Abstract
Background
Panic is characterized as a disorder of interoceptive physiological hyperarousal, secondary to persistent anticipation of panic attacks. The novel aim of the present research was to investigate whether severity of agoraphobia within panic disorder covaries with the intensity of physiological reactions to imagery of panic attacks and other aversive scenarios.
Methods
A community sample of principal panic disorder (n=112; 41 without agoraphobia, 71 with agoraphobia) and control (n=76) participants imagined threatening and neutral events while acoustic startle probes were presented and the eye-blink response (orbicularis oculi) recorded. Changes in heart rate, skin conductance level, and facial expressivity were also measured.
Results
Overall panic disorder patients exceeded controls in startle reflex and heart rate during imagery of standard panic attack scenarios, concordant with more extreme ratings of aversion and emotional arousal. Accounting for the presence of agoraphobia revealed that both panic disorder with and without situational apprehension showed the pronounced heart rate increases during standard panic attack imagery observed for the sample as a whole. In contrast, startle potentiation to aversive imagery was more robust in those without versus with agoraphobia. Reflex diminution was most dramatic in those with the most pervasive agoraphobia, coincident with the most extreme levels of comorbid broad negative affectivity, disorder chronicity, and functional impairment.
Conclusions
Principal panic disorder may represent initial, heightened interoceptive fearfulness and concomitant defensive hyperactivity, which through progressive generalization of anticipatory anxiety, ultimately transitions to a disorder of pervasive agoraphobic apprehension and avoidance, broad dysphoria and compromised mobilization for defensive action.
Keywords: imagery, anxiety, panic, agoraphobia, comorbidity, depression, anhedonia, anxiety sensitivity, chronicity, emotional reactivity, narrative imagery, diagnostic subtypes, psychophysiology, startle, heart rate, facial expressivity, skin conductance, corrugator, EMG, SCL
Introduction
Panic disorder is characterized by unexpected panic attacks and concern about their recurrence and/or injurious consequences (1), further specified by the presence or absence of agoraphobia—apprehension and/or avoidance of situations in which panic attacks are presumed likely. Although findings are mixed (2), panic disorder with agoraphobia is typically associated with more profound pathology (3), indexed in elevated apprehension about panic recurrence (4) and consequences (5), generalized symptomatology (e.g., non-specific anxiety) (6), comorbidity (7–9), socio-occupational impairment (9–10) and poorer treatment outcome (11). Although reduced to a dichotomous distinction in the current nosology (DSM-IV), agoraphobia severity was previously qualified (none, mild, moderate, severe; DSM-III-R) (12) and contemporary investigations indicated a spectrum of increasing distress, least in panic disorder without agoraphobia and most marked in patients with severe situational avoidance (13). The current investigation of panic disorder examines whether the presence/absence as well as finer gradations of agoraphobia reflect differences in defensive reflex physiology during narrative imagery.
Script-driven emotional imagery is a valuable tool in studies of anxiety disorders, permitting presentation of both standard and idiographic threat challenges, paralleling methods of imaginal exposure therapy (14–15). Physiological arousal during aversive imagery parallels anticipatory reactions to threatening events (16), similarly mobilizing the autonomic nervous system (e.g., heart rate, skin conductance), communicating threat through facial musculature (e.g., corrugator “frown” muscle), and prompting somatic reflexive action (e.g., startle potentiation) (17–18). Animals confronting survival threat show similar reactions, mediated by the brain’s defense circuit (centered on the amygdala) (19–20) and neuroimaging studies suggest a comparable circuit (21–23) underlies human fear.
Whereas imagery has been a productive methodology for characterizing the physiology of posttraumatic stress disorder (PTSD) (24–25), a parallel database on panic disorder has yet to accumulate. In one of the first studies reported (26), panic patients relative to controls showed elevations in blood pressure during idiographic aversive imagery, whereas other autonomic measures did not differ. In a subsequent study (27), sympathetic increases were modest in nocturnal, compared to diurnal, panickers during both standard aversive and neutral imagery, whereas facial expressivity and heart rate patterns were equivalent. Notably, in both studies—consistent with the vast majority of psychophysiological investigations (26–33)—patient groups were combined samples of panic disorder with and without agoraphobia. Although this grouping could be a constraint of sample size or an effort to accord with the International Classification of Diseases (ICD-10) (34–35), pathologically significant differences in emotional reflex patterns could be obscured.
In a series of imagery investigations, Lang and colleagues (17,36–44) explored evoked defensive arousal differences across anxiety disorders: specific and social phobia patients demonstrated the greatest autonomic and startle responses. Paradoxically, patients with more pervasive and diffuse anxiety symptomatology—generalized anxiety disorder (GAD), PTSD secondary to repeated traumatization—showed less robust reflex potentiation (despite reports of intense fear). Importantly, panic disorder with agoraphobia was located at the latter extreme. Furthermore, reflex blunting was consistently more pronounced across and within diagnoses, coincident with increased clinician-rated severity, poorer prognosis, greater comorbidity (depression and anxiety), elevated questionnaire-based indices of negative affectivity, and lengthier disorder chronicity, suggesting that defensive engagement during imagery might be compromised by prolonged hyperarousal, apprehension, and accompanying dysphoria (43–44).
In the current study, a similar distress-related reflex pattern was expected within panic disorder. Similar to phobic disorders (36–43) panic patients without agoraphobia were expected to endorse the least symptomatic distress and show the most robust physiological reactivity (i.e., potentiated startle and enhanced autonomic and facial muscle action) during aversive imagery of panic attacks. Patients without agoraphobia and controls were expected to react similarly during threatening imagery for which defensive mobilization is normal and adaptive (e.g., facing an attacking animal). In contrast, severe agoraphobia was expected to show higher comorbidity, broader negative affectivity, longer disorder duration, and concomitantly, attenuated mobilization for defensive action.
Method
Participants
Participants were assessed at the University of Florida Fear and Anxiety Disorders Clinic: 112 treatment-seeking adults with principal diagnoses of panic disorder without agoraphobia (PD; N=41) and with agoraphobia (PDA; N=71) and 76 healthy community controls.
Diagnostic Classification
Diagnostic groups were established using the Anxiety Disorder Interview Schedule for DSM-IV (ADIS-IV; 45), a semi-structured interview for assessing current anxiety, mood, substance use, and somatoform disorders and for screening psychosis and major physical disease. For multiple Axis I disorders, diagnostic primacy was determined by clinician-rated severity (ranging from 0, No features present, to 5, Diagnosis present; severe) reflecting both distress and interference. Controls denied current or lifetime diagnoses of psychiatric illness. Inter-rater reliability (via videotape) was calculated for 25% of patients, yielding 100% agreement for principal diagnosis among three masters- or doctoral-level clinicians.
The ADIS-IV enables assessment of agoraphobia extent by querying apprehension and avoidance of 22 standard situations (0, No avoidance/apprehension, to 8, Very Severe avoidance/apprehension), specifically due to fears of panic induction and consequent difficulty escaping and/or securing assistance from others. In the current study, presence of agoraphobia was defined as moderate to very severe apprehension and/or avoidance of at least two types of situations (i.e., transportation, shopping or recreational facilities, crowds, open spaces, being alone, enclosed spaces, work).1 Patients with agoraphobia were further classified by a median split on the sum of their situational apprehension and avoidance ratings from the ADIS-IV, yielding 34 with moderate and 37 patients with severe agoraphobia.2
Procedure
The University of Florida Institutional Review Board (IRB-01) approved the study. Participants provided informed consent, completed questionnaires and interview in the morning; psychophysiological assessment and clinical debriefing followed in the afternoon.
