang Y, Wen J. Expression of IL-10 and TNF-alpha in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell Mol Immunol. 2009;6:207–13. doi: 10.1038/cmi.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Z, Li H, Li X, Yu X, Wang H, Tang P, et al. In vitro characteristics and in vivo immunosuppressive activity of compact bone-derived murine mesenchymal progenitor cells. Stem Cells. 2006;24:992–1000. doi: 10.1634/stemcells.2005-0224. [DOI] [PubMed] [Google Scholar]
- 30.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 31.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716–26. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 32.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 33.Urban VS, Kiss J, Kovacs J, Gocza E, Vas V, Monostori E, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–53. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 34.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–97. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 35.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 36.Korblingand M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003;349:570–82. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 37.Emamaullee JA, Rajotte RV, Liston P, Korneluk RG, Lakey JR, Shapiro AM, et al. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes. 2005;54:2541–8. doi: 10.2337/diabetes.54.9.2541. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Hao J, Metzger DL, Mui A, Ao Z, Verchere CB, et al. Local expression of B7-H4 by recombinant adenovirus transduction in mouse islets prolongs allograft survival. Transplantation. 2009;87:482–90. doi: 10.1097/TP.0b013e318195e5fa. [DOI] [PubMed] [Google Scholar]
- 39.Chuah MK, Brems H, Vanslembrouck V, Collen D, Vandendriessche T. Bone marrow stromal cells as targets for gene therapy of hemophilia A. Hum Gene Ther. 1998;9:353–65. doi: 10.1089/hum.1998.9.3-353. [DOI] [PubMed] [Google Scholar]
- 40.Allay JA, Dennis JE, Haynesworth SE, Majumdar MK, Clapp DW, Shultz LD, et al. LacZ and interleukin-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Hum Gene Ther. 1997;8:1417–27. doi: 10.1089/hum.1997.8.12-1417. [DOI] [PubMed] [Google Scholar]
- 41.Satoh M, Yasunami Y, Matsuoka N, Nakano M, Itoh T, Nitta T, et al. Successful islet transplantation to two recipients from a single donor by targeting proinflammatory cytokines in mice. Transplantation. 2007;83:1085–92. doi: 10.1097/01.tp.0000260161.81775.58. [DOI] [PubMed] [Google Scholar]
- 42.Longoni B, Szilagyi E, Quaranta P, Paoli GT, Tripodi S, Urbani S, et al. Mesenchymal stem cells prevent acute rejection and prolong graft function in pancreatic islet transplantation. Diabetes Technol Ther. 2010;12:435–46. doi: 10.1089/dia.2009.0154. [DOI] [PubMed] [Google Scholar]
- 43.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, et al. Mesenchymal Stem Cells Enhance Allogeneic Islet Engraftment in Nonhuman Primates. Diabetes. 2010 doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manning E, Pham S, Li S, Vazquez-Padron RI, Mathew J, Ruiz P, et al. Interleukin-10 delivery via mesenchymal stem cells: a novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum Gene Ther. 2010;21:713–27. doi: 10.1089/hum.2009.147. [DOI] [PubMed] [Google Scholar]
- 45.Yan C, Lian X, Dai Y, Wang X, Qu P, White A, et al. Gene delivery by the hSP-B promoter to lung alveolar type II epithelial cells in LAL-knockout mice through bone marrow mesenchymal stem cells. Gene Ther. 2007;14:1461–70. doi: 10.1038/sj.gt.3303006. [DOI] [PubMed] [Google Scholar]
- 46.Cheng G, Zhu L, Mahato RI. Caspase-3 gene silencing for inhibiting apoptosis in insulinoma cells and human islets. Mol Pharm. 2008;5:1093–102. doi: 10.1021/mp800093f. [DOI] [PubMed] [Google Scholar]
- 47.Noguchi H, Nakai Y, Matsumoto S, Kawaguchi M, Ueda M, Okitsu T, et al. Cell permeable peptide of JNK inhibitor prevents islet apoptosis immediately after isolation and improves islet graft function. Am J Transplant. 2005;5:1848–55. doi: 10.1111/j.1600-6143.2005.00985.x. [DOI] [PubMed] [Google Scholar]
- 48.Omori K, Mitsuhashi M, Todorov I, Rawson J, Shiang KD, Kandeel F, et al. Microassay for glucose-induced preproinsulin mRNA expression to assess islet functional potency for islet transplantation. Transplantation. 2010;89:146–54. doi: 10.1097/TP.0b013e3181c4218d. [DOI] [PMC free article] [PubMed] [Google Scholar]