Experimental Stimuli
Twenty-four narrative imagery texts were used (46). Analyses focused on two idiographic, “personal” threat narratives representing each patient’s primary clinical fear (i.e., panic attacks); or for controls their “worst fear” experiences (Table S1 in the Supplement). Standard scenes included: two panic attack (crowded checkout line, driving alone), four survival threat (physical attack by animal/human), two neutral (watching documentary, reading magazine) events. Filler scripts were low arousal or engaging pleasant scenes to impede an overall unpleasant arousal context. Scripts were ~20 words designed to quickly reveal affect and reflect active participation. A woman recorded the scenes using minimal prosody for presentation over earphones (Telephonics TDH-49, Telephonics Corporation, Huntington, New York).
Imagery Assessment
Seated in a quiet, dimly lit room, with electrodes placed, participants were instructed to listen to the auditory scripts with eyes closed, vividly imagining the events described, as if actively involved. Throughout the recording session, soft tones cued participants to relax, breathe slowly, and silently repeat the word “one” to stabilize between-trial physiological activity (47). Imagery scripts were interspersed every 36 seconds in the tone series, with content pseudorandomized so that no more than 2 stimuli of the same hedonic valence (pleasant, neutral, unpleasant), or content category (e.g., survival threat) were presented consecutively. The script series was repeated in a counterbalanced order.
Trials consisted of a 1-second baseline, a 6-second auditory script, and 12 seconds of imagery. Startle probes (50-ms 95 dB(A) white noise, instantaneous rise-time) were presented at 4–5.5 or 10–11.5 seconds post-script onset, or both, and on 25% of inter-trial intervals, at 22–23.5 seconds post-imagery offset.
Following imagery assessment (approximately 45 minutes) participants rated each scene for experienced pleasure and emotional arousal (48).
Experimental Control and Data Collection
A computer running VPM software (49) controlled stimulus presentation and data acquisition. Bioamplifiers recorded electromyograph (EMG) potentials at left orbicularis occuli and corrugator supercilii, skin conductance level (SCL) and electrocardiogram (EKG) as reported previously (36).
Data Reduction and Analysis
Univariate ANOVAs and Tukey Honestly Significant Difference (HSD) tests for planned comparisons determined group differences in demographic and questionnaire data.
Using VPM software EMG, SCL [ log[SCL+1]], and EKG r-r intervals [converted to beats-per-minute] were reduced into half-second bins. Responses were determined by subtracting amplitude during the 1 second prior to script presentation from averages during the 12-second imagery period.
Startle blinks from orbicularis oculi EMG represented the magnitude difference between onset and peak muscle potential (50), standardized within subject in relation to the mean and standard deviation of inter-trial probe responses (36).
Using SPSS (SPSS, Inc., Chicago, Illinois), omnibus repeated measures ANOVAs were conducted separately for each physiological measure, with diagnostic status as a between-subjects factor and imagery content as a within-subjects factor. Consistent with the majority of preceding physiological investigations of panic disorder (26–33), analyses were initially performed with control versus patient (irrespective of agoraphobia status) as a between-subjects factor. To consider diagnostic distinctions delineated in the DSM-IV and DSM-III-R respectively, subsequent analyses considered presence or absence as well as severity of agoraphobia. Startle and autonomic reactivity during imagery have been shown to strongly covary with rated emotional arousal (43–44), thus contents were entered according to the mean linear increase in arousal reported by the patients (i.e., neutral, panic attack, survival threat, idiographic/personal threat). Significant overall group effects were followed up with between-group tests by contents to specify which imagery scenarios evoked different sensitivities in patients and controls, facilitating comparisons to preceding studies that utilized different prompts (26–27,37–38). Within-group comparisons explicated interactions. Analyses were repeated for presence/absence of agoraphobia (i.e., control, PD, PDA) and subtypes (i.e., PD; PDA-moderate; PDA-severe). Wilks’ lambda addressed sphericity issues (51).
Results
Panic Disorder and Control Groups
Affective Judgments
Across groups rated displeasure reliably increased from neutral, to panic attack, survival threat and personal threat at the extreme, F(3,179)=336.95 p<.001 (Figure 1). Furthermore, controls rated personal and survival threat scenes as equally aversive, all ns; whereas patients rated personal threat as more aversive than all other contents, all ps<.001; Content × Diagnosis interaction, F(3,179)=14.06, p<.001; Diagnosis F(1,181)=19.41, p<.001. Patients rated panic attack and personal threat scenes more unpleasant than controls, ps<.001.
Figure 1.
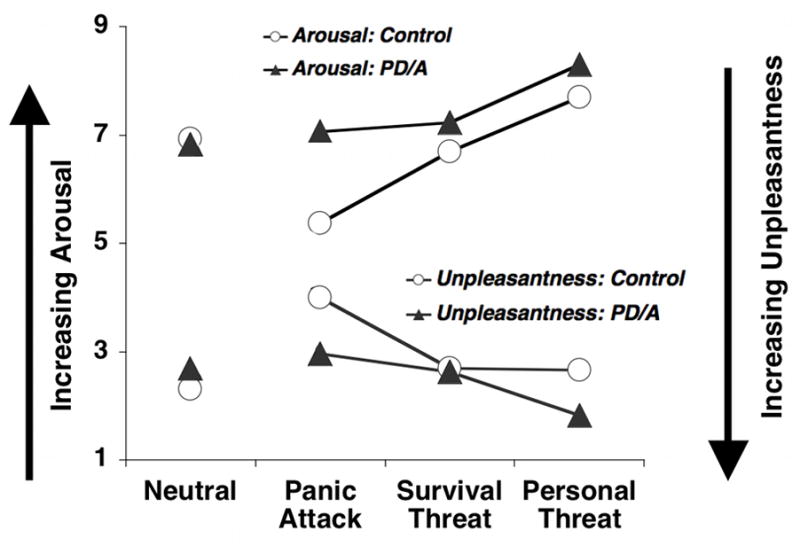
Mean ratings of experienced unpleasantness and emotional arousal for neutral, panic, survival threat, and personal threat imagery for control and panic disorder (with and without agoraphobia, PD/A) groups.
Controls rated personal threat scenes most emotionally arousing followed by survival threat, panic attack and neutral scenes. Patients were similar except panic attack was as intense as survival threat, Content F(3,180)=344.57, p<.001; Content × Diagnosis F(3,180)=10.80, p<.001; Diagnosis F(1,182)=28.43, p<.001. For all aversive scenes, patients endorsed higher arousal than controls, ps<.01.
Baseline Physiology
No group differences emerged for blink magnitude to intertrial startle probes or for baseline SCL or corrugator activity, Fs=0.02–0.63. Consistent with preceding studies (30,50–51) heart rate was higher for panic disorder patients (M=74.85, SD=11.34) than controls (M=65.31, SD=9.95)3, Diagnosis F(1,182)=34.51, p<.001.
Startle Reflex Potentiation
Blink magnitude (Figure 2 top panel) was larger during all unpleasant compared to neutral imagery, Content F(3,165)=14.66, p<.001 (all unpleasant versus neutral comparisons, ps<.001), similarly across groups, Content × Diagnosis F(6,328)=1.04, ns. Panic patients tended to exceed controls, Diagnosis F(1,167)=2.63, p=.05†, attributable to greater patient reactivity, specifically during standard panic attack imagery, F(1,167)=3.97, p<.05.
Figure 2.
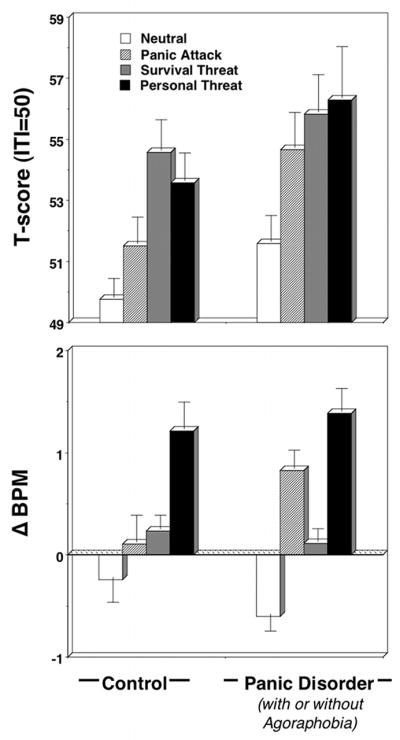
Mean startle reflex responses (standardized to the distribution of responses during intertrial intervals; top panel) and heart rate change (residuals; bottom panel) during neutral, panic attack, survival threat, and personal threat imagery for control and panic disorder (with and without agoraphobia) groups. Error bars refer to standard error of the mean.
Autonomic and Facial Responses
Heart rate accelerated during panic attack, survival and personal threat relative to neutral imagery, Content F(3,180)=14.76, p<.001, individual ps<.001. Contents varied similarly between groups, Content × Diagnosis F(3,180)=2.30, ns, Diagnosis, F(1,182)=0.85, ns, except controls showed a significant linear increase from neutral, with personal threat most extreme, followed by survival threat; conversely patients showed the second largest increase to imagery of panic attacks—an acceleration that far surpassed responding during survival threat imagery (ps<.05), Content × Diagnosis (Cubic Contrast), F(1,182)=5.68, p<.05. A posthoc univariate test revealed a pattern similar to startle responses—patients’ heart rate accelerated more than controls, specifically during standard panic attack imagery, F(1,182)=5.64, p<.05. Whereas acceleration patterns for controls closely paralleled rated arousal, patients showed the strongest increases to scenarios depicting uncontrollable panic attacks, foremost to idiographic narratives (Figure 2 bottom panel).
In contrast to the heart rate sensitivities to clinically-relevant imagery in panic disorder, SCL changes did not differ between groups, Content F(3,180)=21.74, p<.001, Content × Diagnosis F(3,180)=1.15, ns; Diagnosis F(1,182)=0.42, ns. Overall, panic attack, survival and personal threat increased SCL relative to neutral imagery, Fs=7.80–67.10, ps<.01. Facial expressivity was also similar between groups. Unpleasant imagery augmented corrugator tension relative to neutral imagining, ps<.001, Content F(3,182)=11.57, p<.001, Content × Diagnosis F(3,182)=0.61, ns; Diagnosis, F(1,184)=2.82, ns. For both SCL and corrugator, the largest increases were evident during personal threat followed by survival threat, panic attack, and a relative reduction below baseline during neutral imagery.
Panic Disorder with and without Agoraphobia: Defensive Reactivity
Affective Judgments
As listed in Table 1, PD and PDA showed similar patterns in rated displeasure, with a few exceptions, Content F(3,178)=322.61, p<.001; Content × Diagnosis interaction, F(6,356)=7.37, p<.001; Diagnosis, F(2,180)=10.27, p<.001. Both patient groups rated panic attack and personal threat scenes more unpleasant than controls, ps<.01; PDA endorsed more displeasure than PD specifically to standard panic attack narratives, p<.05†.
Table 1.
Mean Responses and Standard Deviations to Imagery Scenes by Control and Panic Disorder Groups
| Response Modality/Imagery scene | Control | Panic Disorder Without Agoraphobia | Panic Disorder With Agoraphobia |
|---|---|---|---|
| Pleasure (1–9) | |||
| Neutral | 6.93 (1.53) | 6.74 (1.42) | 6.87 (1.66) |
| Panic Attack | 4.00 (1.08)* b c | 3.28 (1.29)* c† | 2.77 (1.31)* a b† |
| Survival Threat | 2.68 (0.99)* | 2.76 (1.19)* | 2.55 (1.14)* |
| Personal Threat | 2.65 (1.41)* b c | 1.88 (1.07)* a | 1.79 (1.16)* a |
| Arousal (1–9) | |||
| Neutral | 2.31 (1.54) | 2.55 (1.54) | 2.78 (1.77) |
| Panic Attack | 5.37 (1.90)* b c | 6.52 (1.67)* a c | 7.38 (1.40)* a b |
| Survival Threat | 6.69 (1.53)* c | 6.84 (1.75)* c† | 7.45 (1.18)* b† |
| Personal Threat | 7.69 (1.67)* c | 8.11 (1.04)* b | 8.39 (1.19)* 1 |
| Startle Reflex (T-score) | |||
| Neutral | 49.76 (5.01) | 51.15 (9.01) | 51.82 (8.43) |
| Panic Attack | 51.51 (7.20)* | 54.93 (9.70)* | 54.50 (12.64)* |
| Survival Threat | 54.57 (8.22)* | 59.06 (16.00)* | 53.97 (9.07)* |
| Personal Threat | 53.58 (7.33)* | 58.73 (20.18)* | 54.88 (14.70)* |
| Heart Rate Δ (bpm) | |||
| Neutral | −0.25 (2.07) | −0.68 (1.92) | −0.56 (1.71) |
| Panic Attack | 0.10 (2.25) | 0.90 (2.00)* | 0.79 (1.79)* |
| Survival Threat | 0.23 (1.16)* | −0.11 (1.21) | 0.24 (1.30)* |
| Personal Threat | 1.21 (2.26)* | 1.60 (2.65)* | 1.25 (2.17)* |
| SCL Δ (log (μS + 1)) | |||
| Neutral | −0.006 (0.036) | −0.009 (0.046) | −0.010 (0.066) |
| Panic Attack | 0.001 (0.041) | −0.002 (0.037) | 0.014 (0.072)* |
| Survival Threat | 0.004 (0.022)* | −0.003 (0.049) | 0.001 (0.051)* |
| Personal Threat | 0.045 (0.086)* | 0.045 (0.054)* | 0.072 (0.122)* |
| Corrugator EMG Δ (μV) | |||
| Neutral | −0.07 (0.79) | 0.03 (0.60) | −0.16 (1.03) |
| Panic Attack | 0.49 (1.40)* | 0.50 (0.82)* | 0.15 (0.67)* |
| Survival Threat | 0.96 (1.98)* | 0.48 (0.89)* | 0.75 (1.72)* |
| Personal Threat | 1.17 (2.60)* | 0.51 (0.80)* | 0.76 (2.00)* |
Note. Pleasure rated on Self-Assessment Manikin (SAM) (50) 1=Completely unhappy, 9=Completely happy; Arousal rated on SAM 1=Completely relaxed, 9=Completely aroused; SCL=skin conductance level; Δ=change; μS=miscrosiemen; bpm=residual beats per minute after removal of baseline effects; EMG=electromyographic; μV=microvolt;
Within-group comparison to neutral significant at p<.05;
one-tailed test; Superscripts = Results of Tukey HSD pairwise comparisons:
Post hoc between-group comparison to control is significant at p < .05;
= Post hoc between-group comparison to PD is significant at p < .05;
= Post hoc between-group comparison to PDA is significant at p < .05.
Ratings for arousal mirrored those for displeasure, Content F(3,179)=328.57, p<.001; Content × Diagnosis F(6,358)=5.89, p<.001; Diagnosis F(2,181)=17.78, p<.001. Both PD and PDA endorsed more intense arousal than controls for panic attack and personal threat scenes, ps<.01, with PDA exceeding PD, again, specifically for panic attack narratives.
Baseline Physiology
Both PD and PDA exceeded controls in heart rate (Table S2 in the Supplement).
Startle Reflex Potentiation
Affective modulation varied between groups (Figure 3), Content × Diagnosis F(6,328)=2.34, p<.05; Content × Diagnosis Linear Trend F(2,166)=3.32, p<.05; Content F(3,164)=18.12, p<.001; Diagnosis, F(2,166)=2.04, ns. Whereas control and PD groups showed similar reflex patterns with the highest magnitudes for survival and personal threat imagery, PDA showed a somewhat diminished overall response with less clear differentiation among contents. To compare with preceding studies (36), startle response differences between neutral and unpleasant imagery (i.e., fear potentiation) were analyzed and suggested overall defensive hyper-responsivity in the PD relative to PDA group, p<.05, Diagnosis, F(2,166)=3.35, p<.05.
Figure 3.
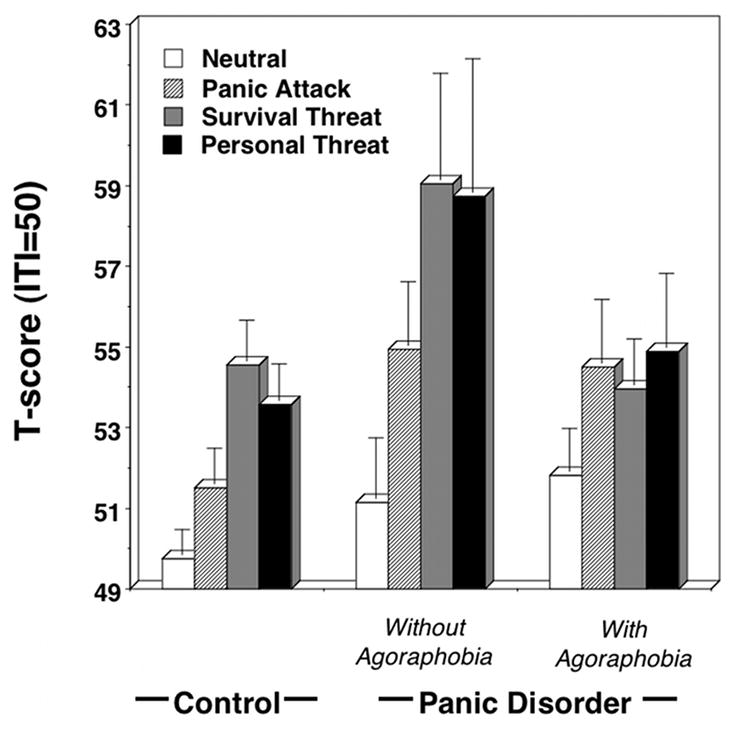
Mean startle reflex responses (standardized to the distribution of responses during intertrial intervals) during neutral, panic attack, survival threat, and personal threat imagery for control and panic disorder without and with agoraphobia groups. Error bars refer to standard error of the mean.
Autonomic and Facial Responses
Heart rate change varied similarly between the three groups, Content F(3,179)=17.24, p<.001; Content × Diagnosis F(6,358)=1.45, ns, Diagnosis, F(2,181)=0.42, ns, except that for both PD and PDA, the second largest response was elicited during panic attack imagery—larger even than to survival threat imagery (ps<.05), Content × Diagnosis (Cubic Contrast), F(1,181)=12.52, p<.05 (Table 1).
Parallel to the two group analyses, SCL, Content F(3,179)=21.85, p<.001, Content × Diagnosis F(6,358)=0.97, ns; Diagnosis F(2,181)=1.19, ns, and corrugator changes, Content F(3,181)=9.08, p<.001, Content × Diagnosis F(6,362)=1.28, ns; Diagnosis, F(2,183)=1.41, ns, suggested consonant, reliable sympathetic and facial motor reactivity across groups.
Agoraphobia Severity & Defensive Reactivity
Accounting for agoraphobia severity (i.e., PD; PDA-moderate; PDA-severe) revealed a more pronounced profile of group differences in startle reactivity (Figure 4); Content × Diagnosis Linear Trend F(3,98)=3.38, p<.05. Patient subgroups demonstrated reliable linear contrasts across contents, Fs=5.06–11.11, ps<.05, except for PDA-severe F(1,30)=0.46, ns. Startle response differences between neutral and unpleasant imagery (i.e., fear potentiation) further indicated that the obtunded reactivity observed for PDA overall, in fact, was attributable to patients with the most pervasive agoraphobia, Diagnosis, F(2,98)=3.51, p<0.05, who showed reliably less startle potentiation than PD, p<.05. PDA-moderate response was intermediate.
Figure 4.
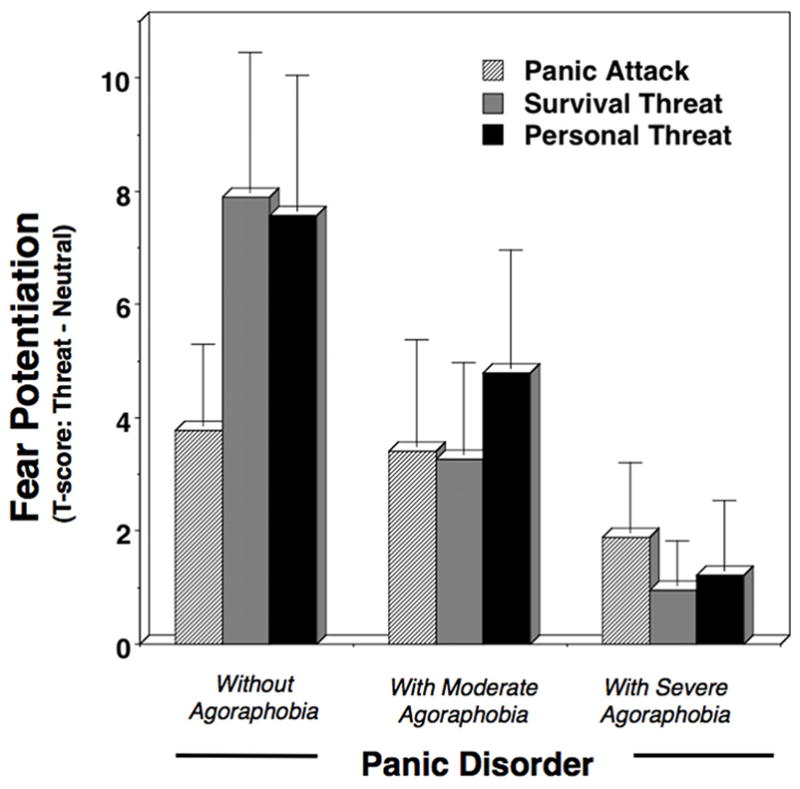
Mean fear potentiation of startle reflexes (startle response magnitude during aversive minus neutral imagery) during panic attack, survival threat, and personal threat imagery for panic disorder patients by severity of agoraphobia. Error bars refer to standard error of the mean.
For affective judgments, SCL, heart rate, and facial action, overall response magnitude and content modulation showed little variation as a function of agoraphobia severity. Furthermore, baseline physiology was commensurate across patients.
Agoraphobia Severity & Comorbid Negative Affectivity
A consistent pattern of elevated broad negative affectivity and functional impairment was observed in the PDA-severe compared to PD (Table 2). Questionnaire measures revealed that, in addition to agoraphobia severity (FSS Agoraphobia), non-specific trait anxiety (STAI), cognitive/somatic symptoms of depression (BDI) as well as anhedonia (MASQ Anhedonia), and functional interference (IIRS)4 reliably increased from controls, to PD, PDA-moderate and PDA-severe at the extreme.5 PDA-severe also surpassed PD in total number of Axis I disorders and frequency of comorbid anxiety, but not mood disorders. To further specify the pattern of comorbidity across the panic spectrum (62), subgroups were compared in terms of presence or absence of single episode versus recurrent major depression, which reflected greater prevalence of only the latter in PDA-severe. Next, comorbid anxiety disorders were defined as predominantly circumscribed fear (i.e., specific phobia, performance phobia) versus broad distress/anxious-misery (i.e., GAD, PTSD) drawing on the findings of epidemiological (63–68) as well as psychophysiological (42–44) studies. Similar to the presence of more intractable depression, the frequency of distress disorders (but not circumscribed fear disorders) was greatest in PDA-severe. This overall pattern of broad dysphoria in the severe agoraphobia group (indexed both dimensionally and categorically) was consistent with clinician-conferred ratings of poorer treatment prognosis.
Table 2.
Questionnaire and interview responses (means and standard deviations) for Control and Panic Disorder Groups
| Measure | Control | Panic Disorder without Agoraphobia | Panic Disorder with Moderate Agoraphobia | Panic Disorder with Severe Agoraphobia | Group Effect |
|---|---|---|---|---|---|
| Questionnaire Measures | |||||
| STAI-Trait (20–80) | 30.80 (8.57)b c d | 49.12 (10.17)a d | 51.65 (9.29)a | 55.26 (10.11)a b | F(3, 181) = 71.61, p < .001 |
| BDI Total (0–63) | 3.51 (4.55)b c d | 15.30 (10.19)a d | 15.24 (6.91)a d | 20.77 (10.55)a b | F(3, 182) = 47.40, p < .001 |
| MASQ Anhedonia (22–110) | 46.26 (13.32)b c d | 67.50 (16.02)a d | 69.85 (11.81)a | 77.03 (14.55)a b | F(3, 182) = 50.27, p < .001 |
| IIRS Total (13–91) | 18.24 (11.64)b c d | 45.49 (18.20)a d | 46.97 (19.96)a | 57.31 (17.81)a b | F(3, 171) = 55.41, p < .001 |
| FSS Agoraphobia (15–75) | 20.03 (5.10)b c d | 26.03 (7.10)a c d | 36.06 (11.27)a b d | 43.47 (12.51)a b c | F(3, 183) = 69.48, p < .001 |
| ASI Total (0–64) | 9.06 (6.83)b c d | 36.82 (11.59)a | 36.84 (13.55)a | 41.00 (10.85)a | F(3, 175) = 110.55, p < .001 |
| MASQ Anxious Arousal (17–85) | 18.62 (2.68)b c d | 37.38 (13.73)a | 38.84 (14.51)a | 42.76 (12.48)a | F(3, 182) = 57.69, p < .001 |
| QMI Total (35–245) | 82.96 (29.70) | 87.15 (19.74) | 89.12 (31.93) | 84.66 (27.74) | F(3, 181) = 0.45, ns |
| Interview Measures of Panic Disorder & Agoraphobia | |||||
| Agoraphobic apprehension & avoidance (0–352) | 15.62 (25.27)c d | 67.28 (25.63)b d | 167.94 (48.47)b c | F(2, 109) = 190.74, p < .001 | |
| Full symptom panic attack intensity (0–112) | 55.38 (18.93)d† | 61.26 (15.17) | 65.49 (20.94)b† | F(2, 109) = 2.98, p =.06 | |
| Full symptom panic attacks last month (Count) | 6.38 (10.76) | 18.40 (53.87) | 18.65 (37.09) | F(2, 109) = 0.19, ns | |
| Current nocturnal panic attacks (%) | 26.83 | 23.53 | 13.51 | X2 (2) = 2.18, ns | |
| Panic disorder severity (0–5) | 3.39 (0.59)d | 3.68 (0.94) | 4.16 (1.04)b | F(2, 109) = 7.78, p < .01 | |
| Prognosis (1–4) | 1.98 (0.61)d | 1.97 (0.52)d | 2.46 (0.80)b | F(2, 109) = 6.77, p < .01 | |
| Age of panic disorder onset (Years) | 25.80 (9.48) | 30.50 (13.74) | 28.00 (12.36) | F(2, 109) = 1.46, ns | |
| Panic disorder chronicity (Years) | 3.29 (5.33)d | 6.41 (8.09) | 7.22 (8.56)b | F(2, 109) = 3.09, p < .05 | |
| Interview Measures of Comorbidity | |||||
| Axis I disorders (Count) | 2.00 (1.02) d | 1.74 (0.83) d | 2.69 (1.27)b c | F(2, 109) = 7.06, p < .01 | |
| Comorbid anxiety disorder (%) | 32.0d | 26.47d | 62.16b c | X2 (2) = 11.32, p < .01 | |
| Comorbid anxiety: fear disorder (%) | 21.95 | 20.59 | 35.14 | X2 (2) = 2.48, ns | |
| Comorbid anxiety: distress disorder (%) | 12.20d | 8.82d | 32.43b c | X2 (2) = 8.15, p < .05 | |
| Comorbid mood disorder (%) | 34.15 | 38.23 | 48.65 | X2 (2) = 1.78, ns | |
| Comorbid single major depressive disorder (%) | 7.31 | 5.88 | 5.45 | X2 (2) = 0.13, ns | |
| Comorbid recurrent major depressive disorder (%) | 17.07d | 17.65d | 40.54b c | X2 (2) = 7.12, p < .05 | |
| Demographics | |||||
| Age at assessment (Years) | 31.79 (11.61) | 29.10 (10.80)c | 36.91 (14.68)b | 35.22 (12.46) | F(3, 184) = 3.19, p < .05 |
| Gender (% Female) | 65.79 | 63.41 | 64.71 | 62.16 | X2 (3) = 0.16, ns |
| Race (% Caucasian) | 84.21 | 75.61 | 85.29 | 86.49 | X2 (3) = 2.11, ns |
| College graduate (%) | 60.53b d | 41.46 a | 52.94d | 24.32a c | X2 (3) = 14.12, p < .001 |
Note. STAI-Trait = Trait scale of State Trait Anxiety Inventory (54); BDI =Beck Depression Inventory (55); MASQ Anhedonia = Anhedonia subscale of the Mood and Anxiety Symptom Questionnaire (56); IIRS = Illness Intrusiveness Rating Scale (57); ASI = Anxiety Sensitivity Index (58); QMI Total = Questionnaire on Mental Imagery (59); Agoraphobic Apprehension & Avoidance = sum of severity ratings from ADIS-IV (9-point scale ranging from 0, None, to 8, Very severe) regarding apprehension and avoidance of 22 situations due to fears of panic (43). Full symptom panic attack intensity = sum of severity ratings from ADIS-IV (9-point scale ranging from 0, None, to 8, Very severe) regarding 11 physiological and 3 cognitive panic attack symptoms. Full symptom panic attacks last month = count of full symptom panic attacks in last month from ADIS-IV. Panic disorder severity = clinician-rated severity (6-point scale ranging from 0, No features present, to 5, Diagnosis present; severe) reflecting both distress and interference; Prognosis = clinician-rated estimate of treatment prognosis (4-point scale ranging from 1, Excellent, to 4, Poor); Age of onset = patient-reported onset of principal diagnosis; Chronicity of principal disorder = years from patient-reported onset of diagnosis to assessment; Superscripts = Results of Tukey HSD pairwise comparisons:
Post hoc between-group comparison to control is significant at p < .05;
Post hoc between-group comparison to PD is significant at p < .05;
Post hoc between-group comparison to PDA-moderate significant at p < .05.
Post hoc between-group comparison to PDA-severe is significant at p < .05;
One-tailed test based on directional hypothesis that PDA-severe agoraphobia group would exceed PD group.
In terms of panic-related symptoms that might contribute to the pattern of broad negative affectivity and vice versa, reports of dispositional interoceptive sensitivity (i.e., ASI, MASQ Anxious Arousal) suggested equivalently heightened awareness of physiological cues irrespective of agoraphobia. Patients also reported commensurate frequency in the last month of full-blown panic attacks and prevalence of nocturnal panic attacks but recalled more severe physiological and cognitive symptoms amidst a prototypical panic attack, consistent with more extreme clinician-conferred ratings of panic disorder severity.
PDA-severe patients recalled disorder-level dysfunction that endured more than twice as long as PD patients, with the PDA-moderate group again intermediate. The majority of PDA patients (76%) recalled near simultaneous onset of panic disorder and agoraphobia (within 1 year), whereas the remainder developed situational apprehension and avoidance within three years (M=2.47, SD=1.84)6. Consistent with protracted functional interference (13), the PDA-severe group reported the lowest educational attainment (Table 2).7
Self-reported ability to generate vivid mental imagery was equivalent across participants. Furthermore, medication and substance use patterns exerted no reliable influence on physiological patterns.8
Discussion
Consistent with the primacy of panic-related fears in their diagnostic profile, the total cohort of panic disorder patients showed hyperarousal during imagery of panic attacks. Specifically, patients demonstrated greater heart rate acceleration and startle potentiation than controls during imagery of standard panic attack scenarios, concordant with more extreme ratings of aversion and emotional arousal. Both patient and control groups rated their personal, “worst” threatening scenes most arousing and evinced their largest physiological increases during imagining. Notably, patients and controls showed similar magnitude reflex responses during standard survival and idiographic threat imagery, suggesting that the exaggerated reactivity to standard panic contents likely does not reflect broader sensitization of fear circuitry, consistent with limited functional neuroimaging data on this syndrome (74–75). Furthermore, patients and controls showed equivalent affective modulation of facial expressivity and sympathetic activation.
Panic disorder with and without agoraphobia showed similarly palpable heart rate increases during panic attack imagery. In fact, regardless of agoraphobia severity (i.e., none, moderate, severe), panic patients showed exaggerated heart rate and subjective distress during standard panic imagery, coupled with normative sympathetic and facial motor activation across contents. Conversely, startle potentiation during aversive imagery was generally more pronounced in PD than PDA—a pattern even more dramatic when PD was compared to the severe agoraphobia subgroup (the moderate group was intermediate). Seemingly paradoxically, the panic disorder group with severe agoraphobia failed to show reflex responses greater than those to neutral during aversive imagery of any content. Essentially, the most distressed patients were characterized by physiological system discordance—simultaneous heightened, normative, and even dampened responses in different domains—suggesting not only magnitude changes but also disruption of output coordination in extreme manifestations of apprehension and avoidance.
Concomitant with blunted defensive startle reflexes, panic patients with severe agoraphobia also endorsed the most intense prototypical panic attacks and received the highest clinician ratings of disorder severity and poor treatment prognosis. Importantly, group differences were most robust for disorder non-specific features—that is, functional interference and broad negative affectivity (i.e., trait anxiety, cognitive and somatic depressive symptoms, anhedonia). Notably, negative affectivity has been implicated in the vulnerability and perpetuation of pathological panic and agoraphobia (76). In fact, relative to non-clinical community samples (54–56), the severe agoraphobia patients endorsed symptom levels on the BDI and STAI beyond the 99th percentile. Axis I comorbidity mirrored dimensional measures; however, analyses refined by disorder focus revealed that severe agoraphobia patients exceeded the other groups not in circumscribed fearfulness or transient, single episode major depression, but rather in intractable and chronic broad anxious distress and major depression. Complementing this profile of diffuse, protracted dysfunction, panic disorder with severe agoraphobia recalled a history of distress more than twice as long as the PD group.
Interestingly, whereas non-specific distress and interference reliably increased with agoraphobia severity, trait indices putatively specific to panic did not. Measures of interoceptive hypersensitivity were similarly elevated across patient groups, corresponding to their consistent heart rate acceleration during clinically-relevant imagery. In contrast, differences in startle potentiation clearly discriminated between patients with focused distress and those with high comorbidity and long disorder duration. These data recall Melzig et al. (28) who observed that although heart rate patterns were similar, non-depressed panic patients showed robust startle potentiation during threat of shock whereas panic patients with comorbid depression failed to do so. Similar response system discordance was observed by McTeague et al. (43) in depressed, generalized social phobia patients during aversive imagery. Given that the startle effectors are somatic, reduced potentiation may reflect psychomotor retardation and behavioral inhibition associated with comorbid negative affectivity, while augmented autonomic outputs from the defense system persist.
In the current sample, diminished startle reactivity was only evident in patients who endured the longest-standing disorder and the highest comorbid negative affectivity, whereas less chronic and broadly symptomatic patients demonstrated robust fear potentiation. Following contemporary learning theories of panic disorder (32,76), propensity for interoceptive and exteroceptive fear conditioning may initially result in heightened, overgeneralization of defensive responding. Grillon and colleagues have demonstrated that panic disorder in the absence of significant comorbid broad distress is characterized by fear-conditioned overgeneralization indexed by both subjective report and startle potentiation (29–32). Notably, in these samples the comorbidity rates and symptom measures (e.g., mean BDI range 6.9–11.3) were markedly lower than the comorbidly depressed panic patients in the Melzig et al. (28) sample and even the no agoraphobia group in the current study, suggesting that these hyper-reactive samples are predominantly a purer panic disorder.
Longitudinal examination is warranted to rule out the possibility that these are stable, time-invariant defensive profiles throughout the trajectory of panic disorder. However, the current findings encourage the speculation that in some patients focused panic disorder may evolve, as fear becomes progressively generalized and resistant to extinction (34), to agoraphobia and finally chronic stress and a comorbid constellation of negative affectivity, in which defensive action (startle) may be impaired. Animal research varying stressor duration (77–81) has provided insights that chronic anxiety and depression may relate to such somatic hyporeactivity. For example, rats exposed to brief and/or less threatening stress demonstrate hypervigilance and hyperarousal, whereas rats exposed to longer duration stress develop more generalized anxiety and depressive-like symptoms, including passivity and reduced movement and communication behaviors (79–81).
Epidemiological phenotypic and genotypic studies of anxiety and mood disorder comorbidity (63–68) have emphasized a single, core internalizing dimension comprised of two classes of disorders, fear and anxious-misery/distress. While in some investigations (63–65) panic disorder as well as agoraphobia appear most akin to the circumscribed fearfulness of specific phobias, in others (66–68) these disorders resemble the diffuse apprehension of GAD, PTSD and depression. Importantly in these epidemiological studies, the finer distinction of panic disorder with and without agoraphobia is often not considered. The current data, based on structured interview by clinicians, suggest that while chronic panic with agoraphobia may be more similar to distress disorders, the absence of agoraphobia may reflect a shorter duration, more focal fearfulness.
Finally, in the current research strong defensive startle reactivity characterized principal panic disorder, but was progressively diminished with agoraphobia, overall morbidity, and disorder duration. This pattern was previously observed in imagery investigations of social phobia (43) and PTSD (44), highlighting a fundamental dimension of anxiety spectrum pathology. Although these samples were phenotypically dissimilar in foremost clinical complaint (i.e., interpersonal apprehension, trauma-cue sensitivity, interoceptive hyperawareness) and associated principal disorder, symptom profiles suggesting more circumscribed fear showed startle potentiation specific to fear-relevant imagery, whereas at profound levels of broad negative affectivity and comorbidity, startle responses diminished to all aversive imagery contents. Taken together, startle potentiation during fear imagery is a meaningful endophentotype—a biomarker of internalizing symptomatology (82–83).
Conclusion
The psychophysiological and phenotypic profiles obtained here suggest that principal panic proneness may, at one extreme, prompt enhanced defensive startle reactivity in the absence of significant agoraphobia. However, once one has developed broad conditioned avoidance secondary to anxious anticipation of panic, the consequent stress of chronic hyperarousal and accompanying dysphoria may function over time to attenuate or even impair active defensive responding. As found in our previous studies of social phobia and PTSD, when an anxiety disorder transitions to a state of prolonged stress and broad comorbid negative affectivity, its biomarker is blunted fear-startle potentiation—implicating a compromised underlying fear-defense brain circuitry.
Supplementary Material
Acknowledgments
This work was supported in part by a National Institute of Mental Health grant (P50 MH 72850) to the Center for the Study of Emotion and Attention (CSEA), University of Florida, Gainesville, FL (P.J. Lang Director & Principal Investigator) and NRSA Research Fellowship (F31 MH069048) to the first author. Special thanks to Bruce N. Cuthbert for assistance with study design and implementation and critiques of manuscript drafts. Special thanks to the following individuals for their assistance in data collection: Cyd C. Strauss, Denise M. Sloan, Eleni Dimoulas, Greg Perlman, Bethany C. Wangelin, Joshua R. Shumen, and Hailey W. Bulls.
Footnotes
Similar to preceding studies (2–3), agoraphobia without a history of panic disorder was rarely diagnosed (N=1) in this sample and hence precluded analysis.
Although discussion continues concerning the diagnostic utility of agoraphobic avoidance versus distress (2–4), the 22 ratings of situational apprehension and avoidance were very highly correlated in the current sample, r=0.92, p<.001, and did not warrant separate analysis. Furthermore, convergent evidence that the defined groups reflected progressive increases in severity was observed in the agoraphobia subscale scores of the Fear Survey Schedule (Table 2). Relative to the control sample: PD, z=1.18; PDA moderate, z=3.54; PDA severe, z=4.54.
Similar to preceding studies (42–43), analyses for heart rate change were calculated on residuals secondary to removing the trial-specific baseline (1-second average prior to script onset) effects via linear regression.
Similar to other studies that endeavored to utilize the IIRS for assessing functional interference in lifestyle domains associated with mental as opposed to physical health conditions (60–61), the IIRS was worded: “How much do your mental health problems (e.g., anxiety, depression) and/or their treatment interfere with your?…”
For ease of comparison with the extant literature, the BDI and STAI scores were presented in conjunction with the MASQ Anxious Arousal and Anhedonia subscales. The other 3 MASQ subscales (Mixed Anxiety/Depression Symptoms, General Anxiety, General Depression) showed the same pattern across groups as the BDI and STAI and, not surprisingly, all five measures were highly intercorrelated, rs=0.73–0.89, ps<.001.
Consistent with preceding studies (69) none of the PDA patients recalled agoraphobia symptoms that preceded the development of recurrent panic attacks.
The PD group was younger than the PDA-moderate group, whereas the groups with the most pronounced differences in physiological and symptom profiles—PD and PDA-severe—did not differ in age.
Seventy-seven of the 112 patients indicated current use of medication for alleviating mental health symptoms. Most commonly, these medications were selective serotonin-reuptake inhibitors (SSRIs; PD 42.5%; PDA-moderate 44.1%; PDA-severe 32.4%, X2 (2) = 1.23, ns) and/or benzodiazepines (PD 50.05%; PDA-moderate 56.3%; PDA-severe 48.6%, X2 (2) = 0.44, ns), utilized equally across patient subgroups. The effects of these and less frequently endorsed compounds (e.g., beta blockers, 2.7%, tricyclics 1.9%, norepinephrine-dopamine reuptake inhibitors 1%) were assessed by comparing resting and imagery reactivity among the medicated and non-medicated patients. Considering either general psychotropic usage or more specific classes of drugs, no reliable effects emerged, consistent with prior psychophysiological studies of anxiety and depression (70–72). Reported usage of both prescription and over-the-counter medications for promoting physical health, as well as recreational substance use were also collected but low frequencies of endorsement precluded statistical analysis. As previously demonstrated (73), panic disorder patients were more likely than controls (6.6%) to be current smokers, but no differences were revealed between patient subtypes (PD 23.1%; PDA-moderate 15.6%; PDA-severe 24.3%; X2 (2) = 0.89, ns). Further, no physiological effects were observed comparing resting and imagery reactivity of smokers and non-smokers.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. (DSM-IV) [Google Scholar]
- 2.Wittchen HU, Gloster AT, Beesdo-Baum K, Fava GA, Craske MG. Agoraphobia: a review of the diagnostic classificatory position and criteria. Depress Anxiety. 2010;27:113–33. doi: 10.1002/da.20646. [DOI] [PubMed] [Google Scholar]
- 3.White KS, Barlow DH. Panic disorder and agoraphobia. In: Barlow DH, editor. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic. New York, New York: Guilford Press; 2002. pp. 328–379. [Google Scholar]
- 4.Schmidt NB, Cromer KR. Assessing the clinical utility of agoraphobia in the context of panic disorder. Depress Anxiety. 2008;25:158–66. doi: 10.1002/da.20285. [DOI] [PubMed] [Google Scholar]
- 5.Telch MJ, Brouillard M, Telch CF, Agras WS, Taylor CB. Role of cognitive appraisal in panic-related avoidance. Behav Res Ther. 1989;27:373–83. doi: 10.1016/0005-7967(89)90007-7. [DOI] [PubMed] [Google Scholar]
- 6.Uhlenhuth EH, Leon AC, Matuzas W. Psychopathology of panic attacks in panic disorder. J Affect Disord. 2006;92:55–62. doi: 10.1016/j.jad.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Andrews G, Slade T. Agoraphobia without a history of panic disorder may be part of the panic disorder syndrome. J Nerv Ment Dis. 2002;190:624–30. doi: 10.1097/00005053-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110:585–99. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- 9.Kessler RC, Chiu WT, Jin R, Ruscio AM, Shear K, Walters EE. The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2006;63:415–24. doi: 10.1001/archpsyc.63.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittchen HU, Nocon A, Beesdo K, Pine DS, Hofler M, Lieb R, Gloster AT. Agoraphobia and panic. Prospective-longitudinal relations suggest a rethinking of diagnostic concepts. Psychother Psychosom. 2008;77:147–57. doi: 10.1159/000116608. [DOI] [PubMed] [Google Scholar]
- 11.Slaap BR, den Boer JA. The prediction of nonresponse to pharmacotherapy in panic disorder: A review. Depress Anxiety. 2001;14:112–22. doi: 10.1002/da.1053. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3-revised. Washington, DC: American Psychiatric Press; 1987. (DSM-III-R) [Google Scholar]
- 13.Cox BJ, Endler NS, Swinson RP. An examination of levels of agoraphobic severity in panic disorder. Behav Res Ther. 1995;33:57–62. doi: 10.1016/0005-7967(93)e0029-5. [DOI] [PubMed] [Google Scholar]
- 14.Craske MG, Barlow DH. Mastery of Your Anxiety & Panic: Therapist Guide (Treatments That Work) 4. New York: Oxford University Press; 2006. [Google Scholar]
- 15.Foa E, Hembree E, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide (Treatments That Work) New York: Oxford University Press; 2007. [Google Scholar]
- 16.Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: Conceptual structure and pattern of somato-visceral response. Psychophysiology. 1980;17:179–92. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 17.Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: The problem of affective response integration. J Abnorm Psychol. 1983;92:276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- 18.Vrana SR, Lang PJ. Fear imagery and the startle-probe reflex. J Abnorm Psychol. 1990;99:189–97. doi: 10.1037//0021-843x.99.2.189. [DOI] [PubMed] [Google Scholar]
- 19.Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 20.Lang PJ, Davis M. Emotion, motivation, and the brain: Reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- 21.Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–70. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 23.Costa VD, Lang PJ, Sabatinelli D, Versace F, Bradley MM. Emotional imagery: Assessing pleasure and arousal in the brain’s reward circuitry. Hum Brain Mapp. 2010;31:1446–57. doi: 10.1002/hbm.20948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–5. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 25.Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, Hsieh FY, Lavori PW. Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: Results from a Department of Veterans Affairs Cooperative Study. J Consult Clin Psychol. 1998;66:914–23. doi: 10.1037//0022-006x.66.6.914. [DOI] [PubMed] [Google Scholar]
- 26.Bystritsky A, Maidenberg E, Craske MG, Vapnik T, Shapiro D. Laboratory psychophysiological assessment and imagery exposure in panic disorder patients. Depress Anxiety. 2000;12:102–8. doi: 10.1002/1520-6394(2000)12:2<102::AID-DA7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Tsao JC, Craske MG. Reactivity to imagery and nocturnal panic attacks. Depress Anxiety. 2003;18:205–13. doi: 10.1002/da.10091. [DOI] [PubMed] [Google Scholar]
- 28.Melzig CA, Weike AI, Zimmermann J, Hamm AO. Startle reflex modulation and autonomic responding during anxious apprehension in panic disorder patients. Psychophysiology. 2007;44:846–54. doi: 10.1111/j.1469-8986.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 29.Grillon C, Lissek S, McDowell D, Levenson J, Pine DS. Reduction of trace but not delay eyeblink conditioning in panic disorder. Am J Psychiatry. 2007;164:283–9. doi: 10.1176/ajp.2007.164.2.283. [DOI] [PubMed] [Google Scholar]
- 30.Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, Pine DS, Grillon C. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behav Res Ther. 2009;47:111–8. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michael T, Blechert J, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in panic disorder: Enhanced resistance to extinction. J Abnorm Psychol. 2007;116:612–7. doi: 10.1037/0021-843X.116.3.612. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders – Diagnostic Criteria for Research. Geneva: World Health Organization; 1993. [Google Scholar]
- 35.Andrews G, Slade T. Panic and agoraphobia: sources of dissonance between ICD-10 and DSM-IV. Int J Meth Psych Res. 2007;7:156–161. 7. [Google Scholar]
- 36.Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology. 2003;40:407–22. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- 37.Cook EW, 3rd, Melamed BG, Cuthbert BN, McNeil DW, Lang PJ. Emotional imagery and the differential diagnosis of anxiety. J Consult Clin Psychol. 1988;56:734–40. doi: 10.1037//0022-006x.56.5.734. [DOI] [PubMed] [Google Scholar]
- 38.McNeil DW, Vrana SR, Melamed BG, Cuthbert BN, Lang PJ. Emotional imagery in simple and social phobia: Fear versus anxiety. J Abnorm Psychol. 1993;102:212–25. doi: 10.1037//0021-843x.102.2.212. [DOI] [PubMed] [Google Scholar]
- 39.Weerts TC, Lang PJ. Psychophysiology of fear imagery: Differences between focal phobia and social performance anxiety. J Consult Clin Psychol. 1978;46:1157–9. doi: 10.1037//0022-006x.46.5.1157. [DOI] [PubMed] [Google Scholar]
- 40.Lang PJ, McTeague LM, Cuthbert BN. Fearful imagery and the anxiety disorder spectrum. In: Rothbaum BO, editor. Pathological Anxiety: Emotional Processing in Etiology and Treatment. New York: Guilford Press; 2005. pp. 56–77. [Google Scholar]
- 41.Lang PJ, McTeague LM, Cuthbert BN. Fear, anxiety, depression, and the anxiety disorder spectrum: A psychophysiological analysis. In: Treat T, Baker T, editors. Psychological Clinical Science: Recent Advances in Theory and Practice. Integrative Perspectives in Honor of Richard M. McFall) Mahwah, NJ: Lawrence Erlbaum Associates; 2007. pp. 167–195. [Google Scholar]
- 42.Lang PJ, McTeague LM. The anxiety disorder spectrum: Fear imagery, physiological reactivity, and differential diagnosis. Anxiety Stress Coping. 2009;22:5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: Generalization, comorbidity, and physiological reactivity. Biol Psychiatry. 2009;65:374–82. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: Trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry. 2010;67:346–56. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown TA, Barlow DH, DiNardo PA, Barlow DH. The Anxiety Disorder Interview Schedule for DSM-IV: Adult Version. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 46.Bradley MM, Lang PJ. Techical Report. D-1. Gainesville, FL: University of Florida; 2007. Affective Norms for English Text (ANET): Affective ratings of text and instruction manual. [Google Scholar]
- 47.Benson H. The relaxation response. New York: Morrow; 1975. [Google Scholar]
- 48.Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 49.Cook EW., 3rd . VPM reference manual. Birmingham, AL: 2000. [Google Scholar]
- 50.Globisch J, Hamm A, Schneider R, Vaitl D. A computer program for scoring reflex eyeblink and electrodermal responses written in Pascal. Psychophysiology. 1993;39:S30. [Google Scholar]
- 51.Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: A multivariate solution. Psychophysiology. 1987;24:479–86. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 52.Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: Application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000;96:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- 53.Martinez JM, Garakani A, Kaufmann H, Aaronson CJ, Gorman JM. Heart rate and blood pressure changes during autonomic nervous system challenge in panic disorder patients. Psychosom Med. 2010;72:442–9. doi: 10.1097/PSY.0b013e3181d972c2. [DOI] [PubMed] [Google Scholar]
- 54.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 55.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 56.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 57.Devins GM. Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. J Psychosom Res. 2010;68:591–602. doi: 10.1016/j.jpsychores.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 59.Sheehan PW. A shortened form of Betts’ questionnaire upon mental imagery. J Clin Psychol. 1967;223:380–89. doi: 10.1002/1097-4679(196707)23:3<386::aid-jclp2270230328>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 60.Beiling PJ, Rowa K, Summerfeldt LJ, Swinson RP. Factor structure of the Illness Intrusiveness Rating Scale in patients diagnosed with anxiety disorders. J Psychopathol Behav. 23:223–230. [Google Scholar]
- 61.Wuyek LA, Antony MM, McCabe RE. Psychometric properties of the panic disorder severity scale: clinician-administered and self-report versions. Clin Psychol Psychother. 2010 doi: 10.1002/cpp.703. in press. [DOI] [PubMed] [Google Scholar]
- 62.Angst J, Gamma A, Rössler W, Ajdacic V, Klein DN. Long-term depression versus episodic major depression: Results from the prospective Zurich study of a community sample. J Affect Disord. 2009;115:112–21. doi: 10.1016/j.jad.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 63.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–6. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 64.Slade T, Watson D. The structure of common DSM-IV and ICD-10 mental disorders in the Australian general population. Psychol Med. 2006;36:1593–600. doi: 10.1017/S0033291706008452. [DOI] [PubMed] [Google Scholar]
- 65.Vollebergh WA, Iedema J, Bijl RV, de Graaf R, Smit F, Ormel J. The structure and stability of common mental disorders: The NEMESIS study. Arch Gen Psychiatry. 2001;58:597–603. doi: 10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- 66.Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62:182–9. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 67.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 68.Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103:133–69. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 69.Craske MG, Barlow DH. A review of the relationship between panic and avoidance. Clin Psychol Rev. 1988;8:667–85. [Google Scholar]
- 70.Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early- and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41:433–40. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- 71.Forbes EE, Miller A, Cohn JF, Fox NA, Kovacs M. Affect-modulated startle in adults with childhood-onset depression: relations to bipolar course and number of lifetime depressive episodes. Psychiatry Res. 2005;134:11–25. doi: 10.1016/j.psychres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. J Abnorm Psychol. 2005;114:627–39. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- 73.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 74.Pillay SS, Gruber SA, Rogowska J, Simpson N, Yurgelun-Todd DA. fMRI of fearful facial affect recognition in panic disorder: The cingulate gyrus-amygdala connection. J Affect Disord. 2006;94:173–81. doi: 10.1016/j.jad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 75.Tuescher O, Protopopescu X, Pan H, Cloitre M, Butler T, Goldstein M, Root JC, Engelien A, Furman D, Silverman M, Yang Y, Gorman J, Ledoux J, Silbersweig D, Stern E. Differential activity of subgenual cingulate and brainstem in panic disorder and PTSD. J Anxiety Disord. doi: 10.1016/j.janxdis.2010.09.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- 77.Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–87. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci. 2010;30:15007–18. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Avgustinovich DF, Kovalenko IL, Kudryavtseva NN. A model of anxious depression: Persistence of behavioral pathology. Neurosci Behav Physiol. 2005;35:917–24. doi: 10.1007/s11055-005-0146-6. [DOI] [PubMed] [Google Scholar]
- 80.Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav Brain Res. 2005;162:127–34. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Dwivedi Y, Mondal AC, Payappagoudar GV, Rizavi HS. Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: Role in learned helplessness behavior. Neuropharmacology. 2005;48:204–14. doi: 10.1016/j.neuropharm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Insel TR, Cuthbert BN. Endophenotypes: Bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66:988–9. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 83.Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PS, Cuthbert BN. Developing constructs for psychopathology research: Research domain criteria. J Abnorm Psychol. 2010;119(4):631–9. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


